Abstract
Hypoxia-inducible factor 1α (HIF-1α) is upregulated by hypoxia and oncogenic signalling in many solid tumours. Its regulation and function in thyroid carcinomas are unknown. We evaluated the regulation of HIF-1α and target gene expression in primary thyroid carcinomas and thyroid carcinoma cell lines (BcPAP, WRO, FTC-133 and 8505c). HIF-1α was not detectable in normal tissue but was expressed in thyroid carcinomas. Dedifferentiated anaplastic tumours (ATCs) exhibited high levels of nuclear HIF-1α staining. The HIF-1 target glucose transporter 1 was expressed to a similar level in all tumour types, whereas carbonic anhydrase-9 was significantly elevated in ATCs. In vitro studies revealed a functionally active HIF-1α pathway in thyroid cells with transcriptional activation observed after graded hypoxia (1% O2, anoxia) or treatment with a hypoxia mimetic cobalt chloride. High basal and hypoxia-induced expression of HIF-1α in FTC-133 cells that harbour a phosphatase and tensin homologue (PTEN) mutation was reduced by introduction of wild-type PTEN. Similarly, pharmacological inhibition of the phosphoinositide 3-kinase (PI3K) pathway using LY294002 inhibited HIF-1α and HIF-1α targets in all cell lines, including those with B-RAF mutations (BcPAP and 8505c). In contrast, the effects of inhibition of the RAF/MEK/extracellular signal-regulated kinase pathway were restricted by environmental condition and B-RAF mutation status. HIF-1 is functionally expressed in thyroid carcinomas and is regulated not only by hypoxia but also via growth factor signalling pathways and, in particular, the PI3K pathway. Given the strong association of HIF-1α with an aggressive disease phenotype and therapeutic resistance, this pathway may be an attractive target for improved therapy in thyroid carcinomas.
Introduction
Thyroid cancers represent the most common malignancy of endocrine organs and their incidence is increasing (Kondo et al. 2006). The precise molecular mechanisms promoting the development and progression of the disease are only partially known. Available data suggest that the most frequent mutational events in these carcinomas are mutations in B-RAF, leading to activation of the RAF/MEK (mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase)/ERK pathway and in phosphoinositide 3-kinase (PI3K)/AKT signalling (Kimura et al. 2003, Hou et al. 2007). A recent summary suggests that whilst B-RAF mutations represent an early stage event, particularly in the development of papillary thyroid carcinomas, changes in the PI3K pathway occur later and are associated with aggressive transition (Paes & Ringel 2008). To date, there has been no comprehensive analysis of the potential role of signalling mediated via hypoxia-induced transcription cascades.
Hypoxia triggers a coordinated transcriptional response, which impacts on the activation of many genes involved in the physiological adaptation of tumour cells. This promotes tumour cell survival and disease progression. Examples of such target genes include the glucose transporter 1 (GLUT1), which increases glucose uptake into cells for anaerobic respiration (glycolysis), genes involved in the maintenance of the intracellular milieu, for example, carbonic anhydrase-9 (CA-9), MCT-4 and NHE-1/-6, and genes that increase or restore vascular supply to the cells such as vascular endothelial growth factor (VEGF; Pouyssegur et al. 2006). These adaptive processes are controlled by hypoxia-inducible factor 1 (HIF-1), which is composed of two subunits, the oxygen-sensitive HIF-1α and the constitutively expressed HIF-1β. Under normoxic conditions, HIF-1α is rapidly inactivated through hydroxylation, which enables binding of the molecule to von Hippel–Lindau protein. Subsequently, the complex is degraded in the proteasome via the ubiquitination pathway (Pouyssegur et al. 2006). In hypoxia, HIF-1α is stabilized, translocates to the nucleus and, following heterodimerization with HIF-1β and cofactors like CREB-binding protein (CBP)/p300, activates HIF-responsive genes.
Hypoxia is a characteristic hallmark of aggressive malignancies, and HIF-1α signalling is upregulated in many tumours. It is important to note that HIF-1α signalling is activated not only by lowered oxygen tension within the tumour but also by oncogenic stimulation through aberrant growth factors or through the loss of tumour suppressor gene activity, e.g. phosphatase and tensin homologue deleted on chromosome ten (PTEN), and studies have implicated both the MEK/ERK and PI3K pathways in HIF-1 regulation (Bardos & Ashcroft 2005, Shafee et al. 2009). Both stimuli induce cellular adaptation to an anaerobic state and substantially change cell physiology (Garcia 2006). HIF-1α-dependent activation of target genes has major implications on tumour aggressiveness and local or distant invasiveness. In addition, HIF-1α may reduce the effectiveness of cancer treatment because it has been shown to play a role in therapeutic resistance to radiotherapy and some forms of chemotherapy (Unruh et al. 2003, Williams et al. 2005, Brown et al. 2006, Chiche et al. 2009). As radioiodine treatment or external beam radiation is a first line non-surgical therapy in disseminated differentiated or undifferentiated thyroid tumours, the effect on radiation resistance is of high therapeutic relevance in these aggressive carcinomas.
Previous data characterizing the potential role of HIF-1α in thyroid carcinomas are limited. In vitro studies have shown that stimulation of VEGF production in SW579 thyroid tumour cells is dependent on HIF-1α and AP-1 (Poulaki et al. 2003). In a clinical study, low levels of HIF-1α mRNA were observed in thyroid carcinomas using a microarray approach (Jubb et al. 2004).
However, expression of HIF-1 target genes like GLUT1 (Yasuda et al. 2005) and VEGF (Tuttle et al. 2002) was observed and appeared to be related to tumour pathological grade/type.
