Abstract
The ventral tegmental area (VTA) plays a critical role in motivated behavior. However, it remains unclear whether intact VTA function is necessary for motivated behavior to seek contexts repeatedly paired with natural stimuli and/or pharmacological stimuli. In the present study, conditioned place preference (CPP) was induced using highly salient natural or drug stimuli attributed with strong incentive-motivational value in each of two female models: postpartum females were conditioned to associate one unique context in the CPP apparatus with young offspring (pups) and a second context with a neutral stimulus, and virgin females were conditioned to associate unique contexts with cocaine (5mg/kg i.p.) and saline injections. Immediately prior to CPP testing, each female received a microinfusion of bupivacaine bilaterally into the VTA to transiently inactivate the region; subjects were also tested following saline microinfusion into the VTA. Postpartum females’ preference for the pup-paired context was abolished by VTA inactivation but was restored to high control levels following saline microinfusion. In separate tests, VTA inactivation also reduced motivated pup licking and pup retrieval in postpartum females, suggesting that intact VTA function is required for the expression of both pup CPP and motivated pup-directed behaviors. Cocaine CPP remained unaffected by VTA inactivation. Locomotion was not affected by VTA microinfusions but was severely impaired by bupivacaine microinfusions into the substantia nigra. We conclude that the VTA is differentially involved in the expression of conditioned preference for contexts paired with pups, a salient natural stimulus, and contexts paired with cocaine.
Keywords: conditioned place preference, ventral tegmental area, inactivation, maternal, cocaine
Introduction
Extensive research has provided important insights into the unique motivational state of the female mammal. In rats, as well as virtually every other mammalian species, the female expresses remarkable motivation to seek out and interact with unique natural stimuli, such as offspring, during her natural lifecycle (Lee et al., 2000; Seip and Morrell, 2008; Wansaw et al., 2008). Female rats are also highly responsive to pharmacological stimuli, such as cocaine, and are consistently more motivated to seek out and consume drugs of abuse compared to their male counterparts (Lynch and Taylor, 2004; Becker and Hu, 2008; Seip et al., 2008). However, limited research has explored how discrete components of the extended neural circuitry participate in females’ motivation to seek out natural and/or drug stimuli and the contexts that predict them.
Motivated behavior directed toward natural and drug stimuli is mediated by an extended, overlapping neural circuitry that includes the ventral tegmental area (VTA) and its ascending mesocorticolimbic projections (Ikemoto and Panksepp, 1999; Carelli et al., 2000; Carelli and Ijames, 2001; Carelli, 2002, 2004; Phillips et al., 2003b; Roitman et al., 2004; Wise, 2004; Yun et al., 2004; Salamone et al. 2005, 2007; Fields et al., 2007; Berridge 2007). Subpopulations of VTA neurons respond to appetitive stimuli attributed with positive incentive value (Kiyatkin and Rebec, 1997; Brodie et al., 1999) and to cues or contexts associated with appetitive stimuli (Fields et al., 2007; Sombers et al., 2009). Disrupting VTA function, conversely, impairs an animal’s ability to acquire and express effortful stimulus-seeking behavior directed toward stimulus-predictive cues (Yun et al., 2004; Zellner et al., 2009). It is thus likely that intact VTA function is required for animals to seek out contexts associated with appetitive natural and/or drug stimuli.
Well-established research indicates that the VTA participates in animals’ behavioral and physiological responses to a highly appetitive drug, cocaine, and to the expression of cocaine-seeking behavior. Both cocaine and cocaine-seeking behavior can rapidly alter VTA activity (Einhorn et al., 1988; Le Foll et al., 2002; Febo et al., 2004; Borgland et al., 2004) and transiently increase accumbal dopamine (DA) via VTA-dependent mechanisms (Carelli et al., 2000; Carelli and Ijames, 2001; Carelli, 2002, 2004; Sombers et al., 2009). Both operant and conditioned place preference (CPP) responses to cocaine rely on ascending DAergic projections originating in the VTA (Tzschentke, 2000; Marinelli and White, 2000; Harris and Aston-Jones, 2003; Beninger and Gerdjikov, 2004; Ishikawa et al., 2008; Sombers et al., 2009), suggesting that cocaine’s incentive value involves intact VTA function.
The VTA also participates in the performance of motivated behavior directed toward natural stimuli, including offspring (Fahrbach et al., 1986; Numan and Insel, 2003; Numan and Stolzenberg, 2008, 2009). Young pups elicit strongly motivated responses from maternal females: females will bar-press at high rates for pups (Lee et al., 2000) and prefer a pup-paired chamber over neutral or even cocaine-paired chambers (Fleming et al., 1994; Mattson et al., 2001; Seip and Morrell, 2007, 2008; Wansaw et al., 2008). Not surprisingly, the VTA of maternal females is activated by exposure to young pups and their associated sensory stimuli (Lin et al., 1998; Komisaruk et al., 2000; Febo et al., 2005; Hernández-González et al., 2005b) and during the expression of maternal behavior toward pups (Hernández-González et al., 2005a). Intact DAergic transmission from the VTA is required for females to express maternal behavior (Hansen et al., 1991) and accumbal DA is transiently elevated during pup licking and retrieval (Hansen et al., 1993; Champagne et al., 2004; Afonso et al., 2008), likely via DAergic projections from the VTA. The incentive value of pups is also mediated, at least in part, by DA, as preference for a pup-paired context can be disrupted by blocking DA systemically (Fleming et al., 1994). We posit that the VTA is required for a female to seek out a pup-paired context; to date, no work has directly assessed this hypothesis.
The present study uses a conditioned place preference (CPP) paradigm to assess females’ motivation to seek out contexts paired with salient natural (pup) or drug (cocaine) stimuli (Tzschentke, 1998, 2007). To deliberately simplify our methodology, postpartum females were conditioned and tested for pup CPP and virgin females for cocaine CPP. Although virgin females will express maternal behavior if given prolonged exposure to young pups (Seip and Morrell, 2008), we chose to assess pup CPP in postpartum females, which provided the most simple and naturally occurring maternal model. As virgin and postpartum females also express identical cocaine CPP across a wide range of doses (0.5–10mg/kg i.p., Seip et al., 2008; Pereira and Morrell, unpublished data), we selected virgin females as the most straightforward population in which to examine cocaine CPP and to provide highly translatable data across female groups.
In the present study, females learned to associate one unique cue-decorated CPP chamber (i.e., context) with either pups or cocaine and a second unique context with a neutral stimulus. As this associative learning occurs rapidly in females (Russo et al., 2003), CPP provides a useful measure of motivated behavior when females’ postpartum state and pups’ development are both progressing (Wansaw et al., 2008). During the CPP test, each female was allowed to freely access each stimulus-paired context for 1hr. As primary stimuli were absent, the time that a female spent in each stimulus-paired context reflected her preference for the context, i.e., motivation to seek out (or avoid) the context.
To assess whether intact VTA function was required for the expression of pup and/or cocaine CPP, each female received a bilateral microinfusion of bupivacaine into the VTA immediately prior to CPP testing. This site-specific inactivation lasted for the duration of CPP testing (Coyle and Sperelakis, 1987; Tucker and Mather, 1998). Females were also tested for CPP when VTA function was intact (i.e., following saline microinjections). As guide cannulae were implanted prior to the start of the CPP paradigm and microinjections were not administered during CPP conditioning, conditioning (i.e., learning to associate a stimulus with a unique CPP context) occurred with an intact and fully functional VTA. Then, at CPP test, the VTA was transiently inactivated to determine whether the VTA was critically involved in the expression of conditioned preference for contexts paired with these natural and/or drug stimuli.
