Abstract
Ghrelin is a peptide hormone that has been implicated in the regulation of food intake and energy homeostasis. Ghrelin is predominantly produced in the stomach, but is also expressed in many other tissues where its functions are not well characterized. In the rodent and human pancreas, ghrelin levels peak at late gestation and gradually decline postnatally. Several studies have suggested that ghrelin regulates beta cell function during embryonic development and in the adult. In addition, in a number of mouse models, ghrelin cells appear to replace insulin and glucagon-producing cells in the islet. In this analysis, we investigated whether the absence or overexpression of ghrelin influenced the development and differentiation of the pancreatic islet during embryonic development. These studies revealed that ghrelin is dispensable for normal pancreas development during gestation. Conversely, we demonstrated that elevated ghrelin in the Nkx2.2 null islets is not responsible for the absence of insulin- and glucagon-producing cells. Finally, we have also determined that in absence of insulin, ghrelin cells form in their normal numbers and ghrelin is expressed at wild type levels.
Introduction
The adult endocrine pancreas consists of four hormone-producing cell types organized in islets of Langerhans. In the rodent, islets are comprised of a large core of insulin-producing beta cells surrounded by smaller numbers of glucagon-producing alpha cells, somatostatin-producing delta cells, and pancreatic polypeptide-producing PP cells. During embryonic development, the epsilon cell population and a subset of alpha cells produce a fifth hormone, ghrelin [1–4].
Ghrelin is a 28-amino acid peptide hormone that was originally isolated as an activator of the growth hormone secretagogue receptor (GHSR1a) [5]. In adults, serum ghrelin levels are primarily dependent on secretion by the X/A-like cells in the posterior stomach [6, 7]. Serum ghrelin levels peak shortly before normal feeding times and is higher in fasting animals. Cerebral administration of ghrelin in rats acutely stimulated food intake and increased long-term weight gain [8–11]. IV injection of ghrelin into human patients also resulted in increased food intake [12]. Surprisingly, ghrelin null mice show few significant differences with respect to bone mineral density, fat content, body weight, food intake, and serum leptin or glucose concentration before or after fasting [13, 14]. Therefore, it is possible that ghrelin is sufficient, but not necessary to stimulate appetite.
In addition to the stomach, ghrelin expression has been reported in a variety of tissues including the small intestine, lymphocytes, placenta, lung, kidney, brain, and gonads [15, 16]. In many of these tissues, ghrelin exerts a variety of endocrine and nonendocrine effects. For example, ghrelin expression in the testes has been shown to function as a paracrine signal to control the replication of immature Leydig cells, steroidogenesis, and stem cell factor (SCF) expression in sertoli cells [17, 18]. Furthermore, there is emerging evidence to suggest that ghrelin modulates cell proliferation and/or differentiation of osteoblasts, adipocytes and neuronal cells [19–22]. Therefore, ghrelin may play an important role in regulating development of specific tissues.
In the developing mouse pancreas, ghrelin can be detected as early as e9.5 and continues to be expressed throughout gestation, with levels peaking shortly before birth [23]; supplemental figure 1). Postnatally, ghrelin levels gradually decline until little pancreatic ghrelin can be detected in the adult islet [24]. Interestingly, the number of ghrelin positive cells is significantly increased in the Pax4, Pax6 and Nkx2.2 knockout models at the expense of many of the other hormone-producing cell populations [1, 25]. Given the potential role for ghrelin in regulating cell differentiation in other tissues, it is possible that ghrelin may influence the development and differentiation of the pancreatic endocrine cell populations during development. In this study we analyzed the ghrelin null phenotype in the embryonic pancreas at various stages of development. Our data show that the number of insulin- and glucagon- producing cells is unchanged in ghrelin null mice. Furthermore, RNA levels of insulin, glucagon, somatostatin and pancreatic polypeptide as well as the transcription factors Nkx2.2 and Pdx1 are unaffected. We also demonstrate that the upregulation of ghrelin is not responsible for the loss of insulin and glucagon in Nkx2.2 null embryos. Overall, this study suggests that ghrelin is dispensable for the development and differentiation of the pancreas during embryogenesis.