Currently, little is known about the hypoxic regulation of HIF-1α and the potential interaction with oncogenic pathways in thyroid carcinomas. Thus, the aim of the present study was to determine whether HIF-1α and its target genes, CA-9 and GLUT1, are expressed in primary tumours and cell lines derived from thyroid carcinomas. The cell line panel included those derived from papillary (BcPAP), follicular (WRO and FTC-133) and anaplastic (8505c) thyroid tumours. They reflected the common genotype aberrations associated with these subtypes with the highly prevalent B-RAF V600E mutation present in the BCPAP and 8505c cell lines (Xu et al. 2003, Elisei et al. 2008), and the FTC-133 harbouring a PTEN mutation (Weng et al. 2001).
To elucidate functional HIF-1α regulation, we exposed thyroid cancer cell lines to graded hypoxia or stimulation by a biochemical hypoxia mimetic cobalt chloride (CoCl2) and tested the impact of inhibitors on the relevant genetically altered pathways in these cells. We show that HIF-1α is expressed in both differentiated and dedifferentiated primary thyroid carcinomas as well as in thyroid cancer cell lines. HIF-1α protein levels and reporter gene activity are upregulated by lowering the oxygen tension and by chemical stimulation, paralleled by increased levels of the HIF-1α target genes, CA-9 and GLUT1. Basal and induced expression of HIF-1α in FTC-133 cells bearing a PTEN mutation is high. Transfection of wild-type PTEN (WTPTEN) as well as silencing the PI3K/AKT pathway via pharmacological inhibition decreased both HIF-1α and target gene expression. This supports an important link between PI3K/AKT and HIF-1α, which may have particular relevance to disease progression in thyroid carcinomas.
Materials and methods
Patients and cell lines
Thyroid tissues obtained from patients undergoing surgery for thyroid carcinomas (12 dedifferentiated anaplastic tumours (ATC) and 25 differentiated carcinomas, 13 follicular (FTC) and 12 papillary (PTC) carcinomas) were snap frozen in liquid nitrogen and stored at −80 °C.
Histopathological diagnosis and classification were performed at the Department of Pathology of the Medical School of Hannover and of Halle University. We compared these results with 13 normal thyroid tissues obtained during surgery from patients with parathyroid adenomas. All patients involved in this study gave informed consent, and the use of the material was approved by the respective Ethical Committees. Snap frozen tissue was paraffin embedded and stained by standard immunohistochemistry techniques.
For functional studies, we used thyroid carcinoma cell lines derived from papillary (BcPAP), follicular (WRO and FTC-133) and anaplastic thyroid carcinomas (8505c) (Schweppe et al. 2008). WRO, BcPAP and 8505c were cultured in RPMI supplemented with 2 mM glutamine (Gibco; Invitrogen Ltd) and 10% (v/v) FCS (Biosera, East Sussex, UK). The parental FTC-133 cells were cultured in DMEM/HAM'S F-12 with 2 mM glutamine and 10% FCS (v/v) (PAA Laboratories GmbH, Haidmanweg, Austria). Medium for the stably transfected FTC-133 PTEN and PCI NEO derivatives was supplemented with 1.44 mM G418. Additionally, we transfected normal thyroid cells with SV40 to obtain stable cell lines as previously described (Belge et al. 1995), as a model for normal thyroid function.
Experimental protocol for hypoxia/anoxia
Exposures to anoxia were undertaken by placing cells in an anaerobic chamber (Bactron anaerobic chamber, Sheldon Manufacturing, Cornelius, OR, USA) in which a gas mixture of 5% CO2:5% H2:90% N2 is passed over a palladium catalyst to remove residual oxygen. Other oxygen environments were generated by continuous gassing of sealed chambers containing cell cultures with gas mixtures containing the appropriate oxygen tension with 5% CO2 and N2 balance. As an alternative, cells were treated with the CoCl2 (100 μM; Sigma) for 18 h. To modulate PI3K or ERK pathways, the cells were treated for 18 h with 50 μM LY294002 or 50 μM PD98059 respectively (Sigma–Aldrich).
Primary antibodies
The following antibodies were used: mouse monoclonal anti-HIF-1α (BD Transduction Laboratories, Oxford, UK), rabbit polyclonal anti-von Willebrand Factor (vWF; DAKO, Ely, UK), rabbit polyclonal anti-GLUT1 (Chemicon (Millipore), Temecula, CA, USA), monoclonal anti-CA-9 (kindly provided by Dr Jaromir Pastorek, Bratislava, Slovakia), mouse monoclonal anti-β-actin (Sigma).
Western blot analysis
Cells were lysed in 100 μl lysis buffer (10 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100 v/v, 0.5% Nonidet P-40 v/v, 2 mM leupeptin, 0.15 mM aprotinin, 1.46 mM pepstatin, 1 mM phenylmethansulfonyl fluoride). For western blot analysis, proteins (20 μg/lane) were resolved on a 7.5% SDS-PAGE gel and electrotransferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Schwalbach, Germany) using standard procedures. After blocking with 5% non-fat dry milk w/v in PBS-T (137 mM NaCl, 2.7 mM KCl, 4.3 mM di-sodiumhydrogenphosphate, 1.4 mM potassium-di-hydrogenphosphate, 0.1% Tween20 v/v) overnight at 4 °C, the blot was probed with primary antibodies. The blot was then reacted with suitable secondary alkaline phosphatase-conjugated antibodies (Dianova, Hamburg, Germany), followed by detection of the protein with CDP-Star chemiluminescent reagent (Applied Biosystems, Foster City, CA, USA). Finally, the blot was stripped and reprobed for β-actin to ensure equal loading and transfer of proteins.
Immunohistochemistry
Sections (3 μm thick) of paraffin-embedded normal thyroid, FTC, PTC and ATC tissues were dewaxed, rehydrated and subjected to antigen retrieval in citrate buffer (pH 6.0) or Tris–EDTA buffer (pH 8.5) in a PT module (Lab Vision, Suffolk, UK) for 30 min at 98 °C. The slides were incubated for 1 h in PBS with 10% goat or rabbit serum to block non-specific antibody binding followed by 1-h incubation with the primary antibody (see above). After washing, the sections were incubated with a suitable biotinylated secondary antibody (1:300 dilution) for 1 h. Antigen–antibody complexes were visualized by applying a standard streptavidin–biotin complex (Vector Laboratories, Peterborough, UK) for 35 min followed by 3,3-diaminobenzidine (DAB) (Sigma) for 6–8 min. Sections were counterstained with haematoxylin. Control sections were incubated with rabbit or mouse IgG as the primary antibody. The extent of immunostaining was evaluated by the method described by Allred et al. (1993). The mean scores of three independent reviewers are shown.
