Abstract
Purpose
Reports on biochemical recurrence after prostate cancer (PCa) primary therapy have shown differences between Gleason 4+3 and 3+4 tumors. These findings have not been explored for PCa-specific mortality (PCSM). In this population-based cohort, we determine PCa outcomes at different Gleason scores, in particular the different Gleason 7 patterns.
Methods
Men aged 40–64 diagnosed with PCa between 1993–1996 in King County, Washington comprised the cohort. Recurrence/progression was determined by follow-up survey and medical records review. Mortality and cause of death were obtained from the SEER registry. Outcomes were determined with Cox proportional hazards regression analysis.
Results
Of 753 men with PCa, 65 PCa-specific deaths occurred during a median follow-up of 13.2 years. The 10-year PCa-specific survival rates for Gleason ≤6, 3+4, 4+3, and 8–10 were 98.4%, 92.1%, 76.5% and 69.9%, respectively. Compared to patients with Gleason 3+4 disease, those with Gleason 4+3 tumors had an increased risk of PCSM in both the unadjusted (HR 2.80, 95%CI 1.26 – 6.18) and multivariate models (HR 2.12, 95% CI 0.87–5.17, p=0.1). In men undergoing curative therapy with radical prostatectomy or radiation therapy, there was an increased risk of recurrence/progression (HR 2.1, 95% CI 1.1–4.0) and PCSM (HR 3.2, 95% CI 1.0–9.7) in those with Gleason 4+3 compared to 3+4 tumors in the multivariate models. No difference in PCSM was seen between Gleason 4+3 and 8 – 10 tumors.
Conclusion
Gleason 7 PCa tumors exhibit a heterogeneous behavior with Gleason 3+4 and 4+3 tumors conferring different PCSM. These data provide important information for counseling patients with Gleason 7 PCa on the natural history of their disease and may inform treatment decisions.
Keywords: prostate cancer, Gleason score, survival, population-based, Gleason 4+3
Introduction
One factor that defines the aggressiveness of prostate cancer (PCa) is the Gleason score. Studies have consistently shown the Gleason score to be a powerful predictor of both disease progression and mortality.1–3 The composite Gleason score is traditionally comprised of a primary and a secondary pattern. Historically, Gleason score was categorized into groups of 2 – 6, 7, and 8 – 10. Gleason 7 tumors include those with a predominant Gleason 4 pattern (Gleason 4+3) and those with a predominant Gleason 3 pattern (Gleason 3+4).
Recent work has demonstrated the heterogeneity of Gleason 7 tumors, with significantly higher biochemical recurrence (BCR) rates seen in those with Gleason 4+3 disease as opposed to Gleason 3+4 tumors.4–7 However, BCR represents an early event in the natural history of PCa. Patients with BCR have heterogeneous outcomes, and BCR has not been accepted as a surrogate for PCa-specific mortality (PCSM).8, 9 For example, only men with BCR and a short PSA doubling time or rapid time to BCR from primary treatment have been shown to have worse PCSM.10, 11 Additionally, BCR is limited to men undergoing primary curative therapy and cannot be applied to men presenting with regional or distant stage disease, men undergoing primary androgen deprivation therapy (ADT), or the watchful waiting/active surveillance population. Lastly, the data on BCR after primary treatment often come from single institution case series undergoing radical prostatectomy (RP) and may not reflect the diversity of PCa due to selection and referral biases.
To date, studies that examine PCSM in patients with Gleason 7 PCa stratified by primary pattern have not been reported, probably owing to the low case fatality rate of PCa. In this report, using a well-established, population-based cohort of PCa cases with long follow-up, we explore the impact of Gleason score on outcomes with particular attention to differences between Gleason 4+3 and Gleason 3+4 tumors.
Methods
Study participants
Men with newly diagnosed PCa who participated in a population-based, case-control study comprise this study cohort. Details of the study participants and data collection have previously been described.12 Briefly, residents of King County, Washington, aged 40–64 with newly diagnosed, histologically confirmed PCa identified through the Seattle-Puget Sound Surveillance, Epidemiology and End Results (SEER) cancer registry between January 1, 1993 and December 31, 1996 were considered eligible. The study was designed to evaluate whether or not vasectomy was associated with risk of PCa. Of 915 eligible cases, 753 (82%) agreed to participate. Non-response included patient refusal (12.5%), physician’s refusal to allow contact (2.6%), inability to locate (1.5%), illness (0.4%) and death (0.2%).