Materials and Methods
Animals
Nkx2.2 and ghrelin heterozygous mice were generated by homologous recombination as previously described [14, 26]. Nkx2.2+/− and ghrelin +/− or −/− animals were maintained in a Swiss Black (Taconic) background. Double heterozygous mice were mated to obtain Nkx2.2−/−;ghrelin−/− (DKO) mice. Genotyping of mice and embryos was performed by PCR analysis as described [14, 26]. Animals were housed and treated according to UCHSC and Columbia University Institutional Review Board approval protocols. Ins1/Ins2 DKO embryos and their wild type controls were provided by Dr. A. Pugliese for these studies [27].
Immunofluorescence
Immunofluorescence was performed on cryopreserved tissue fixed with 4% paraformaldehyde for 3 hours (Pdx1 and Nkx6.1) or overnight, as previously described [28]. Staining was performed on 10 μm sections. Antibodies used were rabbit anti-amylase (1:1000, Sigma), rabbit anti-ghrelin (1:200, Phoenix peptide), guinea pig anti-glucagon (1:3000, Linco), rabbit anti-glucagon (1:1000, Phoenix peptide), guinea pig anti-insulin (1:1000, Linco), mouse anti insulin (1:500, Sigma), rabbit anti-Nkx6.1 (1:800, Beta Cell Biology Consortium (BCBC)), rabbit anti-Pdx1 (1:1000, Chemicon), guinea-pig anti-pancreatic polypeptide (1:500, Linco), and rabbit anti-somatostatin (1:200, Phoenix peptide). Secondary antibodies used were Alexafluor-488 anti-rabbit (1:400), Alexafluor-488 anti-guinea pig (1:400), Alexafluor-488 anti-mouse (1:200), Alexafluor-594 anti-guinea pig (1:400), Alexafluor-594 anti-mouse (1:200), Alexafluor-594 anti-rabbit (1:400) and Cy5-anti-rabbit. Confocal images were processed with a Zeiss Microscope, LSM 510 META at 25X magnification. Fluorescent images were obtained with a Nikon Eclipse 80i microscope, Q-Image camera and ImagePro software from Media Cybernetics.
Morphometric analysis
Wild type and ghrelin null embryos were processed as above. Every fifth section (e13.5) or every tenth section (e15.5 and e18.5) was collected and stained by immunofluorescence for insulin and glucagon. DAPI staining was used to visualize the nuclei to aid in cell counting. The entire pancreas from each wild type and mutant embryos n = 4 (e13.5), n=4 (e15.5), n=3 (e18.5) was used to obtain a representative number of hormone positive cells per embryo. The respective immunofluorescent-positive cells on glucagon+, insulin+ sections were counted. Total islet area was calculated using Image Pro Plus 5.1 software using amylase immunofluorescence staining to delineate the islet. Islet area was unchanged in the ghrelin null embryos (data not shown).
Quantification of mRNA
Total RNA was extracted from pancreatic tissue and prepared using a Qiagen RNeasy Micro kit. cDNAs were prepared with random hexamer primers and Superscript III (Invitrogen). Real-time PCR was performed using Taqman probes (ABI Assays on Demand) for Gapdh (4352932E), ghrelin (Mm00445450_m1), glucagon (Mm00801712_m1), insulin 2 Mm00731595_gH), somatostatin (Mm00436671_m1), and pancreatic polypeptide (Mm00435889_m1), Pdx1 (Mm00435565_m1), on the ABI 7000. Taqman probes and primers were designed for Nkx2.2 (FAM-CCATTGACTCTGCCCCATCGCTTCT, Forward: CTCCCCGAGTGGCAGAT, Reverse: GAGTTCTATCCTCTCCAAAAGTTCAAA). Taqman probes and primers were designed for Cyclophilin B (FAM –TGGTACGGAAGGTGGAG, Forward: GCAAAGTTCTAGAGGGCATGGA, Reverse: CCCGGCTGTCTGTCTTGGT). Each gene was normalized to Gapdh (Figure 5). Ghrelin levels in the Supplemental figure 1 were normalized to cyclophilin B. Samples were quantified with ABI prism software. An n=5 was obtained for wild type and ghrelin null mice. All values are expressed as means +/− SEM. Statistical analysis was performed with a two-tailed Student’s unpaired t test. Results were considered significant when P < 0.05.