Immunofluoresence and cell imaging
Cells were seeded at a density of 5×103 cells/well in a 96-microplate ‘CytoWell’ (Nunc, Wiesbaden, Germany). Cells were fixed in 4% paraformaldehyde v/v in PBS for 10 min and permeabilized in 0.1% Triton X-100 v/v in PBS for 5 min at room temperature. Cells were incubated in a blocking buffer, containing 3% BSA w/v in PBS for 30 min. Incubations with primary antibodies were followed by FITC-conjugated secondary antibody (Dianova) for 1 h at 37 °C. Finally, cells were embedded in ProLong Gold Antifade Reagent with DAPI (Invitrogen) and viewed by fluorescent microscopy (DeltaVision, Washington, DC, USA). Immunofluorescence images were acquired with an Olympus BX71 microscope.
Assessment of HIF-1 proficiency
Cells were infected with an adenovirus containing a firefly luciferase reporter construct linked to trimers of the lactate dehydrogenase (LDH) hypoxia-responsive element (HRE) sequence (Cowen et al. 2004) 5 h prior to exposure to the respective condition. The multiplicity of infection (MOI; number of viral particles per cell) used was 20. Cells were then trypsinized and plated in triplicate in a 96-well plate. Cells received either 50 μM LY294002 or 50 μM PD98059, or no treatment and were subjected to normoxia, 1% hypoxia, anoxia or 100 μM CoCl2 for 18 h. Cells were then harvested and luciferase activity was analysed using a manufactured kit (Promega UK Ltd) following the recommended guidelines. A MicroLumat LB 96 luminometer (EG & G Berthold Technologies, Harpenden, UK) was used to measure luminescence. The efficiency of adenoviral infection was tested by infecting cells with an adenovirus expressing green fluorescent protein (GFP) under the control of a constitutively active viral promotor (cytomegalovirus (CMV)); a kind gift from Dr Nasreen Akhtar, Manchester, UK.
Silencing of HIF-1α
Cells were transfected with 100 nM HIF-1α siRNA (5′-AGGTGGATATGTCTGGGTTtt-3′) (Dharmacon Research, Inc., Surrey, UK) and control siRNA (Santa Cruz Biotech Inc, Santa Cruz, CA, USA) for 4 h and were then exposed to 1% hypoxia, anoxia or normoxia for 18 or 24 h. Cells lysates were collected and western blotting was performed, as previously described.
Vascular endothelial growth factor ELISA
Cells were incubated in normoxia or anoxia for 18 h, and the media was removed for the analysis of VEGF levels. Cells were harvested and the protein concentration was determined as previously described (Williams et al. 2005). The concentration of secreted VEGF was determined using a Duoset Human VEGF ELISA kit (R&D Systems, Abingdon, UK) according to the manufacturer's instructions.
Statistical analysis
For analysis between the various stimulation conditions, the paired t-test and a one-way ANOVA with post test Tukey were used.
Results
HIF-1α protein is expressed in differentiated and dedifferentiated thyroid tumours but absent from normal thyroid tissue
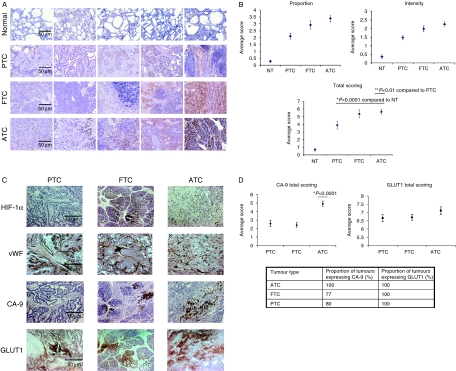
The expression of the HIF-1α protein was first studied in snap frozen normal thyroid and differentiated and dedifferentiated primary thyroid carcinomas. Normal thyroid tissue did not show HIF-1α immunostaining, whereas 11 out of 12 dedifferentiated ATCs studied showed strong HIF-1α staining. In contrast, differentiated PTCs and FTCs showed variable distribution and intensity of staining for HIF-1α (Fig. 1A). This was supported by a semiquantitative evaluation of HIF-1α expression in these tumours (Fig. 1B). Within the tumour, the staining pattern varied between differentiated and dedifferentiated tissue. Positive staining was diffusely distributed in ATCs compared with PTCs and FTCs where HIF-1α expression varied considerably from very intense, localized staining to almost no staining. HIF-1α expression was significantly greater in all tumour types than in normal tissue and ATC and FTC tumours showed the highest levels of expression (Fig. 1B).
Figure 1.
HIF-1α protein is expressed in primary thyroid tumours and can be associated with CA-9 and/or GLUT1 expression but not vWF. (A) Immunohistochemical staining for HIF-1α (brown staining) in primary thyroid carcinomas and normal thyroid tissue. Five examples of normal thyroid, papillary (PTC), follicular (FTC), and anaplastic (ATC) carcinomas are displayed, which show the variation of HIF-1α expression from the lowest (left) to highest (right) expression observed in each subtype. (B) Semiquantitative analysis of HIF-1α immunostaining performed according to Allred et al. (1993). Total scoring is derived from the intensity and proportion plots. Data represent the mean±s.e.m. of three independent scorers. (C) Representative immunohistochemical staining for HIF-1α, vWF, CA-9 and GLUT1 (each staining brown) in papillary, follicular and anaplastic thyroid carcinomas (D) Semiquantitative analysis of CA-9 and GLUT1 expression showing total scoring only. The proportion of tumours showing positive staining is given below the figures. The significance value given on the CA-9 plot is versus PTC and FTC.