Given the role of the VTA in maternal behavior (Numan and Insel, 2003), we also inactivated the VTA via bupivacaine microinfusions prior to testing postpartum females’ maternal behavior. To our knowledge, this is the first study to assess whether intact VTA function is required for the expression of preference for a pup-paired context and, in separate tests, the expression of maternal behavior.
Bupivacaine transiently inactivates neurons at the discrete microinjection site by reversibly blocking voltage-gated sodium (Na+) channels (Hille, 1966; Tucker and Mather, 1998). Like the structurally similar amide anesthetic lidocaine (Sandkühler et al., 1987; Martin and Ghez, 1999; Tehovnik and Sommer, 1997; Boehnke and Rasmussen, 2001; Pereira de Vasconcelos et al., 2006), bupivacaine depresses neuronal firing within 2–5min post-injection but lasts up to 75min (Coyle and Sperelakis, 1987; Tucker and Mather, 1998; Fukuda et al., 2005), providing a sufficient time window for testing a range of complex behaviors (e.g., CPP, maternal behavior) before recovery of neuronal function. Importantly, transient inactivation also minimizes long-term compensatory neural and behavioral adaptations induced by permanent lesions (e.g., Luhmann, 1996). Given the heterogeneity of VTA microcircuitry (Fields et al., 2007), inactivation of the entire VTA is the first step in determining whether the VTA is critical to an individual’s motivation to seek out contexts paired with a natural stimulus (pups) and/or cocaine.
Methods
Animals
Subjects (n=78) were 90–120 day old female Sprague-Dawley rats raised in a colony maintained at the Laboratory Animal Facility at Rutgers University (Newark NJ), which is accredited by the American Association for Accreditation of Laboratory Animal Care. Subjects were moved into a quiet testing suite two days prior to the experiment and were handled daily. Virgin females (n=37) were sexually naïve. Females’ estrus stage was not identified, as vaginal lavage can elicit CPP and attenuate female’s motoric response to cocaine (Walker et al., 2002). Previous CPP studies from our laboratory consistently reveal low variability in CPP responses in normally cycling females that were not tested for estrus stage (see Seip et al., 2008; Seip and Morrell 2008). Postpartum females (n=41) were primiparous and remained with their culled, eight-pup litter after parturition. Subjects were housed individually in opaque shoebox cages (25.5cm W × 47cm L × 23cm H) with nest material and ad lib food and water, with a 12hr:12hr light:dark cycle (lights on at 0700). All subjects remained healthy and pups developed normally across the experiment. Separate groups of subjects were implanted with bilateral cannulae (postpartum n=22; virgin n=25) or remained non-surgical controls (postpartum n=19; virgin n=12).
Cannula implantation
Subjects were anesthetized with an intraperitoneal injection of ketamine hydrochloride (76.9mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA), xylacine (7.69mg/ml; Lloyd Laboratories, Shenandoah, IA) and acepromazine maleate (1.54mg/ml; AmTech Group, Inc., St. Joseph, MO), at a dose of 0.09ml/100g body weight. When surgical anesthesia was reached, the scalp was shaved, scrubbed repeatedly with Betadine, and a 0.5% marcaine solution was administered (0.5ml s.c.; Hospira, Inc., Lake Forest, IL) before the subject was secured into a stereotaxic apparatus using non-rupture ear bars (David Kopf Instruments, Tujunga, CA) and bite bar set at +/− 3.3mm. Bilateral stainless steel guide cannulae (22 gauge; Plastics One, Inc., Roanoke, VA) were aimed 1.5mm dorsal to VTA (flat skull; AP −5.6mm from Bregma, +/−1.0mm lateral from midline, and −7.0mm ventral from skull surface and cannula pedestal) and secured to the skull using stainless steel screws and dental cement. Coordinates were obtained from Paxinos and Watson (1986). Dummy stylets were inserted through guide cannulae to preserve patency. Surgery occurred on postpartum day 1 in postpartum females. Subjects recovered for a minimum of two days before CPP conditioning began. All subjects gained weight normally, displayed no signs of inflammation or infection at the surgical site, and expressed normal behavior following surgery.
Conditioned place preference
Apparatus
The custom-designed conditioned place preference apparatus consisted of three equal-sized clear Plexiglas chambers (27.5cm W × 21cm L × 20.5cm H). Each side chamber contained distinct striped wallpaper and tactile flooring, which together formed a context unique to that chamber. The center chamber had white walls and a solid grey floor, and was connected to the two side chambers by doors that could be closed manually (Seip and Morrell, 2007, 2008; Seip et al. 2008).
Chamber preference criteria
Stringent quantitative criteria were used to identify whether each individual subject preferred one of the CPP chambers paired with a primary stimulus (i.e., stimulus-paired context) (Seip and Morrell 2007, 2008; Seip et al. 2008). To prefer a stimulus-paired context, a subject must spend at least 30min in one chamber and 25% more time in that chamber than any other chamber. Subjects failing to meet chamber preference criteria were categorized as having no preference. To allow direct comparisons with established CPP literature (Tzschentke, 1998, 2007), the mean time spent in each chamber, averaged across all subjects, is also presented.
Pre-conditioning baseline session
Each female was placed into the center chamber and allowed to freely access all three chambers for 60min, one day prior to conditioning.
Conditioning phase
Subjects were conditioned to associate each uniquely decorated side chamber (i.e., context) with an unconditioned stimulus once a day for four consecutive days. Unconditioned stimuli were selected based on an established history of eliciting strong CPP in each group (Seip et al., 2008; Seip and Morrell, 2008), with cocaine or pups assigned to the side chamber that was least preferred during pre-conditioning.
Virgin females
Conditioning stimuli were intraperitoneal (i.p.) injections of a cocaine (5mg/kg) or saline solution. Cocaine hydrochloride (National Institute of Drug Abuse, Research Triangle Park, NC) was freshly dissolved in a 0.9% saline solution at a 5mg/kg dose, calculated as a salt; this dose establishes strong CPP in both virgin and postpartum females (Seip et al., 2008; Pereira and Morrell, unpublished data). At 1000, females were injected with saline and confined to one cue-decorated chamber for 30min (saline-paired context), then returned to their homecages. At 1200, females were injected with cocaine and confined to the opposite cue-decorated chamber for 30min (cocaine-paired context).
Postpartum females
Conditioning stimuli were pups that were age-matched to the postpartum day of the parturient females [postpartum day (PPD) 4–7 on conditioning days 1–4, respectively]. At 0945, all pups were removed from females’ homecages and placed in small boxes adjacent to each homecage, so that females could smell and hear pups but not interact physically with them (details in Seip and Morrell, 2007, 2008). At 1000, females were confined to one cue-decorated apparatus chamber that contained no unconditioned stimuli (empty context) for 1hr. Females were returned to their pup-less homecages for 1hr. At 1200, five pups were placed into the opposite cue-decorated apparatus chamber and females were confined to that chamber for 1hr (pup-paired context), allowing sufficient time for maternal interactions (Fleming et al., 1994; Seip and Morrell, 2008) with pups that were needy of maternal care (Pereira and Ferreira, 2006).
Post-conditioning test session
Females were tested for conditioned preference for each context on the day after the final conditioning session (e.g., PPD8 for postpartum females). All females were handled and lightly restrained in the experimenter’s lap for 5min on the day before CPP testing to habituate females to the infusion procedures used on the test day. One hour prior to CPP testing, all pups were removed from postpartum females’ homecages and placed in small boxes adjacent to their homecages.