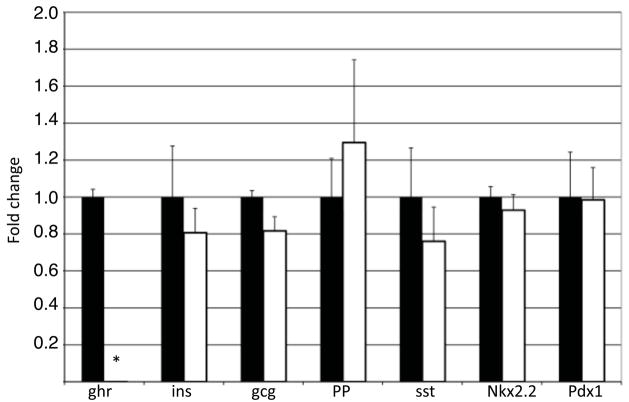
Figure 5. Pancreatic hormone and transcription factor gene expression levels are unchanged in ghrelin null embryos.
mRNA levels were determined by quantitative real time PCR for each pancreatic endocrine hormone and Nkx2.2 and Pdx1 in e18.5 wild type (black bars) and ghrelin null (white bars) embryos. As expected, ghrelin mRNA was undetectable in the ghrelin null pancreas. Expression of the remaining hormones and two essential pancreatic transcription factors, Nkx2.2 and Pdx1, was unaffected in the ghrelin null embryos. n = 5 for each genotype.
Results
Endocrine differentiation in the embryonic mouse pancreas begins at approximately e9.0 with the appearance of glucagon- and ghrelin-positive cells, followed by the formation of a small number of insulin-producing cells soon. Between e13.5 and e15.5 there is a major wave of endocrine cell differentiation and increased endocrine cell numbers. During late gestation, ghrelin-producing epsilon cell numbers decrease while glucagon and insulin cell numbers continue to increase and begin to form islets. To determine the role of ghrelin at these important stages of pancreatic development, we assessed the formation of the insulin- and glucagon-producing cells during in wild type and ghrelin knockout embryos.
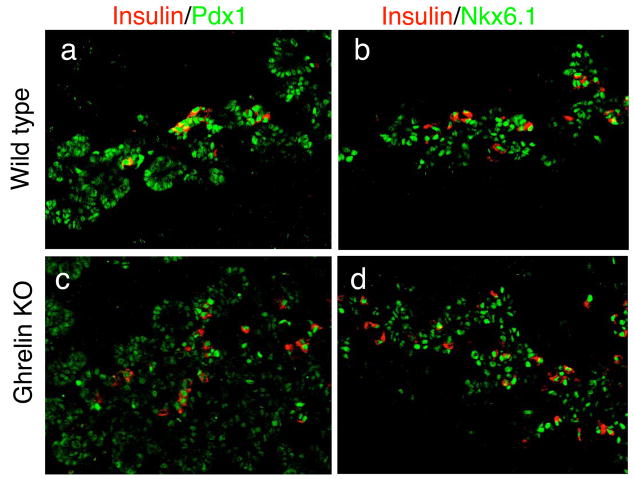
We first analyzed pancreata at e13.5 to determine whether the loss of ghrelin affects early islet cell differentiation. Wild type and ghrelin null mice [14] were stained for glucagon and insulin, as well as the beta cell specific transcription factors Nkx6.1 and Pdx1 (Figure 1). At e13.5, the mouse pancreas consists of a large network of branching epithelium that expresses high levels of Nkx6.1 and Pdx1. In the ghrelin null pancreas, the temporal and spatial expression of Nkx6.1 and Pdx1 is indistinguishable from the wild type littermates (Figure 1). Furthermore, the pancreatic area and the number of glucagon and insulin cells present at this stage appeared to be unchanged (Figure 1 and Figure 2).
Figure 1. Absence of ghrelin does not affect early endocrine cell differentiation.
Immunofluorescence staining of embryonic pancreata in e13.5 wild type (a–c) and ghrelin null (d–f) mice. Nkx6.1 (b and e, green) and Pdx1 (c and f, green) are broadly expressed throughout the pancreatic epithelium in the ghrelin null embryos and the wild type littermates. Glucagon (a and d, red) and insulin (b, c, e and f, red) cells are evident in the ghrelin null embryos in numbers comparable to wild type. Magnification: 40x (a and d) and 25x (b,c,e and f). Confocal microscopy was used for b,c,e and f.
