Abstract
Purpose
Collecting duct renal cell carcinoma (CDRCC) is a rare entity. Recent surgical series on CDRCC presented conflicting results. In this study, we use a United States population-based dataset to describe the survival experience of patients with CRDCC to those with clear cell renal cell carcinomas (CCRCC).
Materials and Methods
Cases of CDRCC and CCRCC were identified from the SEER Program (2001 – 2005). Demographic and pathologic characteristics at time of diagnosis were compared. Differences in disease-specific survival were compared with univariate and multivariate Cox regression analysis.
Results
A total of 160 cases of CDRCC were present in the SEER database from 2001-2005. Over that time period, 33,252 CCRCC cases were diagnosed. CDRCC was more common in African-Americans vs. Caucasians (23% vs. 9%, p<0.001). CDRCC were more commonly T3+ vs. T2/T1 (33% vs. 18%, p<0.001) and metastatic vs. regional/local (28% vs. 17%, p=0.001). Nephrectomy rates were similar (84% and 78%, p=0.06). The 3-year disease-specific survival (DSS) rates were 58% for CDRCC and 79% for CCRCC. In multivariate analysis, there was an increased mortality risk for patients with CDRCC compared to CCRCC (HR 2.42, 95% CI 1.72 – 3.39, p = 0.001).
Conclusions
Compared to CCRCC, patients with CDRCC present at higher stage and more often occurs in African-Americans. Even after adjusting for demographic, surgical and pathologic factors, the DSS is significantly worse for those with CDRCC compared to CCRCC. Further research into the biology of this rare tumor is required to explain these results.
Keywords: collecting duct, renal cell carcinoma, survival, population based
INTRODUCTION
Collecting duct renal cell carcinoma (CDRCC) is a rare entity, occurring in less than 2.0% of cases of renal cell carcinoma (RCC). As a result, our knowledge of CDRCC has primarily come from small case series with a uniformly poor prognosis. Based on these limited data, CDRCC has long been associated with a dismal prognosis when compared with that of other RCC subtypes, including the most common type, clear cell RCC (CCRCC). Recently, two multi-institutional surgical case series from Japan 1 and Europe 2 reported on collecting duct carcinomas. These series found similarities in the high rate of nodal and metastatic disease at presentation, but differed in the rate of pT3 disease, high-grade disease and survival. These series both are limited by the non-population based nature (referral bias) and exclusion of non-surgical patients (treatment bias). In this study, we use a United States population-based dataset to describe the pathological findings and survival experience of patients with collecting duct tumors compared to those with CCRCC.
MATERIALS AND METHODS
Data source
The Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify the cohort of patients for this study. SEER collects cancer incidence and survival data from seventeen population-based cancer registries accounting for approximately 26% of the United States population. Data from 2001-2005 from 17 SEER registries were used as CDRCC was not coded prior to these years 2000 and only 1 case of CDRCC was recorded in 2000.
Study population
Potential subjects were initially identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes for the kidney (C649). CDRCC cases were identified by ICD-O-3 histology code (8319). The comparison cohort consisted of cases with ICD-O-3 histology codes for ‘Clear Cell Adenocarcinoma NOS’ (8310) and ‘Clear Cell Adenocarcinoma, Renal Cell Carcinoma’ (8312).
Data collection and coding
Demographic data included subject age, race, gender and tumor registry. Age was categorized into 10-year age groups. Race was categorized as white, black or other based on SEER coding. Year of treatment and tumor registry were also ascertained. Pathologic data included tumor size (cm), primary T-stage (clinical stage used if pathologic stage not available, i.e., nephrectomy not performed), SEER historic stage (localized, regional, distant), nodal status (negative, positive, not-performed/unknown), metastatic status (present/absent) and tumor grade (well, moderately, poorly/undifferentiated, unknown). Surgical status was recoded (partial/complete nephrectomy), no surgery (biopsy or autopsy confirmation of pathology). Fuhrman grade, chemotherapy, immunotherapy, and co-morbidity data are not available in SEER. Survival was calculated starting at the date of diagnosis to the date of death due to kidney cancer. If death was not observed, patients were censored at the date of last follow-up.
