Abstract
The Wnt genes encode a large family of secreted glycoproteins that play important roles in controlling tissue patterning, cell fate and proliferation during development. Currently, little is known regarding the role(s) of Wnt genes during prostate gland development. The present study examines the role of the noncanonical Wnt5a during prostate gland development in rat and murine models. In the rat prostate, Wnt5a mRNA is expressed by distal mesenchyme during the budding stage and localizes to periductal mesenchymal cells with an increasing proximal-to-distal gradient during branching morphogenesis. Wnt5a protein is secreted and localizes to periductal stroma, extracellular matrix and epithelial cells in the distal ducts. While Wnt5a expression is high during active morphogenesis in all prostate lobes, ventral prostate (VP) expression declines rapidly following morphogenesis while dorsal (DP) and lateral lobe (LP) expression remains high into adulthood. Steroids modulate prostatic Wnt5a expression during early development with testosterone suppressing Wnt5a and neonatal estrogen increasing expression. In vivo and ex vivo analysis of developing mouse and rat prostates were used to assess the functional roles of Wnt5a. Wnt5a−/− murine prostates rescued by organ culture exhibit disturbances in bud position and directed outgrowth leading to large bulbous sacs in place of elongating ducts. In contrast, epithelial cell proliferation, ductal elongation and branchpoint formation is suppressed in newborn rat prostates cultured with exogenous Wnt5a protein. While renal grafts of Wnt5a−/− murine prostates revealed that Wnt5a is not essential for cyto- and functional differentiation, a role in luminal cell polarity and lumenization of the ducts was indicated. Wnt5a suppresses prostatic Shh expression while Shh stimulates Wnt5a expression in a lobe-specific manner during early development indicating that Wnt5a participates in cross-talk with other members of the gene regulatory network that control prostate development. Although Wnt5a does not influence prostatic expression of other Wnt morphogens, it suppresses Wif-1 expression and can thus indirectly modulate Wnt signaling. In summary, the present finds demonstrate that Wnt5a is essential for normal prostate development where it regulates bud outgrowth, ductal elongation, branching, cell polarity and lumenization. These findings contribute to the growing body of knowledge on regulatory mechanisms involved in prostate gland development which are key to understanding abnormal growth processes associated with aging.
Keywords: prostate, prostate gland, prostate development, Wnt5a, estradiol
Introduction
The prostate is a branched exocrine secretory gland of the male reproductive tract that originates from the endodermal urogenital sinus (UGS). In humans, prostate gland development occurs during the second and third trimesters of gestation, whereas in the rodent, a commonly studied model system, budding initiates late in fetal life and branching morphogenesis occurs postnatally (Hayashi et al., 1991; Sugimura et al., 1986). At birth, the rodent prostate consists of solid unbranched, elongating ducts extending into the ventral, lateral and dorsal UGS mesenchyme to form the separate ventral (VP) lateral (LP) and dorsal (DP) prostate lobes with lobe-specific branching patterns (Timms et al., 1994). Mesenchymal and epithelial cell cytodifferentiation with lumen formation is coordinated with branching morphogenesis, beginning in the proximal ducts and slowly extending towards the distal tips between postnatal day (pnd) 10–15 (Prins and Birch, 1995). Functional differentiation with production of secretory proteins by differentiated luminal cells follows thereafter.
Prostate gland development is an androgen-dependent process with the early events regulated through androgen receptors (AR) in mesenchymal cells (Cunha and Chung, 1981; Price, 1936; Prins and Putz, 2008). Other steroid receptors, including estrogen receptors (ERs) and retinoid receptors (RARs and RXRs) are expressed in a cell specific manner during early development and contribute to prostate morphogenesis and differentiation (Prins and Birch, 1997; Prins et al., 2002). As with all branched structures, appendicular patterning and cell differentiation during prostatic development are dictated by common and organ-specific morphoregulatory genes that are expressed in a unique temporal and spatial pattern. These include transcription factors Hoxa13, Hoxb13, Hoxd13, Nkx3.1 and Foxa1 and secreted paracrine factors Fgf10, Shh, Tgfβ1 and Bmp 4&7 {see recent review (Prins, 2008, #3248)}. Several of these developmental genes, cognate receptors and/or downstream signaling molecules are regulated at some level by the various steroids to effect hormone regulated development, although this process is not completely understood. In other branched organs such as the lung, kidney, and mammary gland, the Wnt gene family plays critical roles in branching morphogenesis and cell differentiation (Buhler et al., 1993; Gavin and McMahon, 1992; Mucenski et al., 2003; Okubo and Hogan, 2004; Shu et al., 2002; Stark et al., 1994; Yamaguchi et al., 1999). Despite a large number of studies that have demonstrated a role for aberrant Wnt signaling during prostate carcinogenesis and progression (Chesire and Isaacs, 2003; Yardy and Brewster, 2005), there is little published work on the role(s) of Wnt genes during prostate gland development.
The Wnt genes encode a large, highly conserved family of secreted glycoproteins that play essential roles in controlling tissue patterning, cell fate and proliferation from Drosophila to humans (Cadigan and Nusse, 1997; Nelson and Nusse, 2004). There are 19 mammalian Wnt proteins identified and all bind to frizzled (Fzd) cell surface receptors to initiate cellular responses (Cadigan and Nusse, 1997). Vertebrate Wnts have been divided into two functional groups by reference to their downstream signaling pathways (Moon et al., 1997). In short, the canonical Wnts signal through nuclear β-catenin/Tcf-Lef while the noncanonical Wnts function through alternate signaling cascades that include Ca++/PKC, RhoA/JNK or RhoB/Rab4 (Bejsovec, 2005; Witze et al., 2008). In addition to secreted Wnt glycoproteins, the Wnt signaling network also includes several extracellular secreted regulators that antagonize Wnt actions including secreted frizzled-related proteins (Sfrps), Wnt inhibitory factors (Wif), and the dickkopf (Dkk) proteins which specifically block canonical Wnt signaling (Hsieh et al., 1999; Mao et al., 2001).
Evidence that Wnt signaling is involved in prostate morphogenesis comes from studies analyzing the secreted regulators Sfrp 1&2 and Dkk1&2 whose expression rapidly declines postnatally (Pritchard and Nelson, 2008; Zhang et al., 2006). Addition of recombinant Sfrp1 to rat VP explant cultures increased their growth over 5 days indicating that Wnts normally inhibit this process (Joesting et al., 2005). Further studies with Sfrp1 transgenic overexpressing and null mutant mice confirmed that this Wnt modulator stimulates prostate branching morphogenesis, epithelial cell proliferation and secretory gene expression (Joesting et al., 2008). Studies from our laboratory showed that Dkk1 protein also stimulated growth and branching of cultured newborn rat VP lobes over a four-day period suggesting that canonical Wnts suppress prostate growth (Prins and Putz, 2008). This is supported by another recent study where Wnt3a, a canonical Wnt, reduced ductal branching of cultured neonatal mouse prostates and active canonical Wnt signaling in epithelial progenitor cells maintained their undifferentiated state (Wang et al., 2008). To determine which Wnt genes are actively involved in prostate development, we recently screened rat prostates for Wnt gene expression during early development and identified robust expression of the canonical Wnt2, Wnt2b and Wnt7b genes and the non-canonical Wnt4, Wnt5a and Wnt11 genes. In the present study, we examined in detail the role of Wnt5a in prostate gland development.
Wnt5a is a noncanonical Wnt ligand involved in outgrowth of multiple structures during vertebrate development. Targeted deletion of Wnt5a−/− in mice resulted in perinatal lethality demonstrating a critical role for normal development of structures necessary for life (Yamaguchi et al., 1999). Severe morphological defects observed at the end of gestation include caudal truncation, shortening of limbs along the proximal-distal axis, truncated trachea, overexpansion of the distal airways, absence of the genital tubercle and lack of distal elements in affected structures (Li et al., 2002; Yamaguchi et al., 1999). Together, these phenotypes indicate an essential role for Wnt5a in outgrowth and patterning along the proximal-distal axis. In the current study, we used rats and mice to identify the spatio-temporal expression pattern of Wnt5a during prostate development, to examine its regulation by steroid hormones and to determine its role in budding, ductal outgrowth, branching morphogenesis and cell differentiation. Our results reveal high levels of Wnt5a expression at the distal tips and along the centro-distal periductal mesenchyme during the period of postnatal branching morphogenesis with a rapid decline thereafter in the VP but not the DP and LP. Wnt5a expression in the newborn prostate is suppressed by testosterone and stimulated by early exposure to estradiol. We provide evidence that Wnt5a is involved in determining bud position and size, regulating epithelial cell proliferation and inhibiting ductal outgrowth and branching during prostatic development. Our findings further suggest an essential role for this Wnt ligand in cell polarity and lumen formation within the prostate epithelial ducts.
Materials and Methods
Animals
All animals were handled according to the principles and procedures of the Guiding Principles for the Care and Use of Animal Research and the experiments were approved by the Institutional Animal Care Committee. Timed pregnant female Sprague-Dawley rats from Zivic-Miller (Pittsburgh, PA) were monitored for delivery and the day of birth was designated as day 0. The rats were sacrificed by decapitation on postnatal day (pnd) 1, 3, 6, 10, 30 or 90, the urogenital sinus (UGS)-prostate complexes were removed and the separate prostate lobes were micro dissected at 4C and either frozen in liquid nitrogen or fixed for histology. To examine the effect of developmental estrogen exposure, male pups were treated on pnd 0, 3 and 5 with subcutaneous injections of either 25 μg 17β-estradiol-3-benzoate (Sigma-Aldrich Chemical Co., St. Louis, MO) in 25μl peanut oil or with oil alone as controls.