Data Collection
Participants completed a structured, in-person interview conducted by trained, male interviewers. Demographic and lifestyle characteristics, medical and family history, and PCa screening in the previous 5 years were recorded. Gleason score was obtained from either biopsy reports (30%) or, for men undergoing RP, from surgical pathology reports (70%). Gleason scores were grouped: 2 – 6, 3+4, 4+3, and 8 – 10. The SEER historic stage was used and defined as localized (confined to the prostate, Stages A/B), regional (regional spread outside the prostatic capsule or lymph node involvement, Stage C), and distant (metastatic, Stage D). Primary treatment was categorized as RP, XRT, ADT, watchful waiting and other.
A follow-up survey was sent to all living men in January 2004 who had previously consented to future contact, along with a consent form for access to medical records. The survey queried about any physician’s diagnosis of PCa recurrence/progression, secondary therapies, PSA results, diagnostic procedures performed along with dates and results. A total of 520 (82%) of the 631 eligible patients completed the survey. In addition, next-of-kin provided consent for access to medical records for deceased patients (n=41) who participated in the initial study.
End points
Disease progression was defined, among men with local/regional disease at diagnosis, as the development of metastatic disease. Thirty men who died of PCa before the follow-up survey was completed were considered to have developed metastasis and their date of recurrence/progression was imputed by the technique described by Little and Rubins.13 PSA progression was defined by a PSA value of ≥0.2 in men who had RP as primary therapy and by a nadir PSA+2ng/mL14 for men who received XRT as primary therapy. Progression on watchful waiting was defined as treatment initiation for PSA or biopsy progression. The date of last follow-up for recurrence/progression was December 31, 2005.
PCSM was determined from the SEER registry which links quarterly with the state vital statistics database. Cause of death was verified by a review of death certificates with 99% agreement. The date of last follow-up for survival was December 31, 2008.
Statistical Analysis
Unadjusted PCa-specific Kaplan Meier (KM) survival plots were constructed, and survival rates compared between Gleason categories with the log-rank test with Gleason 3+4 as the referent group. Cox proportional hazards models were created to estimate the hazard ratios (HR) and 95% confidence intervals (95% CI) for PCSM. Crude (unadjusted) HR were determined along with adjusted HR estimates in a multivariate model adjusting for age, race, PCa screening, stage, diagnostic PSA, and primary treatment were created. Subset analysis was performed on patients with non-metastatic disease at diagnosis undergoing treatment with curative intent (surgery/XRT). This sub-analysis was completed to create a more homogenous group in terms of PCSM risk. Additionally, a subset analysis of those undergoing RP was performed as these cases all had pathologic Gleason grading as opposed to biopsy Gleason scores. The proportional hazards assumption for the Cox regression was evaluated with Schoenfeld goodness of fit testing. All statistical analyses were conducted using Stata software, Version 8 (Stata, Inc., College Station, TX).
Results
The cohort of 753 PCa patients had a median survival follow-up of 13.2 years (range 1.26–15.9), during which 65 PCa-specific deaths occurred. Of the 566 patients for whom recurrence/progression data were available, 180 (32%) events occurred. Table 1 gives the clinical and pathological differences between those patients who died of PCa and those who did not. PCa-specific death was more common in younger men (23.9% in men aged 40–49, compared to 6.0–9.0% in those aged 50–65) and African-Americans (18.8% vs. 7.9% for Caucasians). Higher PSA levels and tumor stage were both more commonly observed with PCa-specific death. The majority of cases (78%) staged as regional (extracapsular spread and/or node positivity) underwent RP, suggesting these men initially underwent surgery for clinically localized disease, but were upstaged to regional disease based on surgical pathology.
Table 1.
