Abstract
We used in situ-hybridization on sections to examine the distribution of GAD67-expressing cell populations in the entire forebrain of the adult zebrafish. GAD67 is predominantly expressed in the olfactory bulb (OB), all regions of the subpallium (including dorsal, ventral, central, and lateral nucleus of the area ventralis; = Vd, Vv, Vc, Vl respectively), as well as preoptic (PPa, PPp, PM), pretectal (PPd, PPv, PCN, PSp, PSm), ventral (= pre-) thalamic (I, VM, VL), hypothalamic (Hr, Hi, Hc), preglomerular (P, PGa, PGl, PGm, RT), and posterior tubercular (TPp, TPm) nuclei. Only scattered GAD67-expressing cells are seen in all pallial zones (Dm, Dd, Dc, Dl, Dp) and in the previously unidentified bed nucleus of the stria medullaris (BNSM). The BNSM appears to be the adult teleostean derivative of the larval eminentia thalami (EmT). We identify the GAD67-positive entopeduncular nucleus proper (EN) as being homologous to the entopeduncular nucleus of non-primate mammals. GAD67 is strongly expressed in the anterior thalamic nucleus (A). The anterior thalamic nucleus is laterally bordered by a distinct GAD67-expressing cell population which we interpret as the previously unidentified reticular thalamic nucleus (RTN) of teleosts. Furthermore, we identified a GAD67-positive thalamic nucleus, the intercalated nucleus (IC), which is sandwiched between the GAD67-negative dorsal (DP) and central posterior (CP) thalamic nuclei. Overall, the distribution of GAD67-expressing cells highly resembles the distribution of GABA/GAD67-expressing cells found in the early zebrafish (teleost) forebrain and, thus, allows to propose a prosomeric fate map of GABAergic cell populations.
Keywords: amygdala, basal ganglia, eminentia thalami, EmT, entopeduncular nucleus, GABA, LIM homeobox genes, MGE, migration, thalamic reticular nucleus, pallidum, bed nucleus of the stria medullaris, BNSM, striatum
INTRODUCTION
Comparative analyses of the distribution of various neurotransmitter phenotypes such as γ-aminobutyric acidergic (GABAergic) neurons between mammalian, sauropsidian, and amphibian forebrains have helped to elucidate homologies and evolution of major tetrapod forebrain components, such as the basal ganglia, the amygdaloid complex, as well as ventral and dorsal thalamic nuclei (Mugnaini, 1985; Medina and Reiner, 1995; Marín et al., 1997; 1998b; a; Reiner et al., 1998; Swanson and Petrovich, 1998; González et al., 1999; Katarova et al., 2000; Smeets et al., 2000; Brox et al., 2003). Furthermore, a similar comparative approach of studying expression patterns of regulatory genes during vertebrate forebrain development has led to the proposition of prosomeric models, which serve as developmental paradigms and facilitate interspecies comparisons to uncover homologies between vertebrate forebrains (Bulfone et al., 1993; Puelles and Rubenstein, 1993; Porteus et al., 1994; Eisenstat et al., 1999; Puelles et al., 2000; Puelles and Rubenstein, 2003). Altogether these studies have brought about considerable improvement in the comparative understanding of tetrapod forebrain evolution, for example regarding the ancestral state of basal ganglia circuitry and the organization of the amygdala, as well as the understanding of sauropsidian-avian versus mammalian ventral pallial organization, such as the comparative understanding of the sauropsidian dorsal ventricular ridge (DVR). Similar progress is lacking for most non-tetrapod anamniotes.
A particularly decisive role in dissecting the tetrapod forebrain has been played by the recognition of embryonic and adult distribution of GABAergic phenotypes and there is now growing body of data on these neuronal phenotype distributions in non-tetrapod anamniotes (Pombal and Puelles, 1999; Meléndez-Ferro et al., 2002; Robertson et al., 2007). In the zebrafish in particular, a critical mass of data has accumulated in the past years on the expression of genes and other markers related to the development of the GABAergic system (Blader et al., 1997; Mueller and Wullimann, 2002; Wullimann and Mueller, 2002; Blader et al., 2003; Mueller and Wullimann, 2003; Wullimann and Mueller, 2004b; a; Mueller and Wullimann, 2005; Mueller et al., 2006; Mueller et al., 2008). Theses studies in the developing zebrafish have provided a detailed picture of the spatiotemporal complementary forebrain gene expression patterns of neurogenin1 and neuroD versus Zash-1a and dlx2a during the beginning of secondary neurogenesis around two to three days postfertilization (dpf) (Mueller and Wullimann, 2002; 2003; 2005). The complementary forebrain expression patterns correlate perfectly with the distribution of early GABA-expressing cell populations, which only originate in Zash-1a and dlx2a-positive domains (such as the subpallium, preoptic region, hypothalamus and ventral thalamus) but not in purely neurogenin1/neuroD-positive domains (pallium, eminentia thalami or dorsal thalamus) (Wullimann and Mueller, 2002; 2004a). The GABA/GAD-expressing cells found in the Zash-1a and dlx2a-negative pallium of larval zebrafish originate in a subpallial region homologous to the mammalian medial ganglionic eminence (MGE) from which they invade the pallium by tangential migration resembling the subpallial-pallial migratory streams found in tetrapods (Mueller et al., 2008). Based on the evolutionarily conserved expression patterns of proneural basic helix loop helix genes such as neurogenin1, neuroD, Zash-1a, as well as the distribution of GABA/GAD-expressing cells at early stages of brain development, it was suggested that vertebrates go through a phylotypic stage of GABA cell development (Mueller et al., 2006). In the early zebrafish (teleost) telencephalon, expression patterns of lim homeobox genes indicate a basal ganglia organization resembling that of mammals in the sense that a pallidal primordium (MGE) and striatal primordium (LGE) are present (Mueller et al., 2008). In contrast, in the adult teleost telencephalon, pallidal and striatal elements appear to be intermingled in the dorsal subpallium (Vd) and separate pallidal or striatal components have not been well characterized (Wullimann and Mueller, 2004b).
In contrast to this wealth of developmental information, there are only few studies on GABA/GAD-expressing cells in adult teleostean forebrains. The distribution of GABAergic cells has been investigated in the forebrain of the eel (Medina et al., 1994), trout (Anglade et al., 1999) and goldfish (Martyniuk et al., 2007). These studies focused on the distribution of GABAergic cells in telencephalic and hypothalamic regions and did not lead to an improved anatomical characterization of subpallial units to distinguish among striatum, pallidum and septum as recently discussed (Wullimann and Mueller, 2004b). The distribution of GABAergic cell populations within ventral and dorsal thalamic nuclei of teleosts is far from being well understood. In addition, the important question of how the distribution of GABAergic/GAD-expressing neurons in the adult teleost forebrain relates to the prosomeric distribution of GABA/GAD-expressing cells in the larval teleostean forebrain has yet to be examined. Thus, the recently well documented situation in the larval zebrafish opens a new avenue to better understand the organization of GABAergic systems in the adult zebrafish forebrain and may lead to fundamental insights into the subpallial and thalamic organization of teleosts.
In order to shed light on the evolution and prosomeric organization of the adult teleostean forebrain, we analyzed expression patterns of GAD67-mRNA with in situ-hybridization on complete series of sections of the adult zebrafish forebrain.
Specifically, we asked the following questions:
How does the distribution of GAD67-expression patterns in the adult zebrafish forebrain relate to the distribution of GABA/GAD-expressing cells at early larval stages (Mueller et al., 2006)? Does the distribution of GAD67-expressing cells in the adult zebrafish forebrain support a prosomeric bauplan as has been proposed for the larval teleostean brain?
Does the distribution of GAD67-expressing cells permit the determination of more detailed forebrain homologies such as the distinction between telencephalic subunits for example amygdala, pallidum, striatum and septum?
How does the distribution of GAD67-expressing cells in the adult forebrain compare to the distribution of GABA/GAD in other anamniote and amniote vertebrates? In particular, how does the distribution of GAD67-expressing cells in the adult zebrafish forebrain compare to findings in the brains of lampreys (Meléndez-Ferro et al., 2002; Robertson et al., 2007), lungfish (Trabucchi et al., 2008), frogs (Brox et al., 2003) and mammals (Mugnaini, 1985; Esclapez et al., 1994; Katarova et al., 2000; Hayes et al., 2003)?
We will discuss similarities and differences of the GABAergic system between these species and draw conclusions for the interpretation of vertebrate forebrain evolution especially at the non-tetrapod/tetrapod transition.
MATERIAL AND METHODS
Animals
Zebrafish were kept and bred according to standard procedures (Kimmel et al., 1995). Before perfusion and fixation, adult zebrafish have been anesthetized with MS 222 (Methyl 3-aminobenzoate methanesulfonate, Sigma, St. Louis, MO) dissolved in aquarium water. This study does not involve experiments on living animals. All procedures followed US National Institutes of Health as well as UCSF institutional (IACUC) guidelines for the care and use of animals.
