Abstract
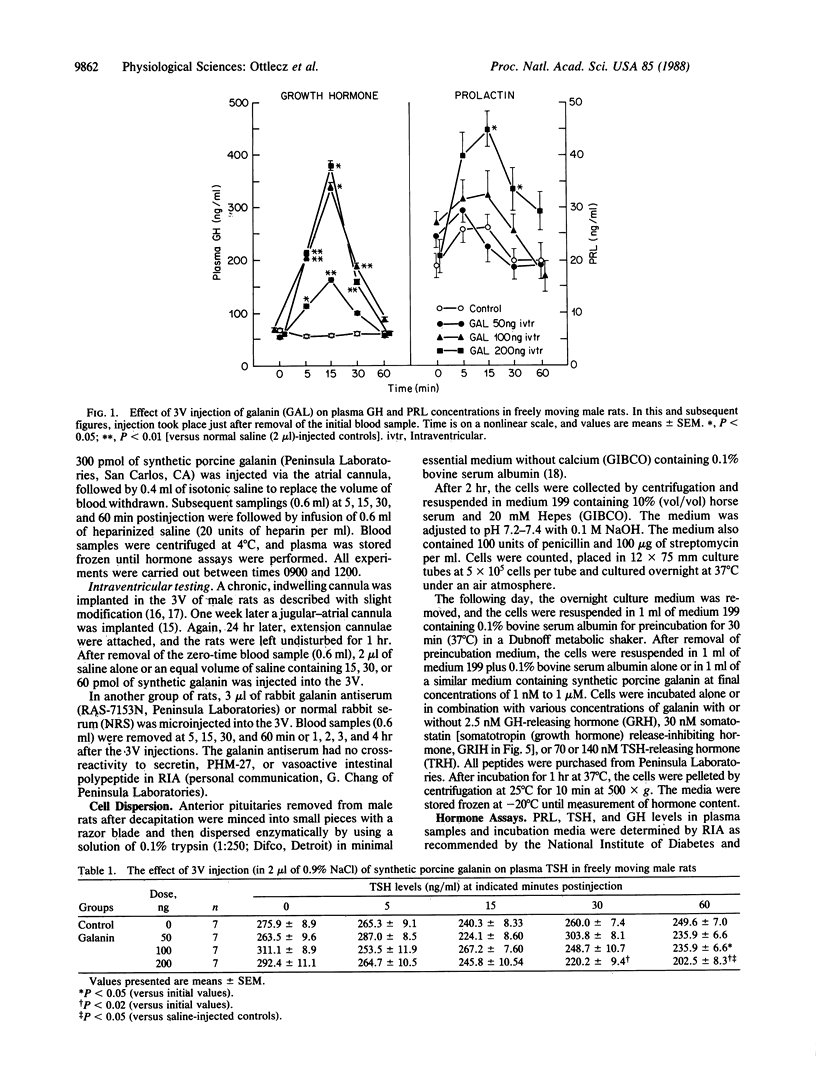
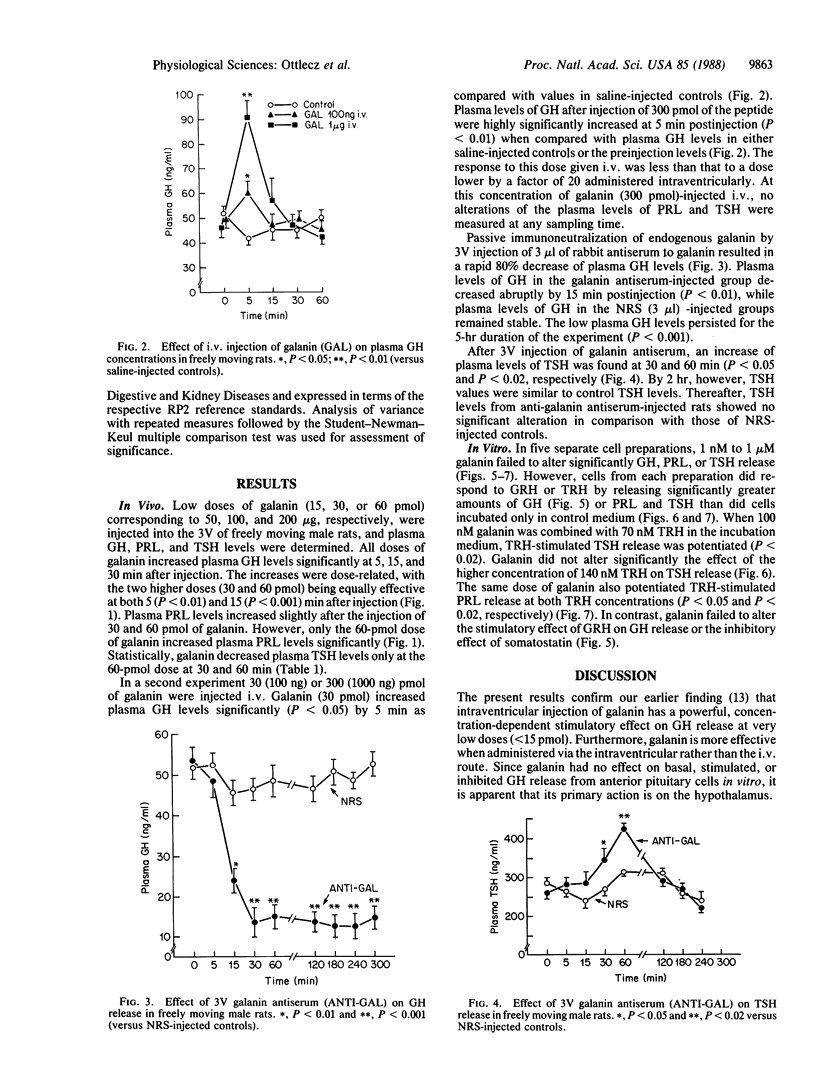
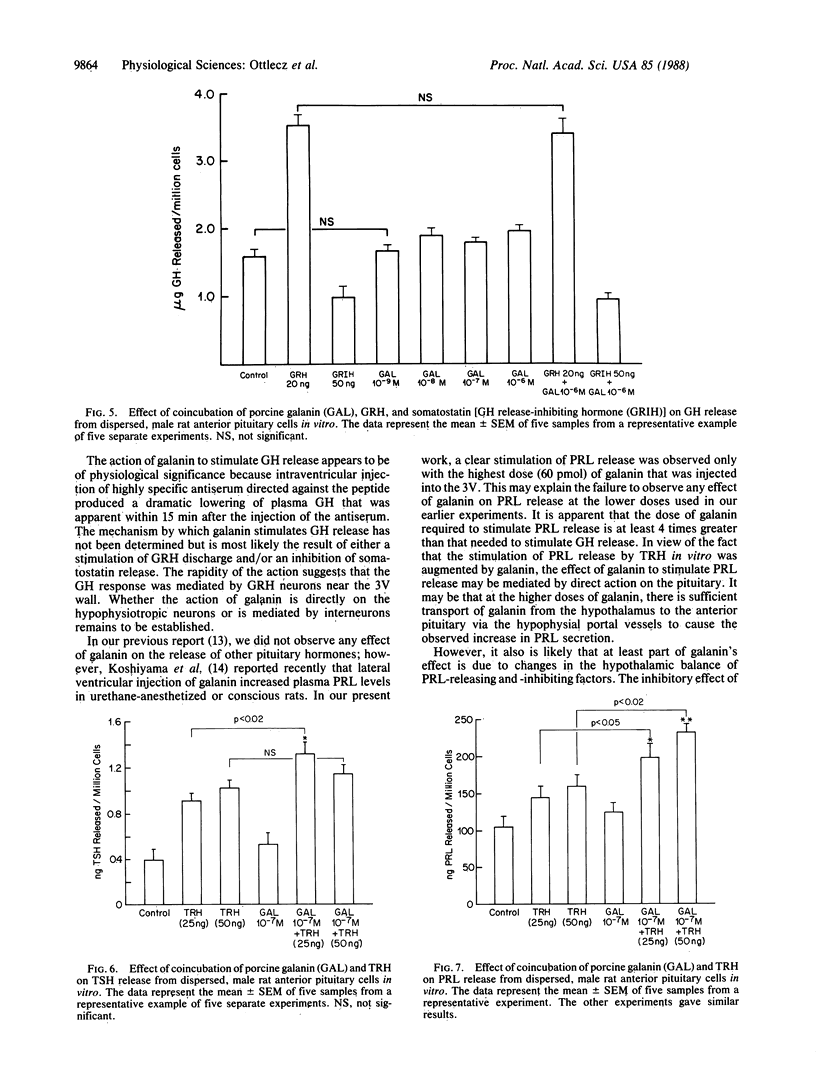
The role of the neuropeptide galanin in the regulation of anterior pituitary function was studied in vivo in conscious male rats and in vitro with cultured anterior pituitary cells. Galanin (50-200 ng; 15-60 pmol) injected into the third cerebral ventricle of rats produced highly significant, dose-related increases of plasma growth hormone (GH) concentrations, whereas galanin increased prolactin (PRL) and decreased thyroid-stimulating hormone (TSH) levels only at the highest dose (60 pmol) tested. Intravenous galanin failed to alter PRL and TSH levels in these rats. In contrast with the results with intraventricular injection of the peptide, intravenous injection of 30 or 300 pmol of galanin produced small, brief, dose-related increases in plasma GH. The response to the 300-pmol dose was less than that induced by a factor-of-20-lower intraventricular dose, which establishes a central action of galanin. Galanin in concentrations ranging from 1 nM to 1 microM failed to alter significantly GH, PRL, or TSH release from dispersed anterior pituitary cells. It also failed to alter GH secretion in response to 100 nM GH-releasing hormone; however, at this dose galanin did potentiate the effect of 100 nM TSH-releasing hormone on TSH and PRL release. Thus, the effects of third-ventricular injection of the peptide are mediated by the hypothalamus. To determine the physiological significance of galanin in control of pituitary hormone release, highly specific antiserum against galanin was injected intraventricularly. Third-ventricular injection of 3 microliter of galanin antiserum resulted in a dramatic decrease in plasma GH values as compared with those of normal rabbit serum-injected controls within 15 min, which persisted until the