To characterize the possible relationship between O2 diffusion-dependent hypoxia and HIF-1α expression, we evaluated microvascular density in these tumours using vWF staining and compared the pattern to HIF-1α (Fig. 1C). No clear relationship between both markers was detectable. In addition, we evaluated the expression of the HIF target genes, CA-9 and GLUT1 (Fig. 1C), which can reflect tissue hypoxia in other epithelial tumours (Airley et al. 2003). GLUT1 was expressed to equivalent levels in all analysed tumours (Fig. 1C and D). CA-9 was expressed in all dedifferentiated ATC tumours compared with 77% of FTCs and 80% of PTCs. In addition to increased frequency of expression, the level of CA-9 observed was significantly higher in ATCs compared with PTC and FTC tumours (Fig. 1D).
Thyroid cell lines grown in culture show a functional HIF-1 response to hypoxia and hypoxic mimetics
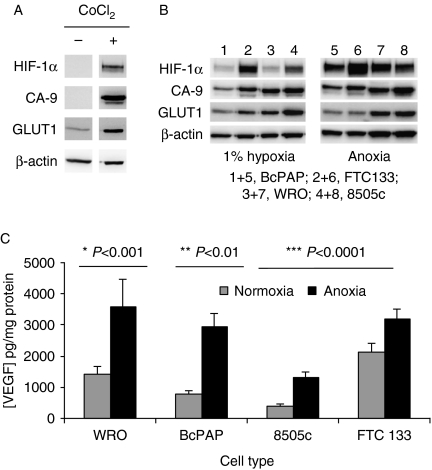
To further investigate the functional regulation of HIF-1α, we examined cell lines derived from normal thyroid tissue and from PTC (BcPAP), FTC (WRO and FTC-133) and ATC (8505c). Cell lines of normal thyroid origin are not immortal. Therefore, to assess HIF-1α response in a genetically normal environment, cells were transfected with SV40 to create an immortal cell line from normal thyroid tissue. Consistent with the immunohistochemical studies of clinical material, the normal thyroid-derived cells had undetectable basal levels of HIF-1α (Fig. 2A). Exposure to the hypoxic mimetic CoCl2 induced both HIF-1α expression and that of the downstream targets, CA-9 and GLUT1. In the thyroid carcinoma cells, the expression of HIF-1α increased with severity of hypoxia, with anoxia inducing a higher level of expression than hypoxia (1% oxygen) in all cell lines. The FTC-133 cell line exhibited the highest levels of HIF-1α expression of the thyroid carcinoma cell line panel used (Fig. 2B). CA-9 and GLUT1 were detected at various levels in all cells exposed to reduced oxygen (Fig. 2B). Whereas basal HIF-1α and CA-9 expression was markedly lower than the induced states for all cell lines, for GLUT1 the relative change in expression was considerably lower (Figs 3C and 4C). The secretion of VEGF was also monitored in response to anoxia. Basal VEGF levels varied across the cell line panel, with the FTC derivatives showing the highest expression. In all cell lines, VEGF expression was significantly increased by exposure to anoxia (Fig. 2C). Consistent with the western blotting data, immunofluorescence studies revealed low cytoplasmic expression of HIF-1α in normoxic conditions. Hypoxia/anoxia HIF-1α expression was induced and was nuclear localized (data not shown).
Figure 2.
HIF-1α and downstream target expression are upregulated in response to hypoxia and hypoxia mimetics in immortalized normal thyroid tissue (A) and cell lines derived from papillary (BcPAP), follicular (WRO and FTC-133) and anaplastic (8505c) thyroid carcinomas (B and C). Normal thyroid cells immortalized using SV40 (according to Belge et al. (1995)), or thyroid cell lines were exposed to hypoxia (1% oxygen), anoxia or CoCl2 (100 μM) for 18 h. Cells were harvested and lysed samples containing 20 μg protein were subjected to western blotting (A and B) using primary antibodies against HIF-1α, CA-9 and GLUT1 with β-actin used as a loading control (see Materials and methods for details). Blots are representative of four independent experiments. For the determination of VEGF, media samples were removed from the cells after 18 h of exposure to condition and the level of VEGF determined by commercial ELISA, corrected to the amount of protein within the cell cultures from which the medium was taken. Data represents the mean±s.d. of four independent experiments. Significance values relate to comparisons between condition and normoxic concentrations of VEGF for each cell type.
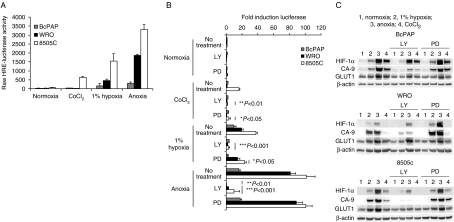
Figure 3.
Inhibition of the PI3K pathway using LY294002 decreases HIF-1 activity in PTC, FTC and ATC cell lines. Thyroid carcinoma cell lines were infected with a HIF-1 reporter virus (A and B) at an MOI of 20. Infected cells were exposed to the relevant condition for 18 h and luciferase activity assessed by luminescence (A and B) respectively. (A) Shows the absolute luciferase activity observed in each condition for each cell line. In (B), fold inductions of luciferase activity are given relative to normoxic untreated cells. Cells were exposed to normoxic or ‘hypoxic’ condition in the presence or absence (no treatment) of 50 μM LY294002 (LY) or PD98059 to inhibit PI3K and MEK signalling respectively. Data in (B) are from a single experiment that is representative of the data observed in three independent repeats and shows average values±s.d. from triplicate wells. (B) Is average data (±s.d.) from three independent experiments. Significance values are given against no treatment data within each specific condition. (C) Protein expression ascertained by western blotting for HIF-1α and downstream targets with β-actin as a loading control (see legend to Fig. 2 for details). Data shown are representative of four independent observations.