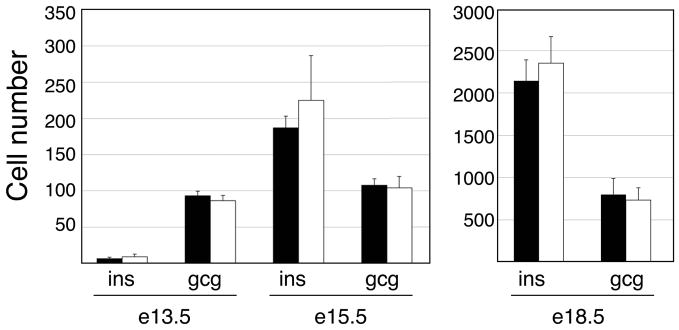
Figure 2. Insulin and glucagon cell numbers are unchanged in the ghrelin null embryos throughout embryogenesis.
Quantification of insulin+ and glucagon+ cells in wild type (black bars) and ghrelin null (white bars) embryos. No significant differences in the cell populations were detectable between the ghrelin null and wild type embryos. n=4 (e13.5 and e15.5), n=3 (e18.5).
We next examined pancreatic development in e15.5 embryos to determine whether the loss of ghrelin affects the major wave of islet cell differentiation. Pancreata from ghrelin null embryos showed no significant difference in Pdx1 and Nkx6.1-expressing populations and the number of insulin-producing cells was comparable to wild type embryos (Figure 2 and Figure 3). Between e15.5 and e18.5, the remaining endocrine cell populations differentiate and the endocrine cells coalesce to form islets. At e18.5, ghrelin cell numbers and ghrelin expression levels peak. In the ghrelin null embryos, notwithstanding the absence of ghrelin cells, we did not detect any perturbation in the islet cell populations and islet structure was indistinguishable from wild type islets (Figure 4 and 5). There were no statistical differences in hormone expression in the ghrelin KO embryos and at least two of the essential islet regulatory factors, Nkx2.2 and Pdx1, were also unchanged (Figure 5). These results indicate that ghrelin is not necessary for normal endocrine development and islet formation.
Figure 3. The loss of ghrelin does not affect the major wave of insulin cell differentiation during the secondary transition of pancreas development.
Immunofluorescence staining of embryonic mouse pancreata in e15.5 wild type (a–b) and ghrelin null (c–d) mice. Pdx1 (a and c, green) and Nkx6.1 (b and d, green) are broadly expressed throughout the pancreatic epithelium, with no apparent differences in cell number or distribution between wild type and ghrelin null embryos. Insulin positive cells (a–d, red) are present in similar numbers in wild type and ghrelin null embryos. Magnification 25x (confocal images).
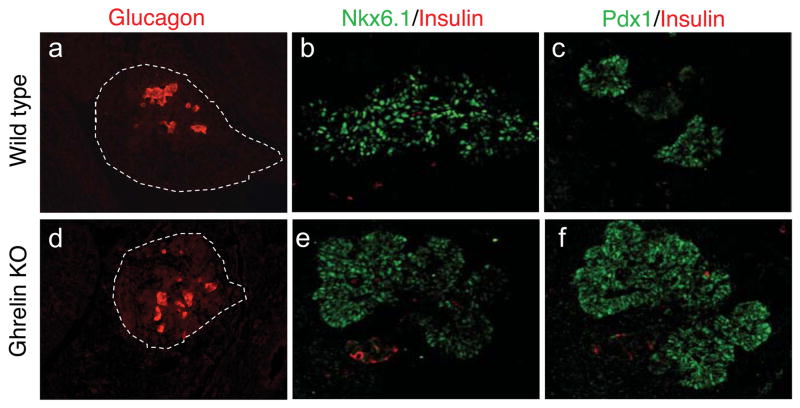
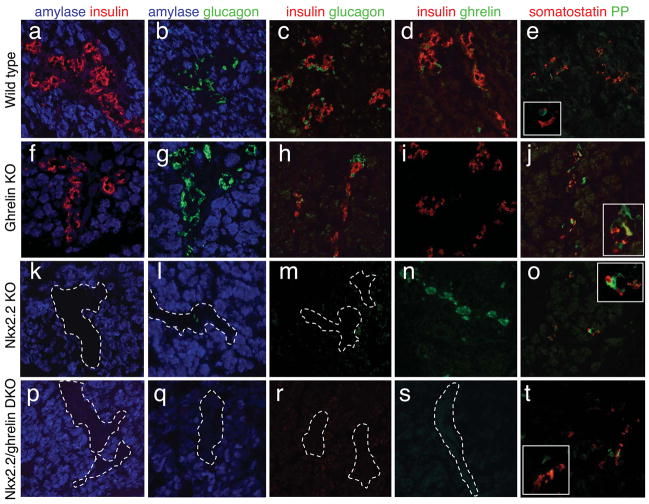
Figure 4. Pancreatic islets form normally in the ghrelin null embryos and ghrelin overexpression is not responsible for the absence of insulin and glucagon cells in Nkx2.2 null embryos.