Wnt5a−/− null mutant mice on a C57BL/6 background were generated as previously described (Yamaguchi et al., 1999). Heterozygote breeders were housed together and the day of vaginal plug was considered gestation day 0.5. Pregnant mothers were anesthetized at gestation day 16.5 (e16.5) or e18.5 and the embryos removed. The UGS with rudimentary prostates were dissected from male pups and immediately used for organ culture or renal graft studies. The Wnt5a −/− embryos were identified visually by a reduced size phenotype and confirmed by PCR while the Wnt5a+/− and Wnt5a+/+ embryos were distinguished solely by PCR as described (Yamaguchi et al., 1999).
Prostate organ culture and imaging
To examine the effect of Wnt5a on normal rat prostate development, rudimentary ventral prostate lobes (VP) were removed on pnd 0 (within 4 hr of birth) and paired, contralateral lobes from a single pup (n=6) were cultured for 6 days in either basal culture medium (BOCM) with 1.0μg/ml murine Wnt5a protein (R&D system) or 1.0μg/ml BSA as controls. The BOCM consisted of DMEM/F-12 (Invitrogen/GIBCO, Carlsbad, CA), 50μg/ml Gentamycin, 1× insulin-transferrin-selenium and 10nM testosterone and was replaced every 48–72 hr. Tissues were floated on Millicell-CM filters (Millipore Corp., Bedford, MA) in 2ml medium in BD Falcon 12-well plates (BD Biosciences, San Jose, CA) inside a closed culture chamber at 37°C with ports for introduction and exhaust of humidified 5% CO2 (Carl Zeiss MicroImaging, Inc., Thornwood, NY). The culture chamber was placed within a heated incubator attached to a Zeiss Axiovert 200 inverted microscope with an automated X-Y-Z stage and Axiocam HRm digital camera. Movement of the automated stage to photograph each cultured prostate in sequence and image acquisition were controlled by AxioVision 2.0.5 and Zeiss Imaging 25A software. Digital photographs of each sample were captured at 30 minute intervals. To generate time-lapse imaging, still images taken over the first 72 hours were converted to a QuickTime format using QuickTime Pro. Morphometry was performed on images taken at 10 hours intervals from 0–70 hr. A color-coded skeleton that denoted branching events over time was produced with AxioVision software. The number and length of segments for each branch generation, the total ductal length, the number of branchpoints and total tip number were measured. To classify branching events as terminal bifed, trifed or lateral, additional images between the 10 hr time points were examined. Data were analyzed by analysis of variance followed by Bonferroni post-hoc tests (Instat ver 3.01, GraphPad Software, Inc., San Diego, CA), using 6–10 samples for each treatment group.
To determine the effect of exogenous Wnt5a protein on rat prostate gene expression, 8 paired VPs were cultured as above and processed for RNA analysis after 18 hr. To examine the effect of testosterone on Wnt5a expression, 8 paired VPs were cultured in BOCM with or without 10nM testosterone and collected after 18 hr exposure.
The UGS complexes from Wnt5a−/− mice and wild-type littermates were collected on16.5 and cultured for 4 days in BOCM containing 10 nM dihydrotestosterone (DHT) in place of testosterone. The cultured tissue was collected and fixed in 10% buffered formaldehyde, paraffin embedded and processed for histology examination.
Renal grafting
The UGS-prostate complexes from e16.5 Wnt5a−/− and wild-type mice were grafted under the renal capsule of 7–8 week old male nude mice as described (http://mammary.nih.gov/tools/mousework/Cunha001/index.html). Wild-type and mutant tissues were grafted to contralateral kidneys of recipient mice which were supplemented with subcutaneous 0.5 cm sialastic capsules filled with crystalline testosterone. After 30 days, the kidneys were removed and grafts were cut out en masse and either fixed in 10% buffered formaldehyde or directly processed for RNA extraction. A small piece of graft tissue was taken before fixation and used for genotyping.
Immunohistochemistry (IHC)
Wnt5a, p63, cytokeratins 8/18 (CK8/18), e-cadherin, AR and secretory DLP protein were localized by IHC in fixed, dehydrated and paraffin-embedded tissues as previously described (Prins and Birch, 1995; Putz et al., 2001). For Wnt5a, tissues were frozen in Tissue-Tek O.C.T. compound (Sakura Finetek, USA, Inc., Torrence, CA) and frozen sections were fixed in 2% paraformaldehyde. All sections were blocked with Superblock (Vector laboratories, Inc., Burlingame, CA) and incubated overnight at 4°C with goat anti-mouse Wnt5a antibody (10μg/ml, R&D system, Minneapolis, MN), rabbit anti-p63 antibody (1:500, sc-8343; Santa Cruz), guinea pig anti-CK8/18 (American Research Products 03-GP11, Belmont, MA), 50 μg/ml mouse anti-E-cadherin (Transduction Laboratories,Lexington, KY), 5 μg/ml rabbit anti-AR (PG-21) (Prins et al., 1991) or rabbit anti-mouse DLP antibody (kindly provide by Dr. AnneMarie Donjacour). The sections were reacted with biotinylated anti-IgG (Vector Laboratories, Inc.) and detected with avidin-biotin peroxidase (ABC-Elite Vector Laboratories) using diaminobenzidine tetrachloride (DAB) as chromagen. For controls, normal goat, rabbit, guinea pig or mouse IgG was substituted for primary antibody. The sections were counterstained with Gill’s #3 hematoxylin (1:4).
To localize BrdU-labeled cells from organ culture studies, the tissues were pulsed with 10 μM BrdU (Sigma-Aldrich) 2 hr prior to culture termination and BrdU was detected by IHC as previously described (Huang et al., 2005). For statistical comparisons, BrdU labeling was analyzed by two-tailed Student’s t-test.
Whole mount in situ hybridization (wmISH)
The rat Wnt5a plasmid was kindly provided by Dr. Yiming Zhu at the University of Chicago. The plasmid was linearized by Hind III and a 802bp anti-sense digoxigenin-labeled RNA probe was prepared by in vitro transcription with T7 RNA polymerase (Roche). WmISH was performed as previously described (Huang et al., 2005) using 0.2 μg/ml digoxigenin-labeled RNA probe. Following overnight incubation with anti-digoxygenin alkaline phosphatase-conjugated antiserum (Roche, Indianapolis, IN), the samples were color reacted with NBT and BCIP (Roche). Pnd 1, 3, 6 prostatic complexes were processed together within each assay to permit direct comparisons of signal intensity for temporal analysis. Six separate wmISH assays were performed on neonatal rats treated neonatally with oil or estradiol. To identify cellular localization of gene expression, wmISH-stained tissues were cross-sectioned at 10μm.
Real-time RT-PCR
Real-time RT-PCR was performed using our published sequences for primers and probes for Wnt5a, Shh, Ptc, Fgf10, Nkx3.1, Hoxb13, Bmp4 and Rpl19 (Huang et al., 2005; Pu et al., 2007; Pu et al., 2004). The exon-spanning primers and dual-labeled probes for Wnts 2, 2b, 4, 7b, 11 and Wif1 are listed in Table 1. Primers were designed across exon boundaries to minimize the effect of potential genomic DNA contamination. The primers and dual-labeled probes were designed on the Primer3 website (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi ) and secondary structure was avoided when possible. The melting temperature (Tm) of primers was 58–60 C and the Tm of dual-labeled probes was 68–70 C. For dual-labeled probes, 5′ reporters were FAM for Shh, Ptc, Fgf10, Hoxb13, Bmp4, Wnt2, Wnt2b, Wnt4, Wnt5a, Wnt7b and Wnt11 and Hex for Rpl19. For Nkx3.1 and Wif1, a SYBR green assay with melting curve was used to ensure no nonspecific amplification. For all genes, plasmids containing each DNA sequence were cloned with TOPO TA cloning kit (Invitrogen) and used for standard curves, which were run in parallel for each reaction. The actual amount of target DNA in each experimental sample was directly calculated from each plasmid DNA standard curve. Ribosomal protein L19 (Rpl19) was quantitated and served as an internal reference gene for normalization. Under these conditions, it was determined that as low as 100 gene copies in a single neonatal prostate lobe could be reliably measured.
Table 1.
Primers and dual-labeled probes used for real-time PCR
| Gene | Sequence | Amplicon size (bp) |
|---|---|---|
| Wnt2 | ||
| Forward primer | tccgaagtagccgggaat | 111 |
| Reverse primer | gatcgcaggaacaggactttaat | |
| Probe | cctttgtttacgccatctcctcagc | |
| Wnt2b | ||
| Forward primer | actgtctttggccgtgctat | 78 |
| Reverse primer | gaccactcctgctgatgaga | |
| Probe | ctcagaagcagtcgggaggcag | |
| Wnt4 | ||
| Forward primer | acgtccgagagagaagcaag | 149 |
| Reverse primer | gcatgtctttacctcacaggag | |
| Probe | aggatgctcggacaacatcgcctat | |
| Wnt7b | ||
| Forward primer | cagcaccagttccgattc | 113 |
| Reverse primer | gtgatggcgtatgtgaagg | |
| Probe | ctccgagtagggagtcgagaggc | |
| Wnt11 | ||
| Forward primer | cccaagccaataaactgatgc | 87 |
| Reverse primer | ggcacttacacttcgtttcca | |
| Probe | tgaagtggggagacaggctctacgt | |
| WIF1 | ||
| Forward primer | tcgctggataaaggcatcatgg | 148 |
| Reverse primer | tgacaatcacattcacttcaaatgc |
Total RNA was extracted with RNeasy (Qiagen, Valencia, CA) and reverse transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed as previously described (Huang et al., 2005; Pu et al., 2004) using an iCycler (Bio-Rad). Each data point was repeated 3–15 times with prostate tissue from different animals. The ratio of Amplicon cDNA copy number of a specific gene to Rpl19 was calculated, and statistical analysis was performed with two-tailed Student’s t test or for multiple comparisons, analysis of variance followed by Bonferroni post-hoc analysis. P<0.05 was considered as statistically significant.