Microinfusions of bupivacaine and saline
Immediately before CPP testing, females were given a bilateral microinfusion of 2% bupivacaine (in 0.9% saline) or saline. Microinfusions were made via bilateral injector cannulae that projected 1.5mm from the tip of the guide cannulae (8.5mm from pedestal; 28 gauge, Plastics One, Inc., Roanoke, VA). Infusions of 0.5µl/side/min were performed using 10µl gastight syringes (1800 Series, Hamilton, Reno, NV) connected to a Harvard infusion pump. The injector cannulae were left in place for 1min after infusions ended. Females were then promptly placed into the center CPP chamber and allowed free access to all chambers for 60min. Unconditioned stimuli (pups or injections) were not present. Females were retested on the next day with the alternate microinfusion (bupivacaine or saline) in a counterbalanced design. Microinfusion order did not elicit any CPP differences, so data were pooled.
Surgical and intact controls
Females with immovable stylets (i.e., surgical controls) and intact controls were lightly restrained for 5min prior to CPP testing to mimic infusions.
After CPP testing ended, all females and pups were returned to their homecages. A positive conditioning effect was identified if, within each group, mean chamber times changed from pre- to post-conditioning. In females given microinfusions, the saline CPP test was used as post-conditioning data.
Locomotion
Locomotion was measured within the CPP apparatus via infrared beams that traverse the chamber floors. New beam breaks were automatically pooled into 5min bins and divided by the time (in seconds) spent in the chamber during those 5min, resulting in a locomotor rate. Locomotor rates during each CPP test session were compared to confirm that microinfusions did not impair subjects’ motor activity.
Maternal behavior testing
In a time frame independent of CPP conditioning and testing, homecage maternal behavior was observed and scored in a subpopulation of VTA (n=9) and intact (n=3) postpartum females on PPD7–8. Behavioral testing commenced at least 3hrs after postpartum females were reunited with pups in their homecages following CPP. One hour prior to behavioral testing, pups were removed from females’ homecages and placed in small boxes adjacent to each homecage (see Seip and Morrell, 2008). VTA females received a microinfusion of bupivacaine or saline (as described above) and were promptly returned to their homecages. Intact controls (PPD8) were handled and lightly restrained for 5min before being returned to their homecages. Eight pups were scattered around each female’s homecage and behavior was observed for 15min. The female’s latencies to retrieve her first pup and all eight pups to her nest, hover over pups, and assume a low crouch position over her pups were recorded. Frequency of anogenital and corporal licking of pups, carrying a pup by mouth to non-nest locations and non-maternal behaviors (sniffing air, self-grooming, eating/drinking, resting, sleeping) were recorded every 10sec. Behavioral criteria are as previously defined (Seip and Morrell, 2008).
Histology
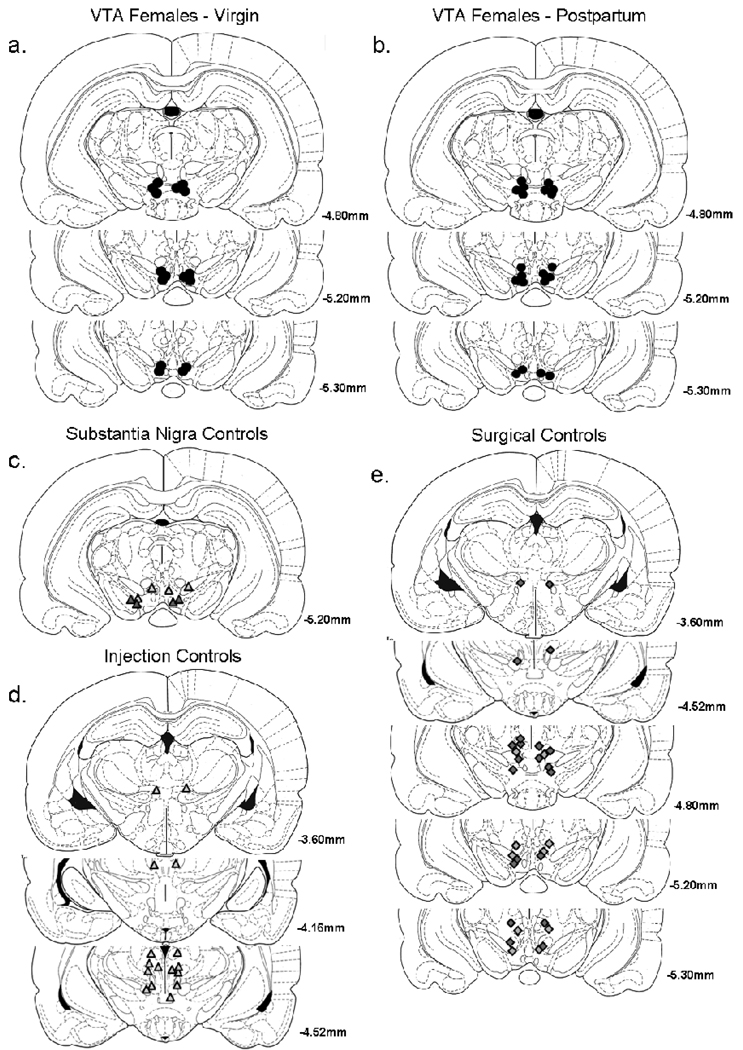
Subjects were deeply anesthetized with Nebutal (1ml) and intracardially perfused with 4% formalin. Brains were removed, sectioned at 30µm using a Bright-Hacker cryostat, and mounted on chrom-alum coated slides. Alternate sections were stained with Cresyl Violet to identify cannulae location, based on neuroanatomy described by Paxinos and Watson (1986), and rinsed with a series of distilled H20 and alcohol washes before coverslipping. Tissue located past the injector cannulae tips did not show signs of lesion or scarring, indicating that injected volumes of solution did not permanently damage tissue. Anatomical location of cannulae. The most caudal tips of injector cannulae marked the presumed anatomical location of microinfusions. Females receiving injections were separated into groups by anatomical location of injector cannulae termination: bilaterally in the VTA (VTA females), unilaterally within or in close proximity to the substantia nigra (Substantia nigra controls), or bilaterally rostrally and/or dorsally to VTA (Injection controls). Additional groups were formed by females with stylets that could not be removed from guide cannula for microinjections (Surgical controls) and by non-surgical females (Intact controls). See Figure 1.
Figure 1.
Histology. The placement of injector tips of bilateral cannulae in individual virgin (a) and postpartum (b) females with cannulae aimed at the ventral tegmental area (i.e., VTA females) or control females with cannulae aimed at the substantia nigra (Substantial Nigra controls; c), dorsally/rostrally to the VTA (i.e., Injection controls; d), and that had unmovable stylets (i.e., Surgical controls; e). Approximate stereotaxic coordinates, represented as millimeters posterior to Bregma, are listed to the right.
Statistics
Statistical analyses were performed using SPSS or manually (Siegel, 1956; Bruning and Kintz, 1987) with P<0.05 as significance level. Chamber preferences are presented as the percentage of individual subjects meeting criteria for each of four preference categories (preference for either stimulus-paired side chamber, center chamber, or no preference). Chamber times (averaged across subjects), locomotor rates and behavior scores are presented as means and standard errors of the mean (SEMs). A Levine’s test for equality of variance and Kolmogorov-Smirnov test preceded all parametric tests; non-parametric tests were used as needed. Within-groups. Pre- and post-conditioning preferences were compared using a chi-square goodness of fit test for specified proportions or a one-tailed test for significance of a proportion. Pre- and post-conditioning times were compared using two-way ANOVAs (chamber and session as repeated measures) and select t-tests. Locomotor rates were compared using two-way ANOVAs (infusion and time as repeated) and select t-tests. Maternal behavior scores were compared using Wilcoxon signed ranks (T) tests. Between-groups. Chamber preferences were compared using one-tailed tests of significance of difference between two proportions. Chamber times and locomotor rates within a session were compared using two-way ANOVAs (chamber or time/day as repeated, respectively) and select t-tests. Maternal behavior scores were compared using Mann-Whitney U tests.