Statistical Analysis
Demographic and pathologic data are reported for the cohort. Kaplan-Meier survival curves were generated to compare the unadjusted survival experience between CRDCC and CCRCC. Multivariable Cox regression was performed to evaluate the disease-specific mortality risk. All covariates were included in the model. SEER historic stage was used in the multivariate analysis rather than the T-stage as SEER does not record a T-stage for patients with metastatic disease. This limitation was mitigated by inclusion of tumor size which was available for most patients (91%). Sub-analyses were performed that only included (1) only patients undergoing surgery, (2) T3a disease or less with non-metastatic disease, (3) excluding non-high grade CCRCC, and (4) those with metastatic disease.
The proportional hazards assumption for the Cox regression was evaluated with Schoenfeld residuals. Stratified Cox regression was used for variables that violated the proportional hazards assumption. Individual variables and the combined model were then tested and met the proportional hazard assumption (p=0.32, where p<0.05 indicates evidence of non-proportionality). In the subset models of only patients with (1) surgical treatment, (2) T3a or less with no distant metastasis and (3) only high grade CCRCC as comparisons to all CDRCC, the proportional hazards assumptions were also met (p=0.28, 0.81, 0.97 respectively). Hazard ratios (HR) are presented along with their 95% confidence intervals (95%CI). All statistical analyses were conducted using Stata software, Version 8 (Stata, Inc., College Station, TX).
RESULTS
A total of 160 cases of CDRCC were identified over the years 2001–2005. Over that same time period, there were 33,252 cases of CCRCC in the SEER database. Table 1 lists the demographic and pathologic characteristics between the two histologies. Those with CDRCC were more commonly male (70% vs. 62%, p=0.03) and African-American (23% vs. 9%, p<0.001). CDRCC accounted for 1.2% of RCC cases in African-Americans compared with only 0.4% of RCC in Caucasians. There was no difference in age at diagnosis (median 63 vs. 64 yrs, p=0.44). Cases of CDRCC presented at higher stage compared to the CCRCC group, as evidenced by higher rates of T3-4 disease (33% vs. 18%, p<0.001), nodal involvement (15% vs. 2%, p<0.001) and distant metastases (28% vs. 17%, p=0.001) in those with CDRCC vs. CCRCC, though mean tumor size was not significantly greater in the CDRCC group (5.9 cm vs. 5.7 cm, p=0.61). Higher grade disease was more common in those with CDRCC (70% vs. 31%, p<0.001), though grade was missing from 38% of patients in each group. Surgery (radical or partial nephrectomy) was performed in 84% of CDRCC and 78% of CCRCC cases respectively (p=0.06). There was no difference in tumor laterality between CDRCC and CCRCC. Bilateral tumors were not reported in CDRCC, but 0.31% (n=102) CCRCC were bilateral. Exclusion of these patients did not change the survival analysis estimates performed below.
Table 1.
Demographic and Pathologic Characteristics Between CDRCC and CCRCC
| |
CDRCC N (%) |
CCRCC N (%) |
p-value |
|---|---|---|---|
|
Total cases |
160 |
33,252 |
|
| Demographics | |||
| Age | 0.44 | ||
| < 50 | 32 (20) | 5,222 (16) | |
| 51 – 59 | 36 (23) | 7,634 (23) | |
| 60 – 69 | 39 (24) | 8,606 (26) | |