Results
Ontogeny and localization of Wnt5a in the developing rat prostate gland
The temporal and spatial pattern of Wnt5a expression was followed in individual lobes during rat prostate development. Expression levels of Wnt5a mRNA in the VP were high between pnd 1–10, rapidly decreased by day 15 and slowly decline thereafter to a nadir at day 90 (Fig. 1). Expression was also high in DP and LP lobes during early development (Fig.1) but in contrast to the VP, did not decline into adulthood (data not shown). During morphogenesis, Wnt5a mRNA localized intensely to the condensed mesenchymal cells surrounding the central-to-distal aspects of the elongating and branching ducts with the most intense signal observed at the distal ductal tips (Fig. 2A–E). Signal intensity was sustained between pnd1–6 and was similar between the separate prostate lobes which corroborates the quantitative RT-PCR results. Cross-sectional analysis of the pnd 3 VP from the wmISH confirmed that Wnt5a mRNA was limited to the periductal mesenchymal cells with no signal observed in the epithelium (Fig. 2E). In contrast, Wnt5a protein localized to periductal mesenchymal cells, the extracellular matrix and epithelial cells in the distal ductal regions documenting its secretion by stromal cells (Fig. 2G–H). Wnt5a protein association with epithelial cells was largely confined to the distal ducts as proximal epithelial cells were mostly negative for Wnt5a (Fig. 2I–J).
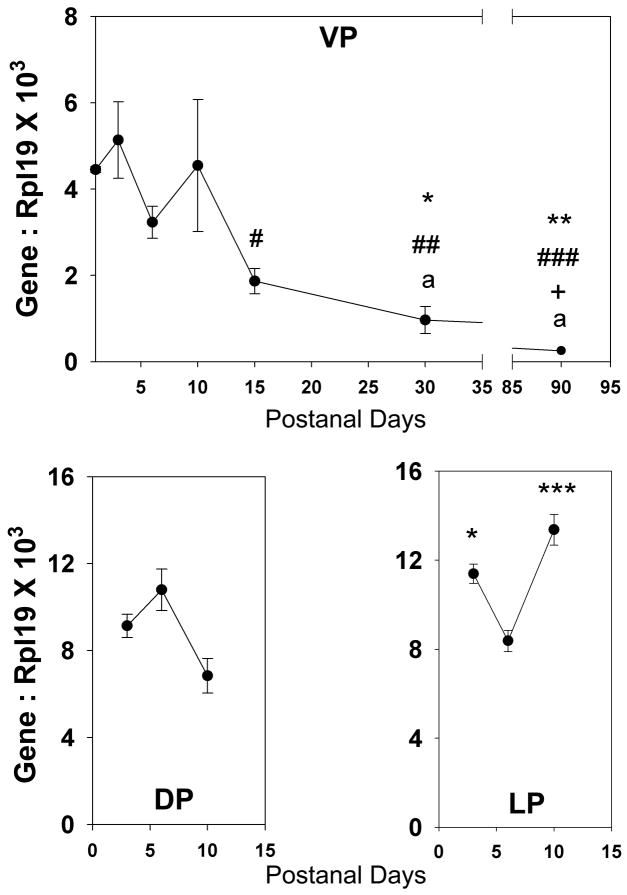
Figure 1.
(A) Ontogeny of Wnt5a mRNA expression in the rat ventral prostate lobes as quantified by real-time RT-PCR. Wnt5a expression in the VP was high at birth and remained at high level until pnd 10. The expression level markedly declined thereafter reaching a nadir in young adulthood. Each point represents mean ± SEM for 3–9 replicates. * = P<0.05 vs pnd 1, ** = p<0.01 vs pnd 1, # = p<0.05 vs pnd 3, ## = p<0.01 vs pnd 3, ### = p<0.001 vs pnd 3, + = p<0.05 vs day 6, a = p<0.05 vs pnd 10. (B) Wnt5a expression levels in the lateral and dorsal prostate are comparable to ventral prostate expression levels during the early developmental stage. Each point represents the mean ± SEM for 3–11 replicates. * = p<0.05 vs. pnd 6, *** = p<0.001 vs pnd 6.
Figure 2.
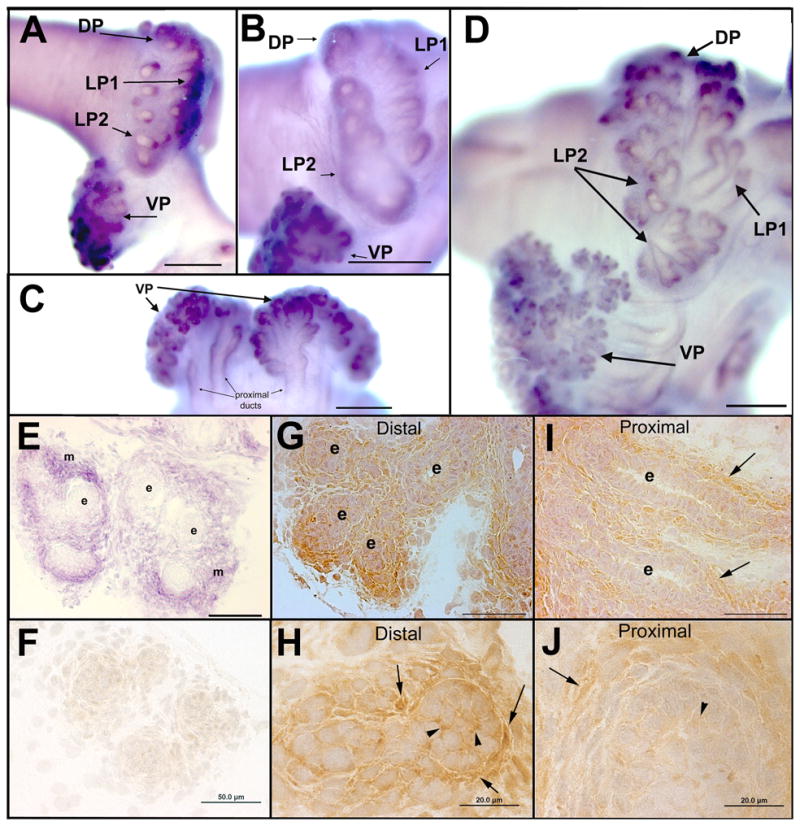
Localization of Wnt5a transcript (A–E) and Wnt5a protein (F–G) in the developing rat prostate gland. Wnt5a mRNA expression was examined by wmISH and exhibited a periductal pattern typical for mesenchyme-expressed genes. To allow direct comparisons of signal strength, tissues from pnd1 (A), pnd 3 (B–C) and pnd 6 (D) were processed together. Wnt5a mRNA expression localized to periductal cells along the ductal length with an increasing gradient from the proximal duct out towards the distal tips where the signal was most intense. (E) Cross-section of pnd 3 VP from wmISH confirms strong Wnt5a expression in the mesenchymal cells (m) immediately adjacent to the ducts but not in the epithelial cells (e). Wnt5a protein was localized by immunohistochemistry in pnd 6 VP tissue sections counterstained with hematoxylin. (F) Negative control IgG in place of Wnt5a on an adjacent section shows lack of signal in the mesenchymal and epithelial cells. (G–H) Low and higher power images, respectively, of Wnt5a protein localized to mesenchymal cells and extracellular matrix (arrows) and to the cell surface of epithelial (e) cells (arrowheads) in the distal VP ducts. (I–J) Wnt5a protein immunostain in the proximal VP ducts was strong in periductal mesenchyme (arrows) but negligible in the epithelium. Scale bars: A–D = 200μm; E–F, G, I = 50μm; H &J = 20μm. VP=ventral prostate, DP= dorsal prostate, LP1= lateral prostate type 1 ducts, LP2=lateral prostate type 2 ducts.
Role of Wnt5a in prostate growth and branching morphogenesis
An organ culture system that recapitulates prostate morphogenesis was used to examine the effects of Wnt5a protein on prostate growth and branching. While culture of newborn rat VPs for 6 days in testosterone resulted in morphogenesis of a complex branched ductal system, addition of Wnt5a markedly inhibited ductal outgrowth and branching (Fig. 3A). Distal tip numbers in images taken at day 6 were reduced from 58 ± 4 in control VPs to 11 ± 2 in lobes cultured in Wnt5a (Fig. 3B). Epithelial cell proliferation, as assessed by BrdU labeling, was significantly suppressed in the central-distal ducts (Fig. 3C–D) but not in the proximal ducts. Apoptotsis, as examined by TUNEL labeling, was rarely observed at pnd 6 in the presence or absence of Wnt5a (data not shown).
Figure 3.