Tissue preparation
Anesthetized adult zebrafish have been perfused with phosphate buffer (PB; pH 7.4) followed by perfusion fixation with 4% paraformaldehyde (PFA) in PB. Brains were removed and kept in the same fixative overnight. For cryoprotection, brains were transferred and kept overnight in phosphate buffer containing 30% (w/v) sucrose. For preparation of cryosectioning, brains were embedded in Tissue Tek (Sakura) and frozen at −80° Celsius. Brains were sectioned on a Leica cryostat at a thickness between 16 and 40 μm and transverse sections were thaw-mounted on superfrost plus slides (fisher) and air-dried for 2 hours or overnight before in situ-hybridization.
In situ-hybridization
The in situ-hybridization on sections was performed according to the protocol of Dorsky et al. (1995). The hybridization step was carried out under high stringency conditions at 55°C. Antisense riboprobes for in situ-hybridization were synthesized as follows: GAD67 (zfin: gad1; nucleotides 151–1964 of GenBank accession number AF017266). Riboprobes were labeleled with digoxigenin-11-UTP (Roche, Mannheim, Germany). The detection of hybridized probes was performed with an alkaline phosphatase (AP) coupled anti-DIG antibody (Roche, Mannheim, Germany; dilution 1:1000). AP staining was developed overnight with 4-nitroblue tetrazolium chloride/phosphate/5/bromo-4-chloro/3-indolyl-phosphate solution (NBT/BCIP, Roche, Mannheim, Germany). Afterwards, slides were washed 3x (15 min each) in PB, fixed in 4% PFA (in phosphate buffer, PB) for 1h and passed, after an additional round of washes in PB (3x 15 min) through an ascending series of graded ethanol solutions (50%, 70%, 80%, 90%, 96%, 100%; 2 min each). Slides were then transferred into xylene (3x 5 min) and coverslipped with Entellan (Merck, Darmstadt, Germany). Around 50% of brain sections (two out of 4 brains) have been counterstained with nuclear fast red which allowed a clear distinction of GAD67-expressing cells versus unstained cells.
Photomicrograph production
Histological sections were analyzed with a Zeiss Axiophot 2 microscope and photographed with Nomarski optics via a Zeiss Axiocam digital camera. Selected sections were slightly adjusted for contrast and sharpness using Photoshop Elements 2.0. Resulting photographs have been arranged and designated using CorelDraw 9.
Principles of interpretation and neuroanatomical nomenclature
Analysis and grouping of GAD67-expressing cells have both been done on nuclear fast red counterstained or non-counterstained sections photographed with Nomarski optics, which allowed a clear distinction between stained and non-stained cells. Furthermore, all sections were analyzed in comparison to the atlas of the adult zebrafish brain (Wullimann et al., 1996), which is based on sections stained with a combined Nissl-Bodian silver protocol.
The interpretation of GAD67 expression in the adult zebrafish brain follows the previously established larval teleostean prosomeric bauplan (Wullimann and Puelles, 1999; Wullimann and Mueller, 2004a). According to this bauplan the pretectum, dorsal thalamus (thalamus proper), and ventral thalamus (prethalamus) are the alar plate derived prosomeres one to three respectively and the nucleus of the medial longitudinal fascicle (NMLF), dorsal posterior tuberculum (PTd) and ventral posterior tuberculum (PTv) are their respective basal plate counterparts.
Neuroanatomical designations for the zebrafish brain largely follow those of the adult zebrafish brain atlas (Wullimann et al., 1996), but have been complemented according to recent studies (Rink and Wullimann, 2001) and our findings in this study.
Results
In the following, we will give a description of the adult zebrafish forebrain with respect to its GAD67 cytoarchitectonic organization.
Expression of GAD67 in the telencephalon
In the zebrafish telencephalon, GAD67-expressing cells are highly abundant in the subpallium (figs. 1A–E; 2A, B), allowing clear discernment of the subpallium from the dorsally adjacent pallium, which shows only sparse and scattered GAD67-expressing cells. This is not merely due to compact clustering of neuronal cell bodies in the subpallium versus more dispersed cell bodies in the pallium, but rather because a much smaller percentage of GAD67-expressing cells is present in the pallium than in the subpallium. This finding is similar to reports on diverse tetrapod model organisms such as rodents (Swanson and Petrovich, 1998; Katarova et al., 2000), chick (Sun et al., 2005), and frog (Xenopus: (Barale et al., 1996). All pallial subdivisions including the medial (Dm), dorsal (Dd), central (Dc), lateral (Dl) and posterior (Dp) zone of the dorsal telencephalic area exhibit scattered GAD67-expressing neurons, but Dl is defined by even less numerous GAD67-expressing cells compared to all other pallial areas. In the subpallium, heavily labeled regions include the dorsal nucleus (Vd; figs. 1A–D), the ventral nucleus (Vv; figs. 1A–D) and the lateral nucleus of the area ventralis telencephali (Vl; figs. 1A-D). Large GAD67-expressing cells originating in the dorsal nucleus of the area ventralis telencephali formed a strip (figs. 1B, C) leading ventrally into the central nucleus of the area ventralis telencephali (Vc). The area of origin of these migrated cells in the ventral part of the dorsal nucleus of the area ventralis telencephali (Vd) suggests that this is part of the adult derivative of the teleostean medial ganglionic eminence (MGE) as recently identified in the larval zebrafish brain (Mueller et al., 2008).
Fig. 1.
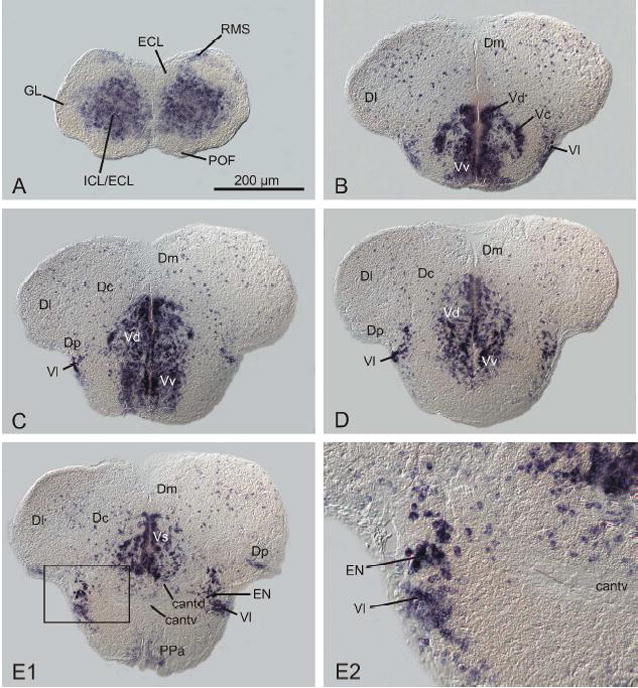
Distribution of GAD67-expressing cells in the zebrafish telencephalon (figs. A-E2). Note that GAD67-expressing cells in the (pallidal) entopeduncular nucleus (EN) are considerably larger compared to GAD67-expressing cells in the (septal) lateral nucleus of the area ventralis telencephali (Vl; fig. E1). For abbreviations, see list.
Fig. 2.
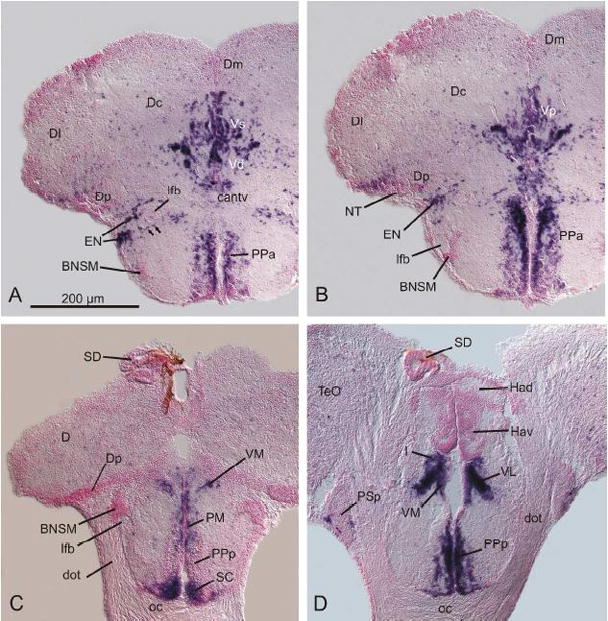
Distribution of GAD67-expressing cells in the zebrafish telencephalon, preoptic region and habenula (A–D). Note the strip of GAD67-expressing cells (arrows in fig. A) reaching from a medial part of the area ventralis towards the entopeduncular nucleus (EN). For abbreviations, see list.
As recently suggested in the context of the partial eversion hypothesis of the teleostean telencephalon (Wullimann and Mueller, 2004b), Vd comprises the basal ganglia and includes the derivative of both medial and lateral ganglionic eminences. Consistent with this interpretation, we did not find differences in the mean size or densities of GAD67-expressing cells in Vd, which supports the notion that striatal and pallidal elements are intermingled and not clearly separable from each other in the adult zebrafish basal ganglia. At the level of the anterior commissure (ac) and caudally to it, abundant GAD67-expressing cells are present in the supracommissural nucleus (Vs; 2A) and the postcommissural nucleus (Vp; 2B) of the ventral telencephalic area which together represent the teleostean subpallial part of the amygdaloid complex (Braford and Northcutt 1983; Wullimann and Mueller, 2004b).
