Abstract
Helicobacter pylori cagA–positive strains exert population-specific risks for gastric cancer. We determined whether variations in CagA phosphorylation motifs were associated with carcinogenic or proinflammatory epithelial phenotypes induced by strains from regions with divergent cancer risks (Colombia and Nashville, TN). Motif number was significantly related to levels of CagA phosphorylation and cytoskeletal abnormalities. Precancerous isolates possessed a higher number of motifs, and precancerous strains from Nashville induced higher levels of IL-8 than Colombian strains. These results indicate that CagA variants are linked with premalignant lesions in distinct populations and that epithelial responses to these strains are selective based upon locale.
Helicobacter pylori infection increases the risk for gastric adenocarcinoma, and one strain-specific virulence constituent that augments cancer risk is the cag pathogenicity island [1]. Several cag genes encode components of a type IV secretion system, and the terminal product of the cag island, CagA, is translocated into gastric epithelial cells following bacterial attachment. Intracellular CagA undergoes tyrosine phosphorylation within motifs containing the amino acid sequence EPIYA [2]. Phospho-CagA subsequently activates a eukaryotic phosphatase (SHP-2), leading to morphological changes (e.g., cellular elongation) that are reminiscent of unrestrained stimulation by growth factors.
Although cag-positive strains are disproportionately represented among hosts who develop cancer, most persons colonized by these strains remain asymptomatic [1]. This paradox has fostered the need for studies that more clearly define mechanisms underpinning the biological activities of CagA proteins that are present in different H. pylori strains. The number and type of CagA EPIYA motifs can vary substantially [3]. Motifs in strains harvested from persons residing in Western countries have been termed A, B, or C on the basis of sequences flanking the EPIYA motif. In contrast, phosphorylation sites in CagA proteins from East Asian H. pylori strains lack the EPIYA-C motif and, instead, contain a different motif, termed D [3]. In vitro, all types of CagA EPIYA motifs can be phosphorylated.
H. pylori strains possessing >3 EPIYA motifs are more highly associated with gastric atrophy, intestinal metaplasia, and gastric cancer, although this has not been replicated in all studies [4–7]. In vitro experiments have shown that the number of EPIYA motifs is associated with the intensity of CagA phosphorylation, cellular elongation, and induction of proinflammatory cytokines, such as IL-8 [6, 8]. We hypothesized that EPIYA motif variations in strains harvested from patients with divergent risks for gastric cancer are associated with epithelial morphological and inflammatory phenotypes that may lower the threshold for carcinogenesis, which may explain, in part, the varying risks for adenocarcinoma conferred by the cag locus.
Materials and methods
AGS human gastric epithelial cells were grown in RPMI medium 1640 (GIBCO/BRL) with 10% FBS (Hyclone) and 20 μg/mL of gentamicin (GIBCO/BRL) under 5% CO2 at 37°C. For Western immunoblot and IL-8 analyses, cells were plated at 5 × 105 cells/well in 6-well plates with 2 mL of culture medium. IL-8 was quantified in coculture supernatants by ELISA (R&D Systems). To assess cytoskeletal projections, cells were examined by microscopy 24 h following coculture with H. pylori. At least 100 cells in 5 randomly selected high-powered fields were examined, and the number of cells with needle-like protrusions >2 μm in length [6] was normalized to the total number of cells counted.
The 32 cag-positive clinical strains in this study were randomly selected from a larger population of isolates harvested from patients enrolled in ongoing prospective studies in Colombia (n = 16) or Nashville (n = 16) focused on H. pylori pathogenesis [9, 10]. A total of 588 and 306 patients have been enrolled to date in Colombia and Nashville, respectively. The prevalence of premalignant lesions (gastric atrophy or intestinal metaplasia) is 42% and 28% in the study populations from Colombia and Nashville, respectively. The estimated age-adjusted incidence rates of gastric cancer are 35.9 cases per 100,000 persons for males and 20.9 per 100,000 persons cases for females in Colombia and 7.6 cases per 100,000 persons for males and 4.8 cases per 100,000 persons for females in Nashville [11, 12]. Protocols were approved by the Universidad del Valle Ethics Committee or the Vanderbilt institutional review board, and informed consent was obtained from each patient. Three gastric biopsy specimens were obtained from the antrum, incisura angularis, and corpus for histological analysis [9, 10]. For culture, additional antral specimens were placed in 250 μL of PBS, homogenized, and incubated for 96 h under microaerobic conditions. For coculture experiments, H. pylori was grown in Brucella broth with 5% FBS for 18 h, harvested by centrifugation, and added to gastric cells at a ratio of 100 bacteria to 1 gastric cell.