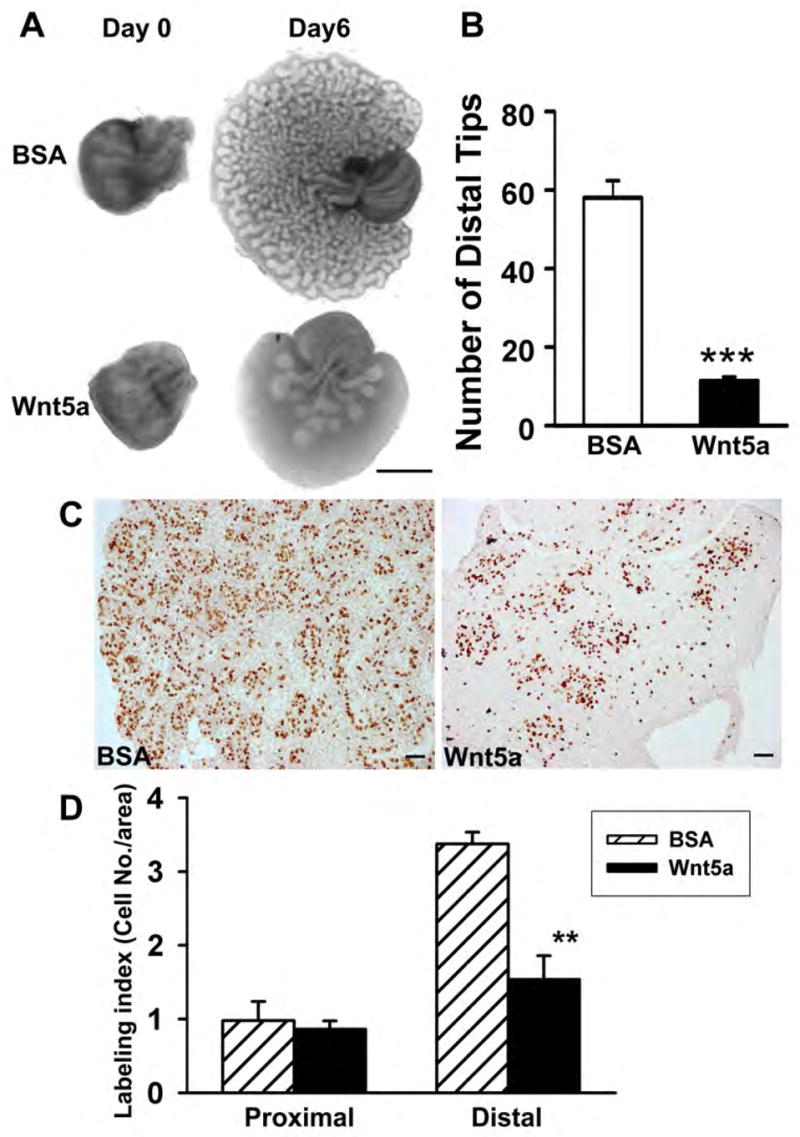
Cultured rat VP following addition of BSA (controls) or Wnt5a protein. Paired lobes from a single rat were treated with either BSA or Wnt5a (1.0 μg/ml) to permit direct comparison of Wnt5a treatment. (A) VP collected on pnd 0 and after culture for 6 days in BOCM with either BSA or Wnt5a protein. Wnt5a markedly inhibited the ductal outgrowth and branching. (B) Number of distal tips observed in 2-D photographs of VPs taken after 6 day culture with or without Wnt5a. Bars represent the mean ±SEM. ***P< 0.0001 versus BSA (n=5). (C) VPs were labeled with BrdU after 6-day culture in BSA or Wnt5a. The number of proliferating epithelial cells was greatly reduced in the central-distal epithelial ducts of Wnt5a-treated prostates as compared to BSA controls. (D) BrdU labeling index of epithelial cells in the proximal and central-distal regions of VP lobes cultured for 6 days with or without Wnt5a. The bar represents the mean ±SEM of 4 replicates. **P<0.05 versus BSA.
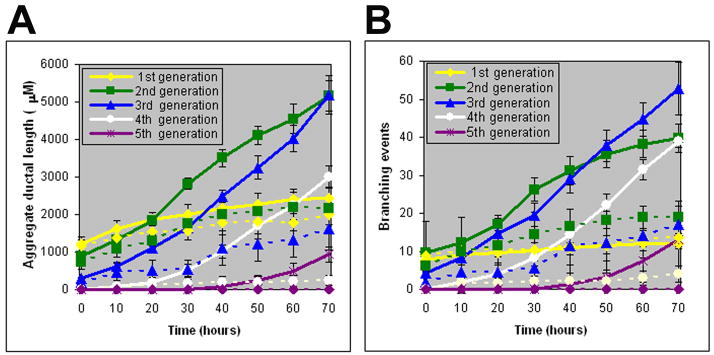
The suppressive effects of Wnt5a on prostatic branching patterns were assessed by real-time imaging. Quick-time movies of contralateral VP lobes simultaneously cultured in the presence of 1μg/ml BSA or Wnt5a protein for 72 hr are shown in Suppl. Fig. 1. While outgrowth of the original ducts with sequential branching of multiple ductal generations was observed in control lobes, ductal elongation and branchpoint formation were markedly reduced by Wnt5a. Kinetic analysis was performed on color-coded skeletonized images (Suppl. Fig. 2). The initial buds that evaginated from the UGS into the VP mesenchymal pad were classified as “original ducts” (4 per VP lobe). The branches that formed by terminal bud division or lateral branches off the original duct were considered “first generation”, branches off the first generation ducts were considered “second” generation and so forth. Frequently, the original ducts had begun to branch at the time of birth and first generation ducts were present. During the first 72 hr of culture, five branch generations developed in control VPs. Each generation progressively elongated (Figure 4A) and produced branchpoints at regular intervals (Fig. 4B) to create similar sized segments throughout the tissue. Branchpoint patterns included terminal bifeds (51%), terminal trifeds (13%) and lateral side branches (36%) resulting in a complex branched network (Suppl. Fig. 3A). Exogenous Wnt5a protein markedly reduced ductal elongation (Fig. 4A) and branching events over time (Fig. 4B) with only three branch generations forming over the 72 hr period. While the total tip number was markedly reduced as early as 20 hr after exposure to Wnt5a (Suppl. Fig. 3B), the pattern of branch point events was not altered (Suppl. Fig. 3A).
Figure 4.
Kinetics of prostatic bud elongation and branching in newborn rat contralateral VPs exposed to BSA (controls) or Wnt5a protein (1 μg/ml) for 72 hr. (A) Aggregate length (μM) of all ductal segments in each successive branch generation over time. Solid lines represent data from control VPs and dotted lines represent data from Wnt5a-exposed VPs. (B) Total number of branchpoint events in each ductal generation as a function of time. Solid lines represent data from control VPs and dotted lines represent data from Wnt5a-exposed VPs. Error bars denote SEM from 6 sets of experiments. Differences between the two treatments were statistically significant (P<0.05) from 20 hr onward for both data sets.
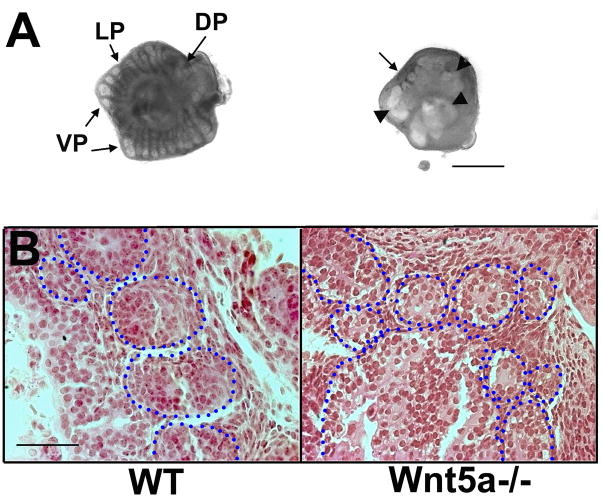
Null mutant Wnt5a−/− mice were used to assess the loss of Wnt5a expression on prostate morphogenesis. Since Wnt5a−/− mice are perinatal lethal, the UGS complex was dissected from e16.5 mice and cultured to examine the role of Wnt5a during the early stages of prostate development. It should be noted that the mouse prostate is less well developed than the rat prostate and forms only secondary with some tertiary branchpoints in vivo at pnd 15 and minimal branching after 6 days in vitro. After 4 days of culture with DHT, loosely corresponding to pnd 2, prostatic buds were observed elongating from the UGS in the ventral, lateral and dorsal directions in the Wnt5a+/+ mice with equivalent widths and even spacing between the emerging ducts (Fig. 4). In sharp contrast, epithelial projections in the Wnt5a−/− UGS were disorganized in position and consisted of large bulbous outgrowths intermingled with short buds projecting in a seemingly random manner (Fig. 4). The failure of Wnt5a−/− prostates to establish orderly growth suggests a critical role for Wnt5a in organizing bud position and controlling proper outgrowth and movement of cells along a proximal-to-distal axis. Heterozygote and wild-type pups maintained through pnd 30 had similar prostate weights and distal tip numbers indicating no Wnt5a+/− haplotype for the prostate gland.