Furthermore, GAD67-expressing medium sized cells form the predominant cell type of the lateral nucleus and ventral nucleus of the area ventralis telencephali (Vl and Vv respectively, figs. 1B–E). These presumably septal cell groups were clearly separable from the dorsally and caudally adjacent GAD67 expressing cells of the entopeduncular nucleus (EN). The GAD67-expressing cells of the EN appeared larger and less densely packed compared to GAD67-expressing cells of Vl (figs. 1E1 and 1E2). The larger size and lesser density of the GAD67-expressing cells in EN suggest that they may be pallidal GABAergic projection neurons. Thus, the delimitation and extent of the entopeduncular nucleus (proper) in this study differs from that of the entopeduncular complex given earlier (Wullimann et al., 1996). The GAD67-positive (dorsal) entopeduncular nucleus (EN = former ENd) appears to be anatomically separate from the GAD67-negative bed nucleus of the stria medullaris (BNSM), which was formerly called the ventral part of the entopeduncular nucleus (ENv; Wullimann et al., 1996). Therefore, the larger entopeduncular complex (ENd plus ENv) can not be seen as having derived in its entirety from the embryonic eminentia thalami (EmT) as proposed recently (Wullimann and Mueller, 2004a). In the present study, the entopeduncular nucleus is exclusively defined as the GAD67-positive portion of the former entopeduncular complex (i.e., the former ENd; Wullimann et al., 1996) which likely is a subpallial derivative homologous to the entopeduncular nucleus of non-primate mammals and internal segment of the globus pallidus in primates.
Expression of GAD67 in the preoptic region
Abundant GAD67-expressing cells are detectable in all subdivisions of the preoptic region (figs. 2A–D). Densely packed neurons expressing GAD67 mRNA are present in the anterior part of the parvocellular preoptic nucleus (PPa; figs. 2A, B), the magnocellular preoptic nucleus (PM) and most parts of the posterior part of the parvocellular preoptic nucleus (PPp). This latter nucleus is free of GAD67-expressing cells in a smaller anterior part of it (PPp in fig. 2C). The suprachiasmatic nucleus (SC) contains both heavily GAD67-positive cells and scattered intermingled GAD67-negative cells (fig. 2C).
Expression of GAD67 in the diencephalon
Bed nucleus of the stria medullaris (BNSM) - Eminentia thalami (EmT)
The ventral nucleus of the former entopeduncular complex (ENv of Wullimann et al., 1996) appears as largely GAD67-negative and displays only a very few number of GAD67-expressing cells and is, thus, the derivative of the larval teleostean EmT as identified earlier (Wullimann and Mueller, 2004). The larval EmT is calretinin-positive and projects to the habenula (Hendricks and Jesuthasan, 2007). These facts together indicate that the largely GAD67-negative derivative of the larval EmT is homologous to the calretinin positive and presumably glutamatergic bed nucleus of the stria medullaris (BNSM) in mammals (Risold and Swanson, 1995; Abbott and Jacobowitz, 1999). The BNSM in the adult zebrafish stands out as a largely GAD67-negative nucleus in close proximity to the lateral forebrain bundle (lfb in figs. 2A–C) and appears at the level of the anterior commissure (cant) in close proximity and ventrally to the lateral forebrain bundle (fig. 2A). Typically, the BNSM surrounds the lateral forebrain bundle at intermediate caudal levels (fig. 2B). At most caudal levels in the region of the optic chiasma (oc), the BNSM is found to be very prominent and is located dorsally of the lfb close to the pallial/subpallial border and anterior to the emerging posterior part of the parvocellullar preoptic nucleus (PSp).
Ventral versus dorsal thalamic nuclei, posterior tuberulum, preglomerular nuclei
All ventral thalamic (prethalamic) nuclei are defined by a predominance of heavily labeled GAD67-expressing cell clusters (VM, VL, I in figs. 2C and D). The GAD67-positive ventromedial thalamic nucleus (VM, fig. 2C) emerges at the level of the optic chiasma somewhat lateral to the caudal remainder of the periventricular subpallial (Vp) expression domain (compare cross section number 114 Wullimann et al., 1996). Somewhat more caudally (fig. 2D), the periventricular ventromedial (VM) and laterally adjacent lateral ventral thalamic (VL) nuclei stand out as heavily GAD67 labeled nuclei in sharp contrast to the dorsally adjacent GAD67-negative habenular nuclei (Had, Hav) and epiphysis (E). This finding is consistent with the notion that these brain regions are part of the dorsal thalamus (alar plate prosomere 2) and glutamatergic.
The zona limitans intrathalamica (ZLI) is an embryonic signaling center and boundary zone, which separates the early postembryonic GABA-free dorsal thalamus from the GABAergic ventral thalamus and itself might be a source of migrating nascent GABAergic cells (Wullimann and Mueller, 2002; Mueller et al., 2006). In the adult zebrafish brain, the ZLI appears ventrally of the most anterior part of the dorsal thalamic anterior nucleus (A; fig. 3A). Consistent with findings in larval stages, the adult ZLI occurs as a horizontal strip of GAD67-negative cells (fig. 3A) dorsally adjacent to a strip of GAD67-expressing cells. This strip of GAD67-expressing cells originates at a periventricular site and protrudes dorsolaterally to surround the anterior thalamic nucleus.
Fig. 3.
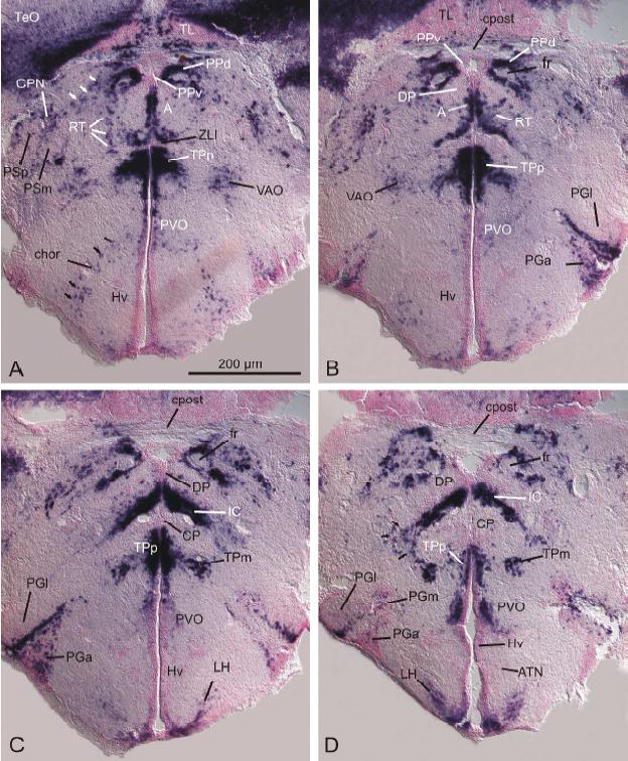
Distribution of GAD67-expressing cells in the zebrafish diencephalon (A–D). Note migrated cells (white arrows) from the dorsal part of the periventricular pretectal nucleus (PPd) to the central pretectal nucleus (CPN) and other adjacent migrated pretectal nuclei such as the parvocellular superficial pretectal nucleus (PSp) and the magnocellular superficial pretectal nucleus (PSm). Also, note the arc of GAD67-expressing cells (black arrows) reaching from the boundary of the paraventricular organ (PVO of Rink and Wullimann, 2001) towards the periphery of the ventral zone of the periventricular hypothalamus (Hv). For abbreviations, see list.
The anterior nucleus, although of dorsal thalamic origin, displays large periventricular clusters of GAD67-expressing cells (figs. 3A and B), whereas GAD67-negative (presumably glutamatergic) cells are located lateral to the periventricular GAD67-expressing cell populations (figs. 3A and B).
The two posterior dorsal thalamic nuclei, namely the dorsal posterior thalamic nucleus (DP) and the central posterior thalamic nucleus (CP), lack GAD67 expression. However, these dorsal thalamic nuclei are separated from each other by a large GAD67-expressing cell mass. We name this previously unidentified GAD67-expressing cell mass the intercalated nucleus (IC, figs. 3C–D). Based on its topology, this GABAergic nucleus most likely is a derivative of the basal plate portion of prosomere two, which has been shown to produce GABAergic cells at larval stages (PTd, Mueller and Wullimann, 2006). The GAD67-expressing cell mass of the intercalated nucleus (IC) appears ventrally of the caudal anterior nucleus (A; fig. 3B) and extends considerably caudally into CP/DP (figs. 3B–D). The intercalated nucleus (IC) protrudes from the periventricular site towards the periphery in a ventrolateral direction and its most peripheral GAD67-expressing cells merge with the migrated nucleus of the posterior tuberculum (TPm), a division of the posterior tuberculum newly described here. However, most GABAergic cells of migrated nucleus of the posterior tuberculum (TPm) appear to be ontogenetically derived from the periventricular posterior tuberculum (TPp). This is indicated by the heavily stained GAD67-expressing cell masses, which form a continuous strip from the periventricular nucleus of the posterior tuberculum (TPp) towards the migrated nucleus of the posterior tuberculum (TPm; nicely seen in fig. 3C). The prosomeric origin of the periventricular nucleus of the posterior tuberculum (TPp) is the larval ventral posterior tubercular area (PTv; Wullimann et al., 1996; Wullimann and Puelles, 1999; Mueller and Wullimann, 2002). Thus, TPm seems to be of multiprosomeric origin.