Immunofluorescence analysis of the five endocrine hormones in e18.5 wild type (a – e), ghrelin null (f – j), Nkx2.2 null (k – o), and Nkx2.2 −/−, ghrelin−/− double mutant embryos (p – t). There are no apparent changes in the number of each hormone-producing cells or the amount of hormone per cell in the ghrelin null embryos (compare a–e with f–j). Hormone expression (or lack of expression) in the double Nkx2.2−/−, ghrelin−/− double knockout embryos appear identical to the Nkx2.2 null mice, with the exception of ghrelin expression (compare k–o with p–t; note the absence of ghrelin the double mutant embryos; s). Insets in e, j, o and t show higher magnification images. White dashed lines encircle islets lacking hormone expression. Insulin (a, c, d, f, h, i, k, m, n, p, r, s; red), glucagon (b, c, g, h, l, m, q, r; green), ghrelin (d, i, n, s; green), somatostatin (e, j, o, t; red), PP (e, j, o, t; green), and amylase (a, b, f, g, k, l, p, q, blue). Magnfication: 20x, insets are 40x.
Nkx2.2 null mice lack insulin- and the majority of glucagon-producing cells; these cell populations are replaced by ghrelin-positive cells [1]; Figure 6). There is some evidence to suggest that ghrelin is able to regulate hormone expression and/or cell differentiation [18] and it has been proposed that the ghrelin over-expression observed in the Nkx2.2 null embryos (Figure 4 and 6) may function to inhibit insulin- and glucagon-cell formation and/or hormone production during embryogenesis. To determine whether the relative changes in hormone-producing populations in Nkx2.2 null embryos is secondary to excessive ghrelin production, we generated Nkx2.2−/−; ghrelin−/− double mutant embryos. In the double mutant embryos, insulin expression remained undetectable at all stages examined (Figure 4 and data not shown), suggesting that the increase in ghrelin production is likely not responsible for the loss of insulin and glucagon in the Nkx2.2 knockout mice.
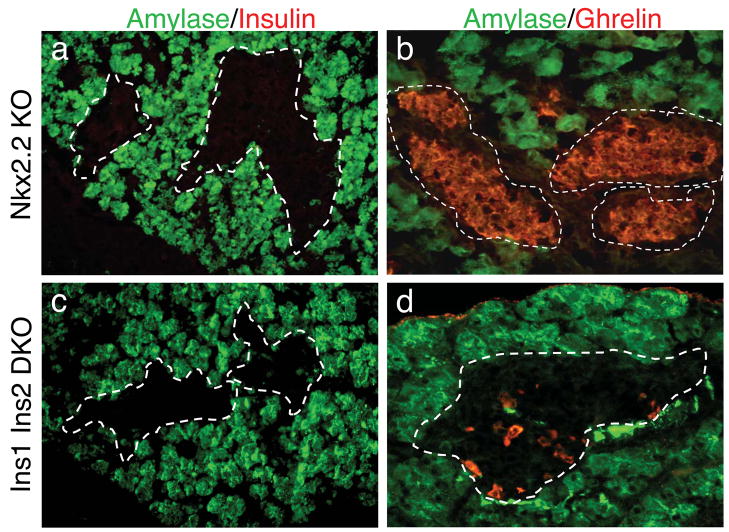
Figure 6. Ghrelin expression is not affected in embryos lacking insulin.
Immunofluorescence analyses of relative insulin and ghrelin expression in Nkx2.2 null (a and b) and Ins1; Ins2 double mutant (c and d) e18.5 embryos. The lack of insulin per se does not contribute to the increase of ghrelin-producing cells observed in the Nkx2.2 null animals. Amylase (a – d; green), insulin (a, c; red), ghrelin (b, d; red). 40x magnification.
It has also been suggested that the ghrelin gene itself is hormonally regulated [29]. To determine whether the lack of insulin in the Nkx2.2 null mice results in the subsequent upregulation of ghrelin, we assessed ghrelin levels in ins1−/−; ins2−/− double mutant e18.5 embryos ([27]; Figure 6). There was no increase in ghrelin expression in the insulin double mutant mice, suggesting that the upregulation of ghrelin in the Nkx2.2 null mice is not simply due to the absence of insulin expression. Taken together, these experiments show that insulin and ghrelin hormone expression do not influence the development and differentiation of the endocrine cell populations during islet development.