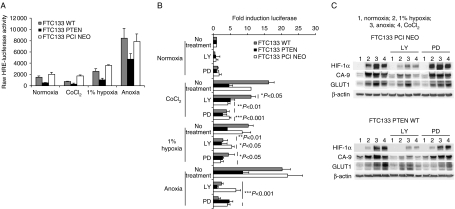
Figure 4.
PTEN restoration inhibits HIF-1 activity and modifies the sensitivity of the HIF-1 pathway to LY294002 and PD98059. FTC-133 parental (WT) cells or derivatives expressing PTEN or control vector (PCI NEO) were infected HIF reporter virus (MOI 20; A and B) and exposed to the stated conditions. Luciferase values are given as raw data (A) or fold inductions (B) in the presence or absence of LY or PD98059 treatment (B) as detailed in the legend to Fig. 3. Protein expression after the same treatments is given in (C) and is representative of four independent experiments. Data in A and B are average values±(s.d.) or a single experiment representative of three repeats (A) or of three independent experiments (B).
Oncogenic signalling via PI3K impacts on HIF-1α activity and expression in thyroid cells
Activating mutations within the RAF/MEK/ERK and PI3K pathways have been associated with oncogenic transformation in thyroid cancer. Similarly both pathways have been implicated in the regulation of HIF-1. To undertake a quantitative assessment of the impact of inhibition of both pathways upon HIF function, we employed an adenoviral-based HIF-1α reporter gene assay. A trimer of the HRE from the LDH gene was linked to the firefly luciferase gene and cloned into an E1/E3-deleted adenoviral backbone. We have previously shown that luciferase expression driven by this construct is robustly upregulated in response to hypoxia in a range of cell lines (Cowen et al. 2004, Chadderton et al. 2005). The MOI used was 20 viral particles per cell, which was sufficient for infection of >80% of the cell population as ascertained using an adenovirus encoding GFP (data not shown). When WRO, BcPAP and 8505c cells were infected with the HRE luciferase virus, graded hypoxia/anoxia-induced HIF-1α reporter gene activity in all cell lines (Fig. 3A). The highest level of expression was observed in the anaplastic 8505c cells in all conditions. Interestingly, neither BcPAP nor WRO cells showed induction following treatment with CoCl2. We next investigated the impact of inhibiting PI3K or MEK activity under the different environmental conditions by exposing the cells to 50 μM LY294002 or 50 μM PD98059 respectively. Treatment with LY294002 reduced basal (normoxic) reporter expression in WRO and 8505c cells, whilst having no effect in BcPAP. PD98059 had no effect in any cell type. Following CoCl2 exposure, the induction observed in 8505c cells was significantly reduced by both LY294002 and PD98059. Under hypoxic/anoxic conditions, LY294002 treatment significantly reduced the expression in all cell lines. In contrast, the effect of PD98059 appeared oxygen and cell line dependent, with inhibitory effects observed only in BcPAP and 8505c under 1% oxygen, and no effects seen in any cell line under anoxia (Fig. 3B). The ‘targeted’ effects of PD98059 against BcPAP and 8505c cells are perhaps in keeping with the mutational status of BRAF in these cells.
Western blotting for HIF-1α, CA-9 and GLUT1 was also undertaken in the LY294002- and PD98059-treated cells (Fig. 3C). In general, the level of HIF-1α expression following hypoxia/anoxia or CoCl2 (where apparent) was reduced in the presence of LY294002. Coincident with this was a reduction in CA-9 expression. GLUT1 appeared reduced only on 8505c cells (Fig. 3C). Interestingly, the significant decreases in reporter gene expression observed for 8505c cells following CoCl2 or hypoxic (1%) exposure in the presence of PD98059 were not related to changes in HIF-1α expression (Fig. 3B and C). In contrast, previous studies undertaken with the B-RAF-mutated putative thyroid line NPA demonstrated a clear correlation between the inhibition of reporter activity and expression of HIF-1α and downstream targets (data not shown). These cells have now been identified as originating from melanoma (Schweppe et al. 2008) where the MAPK pathway has been clearly shown to regulate HIF activity (Kumar et al. 2007).
Genetic restoration of PTEN reduces HIF-1 expression, activity and sensitivity to pharmacological inhibition of the PI3K and MEK pathways
These data strongly implicated PI3K pathway activation in the regulation of HIF-1 and downstream target expression in thyroid cell lines, including those with known B-RAF mutation. To further investigate the role of PI3K, we utilized the FTC-133 model with known PTEN mutation. Consistent with the lack of PTEN, HIF-1α expression is higher in this cell line than the rest of the panel (Fig. 2B). Further basal activation of the luciferase reporter gene in these cells is higher than that observed in BcPAP, WRO and 8505c cells (compare Figs 3 and 4A). This is not due to differences in adenoviral infection efficiency, which is similar across all cells at the MOI used (data not shown). Introduction of wild-type (WT) PTEN into the FTC-133 cells reduced the basal expression of the HRE reporter gene and the level of activity observed in the hypoxic conditions used. Cells expressing the empty vector PCI NEO construct behaved as the parental WT cells (Fig. 4A). Basal reporter expression was reduced by LY294002 in WT and PCI NEO cells, although this did not achieve significance. In hypoxia, significant reductions were restricted to WT and PCI NEO cells, whilst in anoxia LY294002’s inhibitory effects were apparent in all cells. Interestingly, in CoCl2 and hypoxic/anoxic conditions, treatment with PD98059 resulted in significant reduction in HIF-1 reporter expression in WT and PCI NEO cells, whilst having no significant effect in the PTEN-expressing cells (Fig. 4B). Consistent with the reporter gene activity assays, HIF-1α expression was reduced in the PTEN-expressing FTC-133 cells compared with PCI NEO-expressing controls (Fig. 4C). In keeping with the findings in the other thyroid cell lines, LY294002 reduced both HIF-1α and CA-9 protein expression, whilst having minimal effects on GLUT1 and PD98059 had little effect on any of the proteins analysed via western blot (Fig. 4C).