The periventricular nucleus of the posterior tuberculum (TPp) and the related nuclei of the preglomerular complex show many GAD67-positive and -negative portions. At anterior levels, the dorsal part of the periventricular nucleus of the posterior tuberculum (TPp) is formed by heavily stained GAD67-expressing cells, bilaterally dense GAD67-positive cell masses continuously extend to the migrated nucleus of the posterior tuberculum (TPm in figs. 3A–C) up to a caudal level where the migrated nucleus of the posterior tuberculum (TPm) stands out as a round shaped nucleus in some distance to the remaining caudal part of periventricular nucleus of the posterior tuberculum (TPp in fig. 3D). The ventral part of the periventricular nucleus of the posterior tuberculum forms a periventricular strip of GAD67-expressing cells. The GAD67-expressing cells increasingly thin out in density and intermingle with GAD67-negative cells at its outermost ventral part (nicely seen in fig. 3A). At caudal levels (figs. 4A, B), the periventricular nucleus of the posterior tuberculum is GAD67-negative.
Fig. 4.

Distribution of GAD67-expressing cells in the preglomerular complex and hypothalamus (A–F). For abbreviations, see list.
Two nuclei of the preglomerular complex, the posterior thalamic nucleus (P in figs. 4B and C) and the rostral tegmental nucleus (RT in fig. 4E) are heavily labeld with GAD67-expressing cells, whereas the medial preglomerular nucleus (PGm in figs. 4B–D) and tertiary gustatory nucleus (TNG in figs. 4C and D) show some GAD67-expressing cells. The subglomerular nucleus is essentially free of GAD67 expression. The anterior and lateral preglomerular nuclei (PGa and PGl, respectively; figs. 3B–D, 4A and B) display GAD67-positive among GAD67-negative cell clusters, with the lateral preglomerular nucleus showing a characteristic dense basal layer of positive cells. The ontogenetic origin of the majority of GAD67-expressing cells of the anterior, lateral, and medial preglomerular nuclei (PGa/PGl/PGm) is most likely the periventricular nucleus of the posterior tuberculum (TPp), because GAD67-expressing cells are found to form a continuous strip between TPp and PGa/PGl/PGm (figs. 3B–C). The larval pretectum (p1 alar plate) and central posterior thalamic nucleus (originating from PTd, p2 basal plate) may contribute GAD67-expressing cells to the preglomerular complex as stated earlier (Mueller and Wullimann, 2002; Wullimann and Mueller, 2002; Mueller and Wullimann, 2003; 2005). In agreement with this hypothesis, some scattered GAD67-expressing cells are found in the adult zebrafish diencephalon within the white matter between pretectal and preglomerular complex as well as between periventricular/migrated posterior tubercular nuclei and preglomerular complex.
In addition, many GAD67-positive as well as negative cell clusters have been found in the paraventricular organ (PVO) and the posterior tuberal nucleus (PTN) both of which are seen as derivatives of the larval ventral posterior tubercular area (PTv; p3 basal plate). Consistently, the larval PTv is defined by temporally distinct expression of ngn1 and neuroD as well as Zash-1a and many nascent GABAergic cells are produced between two and three days already. The nascent GABAergic cell clusters found in the early larva likely develop into the adult GAD67-expressing cell clusters in the paraventricular organ and the posterior tuberal nucleus as found in this study.
Pretectum
The zebrafish pretectum comprises a periventricular, a central, and a superficial pretectum (Wullimann et al., 1996). In this study, the interpretation of the periventricular nuclei, namely the dorsal and ventral part of the periventricular pretectal nucleus (PPd and PPv respectively) differs somewhat from the interpretation of Wullimann et al. (1996). The smaller and periventricularly located ventral part of the periventricular pretectum (PPv in figs. 3B and C) is largely GAD67-negative whereas numerous GAD67–expressing cells comprise the majority of neurons in the dorsal part of the periventricular pretectum (PPd) which completely surrounds the fasciculus retroflexus (fr; figs. 3A–D) with some scattered GAD67-expressing cells in the periphery beyond the confines of PPd as defined in the atlas of the adult zebrafish brain (Wullimann et al., 1996). Note that according to this interpretation, the border of the dorsal posterior nucleus (DP) of the dorsal thalamus does not touch the fasciculus retroflexus as it does in the atlas (Wullimann et al., 1996). The paracommissural nucleus (PCN; not shown) also shows many GAD67-expressing cells. The central pretectal nucleus (CPN) shows scattered GAD67-expressing cells within a majority of GAD67-negative cells. The same is true for both the parvocellular (PSp) and the magnocellular (PSm) nucleus of the superficial pretecum (fig. 3A). The nucleus of the medial longitudinal fascicle (NMLF) shows GAD67-expressing cells that are intermingled with GAD67-negative cells.
Hypothalamus
The teleostean hypothalamus consists of the median tuberal hypothalamus and a dorsolateral hypothalamus which includes the paired inferior (or lateral) lobes of the hypothalamus (Wullimann et al., 1996). Within the tuberal hypothalamus, the ventral hypothalamic zone extends farthest rostrally with the periventricular nucleus (Hv), which is mostly free of GAD67 in anterior parts (figs. 3A–D). At more caudal levels the periventricular nucleus (Hv) displays numerous small GAD67-expressing cells (figs. 4A, B). The anterior tuberal nucleus (ATN) is devoid of GAD67-expressing cells at all levels (figs. 4A–F). The dorsal zone of the periventricular hypothalamus (Hd) is predominantely populated by heavily labeled GAD67-expressing cells (figs. 4A–F), which suggests that dopaminergic and serotoninergic cells of this nucleus might co-express GABA. The lateral hypothalamic nucleus (LH) shows some GAD67-expressing cells, which are intermingled with GAD67-negative cells that constitute the majority. The caudal periventricular nucleus (Hc) lacks GAD67-expressing cells. It is likely that GAD65 is expressed here in a similar fashion to goldfish (Martyniuk et al., 2007). Both, the central and diffuse nucleus of the inferior lobe (CIL and DIL respectively) show large and scattered GAD67-expressing cells. The torus lateralis (TLa) is predominantly comprised of GAD67-negative cells and a few scattered distributed GAD67-expressing cells (figs. 4A–E). The corpus mamillare (CM) shows a majority of smaller GAD67-negative and a few scattered GAD67-expressing cells.
DISCUSSION
Overview
In this study, we describe the distribution of GAD67-expressing cell populations in the entire forebrain of the adult zebrafish and propose a model of adult teleostean GABAergic forebrain organization (fig. 6). This model relates the distribution of GAD67-expressing cells in the adult zebrafish forebrain to the development of GABAergic cells in the early zebrafish forebrain (Mueller et al., 2006; Mueller et al., 2008), thus providing a hypothetical prosomeric fate map of forebrain areas (fig. 6 and tables 1–3). Our findings are largely in agreement with previous findings regarding the distribution of GAD65- and 67-expressing cells in telencephalic and hypothalamic regions of the goldfish (Martyniuk et al., 2007). Our results show that possibly intermingled pallidal and striatal GABAergic cells in the teleostean dorsal nucleus of the area ventralis telencephali (Vd) are not spatially separable, different from what has been shown in anamniote tetrapods (Brox et al., 2003) and amniotes (Medina and Reiner, 1995; Medina et al., 1999; Smeets et al., 2000). Our results thus confirm that the dorsal nucleus of the area ventralis telencephali (Vd) comprises the teleostean basal ganglia as defined earlier (Wullimann and Mueller, 2004b). However, the distribution of GAD67-expressing cell masses versus GAD67-negative cell masses strongly suggests a reinterpretation of the former teleostean entopeduncular complex (ENd plus ENv of Wullimann et al., 1996). We suggest separating this former entopeduncular complex into a GAD67-positive entopeduncular nucleus proper (EN; homologous to the entopeduncular nucleus of non-primate mammals and the internal segment of the globus pallidus of primates) and a largely GAD67-negative bed nucleus of the stria medullaris (BNSM), which we interpret as being a derivative of the tbr2-positive larval eminentia thalami (EmT), which lies anterior (ventral in the classical coronal interpretation) to the larval ventral thalamus (Wullimann and Mueller, 2004). Furthermore, the bed nucleus of the stria medullaris (BNSM) of the adult zebrafish histogenetically differs from the GAD67-positive ventral thalamic nuclei and lies in a lateral position. We thus interpret the larval thalamic eminence (EmT) and its adult derivative the bed nucleus of the stria medullaris (BNSM) as being anatomically and histogenetically separate from the ventral thalamus in teleost fish, whereas in mammalian/sauropsids, the (pre-) thalamic eminence is meanwhile interpreted in the prosomeric model as a dorsal domain of the ventral thalamus (= prethalamus; Puelles and Rubenstein, 2003).
Fig. 6.