For histological analysis, gastric tissues were fixed in buffered formalin and embedded in paraffin. Sections (thickness, 4 μm) were cut and mounted on ProbeOn-Plus slides (Fisher Scientific) for hematoxylin-eosin staining. Histopathological diagnoses were assessed in accordance with published guidelines [13], and subjects were stratified into those with nonatrophic gastritis and those with precancerous lesions (gastric atrophy and/or intestinal metaplasia).
The 3' end of cagA was amplified from genomic DNA and sequenced either at Vanderbilt University or Louisiana State University. Proteins (20 μg) from gastric cell lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Pall). Levels of total and phosphorylated CagA were determined using anti-CagA antibody (Austral Biologicals) or anti-pY99 antibody (1:300 [Santa Cruz]), respectively, and actin levels were determined using antiactin (C-11) antibody (1:500 [Santa Cruz]). Primary antibodies were detected using goat antirabbit (1:20,000 [Sigma]) or donkey antigoat (1:5000 [Sigma]) horseradish peroxidase-conjugated secondary antibodies, visualized by the ECL detection system (Cell Signaling) and quantified using the ChemiGenius system (Syngene). Densitometric analysis of multiple Western blots was then performed.
Mann-Whitney U and Student t tests were used for statistical analyses of intergroup comparisons. Statistical significance was defined as a P value of <.05.
Results and discussion
H. pylori cag–positive strains exert differing degrees of risk for gastric cancer in geographically distinct regions of the world. To define microbial constituents that may mediate such disparities, we investigated molecular variations within CagA among strains isolated from persons at high (Colombia) or low (Nashville) gastric cancer risk. All strains included in this study contained Western-type EPIYA motifs (A, B, and C), the number of which ranged from 2 to 5. Variation in motif number was solely dependent on differences in the number of C motifs for all strains except for a single Colombian strain, which contained an ABCBC motif sequence.
We first determined whether EPIYA motif number was related to premalignant lesions. To increase the statistical power of this analysis, strains containing 2 or 3 and 4 or 5 motifs were analyzed together. Among the 16 Colombian strains, 10 (63%) contained 3 motifs, 5 (31%) contained 4 motifs, and 1 (6%) contained 5 motifs. Of the 16 Nashville strains, 2 (12%) contained 2 motifs, 11 (69%) contained 3 motifs, and 3 (19%) contained 4 motifs. All 3 Nashville strains and 5 (83%) of 6 Colombian strains that possessed 4 or 5 motifs were isolated from persons with precancerous lesions, compared with only 6 (46%) of 13 Nashville strains and 4 (40%) of 10 Colombian strains with 2–3 motifs, respectively. For the entire group, 89% of strains that possessed 4 or 5 motifs were isolated from persons with precancerous lesions, compared with only 44% of strains with 2–3 motifs (P < .001). These results indicate that, in these populations, the number of phosphorylation sites mirrors the severity of gastric injury.
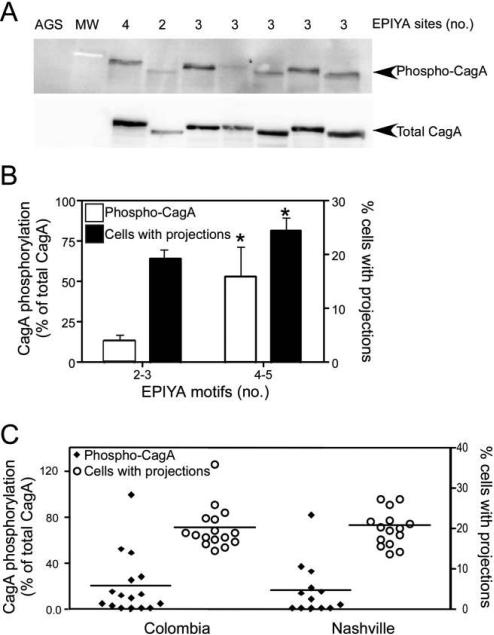
We next investigated whether an increased number of EPIYA motifs was concordant with heightened levels of intracellular CagA phosphorylation in vitro. As shown in figure 1A and 1B, the number of EPIYA motifs was significantly related to levels of CagA phosphorylation (P = .04).