Clinical and Pathologic Characteristics of 753 Population-based Prostate Cancer Cases, King County, WA
| Died of Prostate Cancer |
||
|---|---|---|
| No N (%) |
Yes N (%) |
|
| Age at diagnosis | ||
| 40 – 49 | 35 (76.1) | 11 (23.9) |
| 50 – 54 | 141 (94.0) | 9 (6.0) |
| 55 – 59 | 234 (91.1) | 23 (9.0) |
| 60 – 64 | 278 (92.7) | 22 (7.3) |
| Race | ||
| Caucasian | 649 (92.1) | 56 (7.9) |
| African American | 39 (81.3) | 9 (18.8) |
| Family History | ||
| No | 1,190 (95.4) | 57 (4.6) |
| Yes | 201 (96.2) | 8 (3.8) |
| Year of diagnosis | ||
| 1993 | 169 (88.5) | 22 (11.5) |
| 1994 | 158 (90.8) | 16 (9.2) |
| 1995 | 138 (92.3) | 14 (7.7) |
| 1996 | 193 (93.7) | 13 (6.3) |
| Screening Status * | ||
| No PSA/DRE | 36 (70.6) | 15 (29.4) |
| DRE only | 152 (90.5) | 16 (9.5) |
| PSA | 500 (93.6) | 34 (6.4) |
| Gleason Score | ||
| 2 – 6 | 451 (97.6) | 11 (2.4) |
| 3 + 4 | 168 (90.4) | 19 (10.2) |
| 4 + 3 | 26 (74.3) | 9 (25.7) |
| 8 – 10 | 43 (65.2) | 23 (34.9) |
| PSA at diagnosis | ||
| < 4.0 | 92 (97.9) | 2 (2.1) |
| 4.0 – 9.9 | 371 (96.6) | 13 (3.4) |
| >= 10 | 157 (78.1) | 44 (21.9) |
| Missing | 68 (91.9) | 6 (8.1) |
| Stage | ||
| Local | 537 (96.9) | 17 (3.1) |
| Regional | 146 (83.9) | 28 (16.1) |
| Distant | 5 (20.0) | 20 (80.0) |
| Primary Treatment | ||
| RP | 494 (96.5) | 18 (3.5) |
| XRT (+/− ADT) | 131 (87.3) | 19 (12.7) |
| ADT | 16 (37.2) | 27 (62.8) |
| Watchful waiting | 42 (97.7) | 1 (2.3) |
| Other | 5 (100.0) | 0 (0.0) |
Screening within the five-year period prior to diagnosis date
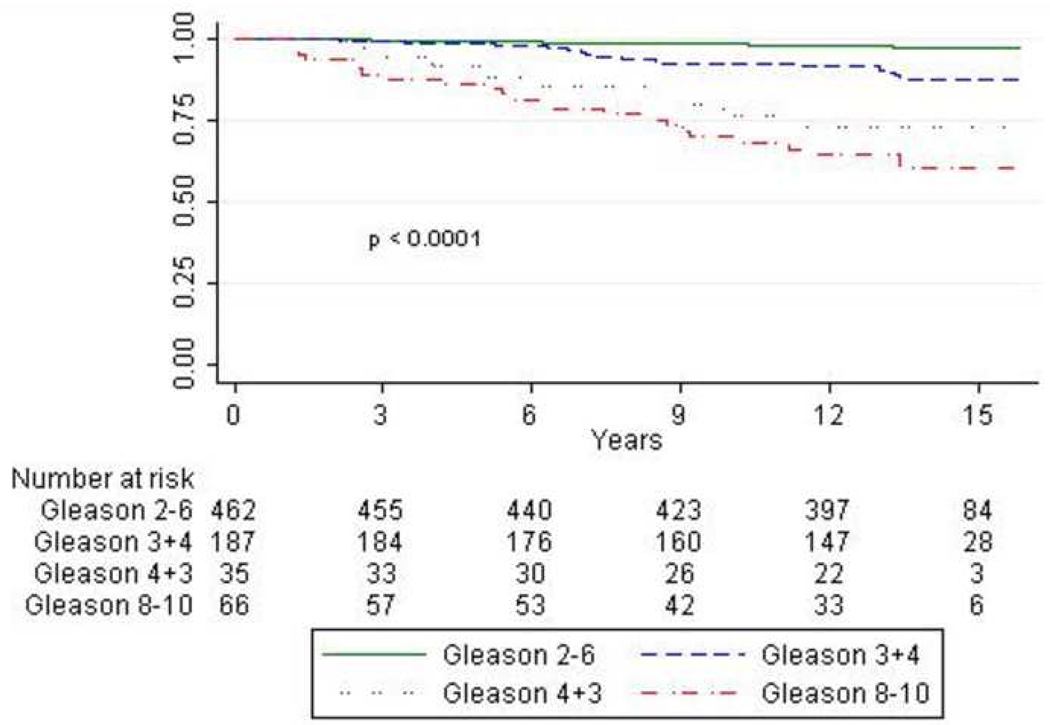
Figure 1 presents Kaplan-Meier curves for PCSM by Gleason score. PCa survival rates were significantly different by Gleason score (p<0.0001) and men with Gleason 3+4 had a significantly higher PCa survival rate than men with Gleason 4+3 (p=0.008). In Table 2, the 5- and 10-year PCa survival rates are shown. The 10-year PCa survival rate for men with Gleason≤6, 3-4, 4-3 and 8-10 were 98.4%, 92.1%, 76.5% and 69.9% respectively. Furthermore, the curve for Gleason 4+3 disease diverges from Gleason 3+4 after 3 years and more closely approximates the curve for Gleason 8–10.