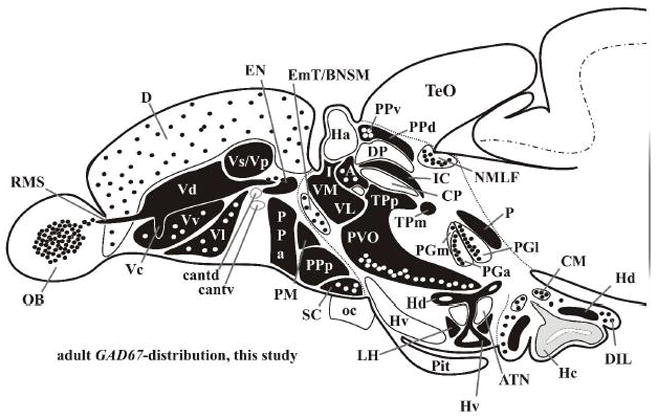
American tribal style schema of the adult zebrafish brain in sagittal view shows distribution of GAD67-expressing cells in the forebrain. Note that, for better understanding of GAD67-distribution, mid- and posterior hypothalamic level are shown in coronal perspective. The caudal hypothalamus (Hc) is free of GAD67-expressing cells but likely comprised of GAD65-expressing cells (indicated in grey) as shown in goldfish (Martyniuk et al., 2007). Black fields: heavily GAD67-labeled regions and nuclei. Black and white dots: scattered GAD67-expressing and GAD67-negative cells, respectively.
Table 1.
Provisional fate map based on the comparison of GAD67-expressing cells in adult telencephalic and preoptic regions versus distribution of GABA/GAD cells in larval primordial regions.
| zf | 3 | dpf | zf adult | suggested GABA origin | |||||
|---|---|---|---|---|---|---|---|---|---|
| olfactory bulb | OB | + | − | + | OB | + | − | + | migrated via RMS |
| pallium | Pa | − | + | − | Dm, Dd, Dc, Dl | − | + | − | 3 dpf, migrated from Sdv, MGE |
| − | + | − | Dp | − | + | − | late, migrated? from striatum? | ||
| subpallium | Sdp | + | + | + | Vp, Vs | + | + | + | 3 dpf, migrated from Sdv/MGE |
| Sdd (LGE) | + | + | + | Vd (basal ganglia) | + | + | + | intrinsic | |
| Sdv (MGE) | + | + | + | ||||||
| (M4) | − | − | − | EN (former ENd) | + | + | + | late, migrated from Sdv/MGE | |
| Sv | + | − | + | Vv | + | − | + | intrinsic | |
| Vl | + | − | + | late, migrated from Vv | |||||
| preoptic region | PO | + | + | + | SC | + | − | + | intrinsic |
| PPa | + | + | + | intrinsic | |||||
| PPp | + | + | + | intrinsic | |||||
| PM | + | + | + | intrinsic | |||||
Table 3.
Provisional fate map based on the comparison of GAD67-expressing cells in adult hypothalamic regions versus distribution of GABA/GAD cells in larval primordial regions.
| zf | 3 | dpf | zf adult | suggested GABA origin | |||||
|---|---|---|---|---|---|---|---|---|---|
| hypothalamus | H | H | |||||||
| periventricular: | |||||||||
| rostral | Hr | + | + | + | Hv | − | + | − | intrinsic |
| intermediate | Hi | − | + | − | Hd | + | + | + | intrinsic |
| caudal | Hc | + | + | + | Hc | + | + | + | intrinsic |
| tuberal: | Hr | not developed | ATN | − | + | − | intrinsic | ||
| lateral | Hr | not developed | LH | + | − | + | intrinsic | ||
| DIL, CIL | − | + | − | ? | |||||
Another unexpected finding is the occurrence of a massive GAD67-expressing cell mass sandwiched caudally (dorsally in the classical coronal interpretation) by the thalamic dorsal posterior nucleus (DP) and rostrally (ventrally in the classical coronal interpretation) the thalamic central posterior nucleus (CP) indicates that this GAD67-expressing cell mass is a discrete and previously unidentified GABAergic nucleus, which we designated the intercalated nucleus (IC).
In sum, our detailed comparative analysis of GAD67-expression patterns in zebrafish with those described in other vertebrates allows an improved understanding of homologies and differences in GABAergic forebrain organization among vertebrates. We hypothesize that the anamniote, non-tetrapod, prosomeric forebrain organization is the result of overall less extensive tangential and interprosomeric migratory activity compared to that described in tetrapods, which display more complexly organized forebrains. For example, the anamniote, non-tetrapod, distinct EmT as present in teleosts, lamprey and lungfish likely does not contribute to hypothalamic and/or preoptic derivatives as seems to be the case in mammals. Furthermore, a ventral migratory stream originating in the ganglionic eminence and contributing to the ventral thalamus (including the reticular thalamic nucleus) as shown for mammals is not present in teleosts and unlikely in lamprey and lungfish. However, there is a distinct strip of GAD67-expressing cells emanating from or a zone above the zona limitans intrathalamica (ZLI) surrounding the anterior thalamic nucleus (A; fig. 3A), which we interpret as being homologous to the mammalian reticular thalamic nucleus (RTN).
Reinterpretation of the former entopeduncular complex
Our results led to the identification of the adult teleostean bed nucleus of the stria medullaris (BNSM), which appears to be a derivative of the larval eminentia thalami (EmT). Both the adult BNSM (this study) as well as the larval EmT (Wullimann and Mueller, 2004a) are largely free of GABA/GAD67-expression. The shape and location of the GAD67-negative cell masses of the adult zebrafish BNSM change in their rostrocaudal extent. At anterior levels, the BNSM is in close proximity to and surrounds the lateral forebrain bundle (lfb). At more caudal levels, the BNSM appears as a solid nucleus dorsally of the lfb and ventrally adjacent to the pallium (ventral part of the entopedunular complex/ENv of section 114 in (Wullimann et al., 1996)) and is consistent with the location of the larval zebrafish EmT as suggested recently (Wullimann and Mueller, 2004a). The location of the larval zebrafish EmT has been confirmed by tract tracing studies showing that calretinin-expressing cells project to the habenula (Hendricks and Jesuthasan, 2007). Therefore, the adult teleostean BNSM appears to be homologous to the BNSM of mammals, where the majority of neurons expresses calretinin and project to the habenula and are suspected to be glutamatergic (Risold and Swanson, 1995; Abbott and Jacobowitz, 1999).
Based on expression patterns of ngn1- and neuroD-mRNA and Hu-proteins, it was suggested that the adult teleostean eminentia thalami (EmT) comprises both parts of the former entopeduncular complex (meaning dorsal and ventral part of the entopeduncular complex = ENd plus ENv) and that an entopeduncular nucleus proper homologous to the entopeduncular nucleus of non-primates is lacking in teleosts (Wullimann and Mueller, 2004a). Our present results do not support this view and rather suggest that the dorsal part of the former entopeduncular complex is comprised of large GAD67-expressing cells comparable to the large GAD67-expressing cells found in pallidal nuclei of Xenopus (Brox et al., 2003). At anterior levels, the large GAD67-expressing cells of the zebrafish entopeduncular nucleus proper (EN) as defined in the present study can be easily distinguished from the small GAD67-expressing cell of Vl (septum). At more caudal levels, the large GAD67-expressing cells of the EN are located a considerable distance away from the ventral and caudal GAD67-negative cell masses of the BNSM. Although a distinction between BNSM and entopeduncular complex has not been made in other teleosts, some GABAergic cells within the entopeduncular nucleus have been recognized in eel (Anglade et al., 1999) and goldfish (Martinoli et al., 1990). Furthermore, it has been shown that in trout neurons of the EN also project to the habenula (Yáñez and Anadón, 1996). This part of the entopeduncular complex in trout as well as in goldfish is comparable with the former dorsal nucleus of the entopeduncular complex (ENd) of zebrafish and does not mix with the bed nucleus of the stria medullaris (BNSM) as defined in zebrafish in this study. Therefore it seems likely that a GABAergic entopeduncular nucleus proper projecting to the habenula is a symplesiomorphic character of teleosts, which is homologous to the amphibian anterior entopeduncular nucleus as well as to the entopeduncular nucleus of non-primate mammals and globus pallidus internus of primates (Nagy et al., 1978; Rajakumar et al., 1994). Furthermore, some of these supposedly GABAergic projection neurons express parvalbumin (unpublished results) and differ in that respect from the projection neurons of the adult BNSM or larval EmT, which express calretinin (Hendricks and Jesuthasan, 2007). In contrast to mammals, the teleostean entopeduncular nucleus and amphibian anterior entopeduncular nucleus do not comprise cholinergic projection neurons, suggesting that the presence of cholinergic projection neurons in the entopeduncular nucleus is a derived character of mammals.
Reinterpretation of the organization of the thalamus
For the first time, our results deliver a precise picture of the distribution of GAD67-expressing versus GAD67-negative cell masses within the teleostean thalamus. In the adult zebrafish brain, the zona limitans intrathalamica (ZLI) - which separates dorsal and ventral thalamus - comprises a thin layer of GAD67-negative cells located ventrally adjacent to the GAD67-expressing cell masses of the anterior thalamic nucleus (A; fig. 3A). The zona limitans intrathalamica is in coronal sections caudally replaced by the emerging central posterior thalamic nucleus (fig. 3B). Furthermore, according to our results the GAD67-expressing cell masses of the previously unidentified intercalated nucleus (IC) separate, in the coronal view, the dorsally adjacent dorsal posterior thalamic nucleus (DP) from the ventrally adjacent central posterior thalamic nucleus (CP). Thus, both DP and CP appear smaller in size compared to former interpretations and do not appose at any level. The comparison of GAD67-expressing cells in the adult (this study) to the larval GABA cell distribution indicates that the central posterior nucleus (CP) and the intercalated nucleus are derivatives of the basal plate of prosomere 2 (PTd), which is dlx2a-negative at larval stages (Mueller et al., 2006; Mueller et al., 2008). Therefore, the central posterior nucleus (CP) and intercalated (IC) nucleus appear not to be part of the dorsal thalamus proper, but rather derivatives of the basal plate portion of the dorsal thalamus (i.e., PTd), while the dorsal posterior thalamic nucleus (DP) is the caudal part of the dorsal alar plate thalamus.