Figure 1.
Relationship between EPIYA motif number, CagA phosphorylation, and epithelial cell morphology. A, AGS cells were cocultured with Helicobacter pylori cag–positive strains, and cell lysates were harvested and subjected to Western blot analysis using an anti–phosphotyrosine antibody or an anti-CagA antibody. A representative blot is shown. AGS, cells incubated with medium alone. B, Levels of CagA phosphorylation and epithelial morphologic aberrations induced by H. pylori, segregated by EPIYA motif number. Left axis, densitometric analysis of multiple Western blot repetitions performed on at least 2 occasions per strain. Levels of phospho-CagA were normalized to total CagA. Right axis, the number of cells with cytoskeletal projections was normalized to the total number of cells counted. H. pylori strains were stratified by number of CagA EPIYA motifs as determined by sequencing. Error bars, SEM. *P < .05 for comparison of strains with 4–5 EPIYA motifs versus those with 2–3 EPIYA motifs for both level of CagA phosphorylation (white columns) and the percentage of cells with projections (black columns). C, Levels of CagA phosphorylation (left axis) and epithelial morphologic abnormalities (right axis) induced by H. pylori, stratified by geographic region of origin. Mean values are shown within scatterplots.
Cellular morphological alterations induced by phospho-CagA include the development of cytoskeletal projections [2]. Therefore, we compared the morphological characteristics of AGS cells following incubation with the 32 cag-positive strains. Similar to CagA phosphorylation, a significantly greater number of cells infected with strains that possessed higher numbers of EPIYA motifs developed cytoskeletal projections (figure 1B). Of interest, when we stratified strains by low-risk and high-risk region, there were no differences in the levels of CagA phosphorylation or the numbers of cytoskeletal projections (figure 1C), indicating that other factors may influence differences in gastric cancer rates observed in these 2 locales.
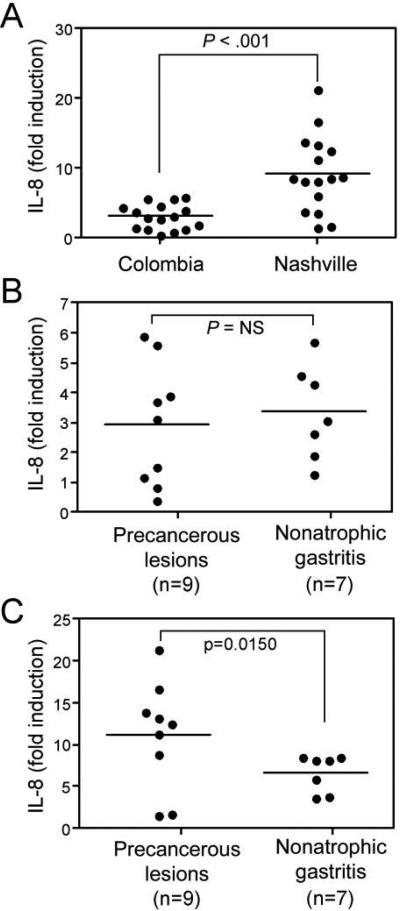
The inflammatory response that develops in response to H. pylori infection is an important mediator of gastric carcinogenesis [1, 2, 14]. Because our epithelial morphological findings did not distinguish between strains harvested from regions with low or high gastric cancer rates (figure 1C) and because recent data have indicated that EPIYA motif number may be related to levels of production of the proinflammatory cytokine IL-8 [8], we next quantified IL-8 levels in coculture supernatants. Levels of IL-8 were significantly higher following infection with strains isolated from Nashville patients, compared with those from Colombian patients (figure 2A).
Figure 2.
IL-8 production is related to cancer precursor lesions in strains harvested from Nashville but not from Colombia. A, AGS cells were cocultured with Helicobacter pylori cag–positive strains, supernatants were harvested, and levels of IL-8 were determined by ELISA. Results are segregated by geographic locale of infecting H. pylori strains. Mean values are shown within scatterplots. B and C, levels of IL-8 induced by strains from Colombia (B) or Nashville (C) stratified on the basis of precancerous lesions or nonatrophic gastritis. Mean values are shown within scatterplots.