CA-9 expression appears to represent HIF-1 activity in thyroid carcinoma cells
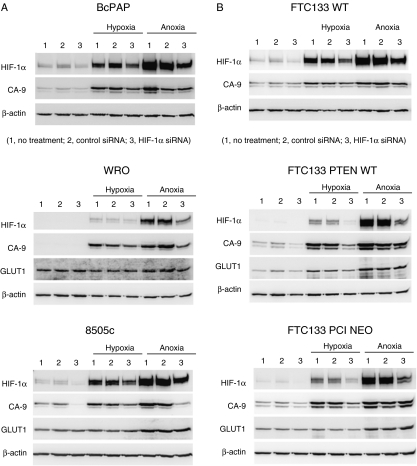
Given the clear differences in response of HIF-1α and its target genes (CA-9 and GLUT1), in response to the PI3K inhibitor, we hypothesize that there is a direct modulation of HIF-1α and particularly its target gene CA-9, by the PI3K pathway. To elucidate the HIF-1α-specific effect, we silenced HIF-1α by treating cells over 18–24 h with HIF-1α siRNA. Where significant knockdown of HIF-1α protein expression was observed, this was co-incident with a reduction in CA-9 expression (Fig. 5). The results clearly demonstrate that there is HIF-1α-dependent regulation of CA-9 in thyroid cell lines representative of each histological subtype (Fig. 5).
Figure 5.
Genetic silencing of HIF-1α reduces CA-9 expression in thyroid carcinoma cell lines. Cells were transfected with 100 nM HIF-1α siRNA (lanes 3) or control siRNA (lanes 2) for 4 h before exposure to normoxia (left panel, lane 1 untreated control), 1% hypoxia or anoxia for 18 or 24 h. Cells lysates were collected, and western blotting was performed as previously described for HIF-1α, CA-9 and β-actin as a control. Data are representative of two independent experiments.
Discussion
Despite thyroid carcinomas being the most common endocrine tumour, the mechanisms involved in disease progression are still poorly understood. Here we report a potential role for HIF-1α that appears to be a key target of the oncogenic signalling pathways associated with advancing tumour grade. HIF-1α protein was expressed in primary thyroid carcinomas but absent from normal thyroid tissue. Consistent with previous studies, the level of HIF-1α protein expression was highest in the aggressive dedifferentiated, anaplastic thyroid tumours. The distribution of HIF-1α was diffuse in these tumours, which may suggest regulation of the protein via a combination of tumour genotype and microenvironment. In contrast, differentiated papillary and follicular tumours commonly showed focal expression, which is perhaps consistent with the presence of diffusion-limited chronic hypoxia. However, analysis of tumour vasculature using vWF was unable to reveal an association between HIF-1α expression and the vessel distribution. Two downstream targets of HIF-1 were also assessed; CA-9 and GLUT1. On the one hand, GLUT1 was expressed to similar levels in all thyroid cancers analysed. On the other hand, CA-9 was detected in 100% of ATC samples, but only in around 80% of FTC and PTCs. In addition, the level of CA-9 expression varied between cancer subtypes, with significantly elevated levels detected in the dedifferentiated ATCs. This suggests that the expression of CA-9 may be associated with a more aggressive disease phenotype in thyroid carcinoma.
Our data raises the question of whether HIF-1α expression (especially in dedifferentiated thyroid carcinomas) is a) of pathophysiological relevance and b) relates to an adaptation to hypoxia-induced invasion and proliferation. The detection of high levels of HIF-1α target genes in dedifferentiated primary thyroid carcinomas supports this notion and agrees with previous work describing high expression levels of CA-9 in anaplastic thyroid carcinomas (Jubb et al. 2004).
The regulation of GLUT1 expression by HIF-1α is also of great interest. This is because overexpression of GLUT1 has been described in thyroid cancers and is linked to tumour aggressiveness. However, the underlying mechanism is not clearly understood (Schonberger et al. 2002, Kim et al. 2006). Recent data (Yasuda et al. 2005) suggest almost 50% of differentiated thyroid tumours express GLUT1 with expression not always being restricted to the membrane. Standard immunohistochemical techniques as used in our study would not allow us to clearly differentiate membranous from cytoplasmic immunostaining. However, our data suggests that differentiated tumours express GLUT1 predominantly in the cytoplasm, in contrast to a predominant membranous staining in dedifferentated carcinomas suggesting the presence of a functionally active transporter.
From our studies with clinical material, we were restricted to making correlative observations between HIF-1α and target protein expressions. To substantiate our observations, we undertook a number of functional studies in thyroid carcinoma cell lines and in immortalized cells derived from normal thyroid tissue. Under non-stimulated conditions, HIF-1α (where detected) was predominantly localized in the cytoplasm, but underwent stabilization with lowering oxygen tension or treatment with the hypoxic mimetic CoCl2 and translocates to the nucleus (data not shown). HIF-1α expression was not observed in immortalized normal thyroid cells, but was markedly induced by CoCl2. This treatment also resulted in induction of CA-9 and GLUT1. This shows that normal thyroid tissue is capable of eliciting a HIF-1-mediated response to hypoxia and suggests that in the clinical samples, the lack of HIF-1α expression was due to a lack of micro-environmental or genetic stimulus.
The thyroid carcinoma cells also showed a functional HIF-1 response as a consequence of hypoxia, identified both from protein expression and HIF reporter activation studies. These observations are consistent with data from other epithelial tumours (Pouyssegur et al. 2006). Interestingly, of the two downstream targets analysed, CA-9 appeared more responsive than GLUT1 to stimulation via HIF-1 and was markedly induced in all cell lines. The level of induction of GLUT1 was much lower, and the modification of GLUT1 expression following treatments that ablated HIF-1α expression was less convincing. The highest levels of HIF-1α activity were apparent in the anaplastic cell line 8505c and the follicular cell line FTC-133. These harbour classic mutations in B-RAF and PTEN respectively leading to activation of oncogenic pathways, which have been reported to influence HIF-1 activity (Bárdos & Ashcroft 2005), and are known to associate with aggressive disease. B-RAF mutations are known to alter the biological status of cancer cells, promoting tumour progression in a range of cancers. Animal models support a high proliferation rate of B-RAF-mutated versus non-B-RAF-mutated thyroid carcinoma cells. BRAF mutations leading to activation of the MAPK/ERK signalling pathway are the most important mutational event in thyroid carcinomas, especially in PTC progression (Durante et al. 2007, Lee et al. 2007, Elisei et al. 2008).