Comparison of early and adult GABA/GAD patterns
Overall, the distribution of adult GAD67-expressing cell masses closely resembles the distribution of GABAergic cell masses in the early larval zebrafish brain (tables 1–3; Mueller et al., 2006) and is in agreement with the prosomeric distribution of proneural and neurogenic basic helix-loop-helix genes in early stages of the zebrafish brain development (Mueller and Wullimann, 2003; Wullimann and Mueller, 2004b; a; Mueller and Wullimann, 2005). The high consistency between adult and larval GABAergic cell distribution suggests that the adult GAD67-expressing cell masses follow the established teleostean developmental prosomeric bauplan (table 1). In particular, the GAD67-positive dorsal part of the periventricular pretectal nucleus (PPd) and the paracommissural nucleus (PCN) as well as the GAD67-negative ventral part of the periventricular pretectal nucleus (PPv) are derivatives of the larval pretectum (alar plate prosomere 1), which shows spatiotemporally distinct expression patterns of ngn1 and neuroD versus Zash1a and GABA indicating the production of both glutamatergic and GABAergic cell masses here, respectively (Mueller and Wullimann, 2002; Wullimann and Mueller, 2002; Mueller and Wullimann, 2003; Mueller et al., 2006). The nucleus of the medial longitudinal fascicle (NMLF) shows a considerable amount of GAD67-expressing cells consistent with the finding of Zash-1a-expressing cells and GABAergic cells in the early larva (Wullimann and Mueller, 2002; Mueller et al., 2006) The GAD67-positive cell masses of the newly identified adult intercalated nucleus (IC) are likely derived from the Zash-1a and GABA positive portion of the larval PTd (basal plate of prosomere 2). In contrast, the GAD67-negative and likely glutamatergic cell masses of the central posterior thalamic nucleus (CP) may be derived from the neurogenin and neuroD expressing portion of the larval PTd (basal plate of prosomere 2). The GAD67-negative habenula and central posterior thalamic nucleus (CP) likely are derived from the neurogenin1 and neuroD expressing habenula and dorsal thalamic area (together comprising alar plate prosomere 2). The considerably large masses of cells expressing GAD67 in the periventricular nucleus of the posterior tuberculum (TPp) and the majority of the migrated nuclei of the preglomerular complex (TPm, PGl, PGm) are likely derived from the GAD67-, GABA- and Zash-1a- positive portion of the larval ventral part of the posterior tubercular area (PTv, basal plate prosomere 3). The GAD67-negative and likely glutamatergic cells in the adult posterior part of TPp and its migrated regions seem to be derived from the neurogenin1 and neuroD expressing portion of the larval PTv. Our results show that all teleostean ventral thalamic nuclei (Vm, Vl, I) are defined by the overwhelming predominance of GAD67-expressing cells, supporting the notion that these nuclei are derived from the ventral thalamic area (alar plate prosomere 3) of the larval zebrafish which is defined by the expression of GAD67, GABA, dlx2a and Zash-1a and the absence of neurogenin1 and neuroD.
Comparison with other vertebrates
The comparison of expression patterns of GAD67 in the forebrain of zebrafish with those of GABA/GAD in other vertebrates sheds some light on similarities and differences in the organization, development and evolution of vertebrate forebrain organization. In particular, our results suggest that the organization of the GABAergic systems in the teleost forebrain is more similar to that in amphibians than previously thought (Brox et al., 2003; Wullimann and Mueller, 2004b). A close comparison between the teleost and amphibian situation thus provides some clues for the understanding of the evolution of the vertebrate diencephalon especially at the anamniote non-tetrapod/tetrapod transition. The comparison of our results in zebrafish with those in Xenopus (Brox et al., 2003) shows that in teleosts as well as in amphibians, the main diencephalic GAD/GABA domains include nuclei of the ventral thalamus, pretectum and hypothalamus, whereas some dorsal thalamic nuclei are defined by the absence of GAD/GABA-expression. In Xenopus, only those dorsal thalamic nuclei in close proximity to the zona limitans intrathalamica (namely the dorsal geniculate nucleus, the anterior nucleus, and the intergeniculate leaflet) comprise large numbers of GAD67-expressing cells (Brox et al., 2003). Similarly, in zebrafish only the anterior nucleus in close proximity to the ZLI shows numerous GAD67-expressing cells, supporting the notion that the anterior nucleus of amphibians and teleosts is homologous and that a GABAergic anterior nucleus is a symplesiomorphic character of ray-finned fishes and tetrapods (Braford and Northcutt, 1983).
In addition, our results show a tangentially oriented strip of GAD67-expressing cells surrounding the anterior nucleus which we consider the reticular thalamic nucleus (RTN) of teleosts. In the classical view, this structure was treated as a part of the anterior nucleus (A) of teleosts (Braford and Northcutt, 1983). The distinct shape of the GAD67-expressing cell mass (this study) suggest different ontogenetic origins for the anterior thalamic (A) and reticular thalamic nucleus (RTN). The GAD67-positive and negative cells of the anterior nucleus protrude laterally indicating an intrinsic, periventricular site of origin followed by radial migration. In contrast, the lateral tangentially oriented strip of GAD67-expressing cells suggests an origin outside the anterior thalamic nucleus followed by tangential migration originating in or running through the ZLI. A comparable situation has been described in the development of the murine dorsal lateral geniculate nucleus, which is the only dorsal thalamic nucleus comprising a large number of GABAergic neurons (Nakagawa and O’Leary, 2001; Hayes et al., 2003). The origin of these GABAergic neurons seems to be the ganglionic eminence giving rise to the ventral migratory stream from which GABAergic cells of the reticular thalamic nucleus and zona incerta (Hayes et al., 2003) are partially derived. Our results indicate that a ventral migratory stream is absent in teleosts and the origin of the possibly tangentially migrated GABAergic neurons surrounding the anterior thalamic nucleus remains an open question, which can only be solved with developmental and fate mapping studies.
The comparison of adult and larval GAD67/GABA cell distributions in the diencephalon (table 2) revealed a heterochronic development of GABAergic cell population, which is comparable to findings in mammals (Hayes et al., 2003; Jones and Rubenstein, 2004). The GABAergic cell populations of the adult ventral thalamus (VM, VL, I) and the newly identified intercalated nucleus appear rather early and are present already in 3 day old zebrafish larvae (Mueller et al., 2006). In contrast, the anterior thalamic nucleus (A; a dorsal thalamic derivative), which is in close proximity to the ZLI, does not show GABAergic cells in 3 day old larval zebrafish, but the periventricular location of these GAD67-expressing cells suggests that these cells are born within this location (intrinsic origin, table 2). It is likely, that in the dlx2-negative dorsal thalamic region other genes like nkx.2.2 influence the determination of GABAergic cells as proposed for Xenopus and birds (Uchikawa et al., 1999; Brox et al., 2003).
Table 2.
Provisional fate map based on the comparison of GAD67-expressing cells in adult diencephalic regions versus distribution of GABA/GAD cells in larval primordial regions.
| zf | 3 | dpf | zf adult | suggested GABA origin | |||||
|---|---|---|---|---|---|---|---|---|---|
| p1 alar plate | Pr | + | + | + | PPd | + | + | + | intrinsic |
| − | − | − | PPv | − | − | − | - | ||
| p1 basal plate | N | + | + | + | NMLF | intrinsic | |||
| p2 alar plate | Ha | − | − | − | Had, Hav | − | − | − | - |
| DT | − | − | − | CP | − | − | − | - | |
| A, RTN | + | − | + | late, intrinsic? | |||||
| p2 basal plate | PTd | + | + | + | IC | + | + | + | intrinsic |
| DP | − | − | − | - | |||||
| p3 alar plate | VT | + | + | + | I, Vm, Vl | + | + | + | intrinsic |
| p3 basal plate | PTv | + | + | + | TPp | + | − | + | intrinsic |
| TPm | + | − | + | migrated from PTv + PTd? | |||||
| − | − | − | PVO | − | + | − | intrinsic | ||
| preglomerular complex | M2 | PGa, PGl, PGm, | + | − | + | migrated, multiprosomeric | |||
| P | + | + | + | migrated, origin unclear | |||||
| SG | − | − | − | ||||||
| RT | + | + | + | tegmentum? | |||||
| former p4 | EmT/M3 | − | − | − | BNSM (former ENv) | − | − | − | − + − origin unclear |
Conclusions
Altogether, this study delivers a comprehensive and detailed picture of the distribution of GAD67-expressing cells within the adult forebrain of a teleost fish. The distribution of GAD67-expressing cells in the adult zebrafish forebrain reveals a prosomeric pattern similar to that found in the larval zebrafish (Mueller et al., 2006). Thus, we can link the adult and larval situation and propose a provisional prosomeric fate map of early versus late developing and migrating GABAergic cell types (tables 1–3). We also propose some previously unidentified homologies between teleosts and tetrapods such as the largely GAD67-negative bed nucleus of the stria medullaris (BNSM) and the GAD67-positive reticular thalamic nucleus (RTN). We show that the former dorsal part of the entopeduncular complex (ENd) is GAD67-positive and likely homologous to the anterior entopeduncular nucleus of amphibians and entopeduncular nucleus of non-primate mammals. Thus, pallidal and striatal elements seem to be at least partially distinguishable from each other in teleost fish and more closely resemble the situation in tetrapods than previously thought (Wullimann and Mueller, 2004b). In summary, this study delivers a foundation for more sophisticated future genetic, fate mapping and hodological studies in order to better understand the evolution of the vertebrate brain and establishing zebrafish as a genetic model for neurological disorders.