Results
Verification of anatomical location of cannulae
VTA females
The tips of injector cannulae terminated bilaterally in the VTA and microinfusions were patent in eight virgin and ten postpartum females (Figure 1a–b).
Substantia nigra controls
In two postpartum and two virgin females, injector cannulae terminated unilaterally within or in close proximity to the substantia nigra (SN; Bregma −5.20mm) and unilaterally in the VTA (Figure 1c). In these SN females, bupivacaine promptly produced severe locomotor impairment, confirming that even unilateral inactivation of a discrete non-VTA site produced observable changes in behavior. No other group of females displayed these locomotor impairments, indicating that, in VTA females, diffusion was spatially restricted and did not extend into the SN. As SN females were unable to move between CPP chambers and remained in the center chamber during the first 15min of the CPP test session, they were omitted from all statistical analyses.
Control females
Non-surgical females (Intact controls), females that had immovable stylets and could not receive microinjections (Surgical controls) and females receiving bilateral injections rostrally and/or dorsally to the VTA (Injection controls) all expressed statistically identical chamber preferences and times during CPP testing. Data from these three groups (now referred to as Control females) were pooled for subsequent analyses.
1. Intact controls
Nineteen postpartum and twelve virgin females provided standard baseline data confirming that conditioning stimuli induced measurable CPP, as expected (Seip et al., 2007; Seip and Morrell, 2008).
2. Surgical controls
In ten postpartum and seven virgin females, guide cannula terminated dorsally and/or rostrally to the VTA but stylets could not be removed for microinjections (Figure 1e). Data from this group confirmed that cannulation surgery (without microinfusions) did not affect the acquisition or expression of CPP.
3. Injection controls
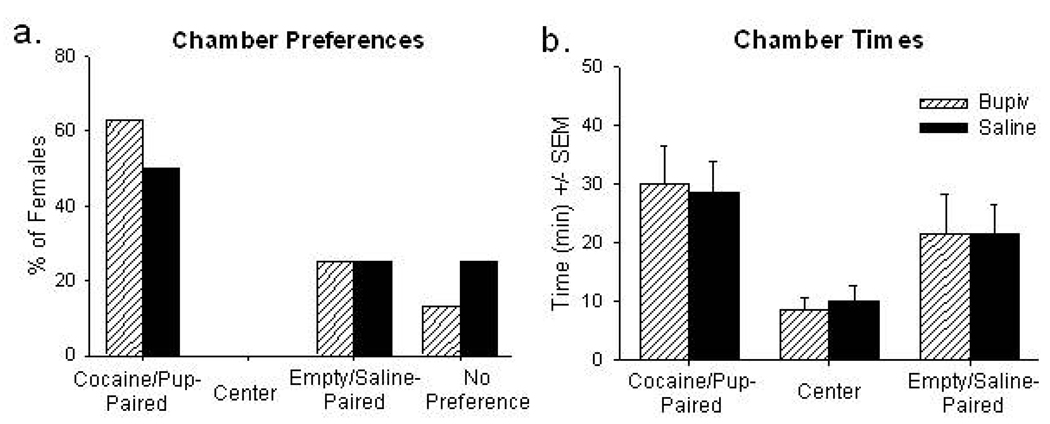
Injector cannulae terminated rostrally and/or dorsally to VTA in seven females (Figure 1d). Females’ chamber preferences and times (i.e., CPP) were identical after bupivacaine and saline microinfusions (Figure 2), confirming bupivacaine microinjections into discrete non-VTA sites do not affect CPP. For simplicity, females’ CPP data after saline microinfusions were used for pooled statistical analyses (above).
Figure 2.
Conditioned preferences (a) and times (b) for each stimulus-paired context following bupivacaine (striped bars) and saline microinfusions (solid bars) in Injection controls, in which cannulae terminated dorsally/rostrally to the ventral tegmental area.
Conditioned place preference
Pre-conditioning responses and effect of conditioning
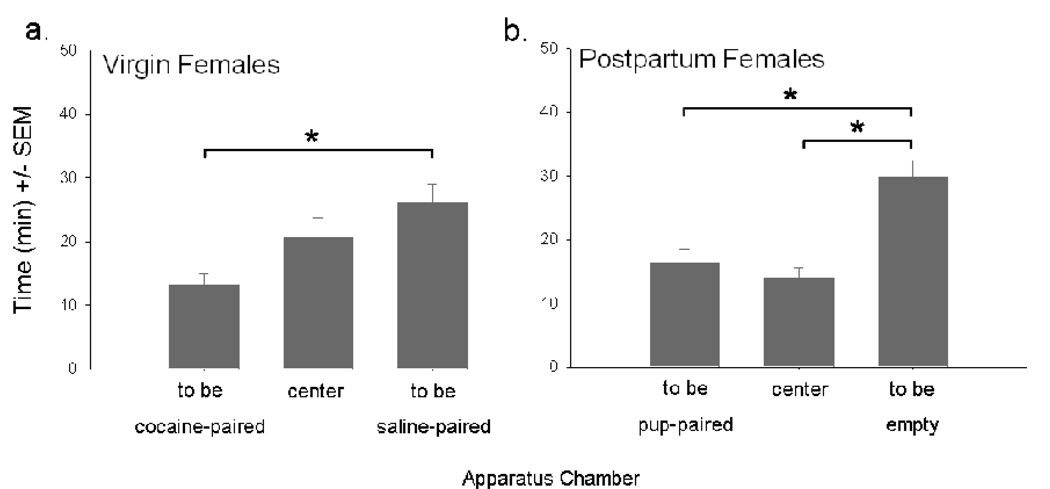
Virgin females spent the least amount of time in the context to be paired with cocaine [compared to the saline-paired context: t(36)=−3.43, P<0.01] and postpartum females spent the least amount of time in the context to be paired with pups [compared to center: t(40)= −4.19; to saline: t(40)=−3.04; both P<0.01] (Figure 3). A positive effect of conditioning was confirmed in all virgin females [VTA females: F(2,14)=3.22, P=0.07; controls: F(2,52)=10.98, P<0.001] and in VTA postpartum females [F(2,18)=4.65, P<0.05].
Figure 3.
Mean time (min) spent in each stimulus-paired context of the place preference apparatus during the pre-conditioning session by virgin (a) and postpartum (b) females. P<0.05 for all within-groups (*) comparisons.