Role of Wnt5a in prostate cell differentiation
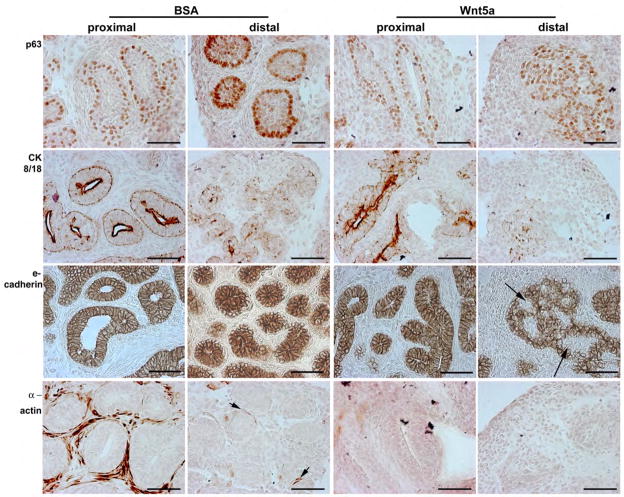
Newborn rat VPs cultured in the absence or presence of exogenous Wnt5a protein were examined with cell differentiation markers. After 6 days, control prostates had undergone cell differentiation that varied along the proximal-to-distal axis as described (Jarred et al., 2000; Prins and Birch, 1995). Proximal ducts had begun to canalize and contained a continuous layer of basal epithelial cells (p63+) underneath a layer of short columnar luminal cells (CK8/18+). The luminal cells exhibited basal-apical polarization as highlighted by lateral surface e-cadherin stain (Fig. 6). A multi-cell layer of smooth muscle cells (α-actin+) surrounded the proximal ducts. In the distal aspects of the branched ducts, differentiation was less advanced. Basal cells (p63+) lined the solid epithelial cords filled with p63+ and CK8/18+ cells that were polarized to form a pinwheel-shaped pattern (see e-cadherin stain). Although periductal mesenchyme occasionally stained for β-actin (Fig. 6, arrows), most cells were negative for markers of smooth muscle differentiation. Rat VPs cultured for 6 days with exogenous Wnt5a protein exhibited signs of delayed maturation and cell differentiation. While proximal duct epithelial cells were similar to controls prostates, the periductal mesenchyme showed no evidence of smooth muscle cell differentiation. In the distal ducts, the enlarged ductal tips were filled with p63+ basal cells with minimal CK8/18+ signal. Pockets of epithelial cells were negative for e-cadherin (Fig. 6, arrow) and the cells that stained for e-cadherin showed a lack of basal-apical alignment. The mesenchymal cells in the distal ducts exhibited no signs of differentiation in the presence of exogenous Wnt5a.
Figure 6.
Immunohistochemistry for p63 (basal cell marker), CK8/18 (luminal cell marker), e-cadherin, and α actin (smooth muscle cell marker) in proximal and distal regions of rat VPs cultured for 6 days with BSA or Wnt5a protein. The proximal prostatic ducts contained a bilayer of epithelial cells in both treatment groups with basal cells (p63+) situated along the basement membrane and differentiating luminal cells positioned above them (apical CK8/18+ stain). Strong e-cadherin stain is observed along the lateral edges of all epithelial cells, producing a pinwheel pattern in the polarized epithelium. While lumen formation was observed in most proximal ducts of BSA-cultured VPs, those treated with Wnt5a showed exhibited limited lumenization. Strong β-actin staining was observed in proximal periductal cells of BSA control VPs indicating their differentiation to smooth muscle cells. This mesenchymal cell differentiation had not occurred by day 6 in the prostates cultured with Wnt5a. In the distal region of BSA-cultured prostates, the solid ducts were nonlumenized but showed appropriate organization and early differentiation with a continuous, single cell layer of p63+ basal cells along the basement membrane, light CK8/18 staining in the central cells. In contrast, the solid epithelial cords in the distal VPs cultured with Wnt5a were largely filled with p63+ basal cells interspersed with CK8/18+ cells and many e-cadherin negative cells (arrows) suggesting altered differentiation and inappropriate epithelial polarization. While early evidence of mesenchymal differentiation to smooth muscle cells was observed by β-actin staining in distal BSA-treated VPs (arrows), this had not occurred in Wnt5a-treated prostates. Scale bar = 50μm.
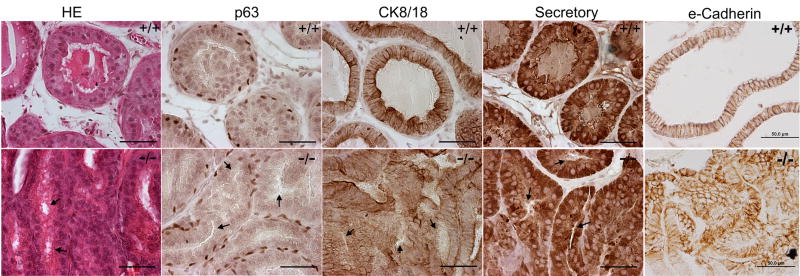
Since the prostate has a limited capacity to fully differentiate in vitro, the role of Wnt5a on prostate maturation and functional differentiation was examined in Wnt5a−/−mouse prostates that were rescued by renal grafts for 4 weeks. Loss of prostatic Wnt5a did not interfere with the capacity of the epithelial cells to fully cytodifferentiate and express secretory proteins as evidenced by the presence of intermittent p63+ basal cells along the basement membrane, luminal columnar epithelial cells (CK8/18+) and strong immunostain for DLP protein in dorsolateral lobes of mutant mice which was similar to wild-type prostates (Fig. 7). Further, there was no difference in proliferation or apoptosis rates or in AR immunostain between the two genotypes at the four week time point (data not shown). However, Wnt5a−/− prostate grafts consistently exhibited defective lumenization of epithelial ducts with apparent loss of luminal cell polarity. In wild-type prostates, acini contained a single cell layer of cuboidal-columnar luminal cells oriented in a basal-apical manner with basally located nuclei, lateral e-cadherin stain and open lumens containing secretions. In contrast, ducts of Wnt5a−/− prostate grafts were typically filled with piled luminal cells that lacked appropriate orientation as visualized by nuclei positioned centrally and apically and e-cadherin stain encircling the piled cells (Fig. 7). Lumen formation either failed to occur or appeared arrested at an early stage of typically observed at day 5–10 of postnatal life (Fig. 7, arrowheads). In total, these findings suggest that while Wnt5a does not permanently affect terminal differentiation, it plays a role in controlling prostate epithelial cell proliferation, organization and polarization.
Figure 7.
Renal grafts of Wnt5a+/+ (top row) and Wnt5a−/− knockout (bottom row) mouse prostates. The UGS/prostate complex was removed at e16.5 and grafted under the renal capsule of nude mice for 30 days. Tissues were sectioned and stained with H&E or immunostained for p63 (basal cell marker), CK8/18 (luminal cell marker), secretory DLP protein and e-cadherin. Arrows point to arrested lumen formation observed throughout sections of mutant Wnt5a−/− prostates. Photos of H&E stain,p63, CK8/18 and e-cadherin are from the VP while DLP protein IHC was taken of DLP regions of the graft. Scale bar = 50μM.
Effects of Wnt5a on prostatic morphoregulatory genes
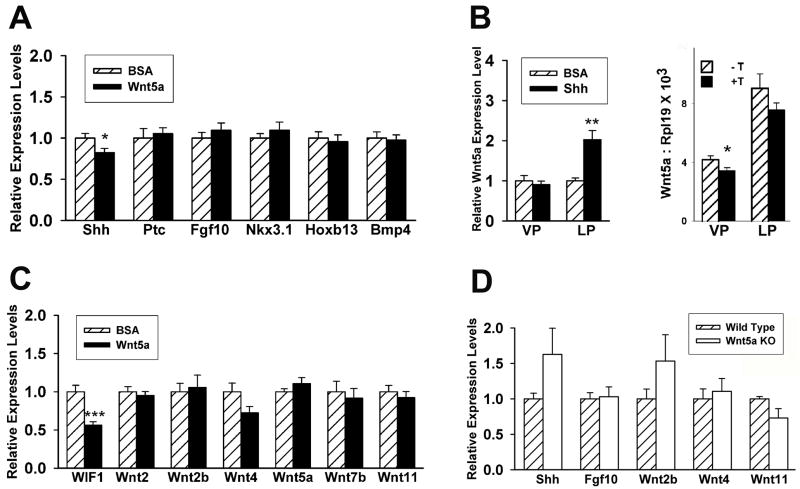
Previous studies have identified a complex regulatory network between developmental genes involved in prostate morphogenesis (Prins & Putz, 2008). Since Wnt5a has been shown to affect the expression of multiple secreted morphogens in other developing structures (Li et al., 2005; Li et al., 2002; Mericskay et al., 2004), the effects of Wnt5a addition or deletion on expression of several prostatic developmental genes were assessed. To evaluate direct effects of Wnt5a on prostate gene expression prior to marked changes in growth (Pu et al., 2007), organ cultures of pnd 0 rat VP lobes with or without Wnt5a protein were terminated after 18 hr and gene expression in individual lobes was determined. As shown in Fig. 8A, Wnt5a did not alter expression of Fgf10, Bmp4, Nkx3.1, Hoxb13 or ptc, the Shh receptor, but did have a small yet significant suppressive effect on prostatic Shh mRNA levels. Since Shh has previously been shown to up-regulate prostatic Bmp4 and down-regulate Fgf10 expression (Pu et al., 2007), the effect of Shh protein on Wnt5a expression was examined in contralateral VP and LP cultures. While Shh had no effect on VP Wnt5a mRNA levels, Wnt5a was significantly up-regulated by Shh in the LP lobes indicating lobe specificity in this regulatory network (Fig. 8B). Testosterone produced a small suppressive effect on Wnt5a mRNA levels in both the VP and LP lobes after 18 hr of exposure (Fig. 8B). To determine if Wnt5a autoregulates its own expression or affects the expression of other Wnt genes during prostate development, their expression was examined after 18 hr of culture in exogenous Wnt5a. While no change was observed in mRNA levels of any Wnts, there was a significant down-regulation of Wif1, a secreted antagonist of canonical and noncanonical Wnts (Fig. 8C). The effect of Wnt5a knock out on gene expression was examined in mouse Wnt5a+/+ and Wnt5a−/− UGS/prostates collected on e18.5 and cultured for 4 days. Loss of Wnt5a resulted in increased Shh and Wnt2b; however, this was not statistically significant due to high variability in Wnt5a−/− specimens as a function of disturbed growth. Nonetheless, these findings support the observations in the rat VP cultures where Wnt5a protein had minimal effects on most developmental genes and only modest down-regulation of Shh levels.