To define the specific factors that may account for these differences, we analyzed levels of IL-8 induced by strains from Colombia or Nashville that were segregated on the basis of histologic lesions. There were no differences in IL-8 production induced by Colombian strains harvested from persons with or without premalignant lesions (figure 2B). In contrast, among Nashville strains, levels of IL-8 were significantly higher following coculture with precancerous strains versus strains associated with gastritis alone (P = .015) (figure 2C). These results suggest that proinflammatory responses differ among H. pylori strain populations and that such responses may exert a stronger influence on the development of intestinal metaplasia and atrophy among persons residing in the United States versus persons from Colombia.
We recognize, however, that CagA phosphorylation is not the singular event in gastric carcinogenesis. Unphosphorylated CagA leads to disruption of apical-junctional complexes, a loss of cellular polarity, and activation of β-catenin, alterations that may also play a role in carcinogenesis [2, 14, 15]. Polymorphisms within host immune response genes heighten the risk for distal gastric adenocarcinoma among H. pylori–infected persons [14]. The risk of gastric carcinoma is also influenced by environmental factors. For example, one environmental factor that has been uniformly associated with an increased risk of gastric cancer is high dietary salt intake. Our results, which demonstrate that higher levels of IL-8 are induced by isolates from a region (Nashville) where cancer risk is lower, suggest that, in certain populations from regions such as Colombia, environmental factors may play a more prominent role than microbial factors in the carcinogenic cascade. Studies that provide more insight into the pathogenesis of H. pylori–induced gastric adenocarcinoma are important, not only to develop more-effective treatments for this common cancer, but also because this infection might serve as a paradigm for the role of chronic inflammation in the genesis of other malignancies that arise in the gastrointestinal tract.
Acknowledgments
Financial support: National Institutes of Health (grants DK 73902, CA 77955, and DK 58587 to R.M.P.; grant CA 028842 to P.C.; and grant DK 058404 to M.B.P.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Digestive Diseases Week, San Diego, CA, May 2008.
References
- 1.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Wessler S, Backert S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 2008;16:397–405. doi: 10.1016/j.tim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99:14428–33. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka Y, El-Zimaity HM, Gutierrez O, et al. Relationship between the cagA 3' repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342–9. doi: 10.1053/gast.1999.0029900342. [DOI] [PubMed] [Google Scholar]
- 5.Azuma T, Yamakawa A, Yamazaki S, et al. Correlation between variation of the 3' region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J Infect Dis. 2002;186:1621–30. doi: 10.1086/345374. [DOI] [PubMed] [Google Scholar]
- 6.Argent RH, Kidd M, Owen RJ, Thomas RJ, Limb MC, Atherton JC. Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology. 2004;127:514–23. doi: 10.1053/j.gastro.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Rota CA, Pereira-Lima JC, Blaya C, Nardi NB. Consensus and variable region PCR analysis of Helicobacter pylori 3' region of cagA gene in isolates from individuals with or without peptic ulcer. J Clin Microbiol. 2001;39:606–12. doi: 10.1128/JCM.39.2.606-612.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argent RH, Hale JL, El-Omar EM, Atherton JC. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between western and East Asian strains, and influences on interleukin-8 secretion. J Med Microbiol. 2008;57:1062–7. doi: 10.1099/jmm.0.2008/001818-0. [DOI] [PubMed] [Google Scholar]
- 9.Bravo LE, van Doorn LJ, Realpe JL, Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? Am J Gastroenterol. 2002;97:2839–42. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 10.Peek RM, Jr, Blaser MJ, Mays DJ, et al. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–31. [PubMed] [Google Scholar]
- 11.Pineros M, Ferlay J, Murillo R. Cancer incidence estimates at the national and district levels in Colombia. Salud Publica Mex. 2006;48:455–65. doi: 10.1590/s0036-36342006000600003. [DOI] [PubMed] [Google Scholar]
- 12.Tennessee Cancer Registry [20 October 2008];Total cancer cases and age-adjusted cancer incidence rates, stomach, 1999–2003. Available at: http://health.state.tn.us/TCR.
- 13.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system. International Workshop on the Histopathology of Gastritis. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–23. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Franco AT, Israel DA, Washington MK, et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]