| 70 – 79 | 31 (19) | 7,853 (24) | |
| 80 + | 22 (14) | 3,937 (12) | |
| Race | < 0.001 | ||
| White | 112 (70) | 28,495 (86) | |
| Black | 36 (23) | 2,849 (9) | |
| Other | 12 (8) | 1,759 (5) | |
| Gender | 0.03 | ||
| Male | 112 (70) | 20,502 (62) | |
| Female | 48 (30) | 12,750 (38) | |
| Year of Diagnosis | 0.44 | ||
| 2001 | 26 (16) | 6,287 (19) | |
| 2002 | 25 (16) | 6,284 (19) | |
| 2003 | 40 (25) | 6,655 (21) | |
| 2004 | 36 (23) | 6,899 (21) | |
| 2005 | 33 (21) | 7,127 (21) | |
| Pathologic Characteristics | |||
|---|---|---|---|
| Tumor size – median (range) | |||
| Stage | < 0.001 | ||
| T1a | 42 (26) | 11,008 (33) | |
| T1b | 17 (11) | 6,669 (20) | |
| T2 | 16 (10) | 4,052 (12) | |
| T3/4 | 53 (33) | 5,952 (18) | |
| No Tstage | 32 (20) | 5,571 (17) | |
| SEER stage | < 0.001 | ||
| Localized | 68 (43) | 20,803 (63) | |
| Regional | 45 (28) | 5,330 (16) | |
| Distant | 44 (28) | 5,705 (17) | |
| Unstaged | 3 (2) | 1,414 (4) | |
| Nodal status | <0.001 | ||
| N0 | 13 (8) | 3,523 (10.6) | |
| N1 | 24 (15) | 765 (2) | |
| Nx | 123 (77) | 28,964 (87) | |
| Metastatic | 44 (28) | 5,705 (17) | 0.001 |
| Grade | < 0.001 | ||
| Well differentiated | 10 (10) | 3,610 (18) | |
| Moderately differentiated | 20 (20) | 10,612 (52) | |
| Poorly/undifferentiated | 70 (70) | 6,384 (31) | |
| Surgery | 135 (84) | 25,969 (78) | 0.06 |
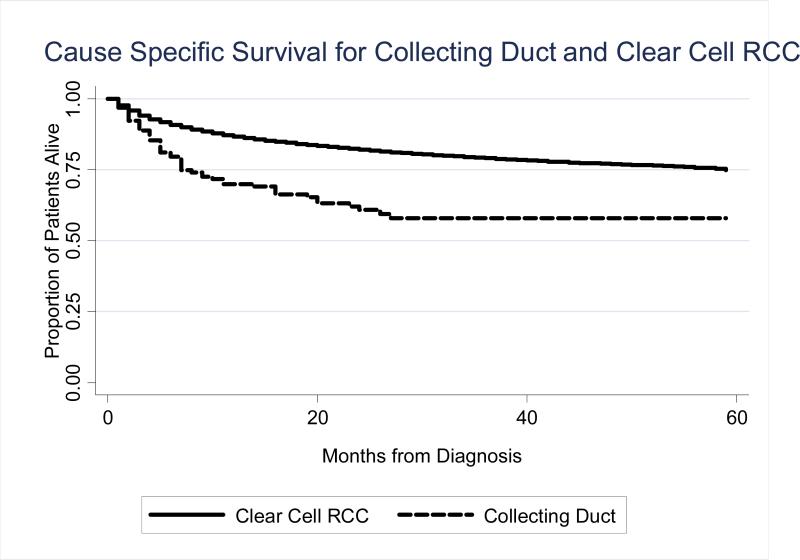
Figure 1 illustrates the unadjusted disease-specific survival difference between those with CDRCC and CCRCC. Survival was worse in those with CDRCC (p<0.001). The 1- and 3-year DSS for CDRCC was 70% (95%CI 62–77%) and 58% (48–67%) respectively. The median follow-up for the entire cohort was 19 months. For CCRCC, the 1- and 3-year disease-specific survival was 87% (95%CI 86–87%) and 79% (95%CI 78–80%). In the multivariate model of DSS (Table 2), adjusting for age, race, gender, year of diagnosis, registry, stage, grade, surgery and tumor size, the risk of renal cell cancer-specific death was more than 2-fold higher in patients with CDRCC compared to CCRCC cases (HR 2.42, 95%CI 1.72–3.39). When the analysis was limited to those undergoing surgery, the risk of death persisted for those with CDRCC (HR 2.72, 95%CI 1.94–3.84). An additional survival analysis was done in patients with T3a or less disease without distant metastasis. This consisted of 110 cases of CDRCC of which 92% underwent surgery. In the multivariate analysis, the risk of death remained for those with CDRCC (HR 2.50, 95%CI 1.81–3.45). When the CCRCC cohort was restricted to those with high grade disease only (poorly or undifferentiated tumors; n=6384) and survival compared to all cases of CDRCC, the survival experience continued to be worse for those with CDRCC (HR 2.15, 95%CI 1.42–3.24). When only high grade cases of CDRCC and CCRCC were compared, the risk was slightly worse for those with CDRCC (HR 2.20, 95%CI 1.46–3.32). Lastly, in patients with metastatic disease at presentation, the median survival for CDRCC was less than for CCRCC (5 months vs. 8 months, p=0.04). In the multivariate survival analysis limited to those with metastatic disease, CDRCC was associated with an increased risk of mortality (HR 1.73, 95%CI 1.20-2.48, p=0.003). There was no evidence for effect modification based on nephrectomy status (likelihood ratio test p=0.82).