Figure 8.
Effect of Wnt5a addition or loss on prostatic gene expression. A–C: Gene expression in newborn rat VP lobes after 18 hr organ culture as measured by real-time RT-PCR. (A) Effect of 1μg/ml Wnt5a protein on expression of multiple developmental genes. Bars represent mean ± SEM for 15 replicates. * P <0.05 BSA vs. Wnt5a protein. (B) Left: Effect of 2 μg/ml Shh protein on Wnt5a expression in the rat VP and LP after 18 h of culture. Shh increased Wnt5a expression in LP but not VP as compared to BSA controls. Bars represent the mean ± SEM for 8 samples. ** P<0.01 Shh vs. BSA. Right: Effect of 10 nM testosterone on Wnt5a expression in the rat VP and LP after 18 hr culture. Testosterone modestly suppressed Wnt5a gene expression in both lobes. Bars represent the mean ± SEM for 8 samples. ** P<0.05 -T vs +T. (C) Effect of 1μg/ml Wnt5a protein on expression of Wnt genes and WIF1 in rat VP. Bars represent mean ± SEM for 15 replicates. ** P <0.001 BSA vs. Wnt5a protein. (D) Expression of Shh, Fgf10, Wnt2b, Wnt4, Wnt5a, Wnt11 and WIF1 mRNA in wild type and Wnt5a−/− knockout mouse UGS/prostate tissues after 4 days of culture. Bars represent mean ± SEM for 6 replicates.
Neonatal estradiol up-regulates prostatic Wnt5a expression
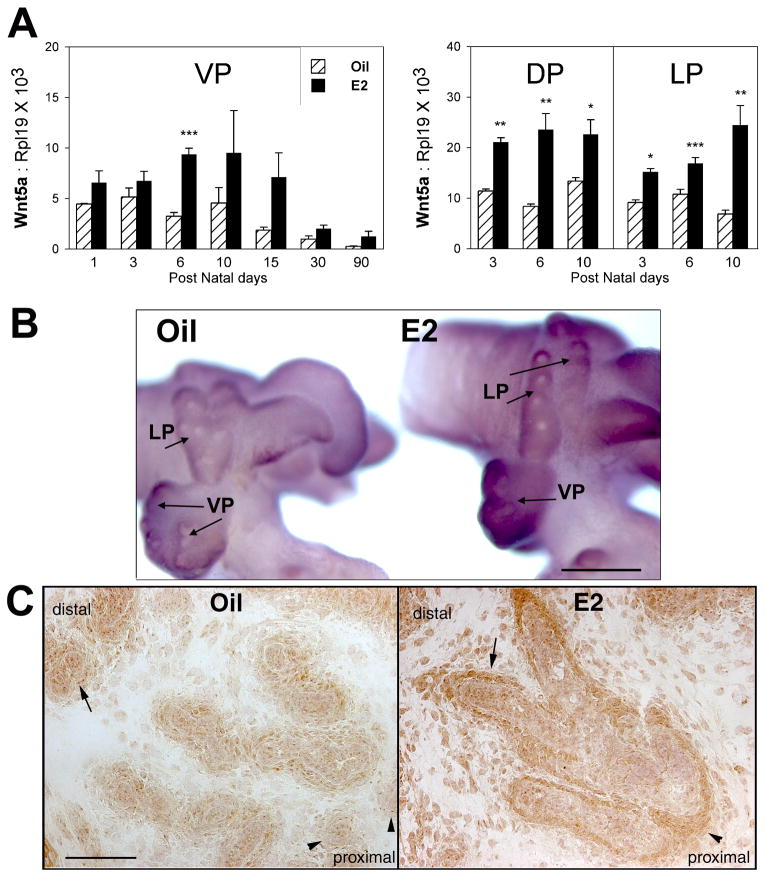
Detailed previous studies in our laboratory have examined the effects of early estradiol exposures on prostate gland morphogenesis and shown that high-doses lead to reduced growth, abnormal development and onset of severe prostatic dysplasia with aging (Prins, 1992; Prins, 1997). This is mediated through early shifts in steroid receptor expression including down-regulation of AR and up-regulation of ERα (Prins, 1992; Prins and Birch, 1995; Prins and Birch, 1997). Down-stream effects of altered steroidal regulation include lobe-specific changes in expression of key developmental genes including Shh, Fgf10, Bmp4, Nkx3.1 and Hoxb13 (Huang et al., 2004; Huang et al., 2005; Prins et al., 2006; Pu et al., 2004). In the present studies, estradiol exposure on pnd 0, 2 and 4 resulted in an immediate and significant increase in mesenchymal Wnt5a mRNA and protein in all three prostate lobes which was sustained through adulthood in the VP (Fig. 9A–C). This increased Wnt5a expression may contribute to the estrogen-induced hypomorphogenesis observed in these tissues. Since elevations in Wnt5a expression have been associated with several malignancies, including prostate cancer (Iozzo et al., 1995; Liu et al., 2008; Yu et al., 2007; Zuidervaart et al., 2007), the elevated Wnt5a in estrogenized prostates may also contribute to the severe dysplasia observed in neonatal estrogenized prostates.
Figure 9.
Effects of neonatal estradiol exposure on prostatic expression of Wnt5a in the separate rat prostate lobes. (A) Real-time RT-PCR of Wnt5a mRNA levels over time in the VP, DP and LP of rats exposed to oil or 25 μg estradiol benzoate on pnd 0, 2 and 4. Estrogen exposure resulted in a significant and sustained increase in prostatic Wnt5a expression. Bars represents the mean ± SEM of 3–10 tissues at each time point. * P< 0.05, ** P < 0.01, *** P< 0.001, Oil vs E2. (B) wmISH of Wnt5a mRNA in the pnd 3 prostatic complex of rats exposed to oil (left) or estradiol (right) on pnd 0 and 2. Tissues were processed together to permit direct comparison of signal intensity. Photos are representatives of six separate sets of tissues. Scale bar = 200μM. (C) Immunohistochemistry of Wnt5a protein in pnd 5 VPs of rats treated with oil (left) or estradiol (right) on pnd 0, 2 and 4. Photos are taken from tissues sections mounted on a single glass slide to permit direct comparisons. Wnt5a signal is observed in periductal mesenchyme (arrows) and epithelial cells in the distal ducts of control prostates and is weaker in the proximal region (arrowhead). In contrast, Wnt5a protein immunostain is increased in neonatal estradiol-exposed prostates with strong periductal signal observed in the distal (arrow) as well as proximal lobes (arrowhead). Scale bar = 100μm.
Discussion
Wnt5a is expressed by distal periductal mesenchymal cells and interacts with distal epithelial cells
The present studies establish an important role for the noncanonical Wnt5a during prostate gland development. Wnt5a is expressed by prostatic periductal mesenchymal cells with focally intense expression at the distal tips. This pattern is similar to distal Wnt5a localization in other developing structures. However, unlike in lungs where Wnt5a is expressed by distal epithelium as well as stroma (Li et al., 2002) and uterus where low but detectable Wnt5a is expressed in epithelium in addition to abundant stromal levels (Mericskay et al., 2004), prostate Wnt5a gene expression is restricted to mesenchymal cells. Clear evidence for Wnt5a secretion and paracrine interaction with epithelial cells is provided by IHC which localizes Wnt5a protein to the periductal mesenchymal cells, extracellular matrix and to epithelial cells in the distal ducts. Thus it appears that Wnt5a is another component of the previously described distal signaling center in the developing prostate where active growth and branching occur through the coordinated actions of a morphoregulatory gene signaling network (Huang et al., 2005; Prins and Putz, 2008).
Role of Wnt5a in prostate duct outgrowth, branching, and epithelial proliferation
Ex vivo and in vivo studies with rat and mouse prostates demonstrate that, similar to its role in other body structures, Wnt5a plays an essential role in prostate duct outgrowth and patterning along the proximal-distal axis. The bud initiation and outgrowth stage of prostatic development was assessed by organ culture of embryonic UGS from Wnt5a−/− mice removed prior to in vivo bud initiation at e16.5. After 4 days of ex vivo culture, Wnt5a−/− UGS exhibited large bulbous outgrowths intermingled with stunted buds projecting in a random manner which sharply contrasted with the evenly spaced protruding prostate buds of equivalent width in wild-type mice. Thus while Wnt5a is not essential for prostate bud initiation, it is required for proper bud position at regular intervals, regulation of bud size and movement of cells along the proximal-to-distal axis.