To study the impact of oncogenic signalling on HIF-1α expression and activity, we used pharmacological inhibitors of the PI3K (LY294002) and RAF/MEK/ERK pathway (PD98059). Reporter gene expression assays revealed that PD98059 could inhibit HIF-1 activity in cell lines with B-RAF mutation (8505c), although this was not seen in anoxia. Interestingly, HIF-1 activity was also inhibited by PD98059 in FTC-133 cells but not the additional FTC cell line WRO. In contrast, inhibition of the PI3K pathway using LY294002 inhibited HIF activity in all cells lines. Reintroduction of PTEN into the FTC-133 line reduced HIF-1 activity and rendered it less susceptible to inhibition via pharmacological manipulation of either the PI3K or RAF/MEK/ERK pathways.
Although PD98059 had some negative effects on HIF activation in the reporter assays, this was not translated into changes in HIF-1α nor downstream target protein expression. The much more substantial changes in reporter activity as a consequence of LY294002 treatment were, however, reflected in reduced expression of both HIF-1α and CA-9. LY294002-mediated effects on GLUT1 appeared both cell line and condition dependent. The differences between reporter and western data potentially reflect the relative sensitivity of the two assays. Furthermore, as suggested by the GLUT1 and CA-9 data, different HIF targets respond in subtly different ways and the reporter assay is based on the regulation of the LDH gene. Our data suggests that HIF-1α is not only hypoxia regulated but also directly modulated by the PI3K pathway, even in cells where one may have anticipated a predominant role for the RAF/MEK/ERK pathway.
A recent systematic analysis suggests that 15% of PTC and ∼50% of FTC have a mutation in the PI3K/AKT signalling pathway (Hou et al. 2007), which has been linked to tumour aggressiveness (Paes & Ringel 2008). Our data showed that the PI3K inhibitor, LY294002, very distinctly decreased both HIF-1α and CA-9 indicating a pronounced reliance of the expression of both proteins upon PI3K signalling. Recent evidence highlights the importance of this interaction on tumour progression, as it has been shown that downregulation of CAs dramatically reduces tumour cell proliferation (Chiche et al. 2009). Here we have shown that HIF-1α silencing by siRNA contribute significantly but not exclusively to the downregulation of CA-9 with additional independent inhibitory effects of PI3K inhibitors on CA-9 but not GLUT1. The relative weak inhibition seen may be explained by the reported long half-live of CA-9 (48 h) and an even longer one of GLUT1 (Heilig et al. 2003, Sobhanifar et al. 2005, Vordermark et al. 2005). This close link between one of the most frequently altered signalling cascades in thyroid carcinomas, PI3K, and HIF-1α further supports an important integrative role of PI3K and HIF-1α in the adaptation and survival of tumour cells. This fits well into the concept of the PI3K pathway being involved in the aggressive behaviour of these cells (Paes & Ringel 2008) and may have important implications in the therapeutic treatment of thyroid tumours. Recent literature supports an impact of small therapeutic molecules that modulate HIF-1α expression in the sensitivity to chemotherapy and more importantly for thyroid tumours, to radiotherapy (Moeller & Dewhirst 2004). Blocking HIF-1α, especially in dedifferentiated thyroid carcinomas, may increase sensitivity to ionizing radiation and thus opens new therapeutic options for the disease (Bardos & Ashcroft 2005). As an obvious next step, these effects would require further testing in vivo to evaluate whether the data offer hope for improving the therapeutic outcome of thyroid carcinomas associated with poor prognosis.
In summary, HIF-1α is expressed in thyroid carcinomas and is regulated not only by hypoxia but also via alteration of growth factor signalling pathways and, in particular, the PI3K pathway. HIF-1α expression appears to be in part responsible for the regulation of CA-9 and GLUT1 in the disease. With the known desensitizing effects of HIF on radiation and chemotherapy, HIF-1α may represent an important target for the treatment of thyroid carcinomas.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Deutsche Krebshilfe (to R von Wasielewski and G Brabant; #106294); the Christie Hospital Endowment Fund (G Brabant), EU FP7 Metoxia Grant agreement no. 222741 (K J Williams), Cancer Research UK (K J Williams C7820/A8696) and by Experimental Cancer Medicine Centre Funding (C M West C1467/A7286).
Acknowledgements
Thanks are due to Dr Sharon Sneddon (Pharmacy and Pharmaceutical Sciences, The University of Manchester) and Dr Nasreen Akhtar (Faculty of Life Sciences, The University of Manchester) who produced and kindly provided the GFP-adenovirus.