Discussion
Ghrelin and the ghrelin receptor (GHSR1a) are widely expressed in mouse and human tissues and are responsible for regulating a variety of metabolic functions, including growth hormone release, food intake and energy metabolism [11, 30–32]. In addition, ghrelin is expressed in the testis where it can modulate the expansion of immature Leydig cells, as well as SCF production in sertoli cells [17, 18]. An alternative role of ghrelin has also been shown in several cancers where ghrelin acts as a paracrine/autocrine factor [33]. There is additional evidence to suggest ghrelin is able to stimulate or inhibit (depending on the cell type) the proliferation and differentiation of other cell populations, including osteoblasts, adipocytes and neuronal cells [19–22]. It has been proposed, therefore, that ghrelin functions as a paracrine signal to control expansion and differentiation of specific cell types.
The ghrelin-producing epsilon population within the rodent pancreatic islet is predominantly present during embryogenesis, with ghrelin expression peaking just before birth [24]; Supplementary figure 1). In at least three different mouse mutant models (Pax4, Pax6 and Nkx2.2), an increase in the amount of ghrelin within the islet corresponds with a loss of either insulin-producing beta cells, glucagon-producing alpha cells, or both cell populations [1, 25]. Although we hypothesized that in the Nkx2.2 null mice the altered ratio of endocrine cell types is due to a switch in cell fates [1], we were not able to rule out that the increase in ghrelin expression acted in a paracrine manner to influence endocrine cell differentiation or insulin and glucagon expression. In this current study, we analyzed the developing pancreas of ghrelin null embryos to determine whether ghrelin is necessary for proper endocrine differentiation and/or islet formation during embryogenesis. Given the mild phenotype associated with the adult ghrelin null mice, we expected to observe a delay in endocrine cell differentiation or perhaps an impairment of islet cell development that would be recovered postnatally. However, we were unable to detect any discernible islet phenotype in the absence of ghrelin, suggesting that ghrelin is dispensable for normal endocrine cell differentiation and development during embryogenesis.
Given that the upregulation of ghrelin has a profound affect on differentiation processes, feeding behavior and metabolic functions [10, 17, 18, 34, 35], it was possible that the increase of ghrelin was responsible for the loss of insulin- and glucagon producing cells in the Nkx2.2 null embryos. However, the elimination of ghrelin from the Nkx2.2 null islets did not restore the insulin- and glucagon- producing cell populations, suggesting that the upregulation of ghrelin is not responsible for the loss of insulin- and glucagon- producing cells in the Nkx2.2 null mice. In addition, we demonstrated that the absence of insulin does not lead to the upregulation of ghrelin. Overall, it would appear that the formation of the different endocrine populations within the islet is not influenced by hormone production and likely occurs independently, although it remains possible that other factors expressed by the ghrelin cell population can influence islet cell development.
The pancreas is one of the major sources of ghrelin in the embryo, yet the function of the ghrelin-producing cells within the developing islet remains elusive. Although ghrelin appears to be dispensable for embryonic pancreas development, the discovery that ghrelin cells often replace the other endocrine populations in the pancreas and intestine [1, 36] suggests there is a lineage relationship between the ghrelin-producing epsilon cells and many of the other hormone-producing populations. This is supported by the evidence that ghrelin cells arise from the common Ngn3+ endocrine progenitor population [25]. Furthermore, the genetic data suggests that the differentiation of epsilon cells is closely linked to the many of the other endocrine cell populations. It remains possible that the ghrelin cells represent an endocrine precursor population or are part of a default lineage pathway that is triggered in the absence of the appropriate regulatory signals. Alternatively, the ghrelin cells may represent a vestigial endocrine cell population in the gut and pancreas that is no longer necessary for organ development or function. Future lineage tracing studies of the ghrelin-expressing population within the pancreas will help resolve these issues and help determine the precise role of islet ghrelin cells during embryogenesis.
Supplementary Material
Supplemental Figure 1. Pancreatic ghrelin mRNA expression throughout embryonic development. Ghrelin mRNA levels were determined by quantitative real time PCR on samples collected daily between e12.5 and PO. n=4 for each stage.