Bupivacaine microinfusions into the VTA disrupt the expression of conditioned preference for a pup-paired context
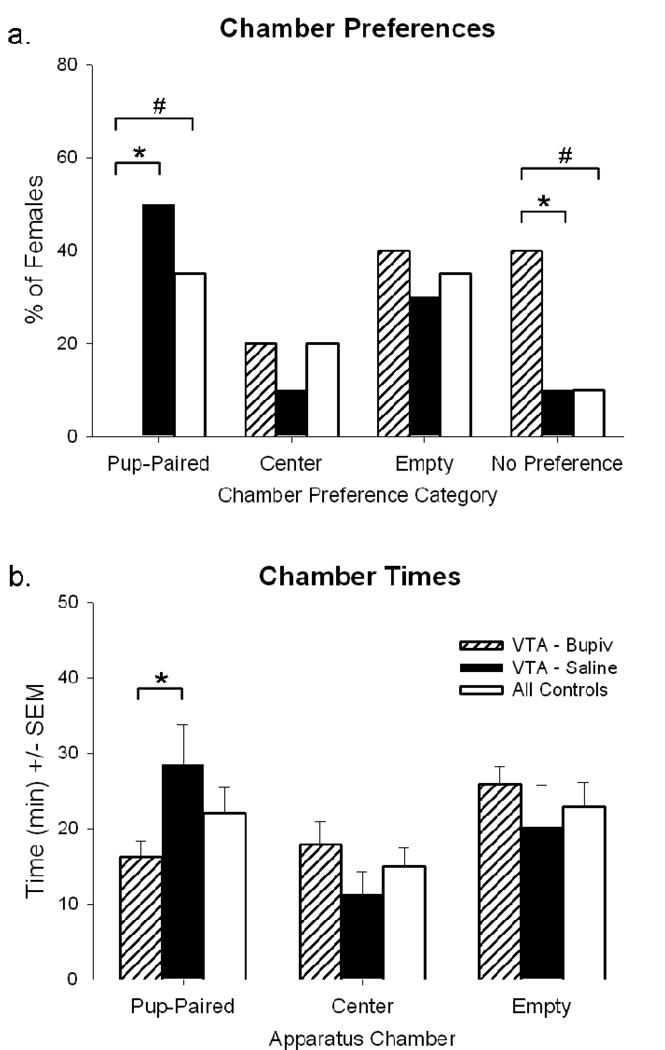
Following saline microinfusion into the VTA, half (50%) of the postpartum females expressed strong preference for the pup-paired context (Figure 4a). VTA females’ preference for the pup-paired context matched the preference expressed by all control females (groups 1–3). Postpartum females given intra-VTA saline also expressed similar preference for the other, non-pup-paired contexts (Figure 4a) and spent similar amounts of time in each chamber (Figure 4b) compared to all control females.
Figure 4.
Individual preferences for each stimulus-paired context (a) and mean time (min) spent in each stimulus-paired context (b) during the post-conditioning test session(s) by postpartum females. Immediately prior to the start of the test session, females were given an intra-VTA microinjection containing 2% bupivacaine (striped bars) or saline solution (solid bars); all control females were handled but no microinjections were administered (open bars). All between-groups (#) and within-groups (*) comparisons, P<0.05.
Following bupivacaine microinfusion into the VTA, however, postpartum females’ preference for a pup-paired context was completely abolished. Significantly fewer females preferred the pup-paired context following intra-VTA microinfusions of bupivacaine than following intra-VTA microinfusions of saline (z=−3.16, P<0.001) and compared to all control females (z=2.13, P<0.05) (Figure 4a); females also spent less time in the pup-paired context after intra-VTA bupivacaine than they did after intra-VTA saline [t(9)=−2.57, P<0.05] (Figure 4b). Intra-VTA bupivacaine also increased the population of females that lacked a chamber preference compared to intra-VTA saline (z=−3.15, P<0.001) and to control females (z=2.13, P<0.05). Time spent in the non-pup-paired contexts (i.e., center/empty contexts) and preference for those contexts, however, remained similar regardless of microinfusion into the VTA and matched the times and preferences of control females.
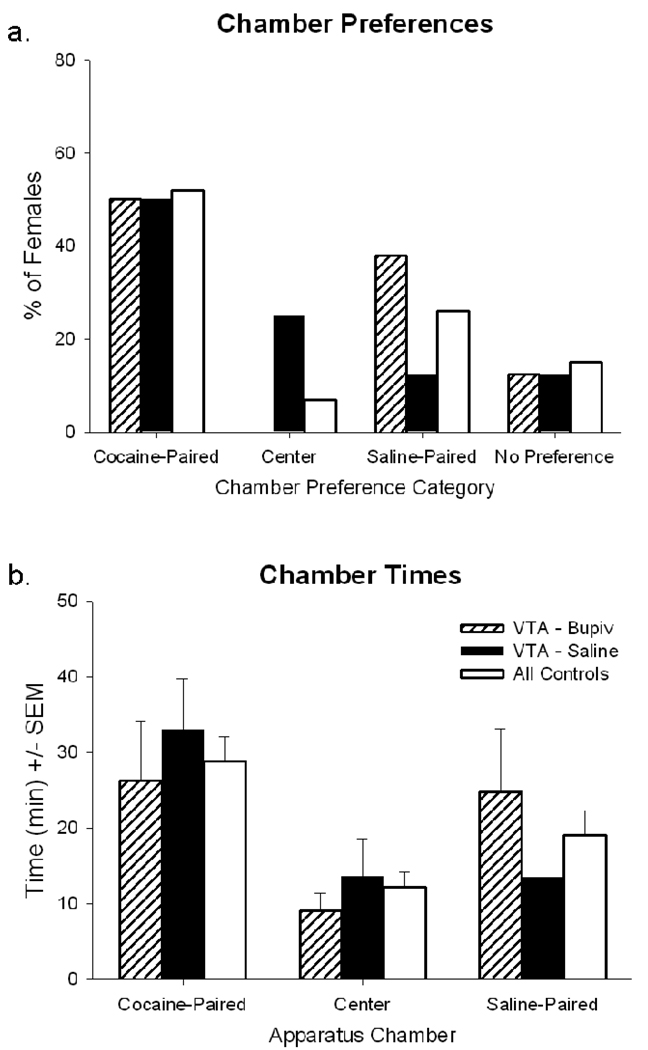
Expression of conditioned preference for a cocaine-paired context is unaffected by intra-VTA bupivacaine
Virgin females’ preference for the cocaine-paired context remaining consistently high (50–52% of females), regardless of whether they had just received microinfusion of bupivacaine or saline into the VTA (Figure 5a). Females also spent similar amounts of time in the cocaine-paired context after each intra-VTA microinfusion (Figure 5b). Females’ preferences for the non-cocaine-paired contexts (Figure 5a) and time spent in those contexts (Figure 5b) did not differ after each intra-VTA microinfusion. In all cases, females’ preferences and times were identical to those of control females (groups 1–3).
Figure 5.
Individual preferences for each stimulus-paired context (a) and mean time (min) spent in each stimulus-paired context (b) during the post-conditioning test session(s) by virgin females. Immediately prior to the start of the test session, females were given an intra-VTA microinjection containing 2% bupivacaine (striped bars) or saline solution (solid bars); all control females were handled but no microinjections were administered (open bars).