Figure 1.
Disease Specific Survival for CDRCC and CCRCC. P < 0.0001
Table 2.
Disease Specific Survival Multivariate Analysis*
| CCRCC | CDRCC | |||
|---|---|---|---|---|
| HR | 95% CI | HR^ | 95% CI | |
| All Patients | 1.00 | referent | 2.42 | 1.72 – 3.39 |
| Surgery patients only | 1.00 | referent | 2.72 | 1.94 – 3.84 |
| ≤T3a and non-metastatic | 1.00 | referent | 2.50 | 1.81 – 3.45 |
| Only high-grade CCRCC | 1.00 | referent | 2.15 | 1.42 – 3.24 |
Adjusted for age, race, gender, year of diagnosis, registry, stage, grade, surgery, tumor size
p < 0.001
DISCUSSION
This report represents the largest series of CDRCC cases and is the only population-based study in the literature. Compared to the other relatively large published series, this present study is unique in that it includes non-operative cases of CDRCC and is from the United States. With three studies now from three different regions of the world, we can begin to draw some conclusions on the demographic and pathological presentation and natural history of this rare disease.
CDRCC are distinct tumors from CCRCC. CDRCC develops from the collecting ducts in the renal medullary pyramid, whereas CCRCC arise from the convoluted tubules. There are no specific CT findings to distinguish CDRCC from CCRCC although medullary location, weak and heterogeneous enhancement, renal sinus involvement, infiltrative growth with preserved renal contours and a cystic component are frequently seen.3 Cytologically, no definitive markers exist for CDRCC although a number of markers suggest CDRCC including UEA-1, PNA, HMW-CK and Fez1 among others. 4-6 As CDRCC accounts for less than 2.0% of renal tumors, early reports in the literature consisted of small case series. In general, CDRCC have a poor prognosis and limited response to immunotherapy 7-11 although less aggressive CDRCC cases have been reported.12, 13 CDRCC shares some cytological similarities with urothelial carcinoma and recently chemotherapeutic agents used for advanced urothelial carcinoma have demonstrated some response, including a phase two trial of gemcitabine and platinum therapy with response rates of 26% in patients with metastatic disease, 14, 15 further supporting a distinction from clear cell renal cell carcinoma.
Because of the rarity of CDRCC, pooled analyses are required to better understand the clinical features and natural history. Tokuda et al surveyed 281 Japanese institutions and 81 cases were reported,1 while Karakiewicz et al identified 41 cases from 17 European institutions.2 Although these two series had many similarities, the European series reported higher T3+ rates (81% vs. 57%), less high grade (78% vs. 99%) and better disease-specific survival (68% vs. 45% at 3 years) versus the Japanese series. Our series is the largest (160 cases) and uses a United States population-based data source (Table 3). Early reports suggested that CDRCC occurred in young men, 7, 10 whereas in these three large studies, the age at diagnosis was 58–61. These early reports may have suffered from the lack of standardized diagnostic criteria for CDRCC and misclassified patients with medullary carcinoma (which is diagnosed commonly in young, black men) as having CDRCC. In our study, we found no difference in age at presentation between CDRCC and CCRCC. Our study also reported higher rates in African-American patients, whereas race was not discussed in the Japanese or European series, most likely due to the lack of racial diversity in these series.
Table 3.
Comparison of largest series on Collecting Duct Carcinoma
| American Series Present study | Japanese Series Tokuda, et al. | European Series Karakiewicz, et al. | |
|---|---|---|---|
| Cases | 160 | 81 | 41 |
| Age (median) | 62 | 58 | 61 |
| Male (%) | 70 | 72 | 76 |
| African American (%) | 23 | not provided | not provided |
| T3 or greater (%) | 38 | 57 | 81 |
| Node positive (%) | 15 | 33 | 49 |
| Distant metastasis (%) | 28 | 32 | 20 |
| High grade (%) | 70 | 99 | 78 |
| Surgical management (%) * | 84 | 99 | 100 |
| 1-yr DSS | 70 | 69 | 86 |
| 3-yr DSS | 58 | 45 | 68^ |
| HR for DSS compared to CCRCC | 2.4 | not provided | 1.1 |
High grade for Japan/European series is Furman Grade 3 or 4; For present series, high grade includes SEER grades poorly and undifferentiated.