end of the experiment (5 hr postinjection). Galanin antiserum did not decrease plasma PRL or TSH levels at any time period after its third-ventricular injection; however, a transient increase of plasma TSH levels occurred after 30 and 60 min in comparison with TSH levels in normal rabbit serum-injected controls. Since there was no effect of the antiserum on plasma PRL and only a transient elevation in TSH, galanin may not be physiologically significant enough during resting conditions to alter PRL and TSH release in the male rat. The results of the experiments with galanin antiserum indicate that endogenous galanin has a tonic action within the hypothalamus to stimulate GH release. The rapidity of onset of the effects of galanin and the antiserum directed against it suggest that it acts to stimulate release of GH-releasing hormone from periventricular structures, which then stimulates the release of GH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes-Rodrigues J., McCann S. M. Water, sodium chloride, and food intake induced by injections of cholinergic and adrenergic drugs into the third ventricle of the rat brain. Proc Soc Exp Biol Med. 1970 Apr;133(4):1464–1470. doi: 10.3181/00379727-133-34713. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Håkanson R., Sundler F., Wahlestedt C. Galanin: neuromodulatory and direct contractile effects on smooth muscle preparations. Br J Pharmacol. 1985 Sep;86(1):241–246. doi: 10.1111/j.1476-5381.1985.tb09455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Rökaeus A., Håkanson R., Sundler F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience. 1985 Oct;16(2):355–363. doi: 10.1016/0306-4522(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Harms P. G., Ojeda S. R. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974 Mar;36(3):391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- Hymer W. C., Evans W. H., Kraicer J., Mastro A., Davis J., Griswold E. Enrichment of cell types from the rat adenohypophysis by sedimentation at unit gravity. Endocrinology. 1973 Jan;92(1):275–287. doi: 10.1210/endo-92-1-275. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Gabriel S. M., Koenig J. I., Sunday M. E., Spindel E. R., Martin J. B., Chin W. W. Galanin is an estrogen-inducible, secretory product of the rat anterior pituitary. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7408–7412. doi: 10.1073/pnas.85.19.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama H., Kato Y., Inoue T., Murakami Y., Ishikawa Y., Yanaihara N., Imura H. Central galanin stimulates pituitary prolactin secretion in rats: possible involvement of hypothalamic vasoactive intestinal polypeptide. Neurosci Lett. 1987 Mar 20;75(1):49–54. doi: 10.1016/0304-3940(87)90073-5. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Dupre J., Tatemoto K., Greenberg G. R., Radziuk J., Mutt V. Galanin inhibits insulin secretion and induces hyperglycemia in dogs. Diabetes. 1985 Feb;34(2):192–196. doi: 10.2337/diab.34.2.192. [DOI] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Rökaeus A., Cuello A. C., Oertel W. H., Verhofstad A., Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986 Dec;6(12):3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Rökaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986 Jun 22;248(4):475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- Melander T., Staines W. A., Hökfelt T., Rökaeus A., Eckenstein F., Salvaterra P. M., Wainer B. H. Galanin-like immunoreactivity in cholinergic neurons of the septum-basal forebrain complex projecting to the hippocampus of the rat. Brain Res. 1985 Dec 23;360(1-2):130–138. doi: 10.1016/0006-8993(85)91228-4. [DOI] [PubMed] [Google Scholar]