Ex vivo cultures of newborn rat prostates found that exogenous Wnt5a protein was capable of inhibiting epithelial cell proliferation at the leading edge of outgrowing ducts resulting in marked suppression of ductal outgrowth and branch point formation. It is noteworthy that while total tip number was significantly reduced by the exogenous Wnt5a, the proportion of distal tip bifeds, trifeds and lateral branching events was unaffected. Together these findings suggest that secreted Wnt5a has a growth and branch point suppressive function at the ductal tips as well along lateral surfaces in the central-distal regions during active branching morphogenesis. Previous studies from our laboratory and others have shown important roles for Fgf10 in stimulating ductal outgrowth (Donjacour et al., 2003; Huang et al., 2005), Shh in inhibiting distal tip growth at specific sites during branch point formation (Pu et al., 2004), and Bmp4 in suppressing bud and duct outgrowth (Lamm et al., 2001; Prins et al., 2006). We propose that Wnt5a contributes to this regulatory network that both stimulates and inhibits growth at specific sites to regulate coordinate outgrowth and branch point formation at regular intervals along the proximal-distal axis resulting in a unique branch pattern during glandular morphogenesis.
While similar roles for Wnt5a have been reported in other branched structures, there are also notable tissue specific differences. The lungs of Wnt5a−/− mice exhibit truncation of the trachea and overexpansion of the distal airways (Li et al., 2002) which bears similarity to the cultured UGS/prostate complex with inhibition of bud elongation and overexpansion into large bulbous structures. While renal grafts of embryonic Wnt5a−/− female reproductive tracts were capable of forming a uterine compartment, they failed to undergo glandulargenesis (Mericskay et al., 2004) which was not the case for grafted Wnt5a−/− prostates in the present study. In the mammary gland, exogenous Wnt5a protein inhibited ductal extension and lateral branching specifically (Roarty and Serra, 2007) which differs from the prostate where branch inhibition was observed at the distal tips as well as at lateral sites. It is possible that differences in Wnt5a action across tissues may be a function of interactions with other genes in an organ-specific manner.
Role of Wnt5a in epithelial cell differentiation, polarity and lumen formation within prostatic ducts
In the present studies, delayed differentiation of epithelial and mesenchymal cells and altered epithelial polarity was observed in rat ex vivo VP cultures exposed to exogenous Wnt5a suggesting that Wnt5a may play a role in suppressing cell differentiation. However, prostates from null mutant Wnt5a−/− mice grafted under the renal capsule showed full differentiation of mesenchyme to stromal cells and epithelium to basal cells and secretory luminal cells indicating that Wnt5a is not essential for cyto- and functional differentiation of the prostate gland. Similar findings were noted in Wnt5a−/−lungs where differentiation of specialized cells was unaffected by loss of this protein (Li et al., 2002). It is noteworthy that postnatal rescue of Wnt5a−/− mouse mammary glands through renal grafts for 1 week resulted in accelerated mammary development (Roarty and Serra, 2007). Since prostatic renal grafts were assessed after 4 weeks in the present studies, we could not determine whether differentiation was accelerated. However, the delayed prostate cell maturation in rat VP organ cultures with exogenous Wnt5a concurs with the mammary results. Since prostatic cell differentiation is temporally coordinated with ductal outgrowth during morphogenesis, we postulate that the observed shifts in glandular maturation with perturbed Wnt5a levels are a function of altered growth rates of prostatic ducts rather than direct effects on cell differentiation pathways.
Despite complete epithelial cytodifferentiation in renal grafted Wnt5a−/− prostates, the ducts consistently exhibited defective and incomplete lumenization with apparent loss of luminal cell polarity and appropriate alignment. Noncanonical Wnt genes affect or control major cell movements and directed migrations in many developing structures that include planar cell polarity and convergent extension (Veeman et al., 2003). Wnt5a has been shown to function in planar cell polarity of hair cells in the inner ear cochlea, a process essential for proper cell alignment and orientation of stereocilia (Qian et al., 2007). Most recently, Wnt5a was found to enhance cell polarization in dispersed melanoma cells through the recruitment of actin, myosin IIB, Frz3 and adhesion molecules into an intracellular structure referred to as W-RAMP (Witze et al., 2008). This structure was shown to distribute in a polarized manner, direct membrane retraction and influence direction of cell movement. In this manner, Wnt5a was demonstrated to control cell polarity and directional movement in response to positional clues from chemical gradients. In light of these findings, it is reasonable to suggest that Wnt5a may play an essential role in directing prostate luminal cell polarity and appropriate lumen formation.
Cross-regulation of Wnt5a with other morphoregulatory genes in the signaling network that dictates prostate gland development
A gene regulatory network exists within the developing prostate gland that organizes normal prostate development through a temporal series of reciprocal signals and feedback lobes (Prins and Putz, 2008). Previous studies with Wnt5a−/− null mutant lungs determined that expression of Shh, ptc, Fgf10 and Bmp4 was increased in the absence of Wnt5a (Li et al., 2002) whereas targeted Wnt5a overexpression in lung epithelium resulted in down-regulation of Shh and ptc expression (Li et al., 2005). Further, direct down-regulation of Shh expression by Wnt5a was demonstrated in lung carcinoma A549 cells cotransfected with Shh promoter reporter and Wnt5a expression constructs (Li et al., 2005). Results from the present study demonstrate that Wnt5a is involved in the prostatic cross-regulatory gene network, most notably through down-regulation regulation of Shh and reciprocal up-regulation of Wnt5a by Shh to form a negative feed-back loop. This is noteworthy since our previous studies showed highly localized differences in Shh expression in distal tip epithelium leading to focal changes in Fgf10 and Bmp4 gene expression and cell proliferation (Huang et al., 2005). It was proposed that these localized shifts in gene expression directly lead to branch point formation in outgrowing prostatic ducts. We herein suggest that Wnt5a modulation of Shh expression may contribute to this signaling network that permits branching morphogenesis to proceed in an organized manner in the developing prostate gland. Interestingly, cross regulation between Wnt5a and Shh was observed in a lobe-specific manner since short-term culture with Shh protein increased Wnt5a expression in rat LP but not VP cultures. The separate prostate lobes have distinctive branching patterns (Hayashi et al., 1991) and it is intriguing to speculate that this may be a function of differential temporal expression of Wnt5a and cross-regulation of key morphoregulatory genes that dictate branching morphogenesis.
While there was no evidence for modulation by Wnt5a of any Wnt morphogens expressed during prostate development or for autoregulation of Wnt5a expression, Wnt5a protein markedly decreased prostatic expression of Wif1 in the neonatal rat prostate. This suggests that in addition to activation of its own downstream signaling pathway, Wnt5a can modulate noncanonical and canonical Wnt signaling through downregulation of this secreted Wnt inhibitor.
Hormone regulation of Wnt5a in the prostate gland
Hormone regulation of Wnt5a expression throughout the body appears to be organ specific. Wnt5a expression is not regulated by estrogen or progesterone in the mammary gland (Humphreys et al., 1997; Weber-Hall et al., 1994) whereas it is up-regulated in the uterus after neonatal exposure to the potent synthetic estrogen, diethylstilbestrol (DES) (Couse et al., 2001; Mericskay et al., 2004). These later results are similar to those of the present study where estradiol permanently up-regulates Wnt5a expression in the prostate. Interestingly, the uterine Wnt5a response to DES was not dependent on ERα since its expression increased in ERα knock out mice treated with DES (Couse et al., 2001). While the requirement of stromal ERα for Wnt5a up-regulation by estradiol was not directly examined in the present study, we have previously shown that neonatal estrogen’s phenotypic effects on the prostate require ERα (Prins et al., 2001). Although a genomic screen identified estrogen response elements in the promoters of Wnt2b, 4 and 10b (Bourdeau et al., 2004), they were not identified in the Wnt5a promoter thus it is possible that estrogen actions on Wnt5a expression are not direct. In the mammary gland, Wnt5a is up-regulated by Tgfβ (Roarty and Serra, 2007). Since we have previously shown that neonatal estradiol up-regulates Tgfβ1 levels in prostatic periductal stromal cells (Chang et al., 1999), it is possible that estradiol effects on prostatic Wnt5a are indirectly mediated through elevated Tgfβ1. Similar to our previous report (Pu et al., 2007), testosterone exposure during early prostate development suppressed Wnt5a expression which supports the hypothesis that down-regulation of growth inhibitory factors by androgens contributes to global prostate morphogenesis. Retinoic acid has also been shown to directly regulate Wnt gene expression and signaling in a variety of developing mammalian organs (Chen et al., 2002; Kudoh et al., 2002) indicating that steroidal regulation of Wnt gene expression is widespread. Since the developing prostate expresses RARs, RXRs and retinoid metabolizing enzymes (Prins et al., 2002), it is possible that retinoids may also be involved in regulation of prostatic Wnt5a expression.
In summary, the present studies establish a role for Wnt5a during prostate gland development. Wnt5a is involved in initial bud positioning, regulation of ductal outgrowth along the proximal-distal axis, branchpoint formation, luminal cell polarity and lumen formation within the prostatic ducts. Evidence suggests that Wnt5a interacts with other morphoregulatory genes in a complex signaling network to tightly control branching morphogenesis and glandular maturation. Expression of Wnt5a is modulated by steroids during prostate development including testosterone and estradiol that suppress and stimulate expression, respectively. This is of particular importance since aberrant expression of WNT5a has been shown in several human malignancies, including elevated expression in prostate carcinomas (Iozzo et al., 1995). These findings add to the growing body of knowledge of the regulatory mechanisms involved in prostate development which are key to understanding abnormal growth regulation associated with prostate cancer.