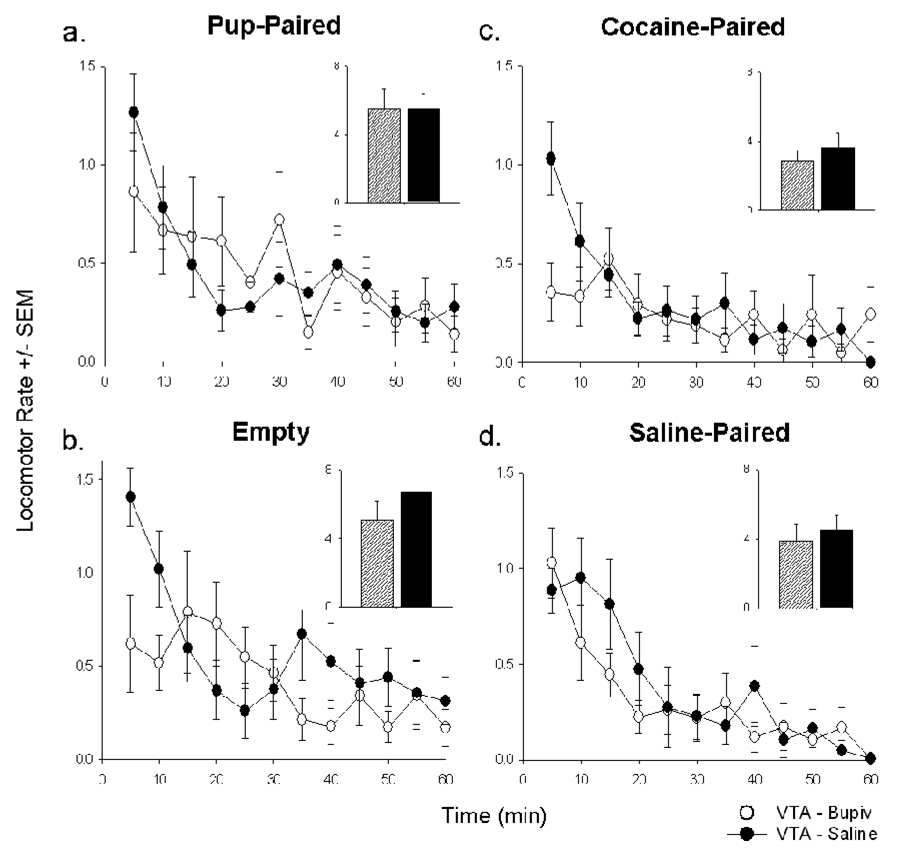
Locomotion
Females’ locomotion was strikingly similar following intra-VTA microinfusions of bupivacaine and saline (Figure 6). After each microinfusion, all VTA females moved easily between CPP chambers and visited each chamber during the first 15min of each CPP test session. Regardless of microinfusion, locomotor rate decreased significantly across the CPP test session in both postpartum females [main rate effect: pup-paired context, F(11,99)=4.03; empty context, F(11,99)=4.12; both P<0.001] (Figure 6a,c) and virgin females [main rate effect: cocaine-paired context, F(11,77)=4.01; saline-paired context, F(11,77)=2.67; both P<0.01] (Figure 6b,d). A significant interaction emerged between session time and microinfusion in the empty side context in postpartum females [F(11,99)=2.70, P<0.01] and in the cocaine-paired context in virgin females [F(11,77)=2.89, P<0.01]. Locomotor rates in these contexts were subsequently collapsed across the first half (0–30min) and last half (30–60min) of the session and compared; bupivacaine only reduced locomotor rate in the empty context [t(9)=3.41, P<0.01].
Figure 6.
Mean locomotor rate (beam breaks per second) of females across the post-conditioning test session, immediately following intra-VTA microinfusions of 2% bupivacaine (open circles) or saline (filled circles). Locomotor rates of postpartum females in the pup-paired (a) and empty (b) contexts and of virgin females in the cocaine-paired (c) and saline-paired (d) contexts are shown. Inset: Mean locomotor rate in each CPP chamber pooled across entire 60min session following intra-VTA microinfusions of bupivacaine (striped bars) or saline (solid bars).
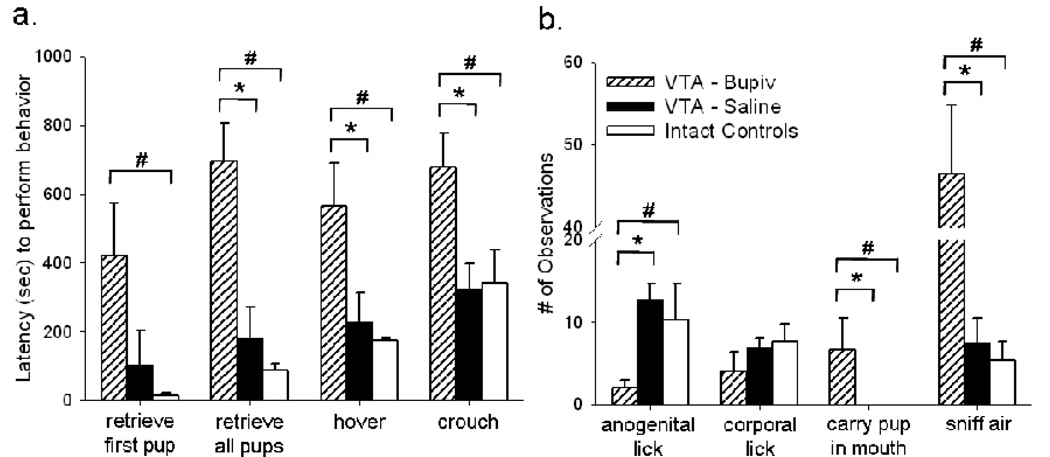
Maternal behavior
In postpartum females, the expression of select maternal behaviors was disrupted by intra-VTA bupivacaine microinfusions (Figure 7). Intra-VTA bupivacaine significantly extended postpartum females’ latency to retrieve all eight pups to the nest [T=2.0, P<0.02], hover over pups [T=5.0, P<0.05] and assume a low crouch position over pups [T=5.0, P<0.05] compared to intra-VTA saline (Figure 7a). Following intra-VTA bupivacaine, females also performed less anogenital licking [T=5.0, P<0.05] and more non-maternal behaviors such as sniffing the air [T=1.0, P<0.01] than they did following intra-VTA saline (Figure 7b). Females were also observed carrying pups in their mouth and/or dropping pups in non-nest locations (i.e., disorganized retrieval) more frequently following intra-VTA bupivacaine than intra-VTA saline [T=0.0, P<0.01]. Compared to control females, females given intra-VTA bupivacaine took longer to retrieve their first pup (U=−8.5, P<0.02) and all eight pups to the nest (U=−12.0, P<0.02) and to hover (U=−9.0, P<0.02) and crouch over pups (U=−10.0, P<0.02). Following intra-VTA bupivacaine, females also performed less anogenital licking (U’=1.0, P<0.05) and sniffed the air more frequently (U=−13.0, P<0.02) than controls. Following intra-VTA saline, maternal behavior expressed by VTA females was identical to that of controls.
Figure 7.
Mean latency to perform (a) and number of observations of (b) various maternal behaviors by postpartum females, immediately following intra-VTA microinfusions of 2% bupivacaine (striped bars) or saline (solid bars). Intact females were handled but no microinjections were administered (open bars). All between-groups (#) and within-groups (*) comparisons, P<0.05.
Discussion
The present study provides novel insight into the motivational state of the female rat by revealing that intact VTA function is required for the expression of CPP for a context paired with a natural (pup) stimulus but not CPP for a context paired with a pharmacological (cocaine) stimulus. Virgin females’ preference for a cocaine-paired context remained intact despite VTA inactivation (via bupivacaine), with preference remaining identical regardless of whether bupivacaine or saline was microinfused into the VTA. Postpartum females’ preference for a pup-paired context, however, was abolished by bupivacaine microinfusions into the VTA. Following saline microinfusions, females’ preference for the pup-paired context matched that of controls (groups 1–3). Postpartum females’ preference for the center and empty side chambers remained similar regardless of VTA function, confirming that disrupted CPP expression was restricted to the context paired with the salient pup stimulus. To our knowledge, this is the first demonstration that intact VTA function is critical for the expression of preference for a unique context paired with an appetitive natural stimulus but not one paired with an appetitive drug.
Chamber preferences following intra-VTA saline microinfusions matched those of control females (groups 1–3), confirming that neither VTA cannulation nor surgery itself altered CPP expression. As surgery occurred prior to the start of the CPP paradigm, any possible surgery-related confounds (e.g., anesthesia, stress, discomfort) or tissue damage caused by cannula implantation (e.g., Benveniste and Diemer, 1987) did not affect CPP acquisition or expression, as reported by others (Herzig and Schmidt, 2007).