Figure 1.
Kaplan Meier prostate-cancer specific survival plots stratified by Gleason score.
Table 2.
Five and 10-year Prostate Cancer-Specific Survival Rates (95% CI) by Gleason Score
| Gleason Score | 5-year | 10-year |
|---|---|---|
| 2 – 6 | 99.1 (97.7 – 99.7) | 98.4 (96.7 – 99.3) |
| 3 + 4 | 98.9 (95.7 – 99.7) | 92.1 (87.1 – 95.3) |
| 4 + 3 | 88.6 (72.3 – 95.6) | 76.5 (58.4 – 87.5) |
| 8 – 10 | 86.2 (75.1 – 92.5) | 69.9 (56.9 – 79.7) |
In the Cox regression models (Table 3), there was an increase in the risk of fatal PCa with higher Gleason score tumors. In the unadjusted model including all patients, the risk of mortality was 78% less for those with Gleason 2–6 (95% CI: 0.10–0.47) tumors whereas there was a 3- and 4-fold increased risk of PCSM for Gleason 4+3 (95% CI: 1.26–6.18) and Gleason 8–10 (95% CI: 2.36–8.00) tumors, respectively, compared to Gleason 3+4 tumors. There was no difference in PCSM between Gleason 4+3 and Gleason 8–10 tumors (HR 1.28, 95% CI 0.58 – 2.82, p=0.55). In the multivariate model, the risks were slightly attenuated with a 70% reduced risk for Gleason 2–6 tumors (95% CI: 0.12–0.65) and a 2- and >2.5-fold increased risk of PCSM for Gleason 4+3 (95% CI: 0.87–5.17) and Gleason 8–10 (95% CI: 1.27–5.57), respectively. The risk for 4+3 in the multivariate model, however, did not achieve statistical significance (HR 2.12, 95% CI 0.87–5.17, p = 0.10). In the model limited to those treated with curative intent (RP or XRT), the risk estimate was significantly elevated with a more than 3-fold increased risk of PCSM in those with Gleason 4+3 compared to Gleason 3+4 tumors (95% CI for HR: 1.04–9.67). There was no difference between the survival experience for those with Gleason 4+3 and Gleason 8–10 tumors in either of the multivariate models (all patients HR 1.25, 95% CI 0.52 – 3.01, p=0.61); RP/XRT only HR 1.41, 95% CI 0.45 – 4.46, p=0.55).
Table 3.