Supplementary Material
Suppl. Figure 1. Quick time movie of contralateral VP lobes from the same newborn rat cultured in the presence of BSA (left) or 1 μg/ml Wnt5a protein (right) over 72 hr. Movie is representative of six separate sets of experiments.
Suppl. Figure 2. Branch generations of cultured contralateral rat VP lobes in the presence of BSA (left) or 1mg/ml Wnt5a (right). Successive images were taken every 30 minutes for 72 hr to track the branching events. A color coded skeleton is used to indicate the generation of branches according to the following convention: The four primary buds emerging from the UGS are considered the original ducts (red); branches that form off the primary ducts are considered the first generation (yellow); branches that form off the first generati on and elongate are considered the second generation (green); third generation (blue); forth generation (white); fifth generation (purple).
Suppl Figure 3. Branch tip number and pattern in contralateral newborn rat VPs cultured in BSA or Wnt5a. (A) Total tip number in 3D analysis as a function of time for VPs cultured in BSA (squares) vs VPs cultured in Wnt5a (triangles). Each data point represents the mean of 6 sets of experiments and bar denotes SEM. * denotes significant differences, p<0.01. (B) Percentage of branching events occurring as terminal bifeds, terminal trifeds or lateral side branches in VPs cultured in BSA (white bars) or Wnt5a (solid bars) for 72 hr. Bar represents the mean ± SEM for 6 sets of experiments.
Figure 5.
Wnt5a+/+ (WT) and Wnt5a−/− mouse UGS-prostatic complex collected on e16.5 and cultured for 4 days in basal medium with 10−8M dihydrotestosterone. (A) The UGS from wild-type mouse (left) developed numerous and symmetric elongating buds in the dorsal (DP), lateral (LP) and ventral (VP) regions. In contrast, epithelial buds from the Wnt5a−/− UGS (right) were nonsymmetrical in position, widely varied in size from bulbous (arrowheads) to small buds (arrow) which failed to elongate. Bar = 500μM. (B) Eosin-stained cross sections of cultured prostatic tissue show numerous prostatic ducts of similar diameter in the wild-type (WT) tissue and irregular bud size and position in tissue from homozygous Wnt5a−/− mice. Blue circles outline the epithelial buds. Bar = 50 μM.
Acknowledgments
The authors thank Dr. Yiming Zhu from the University of Chicago for generously providing the rat Wnt5a plasmid and Ms. Anna Vogelzang for data analysis of prostate morphogenesis in real-time. This work was supported from an NIH grant DK40890 to GSP and American Urologic Association Foundation Fellowship to LH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bejsovec A. Wnt pathway activation: New relations and locations. Cell. 2005;120:11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Molecular Endocrinology. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- Buhler TA, Dale TC, Kieback C, Humphreys RC, Rosen JM. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev Biol. 1993;155:87–96. doi: 10.1006/dbio.1993.1009. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes and Development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Chang WY, Birch L, Woodham C, Gold LI, Prins GS. Neonatal estrogen exposure alters the transforming growth factor-β signaling system in the developing rat prostate and blocks the transient p21cip1/wafl expression associated with epithelial differentiation. Endocrinology. 1999;140:2801–2813. doi: 10.1210/endo.140.6.6833. [DOI] [PubMed] [Google Scholar]
- Chen MH, Antoni L, Tazi-Ahnini R, Cork M, Ward SJ, Bavik CO. Identification of known and novel genes whose expression is regulated by endogenous retinoic acid during early embryonic development of the mouse. Mechanisms of Development. 2002;114:205–212. doi: 10.1016/s0925-4773(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocrine Related Cancers. 2003;10:537–60. doi: 10.1677/erc.0.0100537. [DOI] [PubMed] [Google Scholar]
- Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-α knockout mice exhibit resistance to the developmental effects of diethylstilbestrol exposure on the female reproductive tract. Developmental Biology. 2001;238:224–238. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LWK. Stromal-epithelial interactions: I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/Y) mice. J Steroid Biochem. 1981;14:1317–1321. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha G. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Gavin BJ, McMahon AP. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in prostnatal development of the mammary gland. Molecular and Cellular Biology. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–6. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Andrology. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific supression by neonatal estrogens. Developmental Biology. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Humphreys R, Lydon J, O’Malley B, Rosen J. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endo. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt5a in human malignancy. Can Research. 1995;55:3495–4399. [PubMed] [Google Scholar]
- Jarred R, Cancilla B, Prins GS, Thayer K, Cunha GR, Risbridger G. Evidence that estrogens directly alter androgen-regulated prostate development. Endocrinology. 2000;141:3471–3477. doi: 10.1210/endo.141.9.7648. [DOI] [PubMed] [Google Scholar]
- Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, Rubin JS, Marker PC. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Developmental Biology. 2008;317:161–73. doi: 10.1016/j.ydbio.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Research. 2005;65:10423–30. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–4346. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Developmental Biology. 2001;232:301–314. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li J, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Developmental Biology. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Developmental Biology. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang L, Zhang S, Lin J, Zhang S, Feitelson MA, Gao H, Zhu M. Mutations in the C-terminus of the X protein of hepatitis B virus regulate Wnt-5a expression in hepatoma Huh7 cells: cDNA microarray and proteomic analyses. Carcinogenesis. 2008;29:1207–14. doi: 10.1093/carcin/bgn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:423–424. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-msenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–62. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biological Chemistry. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergance of Wnt, β-catenin and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Hogan BLM. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. Journal of Biology. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am J Anat. 1936;60:79–127. [Google Scholar]
- Price D. Comparative aspects of development and structure in the prostate. In: Vollmer EP, editor. Biology of the Prostate and Related Tissues. Vol. 12. National Cancer Institute; Washington, DC: 1963. pp. 1–27. [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS. Developmental estrogenization of the prostate gland. In: Naz RK, editor. Prostate: Basic and Clinical Aspects. C. R. C. Press; Boca Raton: 1997. pp. 247–265. Chapter 10. [Google Scholar]
- Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland in mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Canc Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143:3628–3640. doi: 10.1210/en.2002-220184. [DOI] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Annals of the New York Academy of Sciences. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;6:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–640. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- Pu Y, Huang L, Birch L, Prins GS. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology. 2007;148:1697–1706. doi: 10.1210/en.2006-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Huang L, Prins GS. Sonic Hedgehog-Patched-Gli Signaling in the Developing Rat Prostate Gland: Lobe-Specific Suppression by Neonatal Estrogens Reduces Ductal Growth and Branching. Developmental Biology. 2004;273:257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz O, Schwartz CB, Kim S, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat: I. Effects on the prostate gland. Biol Reprod. 2001;65:1496–1505. doi: 10.1095/biolreprod65.5.1496. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones CD, Rzadainska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a finctions in plnara cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and Tgf-β-mediated inhibition of ductal growth. Development and Disease. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–1432. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wang BE, Wang XD, Ernst JA, Polakis P, Gao WQ. Regulation of epithelial branching morphogenesis and cancer cell growth of the prostate by Wnt signaling. PLoS ONE. 2008;3:e2186. doi: 10.1371/journal.pone.0002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Hall S, Phippard D, Niemeyer C, Dale T. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Diferentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Bradley A, McMahon A, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer & Prostatic Diseases. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY, Kim KW, Jung JS. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Letters. 2007;257:172–181. doi: 10.1016/j.canlet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang TJ, Hoffman BG, Ruiz de Algara T, Helgason CD. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expr Patterns. 2006;6:310–24. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Zuidervaart W, Pavey S, van Nieuwpoort FA, Packer L, Out C, Maat W, Jager MJ, Gruis NA, Hayward NK. Expression of Wnt5a and its downstream effector beta-catenin in uveal melanoma. LMelanoma Research. 2007;17:380–386. doi: 10.1097/CMR.0b013e3282f1d302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Quick time movie of contralateral VP lobes from the same newborn rat cultured in the presence of BSA (left) or 1 μg/ml Wnt5a protein (right) over 72 hr. Movie is representative of six separate sets of experiments.
Suppl. Figure 2. Branch generations of cultured contralateral rat VP lobes in the presence of BSA (left) or 1mg/ml Wnt5a (right). Successive images were taken every 30 minutes for 72 hr to track the branching events. A color coded skeleton is used to indicate the generation of branches according to the following convention: The four primary buds emerging from the UGS are considered the original ducts (red); branches that form off the primary ducts are considered the first generation (yellow); branches that form off the first generati on and elongate are considered the second generation (green); third generation (blue); forth generation (white); fifth generation (purple).
Suppl Figure 3. Branch tip number and pattern in contralateral newborn rat VPs cultured in BSA or Wnt5a. (A) Total tip number in 3D analysis as a function of time for VPs cultured in BSA (squares) vs VPs cultured in Wnt5a (triangles). Each data point represents the mean of 6 sets of experiments and bar denotes SEM. * denotes significant differences, p<0.01. (B) Percentage of branching events occurring as terminal bifeds, terminal trifeds or lateral side branches in VPs cultured in BSA (white bars) or Wnt5a (solid bars) for 72 hr. Bar represents the mean ± SEM for 6 sets of experiments.