As bupivacaine blocks Na+ channels to induce widespread neuronal inactivation, discrete bupivacaine microinfusions used in the present study presumably inactivated all neuronal types within the VTA. Neurons in the VTA vary widely in their connectivity and neurochemical content. While DAergic VTA neurons projecting to the NAc have received the most experimental attention regarding motivation and goal-directed behavior, VTA neurons containing gamma-amino butyric acid (GABA) (Van Bockstaele and Pickel, 1995; Carr and Sesack, 2000) or glutamate (Yamaguchi et al., 2007) may also participate in motivated behavior and drug-related responsiveness. These non-DAergic subpopulations of VTA neurons send long-range projections to a variety of targets, including the NAc (Van Bockstaele and Pickel, 1995), prefrontal cortex (PFC) (Carr and Sesack, 2000), amygdala (Rosenkrantz and Grace, 2002), hypothalamus and MPOA (Swanson, 1982; Miller and Lonstein, 2006), ventral pallidum (Fields et al., 2007) and lateral habenula (Swanson, 1982), which may contribute importantly to different aspects of reward-related and goal-directed behavior, decision-making and affective processing (McBride et al., 1999; Tzschentke, 2000; Fields et al., 2007; Matsumoto and Hikosaka, 2007, 2009). Unlike blocking specific classes of receptors, bupivacaine depressed the activity of all neurochemical subpopulations of VTA neurons, effectively eliminating VTA input to a broadly distributed neural circuitry participating in motivated behavior.
As voltage-gated Na+ channels are also located at nodes of Ranvier on axonal fibers, bupivacaine also blocks axonal conductance in fibers of passage. One such fiber bundle, the fasciculus retroflexus (FR), projects from the habenula to the interpeduncular and raphé nuclei through the midbrain, just dorsal to the VTA. Though the habenula and its projections respond to the motivational value of stimuli and their predictive cues (Matsumoto and Hikosaka, 2007, 2009), it is unlikely that bupivacaine-induced blockade of the FR within the VTA affected CPP, as our injection controls received bupivacaine into rostral and/or dorsal aspects of the FR yet retained normal CPP.
Given the injected volume and expected diffusion of bupivacaine, we posit that physiological inactivation induced by bupivacaine microinjection was limited to the VTA. The VTA extends approximately 1,000 microns (µm) mediolaterally, 800–1,000µm dorsoventrally, and 600–800µm rostrocaudally. Bupivacaine is structurally similar to the amide anesthetic lidocaine, which diffuses in an estimated sphere of 1000µm from the tip of the injector cannulae per 1µl microinfusion (Sandkühler et al., 1987; Martin and Ghez, 1999; Pereira de Vasconcelos et al., 2006). This sphere of diffusion coincides with a spherical area of depressed physiological activity (Boehnke and Rasmusson, 2001) and local glucose uptake (Martin and Ghez, 1999), with little inactivation extending beyond that perimeter (Tehovnik and Sommer, 1997). As expected for bupivacaine, lidocaine-induced neuronal inactivation is greatest at the center of the microinjection site, where drug concentration is greatest, and decreased gradually with increasing distance from injection site (Martin, 1991; Martin and Ghez, 1999). Given its smaller molecular mass (Tucker and Mather, 1998), bupivacaine is presumed to spread to equal or slightly greater distances than an identical volume of lidocaine. Thus, our bupivacaine microinjections of 0.5µl/side likely diffused about 500–600µm from all directions of the injector cannula. Given the anatomical accuracy of cannula tip placement, we believe that bupivacaine inactivated the majority of the VTA without diffusing into neighboring structures.
The site specificity of microinfusions was also verified behaviorally in our locomotor analyses. In VTA females, locomotor habituation emerged consistently in each chamber, mean locomotor rates did not differ, females moved easily between CPP chambers and postpartum females carried and manipulated pups normally following either microinfusion. In contrast, unilateral inactivation of the adjacent substantia nigra (SN) produced rapid and profound locomotor impairments. Given the normal locomotor behavior observed in VTA females, we posit bupivacaine diffusion into SN was minimal.
Region-specific mesocorticolimbic lesions have been used frequently to alter the acquisition but not the expression of CPP (for review, see Tzschentke, 1998, 2007). Interventions targeting the acquisition of CPP disrupt the ability of a subject to encode the valence of the primary stimulus (e.g., pups) and/or attribute incentive value to a unique context paired with that stimulus. In contrast, interventions targeting CPP expression alter a subjects’ motivation to seek out and spend time in a unique context attributed with a certain value. As mesolimbic manipulations may not affect consolidated learning (Dalley et al., 2005; Berridge, 2007) and thus subjects’ retrieval of learning about the conditioned value of each CPP context, impaired pup CPP following VTA inactivation likely reflects a selective disruption of goal-directed behavior toward unique contexts associated with pups. Only limited work has assessed expression of drug CPP after mesolimbic disruption (Sellings and Clarke, 2003; Moaddab et al., 2008); this is the first study to assess CPP for cocaine- or pup-paired contexts by disrupting VTA function.
These findings extend growing evidence that the incentive value of pups depends upon connections between the maternally responsive medial preoptic area (MPOA) and the mesocorticolimbic system. In females, the MPOA mediates the expression of maternal behavior (Lee et al., 2000; Numan et al., 2007), motivation to reunite with offspring (Perrin et al., 2007) and preference for a unique pup-paired context (Morrell et al., 2008). The MPOA and ventral bed nucleus of the stria terminalis (vBNST) project directly to VTA (Numan and Smith, 1984; Numan and Numan, 1997) and these projections are activated during motivated pup-directed behavior (Numan and Numan, 1997). Maternal behavior is disrupted following MPOA or VTA lesions (Gaffori and LeMoal, 1979; Lee et al., 2000; Perrin et al., 2007) or by blocking select receptor subtypes within the VTA (Pedersen et al., 1994; Thompson and Kristal, 1996; Numan and Stolzenberg, 2009). As DAergic projections from the VTA (Hansen et al., 1991) and their upstream targets (PFC: Afonso et al., 2007; NAc: Lee et al., 2000) must also remain intact for females to express maternal behavior, the VTA may allow maternally relevant input from the MPOA/vBNST to access the mesocorticolimbic system (Numan, 2007; Numan and Stolzenberg, 2008, 2009) to facilitate preference for a pup-paired context.
Select impairments in motivated maternal behavior following VTA inactivation were also revealed in females tested for pup CPP. Transient VTA inactivation selectively disrupted pup retrieval and anogenital licking, though females could still carry/transport pups with their mouths, group and lick pups (see corporal licking data), and assume normal nursing postures (though retrieval impairments extended females’ latency to hover/crouch). As PFC lesions selectively disrupt pup retrieval and licking (Afonso et al., 2007), the specific deficits revealed in the present study suggest that VTA inactivation disabled maternally relevant inputs to the PFC.
While the expression of preference for chambers paired with a natural stimulus required intact VTA function, preference for the cocaine-paired chamber remained intact despite widespread VTA inactivation. Extensive research suggests that the mesolimbic circuitry, including the VTA and NAc, contribute to an animal’s response to cocaine and cocaine-paired stimuli and its expression of cocaine-seeking behavior (Carelli et al., 2000; Carelli and Ijames, 2001; Carelli, 2002, 2004; Phillips et al., 2003a; Roitman et al., 2004; Yun et al., 2004; Sombers et al., 2009). In the present study, however, the expression of cocaine CPP was unaffected by inactivation of the VTA and its projections.
Only one other study used a similar site-specific inactivation procedure, revealing that the expression of morphine CPP was reduced, but not abolished, following bilateral VTA inactivation and remained undisrupted following unilateral inactivation (Moaddab et al., 2008). Compared to the present study, Moaddab and colleagues (2008) used a different strain, sex and age of rats, number of conditioning sessions, and drug, dose and route of administration, each of which can influence CPP (reviewed in Bardo et al., 1995; also, see Russo et al., 2003; Seip et al., 2008). A combination of these variables likely contributed to the difference in magnitude of CPP effects reported in these two studies.