Fig. 5.
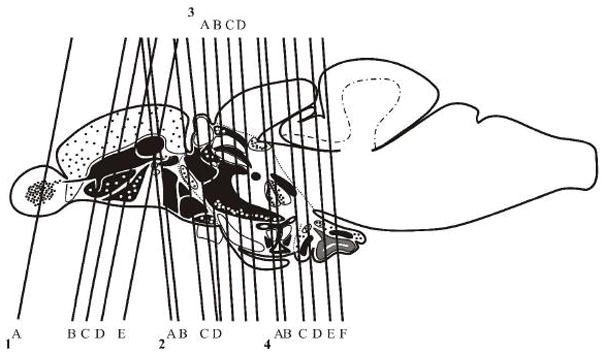
American tribal style schema of the adult zebrafish brain in sagittal view showing level of sections. Note that, for better understanding of GAD67-distribution, mid- and posterior hypothalamic level are shown in horizontal view.
Table 4.
prosomeric diencephalon and EmT GABA/GAD comparisonzebrafish (this study), lamprey (Meléndez-Ferro et al., 2002; Robertson et al., 2007), and lungfish (Trabucchi et al., 2008).
| adult | zebrafish | lamprey | lungfish | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p1 alar plate | PTd | + | + | + | (dorsal pretectum) | + | + | + | Pr | + | + | + |
| PTv | − | − | − | (pretectal N of oc) | − | + | − | |||||
| p1 basal plate | NMLF | + | + | + | NMLF | + | − | + | NMLF | + | + | + |
| p2 alar plate | Had, Hav | − | − | − | − − − | Ha | − | − | − | |||
| CP | − | − | − | ? * Thalamus | DT (Thd) | − | + | − | ||||
| A, RTN | + | − | + | caudal thalamic group* | + | + | + | ? ? ? | ? | ? | ? | |
| p2 basal plate | IC? | + | + | + | ? | part of DT? | + | + | + | |||
| DP | − | − | − | |||||||||
| p3 alar plate | I, Vm, Vl | + | + | + | (rostral thalamic group 1**) + + + | VT | + | + | + | |||
| p3 basal plate | TPp | + | − | + | − − − | ? ? ? | + | − | + | |||
| TPm | + | − | + | not present | not present | |||||||
| PVO | − | + | − | gap DT/H? | − | + | − | |||||
| preglomerular complex | PGl, PGm, PG | + | − | + | not present | not present | ||||||
| former p4 | EmT (ENv) | − | − | − | − − −** | |||||||
data in lamprey appear not completely anatomically resolved, but figures of Meléndez/Ferro et al., (2002) show distinct GABA-groups in the pretectum, dorsal thalamus and ventral thalamus. The caudal thalamic group of Meléndez-Ferro et al. (2002) could be interpreted as a rostralmost dorsal thalamic group consistent with our findings in this study. GABA-negative and –positive portions have not been clearly separated in lamprey.
the ventral thalamus is named as rostral thalamic nucleus, p3 and p4 are grouped together by Meléndez-Ferro and colleagues (2002).
Robertson and colleagues (2007) found some GABA-labeled cells in the EmT of adult lamprey, Meléndez-Ferro and colleagues (2002) did not find GABA-labeled cells in the EmT of larval lamprey.
Acknowledgments
This work is supported by NIH grant NIAAA AA016021. We thank Mario Wullimann for inspiring discussions, Michael Berberoglu, Ed Hurlock, and Priya Mathur for comments on the manuscript and Michael Munchua for practical support and fish maintainance.
Abbreviations
- A
anterior thalamic nucleus
- ac
anterior commissure
- ATN
anterior tuberal nucleus
- BNSM
bed nucleus of the stria medullaris
- cantd
comissura anterior, pars dorsalis
- cantv
comissura anterior, pars ventralis
- chor
commissura horizontalis
- CIL
central nucleus of the inferior lobe
- CM
corpus mamillare
- CP
central posterior thalamic nucleus
- CPN
central pretectal nucleus
- cpost
commissura posterior
- D
area dorsalis telencephali
- DAO
dorsal accessory optic nucleus
- DIL
diffuse nucleus of the inferior lobe
- Dc
central zone of area dorsalis telencephali
- DC
central posterior thalamic nucleus
- Dl
lateral zone of area dorsalis telencephali
- Dm
medial zone of area dorsalis telencephali
- dot
dorsomedial optic tract
- Dp
posterior zone of area dorsalis telencephali
- DP
dorsal posterior thalamic nucleus
- DT
dorsal thalamus
- ECL
external cellular layer of olfactory bulb including mitral cells
- EmT
eminentia thalami
- EN
entopeduncular nucleus
- ENd
former entopeduncular nucleus, dorsal part
- ENv
former entopeduncular nucleus, ventral part
- fr
fasciculus retroflexus
- GL
glomerular layer of the olfactory bulb
- Had
dorsal habenular nucleus
- Hav
ventral habenular nucleus
- Hd
dorsal zone of periventricular hypothalamus
- Hc
caudal hypothalamus
- Hv
ventral zone of periventricular hypothalamus
- I
intermediate thalamic nucleus
- IC
intercalated nucleus
- ICL
internal cellular layer of olfactory bulb
- IGL
intergeniculate leaflet
- lfb
lateral forebrain bundle
- LGE
lateral ganglionic eminence
- LH
lateral hypothalamic nucleus
- LP
lateral pallium
- M2
migrated posterior tubercular (preglomerular) area (larval)
- M3
migrated region of the eminentia thalami (larval)
- M4
early migrated telencephalic area/primordial Vl (larval)
- MGE
medial ganglionic eminence
- NMLF
nucleus of the medial longitudinal fascicle
- OB
olfactory bulb
- oc
optic chiasma
- Pa
pallium (larval)
- P
posterior thalamic nucleus
- Pr
pretectum (larval)
- pc
posterior commissure
- PCN
paracommissural nucleus
- PGa
anterior preglomerular nucleus
- PGm
medial preglomerular nucleus
- PGl
lateral preglomerular nucleus
- pit
pituitary
- PM
magnocellular preoptic nucleus
- Po
preoptic region (larval)
- poc
postoptic commissure
- POF
primary olfactory fiber layer
- PPa
parvocellular preoptic nucleus, anterior part
- PPd
periventricular pretectal nucleus, dorsal part
- PPp
parvocellular preoptic nucleus, posterior part
- PPv
periventricular pretectal nucleus, ventral part
- PSm
magnocellular superficial pretectal nucleus
- PSp
parvocellular superficial pretectal nucleus
- PT
posterior tuberculum
- PTd
dorsal part of posterior tuberculum (larval)
- PTN
posterior tuberal nucleus
- PTv
ventral part of posterior tuberculum (larval)
- PVO
paraventricular organ (after Rink and Wullimann, 2001)
- RMS
rostral migratory stream
- RT
rostral tegmental nucleus
- RTN
reticular thalamic nucleus
- S
subpallium (larval)
- SC
suprachiasmatic nucleus
- SD
saccus dorsalis
- Sd
dorsal division of subpallium (larval)
- Sdd
dorsal subdivision of Sd (larval)
- Sdp
posterior subdivision of Sd
- Sdv
ventral subdivision of Sd (larval)
- SG
subglomerular nucleus
- Sv
ventral division of subpallium (larval)
- TeO
tectum opticum
- TL
torus longitudinalis
- TLa
torus lateralis
- TPp
periventricular nucleus of posterior tuberculum
- TPm
migrated nucleus of posterior tuberculum
- VAO
ventral accessory optic nucleus
- Vc
central nucleus of area ventralis telencephali
- Vd
dorsal nucleus of area ventralis telencephali
- VL
ventrolateral thalamic nucleus
- Vl
lateral nucleus of area ventralis telencephali
- VM
ventromedial thalamic nucleus
- Vp
postcommissural nucleus of area ventralis telencephali
- Vs
supracommissural nucleus of area ventralis telencephali
- VT
ventral thalamus (prethalamus)
- Vv
ventral nucleus of area ventralis telencephali
- ZLI
zona limitans intrathalamica
Contributor Information
Thomas Mueller, Email: MuellerT@pharmacy.ucsf.edu.
Su Guo, Email: su.guo@ucsf.edu.