estimated from Kaplan Meier curve
CDRCC presents at higher stage and grade than CCRCC. Node positivity was reported in 15–49% and distant metastasis in 20–32%. Our series had lower T3+ (33% vs. 57% and 81%) and node positive rates (15% vs. 33% and 49%) than the other two series (Table 3), although our rates were higher than that observed in our series of CCRCC (18% T3+ and 2% node positive, p<0.001). One possible explanation for the differences between the series is the fact that 16% of our patients were managed non-operatively (compared to 1% in the other series) with lack of pathologic staging, probably resulting in some degree of understaging. This theory is supported by the 42% pT3+ and 15% node-positive rates in those undergoing nephrectomy in our series. In addition, the SEER database does not provide a T-stage for patients with metastatic disease. As higher T-stage is associated with risk of metastasis, it is likely that high T-stage disease was present in the 28% of patients in our series with metastatic disease, although not reported by SEER. High grade was reported for 70–99% of cases in these three series, and in our series, this rate was higher than those with CCRCC (31%, p<0.001)
Disease-specific survival appears worse in CDRCC compared to CCRCC. Our series and the Japanese series reported similar 1-yr (70%, 69%) and 3-year DSS (58%, 45%) respectively. The European series cited higher DSS (86% and 68% at 1 and 3 years) and found no difference between a matched cohort of CCRCC. Reasons for this discrepancy are not clear, although there are several potential explanations. First, the European series included cases from 1984–2001, whereas the Japanese and our series included patients only diagnosed after 2000. Precise diagnostic criteria for CDRCC were not well defined until recently, and most rely on the criteria proposed by Srigley and Elbe in 1998.5 Thus, some cases in the European series may have been inappropriately classified as CDRCC. Second, even with recent cases, misclassification occurs as seen in the Japanese series where 39/120 (33%) cases originally reported to be CDRCC were reclassified after central review.1 Given the concern over misclassifying medullary RCC (with its clearly dismal prognosis) as CDRCC in our dataset with the higher rate of African-Americans, we performed an analysis excluding African-Americans and the risk estimates did not change (data not shown). In our series, compared to CCRCC, the disease-specific risk of death was more than 2-fold higher for patients with CDRCC. We tried to mitigate potential confounders in our series through multiple sub-analyses (surgery only, ≤T3a and non-metastatic, only high-grade CCRCC), and the HR remained >2.0 in all scenarios, supporting the worse DSS in CDRCC compared to CCRCC.
This study is not without limitations. The median follow-up time of 19 months limits our comparison of these two subtypes of RCC regarding long term survival. This is a secondary database analysis and an observational cohort study in which random error may bias the results.16 Miscoding, incomplete or missing data cannot be accounted for in the analysis. An example of this is the lack of nodal disease data on the majority of patients in both groups, which may bias the outcomes. For those with reported nodal status, N1 disease is much more common in those with CDRCC than those with CCRCC, similar to the Japanese and European data.
The lack of a centralized pathology review for this rare diagnosis raises the concern for misclassification, especially in light of the Japanese series in which central pathology review resulted in reclassification and exclusion of one-third of cases initially called CDRCC.1 Based on pathology miscoded in that study, it is unclear in which direction this misclassification would affect the data. In that study, papillary RCC was the most commonly misclassified tumor (17 cases), which may potentially lead to longer survival times if not discovered, however invasive TCC was the second most common (10 cases). An additional seven of the 39 excluded cases were due to lack of available tissue for review. The impact of misclassification is also difficult to predict, given the likely differing distributions of the various renal tumor subtypes between the two (Japanese and American) populations. Though we are unable to perform a centralized pathology review on this data, our results corroborate those of the Japanese study that did perform such analyses, suggesting a worse prognosis for CDRCC. No data is available in SEER on comorbidities or the use of chemotherapy/immunotherapy. However, the uniformly poor response to standard adjuvant treatments makes the lack of adjuvant therapy classification unlikely to alter the survival results significantly.
CONCLUSIONS
Despite the limitations outlined above, this population-based analysis represents the largest series reported of CDRCC and helps to define the clinical presentation and natural history of this rare tumor. Patients with CDRCC present at high stage and have worse DSS than those with CCRCC. Although previously thought to occur in younger patients, these data found no difference in age at presentation and suggest that CDRCC may be more common in African-Americans.