Footnotes
(K J Williams and G Brabant contributed equally to this work)
References
- Airley RE, Loncaster J, Raleigh JA, Harris AL, Davidson SE, Hunter RD, West CM, Stratford IJ. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. International Journal of Cancer. 2003;104:85–91. doi: 10.1002/ijc.10904. [DOI] [PubMed] [Google Scholar]
- Allred CD, Clark GM, Elledge R, Fuqua SAW, Brown RW, Chamness GC, Osborne CK, McGuire WL. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. Journal of the National Cancer Institute. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Bárdos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochimica et Biophysica Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Belge G, Garcia E, de Jong P, Bartnitzke S, Bullerdiek J. FISH analyses of a newly established thyroid tumor cell line showing at (1;19) (p35 or p36.1; q13) reveal that the breakpoint lies between 19q13.3-13.4 and 19q13.4. Cytogenetics and Cell Genetics. 1995;69:220–222. doi: 10.1159/000133968. [DOI] [PubMed] [Google Scholar]
- Brown LM, Cowen RL, Debray C, Eustace A, Erler JT, Sheppard FC, Parker CA, Stratford IJ, Williams KJ. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia-inducible factor-1. Molecular Pharmacology. 2006;69:411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- Chadderton N, Cowen RL, Robinson S, Greco O, Scott SD, Stratford IJ, Patterson AV, Williams KJ. Dual responsive promoters to target therapeutic gene expression to radiationresistant hypoxic tumour cells. International Journal of Radiation Oncology, Biology, Physics. 2005;62:213–222. doi: 10.1016/j.ijrobp.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Research. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- Cowen RL, Williams KJ, Chinje EC, Jaffar M, Sheppard FC, Telfer BA, Wind S, Stratford IJ. Hypoxia targeted gene therapy to increase the efficacy of tirapazamine as an adjuvant to radiotherapy: reversing tumor radioresistance and effecting cure. Cancer Research. 2004;64:1396–1402. doi: 10.1158/0008-5472.can-03-2698. [DOI] [PubMed] [Google Scholar]
- Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, et al. Mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. Journal of Clinical Endocrinology and Metabolism. 2007;92:2840–2843. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. Braf V600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. Journal of Clinical Endocrinology and Metabolism. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- Garcia JA. HIFing the brakes: therapeutic opportunities for treatment of human malignancies. Science's STKE. 2006;2006:25. doi: 10.1126/stke.3372006pe25. [DOI] [PubMed] [Google Scholar]
- Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, Zhu M, Weldon S, Wu G, Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. American Journal of Pathology. 2003;163:1874–1885. doi: 10.1016/S0002-9440(10)63546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Liu D, Shan Y, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clinical Cancer Research. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Pham TQ, Hanby AM, Frantz GD, Peale FV, Wu TD, Koeppen HW, Hillan KJ. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. Journal of Clinical Pathology. 2004;57:504–512. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Do IG, Park YK. Expression of the GLUT1 glucose transporter, p63 and p53 in thyroid carcinomas. Pathology, Research and Practice. 2006;202:759–765. doi: 10.1016/j.prp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC–RAS–BRAF signaling pathway in papillary thyroid carcinoma. Cancer Research. 2003;63:1454–1457. [PubMed] [Google Scholar]
- Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nature Reviews. Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, Acs G, Herlyn M, Xu X. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Research. 2007;67:3177–3184. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38–46. doi: 10.1002/cncr.22754. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Dewhirst MW. Raising the bar: how HIF-1 helps determine tumor radiosensitivity. Cell Cycle. 2004;3:1107–1110. [PubMed] [Google Scholar]
- Paes JE, Ringel MD. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinology and Metabolism Clinics of North America. 2008;37:375–387. doi: 10.1016/j.ecl.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulaki V, Mitsiades CS, McMullan C, Sykoutri D, Fanourakis G, Kotoula V, Tseleni-Balafouta S, Koutras DA, Mitsiades N. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I in thyroid carcinomas. Journal of Clinical Endocrinology and Metabolism. 2003;88:5392–5398. doi: 10.1210/jc.2003-030389. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Schonberger J, Ruschoff J, Grimm D, Marienhagen J, Rümmele P, Meyringer R, Kossmehl P, Hofstaedter F, Eilles C. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: an immunohistochemical study. Thyroid. 2002;12:747–754. doi: 10.1089/105072502760339307. [DOI] [PubMed] [Google Scholar]
- Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow L, Copland JA, Smallridge RC, et al. DNA profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. Journal of Clinical Endocrinology and Metabolism. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee N, Kaluz S, Ru N, Stanbridge EJ. PI3K/Akt, activity has variable cell-specific effects on expression of HIF target genes, CA9 and VEGF, in human cancer cell lines. Cancer Letters. 2009;282:109–115. doi: 10.1016/j.canlet.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhanifar S, Aquino-Parsons C, Stanbridge EJ, Olive P. Reduced expression of hypoxia-inducible factor-1A in perinecrotic regions of solid tumors. Cancer Research. 2005;65:7260–7266. doi: 10.1158/0008-5472.CAN-04-4480. [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Fleisher M, Francis GL, Robbins RJ. Serum vascular endothelial growth factor levels are elevated in metastatic differentiated thyroid cancer but not increased by short-term TSH stimulation. Journal of Clinical Endocrinology and Metabolism. 2002;87:1737–1742. doi: 10.1210/jcem.87.4.8388. [DOI] [PubMed] [Google Scholar]
- Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. The hypoxia-inducible factor-1α is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- Vordermark D, Kaffer A, Riedl S, Katzer A, Flentje M. Characterization of carbonic anhydrase IX (CA IX) as an endogenous marker of chronic hypoxia in live human tumor cells. International Journal of Radiation Oncology, Biology, Physics. 2005;61:1197–1207. doi: 10.1016/j.ijrobp.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Weng LP, Gimm O, Kum JB, Smith WM, Zhou XP, Wynford-Thomas D, Leone G, Eng C. Transient ectopic expression of PTEN in thyroid cancer cell lines induces cell cycle arrest and cell type-dependent cell death. Human Molecular Genetics. 2001;10:251–258. doi: 10.1093/hmg/10.3.251. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Telfer BA, Xenaki D, Sheridan MR, Peters HPW, Honess D, Dachs GU, Harris AL, van der Kogel A, Stratford IJ. Enhanced response to radiotherapy in tumours deficient in hypoxia-inducible factor-1α. Radiotherapy and Oncology. 2005;75:89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Research. 2003;63:4561–4567. [PubMed] [Google Scholar]
- Yasuda M, Ogane N, Hayashi H, Kameda Y, Miyagi Y, Iida T, Mori Y, Tsukinoki K, Minematsu T, Osamura Y. Glucose transporter-1 expression in the thyroid gland: clinicopathological significance for papillary carcinoma. Oncology Reports. 2005;14:1499–1504. doi: 10.3892/or.14.6.1499. [DOI] [PubMed] [Google Scholar]