References cited
- Abbott LC, Jacobowitz DM. Developmental expression of calretinin-immunoreactivity in the thalamic eminence of the fetal mouse. Int J Dev Neurosci. 1999;17:331–345. doi: 10.1016/s0736-5748(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Anglade I, Mazurais D, Douard V, Le Jossic-Corcos C, Mananos EL, Michel D, Kah O. Distribution of glutamic acid decarboxylase mRNA in the forebrain of the rainbow trout as studied by in situ hybridization. J Comp Neurol. 1999;410:277–289. doi: 10.1002/(sici)1096-9861(19990726)410:2<277::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Barale E, Fasolo A, Girardi E, Artero C, Franzoni MF. Immunohistochemical investigation of gamma-aminobutyric acid ontogeny and transient expression in the central nervous system of Xenopus laevis tadpoles. J Comp Neurol. 1996;368:285–294. doi: 10.1002/(SICI)1096-9861(19960429)368:2<285::AID-CNE8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Blader P, Fischer N, Gradwohl G, Guillemot F, Strähle U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124:4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- Blader P, Plessy C, Strahle U. Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech Dev. 2003;120:211–218. doi: 10.1016/s0925-4773(02)00413-6. [DOI] [PubMed] [Google Scholar]
- Braford M, Northcutt R. Organization of the Diencephalon and Pretectum of the Ray-finned Fishes. In: Davis RE, Northcutt RG, editors. Fish neurobiology. Ann Arbor: University of Michigan Press; 1983. pp. 203–236. [Google Scholar]
- Brox A, Puelles L, Ferreiro B, Medina L. Expression of the genes GAD67 and Distal-less-4 in the forebrain of Xenopus laevis confirms a common pattern in tetrapods. J Comp Neurol. 2003;461:370–393. doi: 10.1002/cne.10688. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JL. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Rapaport DH, Harris WA. Xotch inhibits cell differentiation in the Xenopus retina. Neuron. 1995;14:487–496. doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- Eisenstat DD, Liu JK, Mione M, Zhong W, Yu G, Anderson SA, Ghattas I, Puelles L, Rubenstein JL. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999;414:217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, Smeets WJ, Marín O. Evidences for shared features in the organization of the basal ganglia in tetrapods: studies in amphibians. Eur J Morphol. 1999;37:151–154. doi: 10.1076/ejom.37.2.151.4752. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Murray KD, Jones EG. Two epochs in the development of gamma-aminobutyric acidergic neurons in the ferret thalamus. J Comp Neurol. 2003;463:45–65. doi: 10.1002/cne.10749. [DOI] [PubMed] [Google Scholar]
- Hendricks M, Jesuthasan S. Asymmetric innervation of the habenula in zebrafish. J Comp Neurol. 2007;502:611–619. doi: 10.1002/cne.21339. [DOI] [PubMed] [Google Scholar]
- Jones EG, Rubenstein JL. Expression of regulatory genes during differentiation of thalamic nuclei in mouse and monkey. J Comp Neurol. 2004;477:55–80. doi: 10.1002/cne.20234. [DOI] [PubMed] [Google Scholar]
- Katarova Z, Sekerkova G, Prodan S, Mugnaini E, Szabó G. Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. J Comp Neurol. 2000;424:607–627. doi: 10.1002/1096-9861(20000904)424:4<607::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Marín O, Smeets WJ, Gonzalez A. Basal ganglia organization in amphibians: catecholaminergic innervation of the striatum and the nucleus accumbens. J Comp Neurol. 1997;378:50–69. doi: 10.1002/(sici)1096-9861(19970203)378:1<50::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Marín O, Smeets WJ, Gonzalez A. Basal ganglia organization in amphibians: chemoarchitecture. J Comp Neurol. 1998a;392:285–312. [PubMed] [Google Scholar]
- Marín O, Smeets WJ, Gonzalez A. Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci. 1998b;21:487–494. doi: 10.1016/s0166-2236(98)01297-1. [DOI] [PubMed] [Google Scholar]
- Martinoli M, Dubourg P, Geffard M, Calas A, Kah O. Distribution of GABA-immunoreactive neurons in the forebrain of the goldfish, Carassius auratus. Cell Tissue Res. 1990;260:277–284. doi: 10.1007/BF00297492. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Awad R, Hurley R, Finger TE, Trudeau VL. Glutamic acid decarboxylase 65, 67, and GABA-transaminase mRNA expression and total enzyme activity in the goldfish (Carassius auratus) brain. Brain Res. 2007;1147:154–166. doi: 10.1016/j.brainres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Medina L, Jiao Y, Reiner A. The functional anatomy of the basal ganglia of birds. Eur J Morphol. 1999;37:160–165. doi: 10.1076/ejom.37.2.160.4735. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of basal ganglia. Brain Behav Evol. 1995;46:235–258. doi: 10.1159/000113277. [DOI] [PubMed] [Google Scholar]
- Medina M, Reperant J, Dufour S, Ward R, Le Belle N, Miceli D. The distribution of GABA-immunoreactive neurons in the brain of the silver eel (Anguilla anguilla L.) Anat Embryol (Berlin) 1994;189:25–39. doi: 10.1007/BF00193127. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Villar-Cheda B, Abalo XM, Rodríguez-Muñoz R, Rodicio MC, Anadón R. Ontogeny of gamma-aminobutyric acid-immunoreactive neuronal populations in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2002;446:360–376. doi: 10.1002/cne.10209. [DOI] [PubMed] [Google Scholar]
- Mueller T, Vernier P, Wullimann MF. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared with mouse. J Comp Neurol. 2006;494:620–634. doi: 10.1002/cne.20824. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. BrdU-, neuroD (nrd)- and Hu-studies reveal unusual non-ventricular neurogenesis in the postembryonic zebrafish forebrain. Mech Dev. 2002;117:123–135. doi: 10.1016/s0925-4773(02)00194-6. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Anatomy of neurogenesis in the early zebrafish brain. Brain Res Dev Brain Res. 2003;140:137–155. doi: 10.1016/s0165-3806(02)00583-7. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Atlas of Early Zebrafish Brain Development: A Tool for Molecular Neurogenetics: Elsevie. 2005. p. 183. [Google Scholar]
- Mueller T, Wullimann MF, Guo S. Early teleostean basal ganglia development visualized by zebrafish Dlx2a, Lhx6, Lhx7, Tbr2 (eomesa), and GAD67 gene expression. J Comp Neurol. 2008;507:1245–1257. doi: 10.1002/cne.21604. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1985. pp. 436–608. [Google Scholar]
- Nagy JI, Carter DA, Lehmann J, Fibiger HC. Evidence for a GABA-containing projection from the entopeduncular nucleus to the lateral habenula in the rat. Brain Res. 1978;145:360–364. doi: 10.1016/0006-8993(78)90869-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O’Leary DD. Combinatorial expression patterns of LIM-homeodomain and other regulatory genes parcellate developing thalamus. J Neurosci. 2001;21:2711–2725. doi: 10.1523/JNEUROSCI.21-08-02711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombal MA, Puelles L. Prosomeric map of the lamprey forebrain based on calretinin immunocytochemistry, Nissl stain, and ancillary markers. J Comp Neurol. 1999;414:391–422. [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Rajakumar N, Elisevich K, Flumerfelt BA. Parvalbumin-containing GABAergic neurons in the basal ganglia output system of the rat. J Comp Neurol. 1994;350(2):324–336. doi: 10.1002/cne.903500214. [DOI] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain research. 1998;28:235–285. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) J Comp Neurol. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Cajal’s nucleus of the stria medullaris: characterization by in situ hybridization and immunohistochemistry for enkephalin. J Chem Neuroanat. 1995;9:235–240. doi: 10.1016/0891-0618(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Robertson B, Auclair F, Menard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Marín O, González A. Evolution of the basal ganglia: new perspectives through a comparative approach. J Anat. 2000;196:501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Wang HB, Laverghetta A, Yamamoto K, Reiner A. The distribution and cellular localization of glutamic acid decarboxylase-65 (GAD65) mRNA in the forebrain and midbrain of domestic chick. J Chem Neuroanat. 2005;29:265–281. doi: 10.1016/j.jchemneu.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Trabucchi M, Trudeau VL, Drouin G, Tostivint H, Ihrmann I, Vallarino M, Vaudry H. Molecular characterization and comparative localization of the mRNAs encoding two glutamic acid decarboxylases (GAD65 and GAD67) in the brain of the African lungfish, Protopterus annectens. J Comp Neurol. 2008;506:979–988. doi: 10.1002/cne.21552. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev. 1999;84:103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Expression of Zash-1a in the postembryonic zebrafish brain allows comparison to mouse Mash1 domains. Brain Res Gene Expr Patterns. 2002;1:187–192. doi: 10.1016/s1567-133x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Identification and morphogenesis of the eminentia thalami in the zebrafish. J Comp Neurol. 2004a;471:37–48. doi: 10.1002/cne.20011. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004b;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Puelles L. Postembryonic neural proliferation in the zebrafish forebrain and its relationship to prosomeric domains. Anat Embryol (Berlin) 1999;199:329–348. doi: 10.1007/s004290050232. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain - A Topological Atlas. Basel: Birkhäuser Verlag; 1996. p. 144. [Google Scholar]
- Yáñez J, Anadón R. Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): an indocarbocyanine dye (DiI) study. J Comp Neurol. 1996;372:529–543. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]