Risk of Prostate Cancer-Specific Mortality by Gleason Score
| Unadjusted HR (95% CI) |
Multivariate HR (95% CI) |
|||
|---|---|---|---|---|
| All Cases | RP/XRT only | All Cases | RP/XRT only | |
| Gleason Score | ||||
| 2 – 6 | 0.22 (0.10 – 0.47) | 0.17 (0.06 – 0.47) | 0.29 (0.12 – 0.65) | 0.17 (0.06 – 0.48) |
| 3 + 4 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| 4 + 3 | 2.80 (1.26 – 6.18) | 3.21 (0.95 – 7.62) | 2.12 (0.87 – 5.17) | 3.17 (1.04 – 9.67) |
| 8 – 10 | 4.35 (2.36 – 8.00) | 4.14 (1.86 – 9.22) | 2.66 (1.27 – 5.57) | 4.49 (1.89 – 10.7) |
Table 4 gives the hazard ratios for PCa recurrence/progression. There was evidence that Gleason 4+3 tumors have a greater risk of recurrence/progression compared to those with Gleason 3+4 tumors in the unadjusted (HR 1.56, 95% CI 0.83–2.91) and multivariate (HR 1.67, 95% CI 0.88–3.17) models, although results for the two Gleason 7 groups were not statistically significantly different. However, in the analyses limited to those undergoing RP/XRT, Gleason 4+3 tumors had significantly increased risk of recurrence/progression compared to those with Gleason 3+4 tumors in the multivariate (HR 2.10, 95% CI 1.08–4.08) models. As in the PCSM models, there was no difference between the recurrence/progression risk for those with Gleason 4+3 and Gleason 8–10 tumors in either of the multivariate models (all patients HR 1.19, 95% CI 0.59 – 2.42, p=0.63, RP/XRT only HR 1.01, 95% CI 0.48 – 2.14, p=0.95).
Table 4.
Risk of Prostate Cancer Recurrence/Progression by Gleason Score
| Unadjusted HR (95% CI) |
Multivariate HR (95% CI) |
|||
|---|---|---|---|---|
| All Cases | RP/XRT only | All Cases | RP/XRT only | |
| Gleason Score | ||||
| 2 – 6 | 0.52 (0.37 – 0.73) | 0.48 (0.33 – 0.68) | 0.49 (0.33 – 0.72) | 0.45 (0.31 – 0.65) |
| 3 + 4 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| 4 + 3 | 1.56 (0.83 – 2.91) | 1.79 (0.95 – 3.35) | 1.67 (0.88 – 3.17) | 2.10 (1.08 – 4.08) |
| 8 – 10 | 2.28 (1.47 – 3.53) | 2.05 (1.26 – 3.32) | 1.98 (1.21 – 3.24) | 2.12 (1.26 – 3.57) |
A final subset analysis was performed limited to those undergoing RP for whom pathologic, rather than biopsy, grade was assigned (n = 512). Of these, 119 cases had Gleason 3+4 and 22 cases had Gleason 4+3 disease. No men (n = 309) with Gleason 2-6 disease died of PCa after RP, whereas the cumulative mortality for Gleason 3+4 and Gleason 4+3 was 4.7% and 13.6%, p < 0.001). In the multivariate model, Gleason 4+3 tumors had a more than 4-fold increased risk of PCSM (HR 4.27, 95% CI 0.99 – 18.4) and a 2-ford increased risk of recurrence/progression (HR 2.13, 95% CI 1.01 – 4.53) compared to Gleason 3+4 tumors.
Discussion
In this population-based cohort of men with PCa, we demonstrate that the different Gleason 7 tumors (Gleason 3+4 vs. Gleason 4+3) have different recurrence/progression and PCSM rates. Although previous studies have reported different BCR rates between these two groups, this report examines the impact of primary pattern on PCSM. In addition, there was no difference observed in mortality risk between Gleason 4+3 and Gleason 8–10, supporting the approach of combining these patients into a high risk group.