A number of alternative, non-VTA dependent mechanisms may exist to maintain cocaine-seeking behavior during the expression of CPP. The basolateral amygdala (BLA) is strongly activated by preference for a cocaine-paired chamber (Mattson and Morrell, 2005) and cocaine-paired cues (Carelli et al., 2003) but is not activated as strongly by preference for a pup-paired chamber (Mattson and Morrell, 2005). Given a sufficiently salient stimulus, such as cocaine, the BLA may be able to sustain CPP during VTA inactivation, possibly by directly modifying accumbal DA release (Floresco et al., 1998; Phillips et al., 2003a). The PFC also contributes to the reinforcing properties of cocaine (Isaac et al., 1989) and participates in goal-directed behavior (Tzschentke, 2000; Hitchcott et al., 2007). As prefrontal disregulation can increase motivation to seek drugs and drug-related stimuli (Jentsch and Taylor, 1999; Tzschentke, 2000) but not necessarily natural stimuli like food (Levy et al., 2007), inactivating the VTA and its projections to the PFC may facilitate responses to cocaine-paired cues to maintain cocaine CPP.
We conclude that the VTA may be differentially involved in motivated seeking behavior directed toward contexts associated with natural stimuli and contexts associated with drug stimuli. As virgin and postpartum females express identical CPP for intraperitoneal cocaine (Seip et al., 2008) and, if sensitized to behave maternally, for a pup-paired chamber (Seip and Morrell, 2008), we fully expect that, following VTA inactivation, cocaine CPP would remain intact in postpartum females and pup CPP would be abolished in maternal virgin females. Together, these findings emphasize the importance of characterizing females’ motivated behavior toward various salient stimuli in her environment. The choice to invest time with a specific stimulus over available alternatives can be evolutionarily advantageous, such as when a female seeks out stimuli that will directly benefit her progeny (e.g., food, maternal care) or maladaptive (e.g., drug-seeking). The present work now reveals that motivated seeking behavior directed toward contexts associated with natural, highly adaptive stimuli may be strikingly vulnerable to loss of VTA function compared to contexts paired with drugs of abuse.
Acknowledgements
This research was supported by NIH DA014025 and March of Dimes #12FY02-05-103 and #12FY05-06-134 awarded to J.I.M. The authors thank the Laboratory Animal Facility staff of Rutgers University, Newark NJ, for animal breeding and care and thank Dr. Mariana Pereira for helpful discussions related to this work.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 2008;1198:115–123. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Gerdjikov T. The role of signaling molecules in reward-related incentive learning. Neurotox Res. 2004;6:91–104. doi: 10.1007/BF03033301. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. 1987;74:234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105:133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24(34):7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23(11):1848–1852. [PubMed] [Google Scholar]
- Bruning JL, Kintz BL. Computational handbook of statistics. 3rd ed. Glenview, IL: Scott Foresman; 1987. [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. 'natural' reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47:180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus "natural" (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci. 2003;23:8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle DE, Sperelakis N. Bupivacaine and lidocaine blockade of calcium-mediated slow action potentials in guinea pig ventricular muscle. J Pharmacol Exp Ther. 1987;242:101–105. [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8(1):100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol. 1986;247:364–382. doi: 10.1002/cne.902470307. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139(2):167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Ann Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nakajima T, Viswanathan PC, Balser JR. Compound-specific Na+ channel pore conformational changes induced by local anaesthetics. J Physiol. 2005;564:21–31. doi: 10.1113/jphysiol.2004.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffori O, Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav. 1979;23:317–323. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105(4):588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Hernández-González M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005a;70:132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Prieto-Beracoechea C, Navarro-Meza M, Ramos-Guevara JP, Reyes-Cortes R, Guevara MA. Prefrontal and tegmental electrical activity during olfactory stimulation in virgin and lactating rats. Physiol Behav. 2005b;83:749–758. doi: 10.1016/j.physbeh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Amygdala cannulation alters expression of cocaine conditioned place preference and locomotion in rats. Addict Biol. 2007;12:478–481. doi: 10.1111/j.1369-1600.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966;210:1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci. 1989;103:345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Activity of presumed dopamine neurons in the ventral tegmental area during heroin self-administration. Neuroreport. 1997;8:2581–2585. doi: 10.1097/00001756-199707280-00032. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Rosenblatt JS, Barona ML, Chinapen S, Nissanov J, O'Bannon RT, Johnson BM, Del Cerro MC. Combined c-fos and 14C-2-deoxyglucose method to differentiate site-specific excitation from disinhibition: analysis of maternal behavior in the rat. Brain Res. 2000;859:262–272. doi: 10.1016/s0006-8993(00)01972-7. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Francès H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not "natural" reinforcementq. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Miyata S, Weng W, Matsunaga W, Ichikawa J, Furuya K, Nakashima T, Kiyohara T. Comparison of the expression of two immediate early gene proteins, FosB and Fos in the rat preoptic area, hypothalamus and brainstem during pregnancy, parturition and lactation. Neurosci Res. 1998;32:333–341. doi: 10.1016/s0168-0102(98)00100-x. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ. Ischemia and lesion induced imbalances in cortical function. Prog Neurobiol. 1996;48:131–166. doi: 10.1016/0301-0082(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neuro. 2009;12(1):77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135:315–328. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Identification of dopaminergic and oxytocinergic projections to the medial preoptic area of lactating rats. Program No. 577.2. [Google Scholar]
- Moaddab M, Haghparast A, Hassanpour-Ezatti M. Effects of reversible inactivation of the ventral tegmental area on the acquisition and expression of morphine-induced conditioned place preference in the rat. Behav Brain Res. 2009;198:466–471. doi: 10.1016/j.bbr.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Seip KM, Pereira M. 2008 Neuroscience Meeting Planner. Washington, D.C: Society for Neuroscience; 2008. Identifying components of the neural circuit mediating maternal motivation and expression of maternal behavior with reversible inactivation. Program No. 795.3. [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel T. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Hypothalamic interaction with the mesolimbic dopamine system and the regulation of maternal responsiveness. In: Bridges RS, editor. Neurobiology of the parental brain. New York: Elsevier Academic Press; 2008. pp. 3–22. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Klur S, Muller C, Cosquer B, Lopez J, Certa U, Cassel JC. Reversible inactivation of the dorsal hippocampus by tetrodotoxin or lidocaine: a comparative study on cerebral functional activity and motor coordination in the rat. Neuroscience. 2006;141:1649–1663. doi: 10.1016/j.neuroscience.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Perrin G, Meurisse M, Levy F. Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Horm Behav. 2007;52:461–473. doi: 10.1016/j.yhbeh.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003a;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68:168–178. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiol Behav. 2008;95:599–608. doi: 10.1016/j.physbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Pereira M, Wansaw MP, Reiss JI, Dziopa EI, Morrell JI. Incentive salience of cocaine across the postpartum period of the female rat. Psychopharmacology. 2008;199:119–130. doi: 10.1007/s00213-008-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw Hill; 1956. [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman MR. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Kristal MB. Opioid stimulation in the ventral tegmental area facilitates the onset of maternal behavior in rats. Brain Res. 1996;743:184–201. doi: 10.1016/s0006-8993(96)01041-4. [DOI] [PubMed] [Google Scholar]
- Tucker GT, Mather LE. Properties, absorption, and disposition of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3rd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 55–96. [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–464. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Horm Behav. 2008;54:294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner MR, Kest K, Ranaldi R. NMDA receptor antagonism in the ventral tegmental area impairs acquisition of reward-related learning. Behav Brain Res. 2009;197:442–449. doi: 10.1016/j.bbr.2008.10.013. [DOI] [PubMed] [Google Scholar]