Acknowledgments
This publication was supported by grant number T32 CA09168 from the National Institute of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Tokuda N, Naito S, Matsuzaki O, Nagashima Y, Ozono S, Igarashi T. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176:40. doi: 10.1016/S0022-5347(06)00502-7. [DOI] [PubMed] [Google Scholar]
- 2.Karakiewicz PI, Trinh QD, Rioux-Leclercq N, de la Taille A, Novara G, Tostain J, et al. Collecting duct renal cell carcinoma: a matched analysis of 41 cases. Eur Urol. 2007;52:1140. doi: 10.1016/j.eururo.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 3.Yoon SK, Nam KJ, Rha SH, Kim JK, Cho KS, Kim B, et al. Collecting duct carcinoma of the kidney: CT and pathologic correlation. Eur J Radiol. 2006;57:453. doi: 10.1016/j.ejrad.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Vecchione A, Galetti TP, Gardiman M, Ishii H, Giarnieri E, Pagano F, et al. Collecting duct carcinoma of the kidney: an immunohistochemical study of 11 cases. BMC Urol. 2004;4:11. doi: 10.1186/1471-2490-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srigley JR, Eble JN. Collecting duct carcinoma of kidney. Semin Diagn Pathol. 1998;15:54. [PubMed] [Google Scholar]
- 6.Kobayashi N, Matsuzaki O, Shirai S, Aoki I, Yao M, Nagashima Y. Collecting duct carcinoma of the kidney: an immunohistochemical evaluation of the use of antibodies for differential diagnosis. Hum Pathol. 2008;39:1350. doi: 10.1016/j.humpath.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Carter MD, Tha S, McLoughlin MG, Owen DA. Collecting duct carcinoma of the kidney: a case report and review of the literature. J Urol. 1992;147:1096. doi: 10.1016/s0022-5347(17)37485-2. [DOI] [PubMed] [Google Scholar]
- 8.Chao D, Zisman A, Pantuck AJ, Gitlitz BJ, Freedland SJ, Said JW, et al. Collecting duct renal cell carcinoma: clinical study of a rare tumor. J Urol. 2002;167:71. doi: 10.1016/s0022-5347(05)65385-2. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Logothetis CJ, Markowitz A, Sella A, Amato R, Ro J. Collecting duct carcinoma of the kidney. Br J Urol. 1993;71:388. doi: 10.1111/j.1464-410x.1993.tb15978.x. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy SM, Merino MJ, Linehan WM, Roberts JR, Robertson CN, Neumann RD. Collecting duct carcinoma of the kidney. Hum Pathol. 1990;21:449. doi: 10.1016/0046-8177(90)90209-n. [DOI] [PubMed] [Google Scholar]
- 11.Kirkali Z, Celebi I, Akan G, Yorukoglu K. Bellini duct (collecting duct) carcinoma of the kidney. Urology. 1996;47:921. doi: 10.1016/s0090-4295(96)00045-3. [DOI] [PubMed] [Google Scholar]
- 12.MacLennan GT, Farrow GM, Bostwick DG. Low-grade collecting duct carcinoma of the kidney: report of 13 cases of low-grade mucinous tubulocystic renal carcinoma of possible collecting duct origin. Urology. 1997;50:679. doi: 10.1016/S0090-4295(97)00335-X. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez-Lavista LG, Uribe-Uribe N, Gabilondo-Navarro F. Collecting duct renal cell carcinoma: two different clinical stages, two different clinical outcomes. Urol Int. 2008;81:116. doi: 10.1159/000137652. [DOI] [PubMed] [Google Scholar]
- 14.Milowsky MI, Rosmarin A, Tickoo SK, Papanicolaou N, Nanus DM. Active chemotherapy for collecting duct carcinoma of the kidney: a case report and review of the literature. Cancer. 2002;94:111. doi: 10.1002/cncr.10204. [DOI] [PubMed] [Google Scholar]
- 15.Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Genitales) study. J Urol. 2007;177:1698. doi: 10.1016/j.juro.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 16.MacMahon S, Collins R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: observational studies. Lancet. 2001;357:455. doi: 10.1016/S0140-6736(00)04017-4. [DOI] [PubMed] [Google Scholar]