Gleason scores have been shown to influence rates of BCR after radical prostatectomy or XRT. 4–7 With a median follow-up of between 2.7 and 6.8 years, the 5- and 10-year progression free survival (PFS) rates range from 70–85% and 60–74% for Gleason 3+4 tumors compared to ranges of 38–63% and 44–55%, respectively, for Gleason 4+3 tumors. Consistent with these previous reports, patients in our series experienced 5- and 10-year PFS rates of 80% (95% CI 72–85%) and 65% (56–73%) for Gleason 3+4 tumors compared to 75% (53–87%) and 40% (14–65%) for Gleason 4+3 tumors. Although BCR often times is associated with substantial morbidity from secondary treatment and development of metastases, most men suffering BCR do not die of PCa. Disease-specific survival has long been the most relevant endpoint for clinical trials and prognostic studies. However, the number of PCa-specific deaths may be small relative to the total number of cases (age-adjusted incidence rate of 163.0 per 100,000 men per year compared to age-adjusted death rate of 26.7 per 100,000 men per year15) requiring that large study populations be followed over longer periods of time to assess differences in PCSM. Here, our unique population-based cohort of men with more than 13 years of follow-up on average allows for exploring PCSM.
The published studies of PCSM include a diverse range of patient populations and treatments. Prior studies of PCSM after definitive RP10 or RP/XRT16 have demonstrated differences in survival based on Gleason score, but did not distinguish between Gleason 3+4 and 4+3. These studies have included men from a single institution10 and two non-population-based data registries.16 Non-population-based datasets and single/multiple institution(s) case series have inherent referral bias and those limited to patients undergoing definitive therapy (RP/XRT) are subject to selection bias. Three large population-based studies of conservatively managed PCa have been performed.1, 3, 17 In each of these, higher Gleason score was positively associated with PCSM, although Gleason grade was extrapolated from the WHO criteria in one study17 and was missing from 29% of cases of another study.3 Two of these studies were in the pre-PSA era1, 17 Our population-based patient cohort includes all eligible men diagnosed with PCa, regardless of clinicopathologic features or primary treatment. This heterogenous nature of our cohort may partially explain why the findings of a more than 3-fold increased risk in PCSM for patients with Gleason 4+3 compared to Gleason 3+4 tumors did not reach statistical significance in the multivariate model of all patients (HR 2.12, 95% CI 0.87 – 5.17, p = 0.1) whereas remaining significant in the RP only and RP/XRT subanalyses. In addition, the relatively small sample size may have limited the statistical power of the analysis.
The present study has several noteworthy differences from the only other population-based study of PCSM that looked at Gleason 3+4 and 4+3. 3 In that study by Cuzick et al. of 1,656 men, no difference was observed between those with Gleason 3+4 vs. Gleason 4+3 in PCSM with 10-year survival of 73% and 68%, respectively. In contrast, our study found significant differences in PCSM between patients with Gleason 3+4 versus Gleason 4+3 tumors with 10-year survival of 92% and 77%, respectively. These differences may be due to a number of factors, including selection bias, population differences, or varying PSA screening practices. Perhaps most importantly, cases in the Cuzick et al. study were limited to those who did not undergo primary curative RP/XRT, whereas our study included all treatment modalities, a factor that may have resulted in different baseline characteristics between the two studies. For example, patients not undergoing treatment with curative intent often have either low risk disease or multiple comorbidities. In the study by Cuzick and colleagues, 80% of men were over the age of 65 at diagnosis and 31% died from other causes during the follow-up period, whereas in our study, all the patients were under age 65 at diagnosis and only 12% died of other causes. In addition, the studies are based in different health care systems (UK vs. USA) and include slightly different eras of enrollment (1990–96 vs. 1993–96). A recent study of survival between these countries has shown a divergence in PCSM starting in 1994, suggesting that PSA screening differences may account for survival imbalances.18
There are limitations to our study. First, central review of pathology was not performed. However, this makes our community-based results more applicable to the realities facing providers and patients. Second, Gleason score was determined using pathological data when available as opposed to biopsy data, such that Gleason score in patients undergoing RP may have changed. However, both upgrading and downgrading occur at RP such that any bias would likely be non-directional. Additionally, we have no data on tertiary pattern, which has been shown to impact BCR rates in men with Gleason 7 tumors.19 Third, our cohort represents men <65 years old at diagnosis with a high proportion undergoing primary curative treatment, which may not completely represent the average PCa patient population. However, the population-based nature of our cohort addresses many of the biases present in other studies. Lastly, misclassification of cause of death is possible, although all deaths in this cohort were confirmed by review of death certificates with 99% agreement.