- Naor Z., Snyder G., Fawcett C. P., McCann S. M. Pituitary cyclic nucleotides and thyrotropin-releasing hormone action: the relationship of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate to the release of thyrotropin and prolactin. Endocrinology. 1980 Apr;106(4):1304–1310. [PubMed] [Google Scholar]
- Nordström O., Melander T., Hökfelt T., Bartfai T., Goldstein M. Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci Lett. 1987 Jan 2;73(1):21–26. doi: 10.1016/0304-3940(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Ottlecz A., Samson W. K., McCann S. M. Galanin: evidence for a hypothalamic site of action to release growth hormone. Peptides. 1986 Jan-Feb;7(1):51–53. doi: 10.1016/0196-9781(86)90060-4. [DOI] [PubMed] [Google Scholar]
- Ottlecz A., Samson W. K., McCann S. M. The effects of gastric inhibitory polypeptide (GIP) on the release of anterior pituitary hormones. Peptides. 1985 Jan-Feb;6(1):115–119. doi: 10.1016/0196-9781(85)90086-5. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Melander T., Hökfelt T., Lundberg J. M., Tatemoto K., Carlquist M., Mutt V. A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett. 1984 Jun 15;47(2):161–166. doi: 10.1016/0304-3940(84)90423-3. [DOI] [PubMed] [Google Scholar]
- Servin A. L., Amiranoff B., Rouyer-Fessard C., Tatemoto K., Laburthe M. Identification and molecular characterization of galanin receptor sites in rat brain. Biochem Biophys Res Commun. 1987 Apr 14;144(1):298–306. doi: 10.1016/s0006-291x(87)80510-7. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985 May-Jun;6(3):509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986 Jul-Aug;7(4):609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Sills M. A., Jacobowitz D. M. Autoradiographic distribution of 125I-galanin binding sites in the rat central nervous system. Peptides. 1986 Nov-Dec;7(6):1029–1042. doi: 10.1016/0196-9781(86)90133-6. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]