Acknowledgments
We thank Morgan Singleton for her help in maintaining the mouse colony. We thank Dr. Lee Niswander for use of the Zeiss confocal microscope. This work was supported by an ADA RRG (LS), NIH Predoctoral Training Awards in Molecular Biology: T32-GM08730 (JS and MD) and Endocrinology: T32-DK07328-29 (JH), and a Naomi Berrie Fellowship (TLM). We would also like to acknowledge support from the DERC (NIH P30 DK63608) and the Naomi Berrie Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierup N, Sundler F. Circulating levels of ghrelin in human fetuses. Eur J Endocrinol. 2004;150:405. doi: 10.1530/eje.0.1500405. [DOI] [PubMed] [Google Scholar]
- 3.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301–10. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 4.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–9. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology. 2003;144:3749–56. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 7.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. The Journal of clinical investigation. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 9.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 11.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 12.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. The Journal of clinical endocrinology and metabolism. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 13.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8227–32. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Molecular and cellular biology. 2003;23:7973–81. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei W, Wang G, Qi X, Englander EW, Greeley GH., Jr Characterization and regulation of the rat and human ghrelin promoters. Endocrinology. 2005;146:1611–25. doi: 10.1210/en.2004-1306. [DOI] [PubMed] [Google Scholar]
- 16.Gualillo 0, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEES letters. 2003;552:105–9. doi: 10.1016/s0014-5793(03)00965-7. [DOI] [PubMed] [Google Scholar]
- 17.Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, Dieguez C, Tena-Sempere M. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod. 2002;67:1768–76. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- 18.Barreiro ML, Gaytan F, Castellano JM, Suominen JS, Roa J, Gaytan M, Aguilar E, Dieguez C, Toppari J, Tena-Sempere M. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology. 2004;145:4825–34. doi: 10.1210/en.2004-0732. [DOI] [PubMed] [Google Scholar]
- 19.Kim SW, Her SJ, Park SJ, Kim D, Park KS, Lee HK, Han BH, Kim MS, Shin CS, Kim SY. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37:359–69. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Maccarinelli G, Sibilia V, Torsello A, Raimondo F, Pitto M, Giustina A, Netti C, Cocchi D. Ghrelin regulates proliferation and differentiation of osteoblastic cells. The Journal of endocrinology. 2005;184:249–56. doi: 10.1677/joe.1.05837. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, Mulholland MW. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–37. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Zhao L, Lin TR, Chai B, Fan Y, Gantz I, Mulholland MW. Inhibition of adipogenesis by ghrelin. Mol Biol Cell. 2004;15:2484–91. doi: 10.1091/mbc.E03-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 24.Chanoine JP, Wong AC. Ghrelin Gene Expression is Markedly Higher in Fetal Pancreas Compared to Fetal Stomach: Effect of Maternal Fasting. Endocrinology. 2004 doi: 10.1210/en.2004-0053. [DOI] [PubMed] [Google Scholar]
- 25.Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Genetic determinants of pancreatic epsilon-cell development. Developmental biology. 2005;286:217–24. doi: 10.1016/j.ydbio.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 26.Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development (Cambridge, England) 1998;125:2213–21. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 27.Duvillie B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Jami J, Joshi RL, Bucchini D. Phenotypic alterations in insulin-deficient mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5137–40. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Developmental biology. 2003;264:323–38. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Barreiro ML, Suominen JS, Gaytan F, Pinilla L, Chopin LK, Casanueva FF, Dieguez C, Aguilar E, Toppari J, Tena-Sempere M. Developmental, stage-specific, and hormonally regulated expression of growth hormone secretagogue receptor messenger RNA in rat testis. Biol Reprod. 2003;68:1631–40. doi: 10.1095/biolreprod.102.008862. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 32.Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. The Journal of clinical endocrinology and metabolism. 2000;85:4908–11. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery PL, Herington AC, Chopin LK. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone-dependent cancer. Cytokine Growth Factor Rev. 2003;14:113–22. doi: 10.1016/s1359-6101(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 34.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell metabolism. 2006;4:323–31. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–12. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 36.Desai S, Loomis Z, Pugh-Bernard A, Schrunk J, Doyle MJ, Minic A, McCoy E, Sussel L. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Developmental biology. 2008;313:58–66. doi: 10.1016/j.ydbio.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Pancreatic ghrelin mRNA expression throughout embryonic development. Ghrelin mRNA levels were determined by quantitative real time PCR on samples collected daily between e12.5 and PO. n=4 for each stage.