In conclusion, this population-based study of men diagnosed with PCa shows higher PCSM in men with Gleason 4+3 tumors compared to those with Gleason 3+4 tumors. We observed no difference in mortality risk between Gleason 4+3 and Gleason 8–10, though a larger scope study is needed before supporting the combining of these into the same high risk group. Further, in men undergoing curative therapy (RP/XRT), patients with Gleason 4+3 disease had a HR for recurrence/progression that was about 2-fold higher relative to patients with Gleason 3+4 disease. These results demonstrate that men with Gleason 4+3 disease should be counseled regarding their higher risk of adverse outcomes, including disease-specific mortality.
Acknowledgments
NIH Grants: R01 CA56678; R01 CA092579; R01 CA097186, T32 CA009168-30; with additional support from the Fred Hutchinson Cancer Research Center.
Glossary
- PCa
prostate cancer
- BCR
biochemical recurrence
- PCSM
Prostate cancer-specific mortality
- RP
radical prostatectomy
- SEER
Seattle-Puget Sound Surveillance, Epidemiology and End Results
- ADT
androgen deprivation therapy
- KM
Kaplan Meier
- HR
hazard ratios
- 95% CI
95% confidence intervals
- PFS
progression free survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293:2095. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.Bianco FJ, Jr, Wood DP, Jr, Cher ML, et al. Ten-year survival after radical prostatectomy: specimen Gleason score is the predictor in organ-confined prostate cancer. Clin Prostate Cancer. 2003;1:242. doi: 10.3816/cgc.2003.n.006. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Fisher G, Kattan MW, et al. Long-term outcome among men with conservatively treated localised prostate cancer. Br J Cancer. 2006;95:1186. doi: 10.1038/sj.bjc.6603411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan TY, Partin AW, Walsh PC, et al. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 5.Herman CM, Kattan MW, Ohori M, et al. Primary Gleason pattern as a predictor of disease progression in gleason score 7 prostate cancer: a multivariate analysis of 823 men treated with radical prostatectomy. Am J Surg Pathol. 2001;25:657. doi: 10.1097/00000478-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Lau WK, Blute ML, Bostwick DG, et al. Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: differences in outcome between primary Gleason grades 3 and 4. J Urol. 2001;166:1692. [PubMed] [Google Scholar]
- 7.Rasiah KK, Stricker PD, Haynes AM, et al. Prognostic significance of Gleason pattern in patients with Gleason score 7 prostate carcinoma. Cancer. 2003;98:2560. doi: 10.1002/cncr.11850. [DOI] [PubMed] [Google Scholar]
- 8.Collette L. Prostate-specific antigen (PSA) as a surrogate end point for survival in prostate cancer clinical trials. Eur Urol. 2008;53:6. doi: 10.1016/j.eururo.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 10.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama. 2005;294:433. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico AV, Moul J, Carroll PR, et al. Surrogate end point for prostate cancer specific mortality in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;173:1572. doi: 10.1097/01.ju.0000157569.59229.72. [DOI] [PubMed] [Google Scholar]
- 12.Stanford JL, Wicklund KG, McKnight B, et al. Vasectomy and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:881. [PubMed] [Google Scholar]
- 13.Little RJA, Rubin DB. Statistical Analysis with Missing data. New York: Wiley; 1987. [Google Scholar]
- 14.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Ries LAG MD, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2005, based on November 2007 SEER data submission, posted to the SEER web site. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 16.Zhou P, Chen MH, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 17.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. Jama. 2004;291:2713. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 18.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9:445. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim HG, Telesca D, Culp SH, et al. Tertiary Gleason pattern 5 in Gleason 7 prostate cancer predicts pathological stage and biochemical recurrence. J Urol. 2008;179:1775. doi: 10.1016/j.juro.2008.01.016. [DOI] [PubMed] [Google Scholar]