Abstract
Background
A limited body of evidence, mostly based on self-report, is available regarding physical activity levels among American-Indian adults.
Purpose
This study aims to examine physical activity levels objectively by pedometer among a large cohort of American Indian adult participants in the Strong Heart Family Study.
Methods
Physical activity levels in 2604 American-Indian adults, aged 18–91 years, from 13 American-Indian communities were assessed using an Accusplit AE120 pedometer over a period of 7 days during 2001–2003. Anthropometric measurements were also assessed. All data analyses were conducted in 2008. Age-adjusted Pearson correlations were used to examine the relationship between average steps per day and age and anthropometric variables. Subjects were placed in age and BMI categories (according to NHLBI cutpoints) to examine trends in PA with increasing age and BMI.
Results
Daily pedometer steps ranged from 1001 to 38,755. Mean step counts by age group for men were: 5384 (18–29 years), 5120 (30–39 years), 5040 (40–49 years), 4561(50–59 years),4321 (60–69 years), and 3768 (≥70 years) and for women: 5038 (18–29 years), 5112 (30– 39 years), 5054 (40–49 years), 4582 (50–59 years), 3653 (60–69 years), and 3770 (>70 years). A significant linear trend in physical activity was noted with increasing age (P= 0.002 for men, P<0.0001 for women) and with increasing BMI (P = 0.05 for men, P = 0.04 for women).
Conclusions
Objectively measured data suggest that inactivity is a problem among American Indian adults and that a majority of American Indian adults in the SHFS may not be meeting the minimum physical activity public health recommendations. Efforts to increase physical activity levels in this population are warranted.
It has been suggested that physical activity provides numerous health benefits including the prevention of many chronic diseases.1–3 Research has demonstrated that habitual physical activity is associated with reduced morbidity and mortality from various chronic diseases and conditions such as cardiovascular disease4–6, diabetes7–9, hypertension10–12, obesity13, and cancer.14,15 Despite this well documented evidence, many individuals continue to lead relatively sedentary lifestyles.16
Physical inactivity appears to be a problem in all facets of the U.S. population, especially among minority populations. A limited body of evidence is available regarding physical activity levels among American Indian adults. The available data do, however, suggest that American Indian adults participate in relatively low levels of physical activity17–24, in many instances, lower than their minority counterparts.21 According to Schoenborn et al, (2004)17 at least 5 in 10 American Indian adults are physically inactive, with women more likely to be inactive than men (55.5% vs 42.5%). This same report suggests that roughly 26.4% of this population do not meet the Surgeon General’s recommendations for physical activity participation.
Unfortunately, most of this evidence is based on physical activity data collected using subjective methods. Self-report measures of physical activity often suffer from reporting bias and may over/underestimate physical activity levels due to the fact that questionnaires often capture only moderate-to-vigorous physical activity and activities that are structured or planned.25 In order for an accurate assessment of activity to be achieved, the assessment tool used must elicit information on the types of physical activities that encompass the greatest proportion of energy expenditure in the study population. In investigations where it can be assumed that low-intensity activities, as well as unstructured activities, are similar across populations, a self report measure may be appropriate. However, in certain subgroups, such as the older adults, injured/impaired individuals, or in individuals where lower-intensity activities may constitute the bulk of their physical activity levels, the use of a subjective measure to assess physical activity may likely miss a substantial portion of activities that make up their total energy expenditure. In this case, an objective measure of physical activity should be considered to better assess total activity including low-intensity and unstructured physical activity.25
The purpose of the current study was to examine physical activity levels in a large cohort of American Indian adult from three geographic locations across the U.S. and to examine trends within this population. Physical activity was assessed with a pedometer, which will allow for objective comparisons across these populations
METHODS
Participants
Strong Heart Family Study
The Strong Heart Family Study (SHFS) is a longitudinal study of cardiovascular disease, its risk factors, and genetic determinants in 13 American Indian communities from three geographic regions in Arizona (AZ), Oklahoma (OK), and North and South Dakota (DA). The SHFS includes two clinical examinations and ongoing mortality and morbidity surveillance. In 2003, the SHFS recruitment and examination of family members was successfully met and included a total of 96 extended families (33–AZ, 36–OK, and 27–DA) totaling 3,665 participants from all three centers ranging in age from 14 to 93 years. As part of the SHFS, participants completed both a personal interview and physical examination. The personal interview solicited information about demographics, health habits, and medical history. The physical examination included measures such as physical activity and anthropometric measurements. All participants gave informed consent for the present study, which was approved by the IRBs at all of the participating institutions.
Physical activity assessment
Physical activity was assessed using an Accusplit AE120 pedometer (Accusplit Inc, San Jose, CA) which has been shown to be a valid and reliable assessment tool for assessing step counts in a variety of laboratory and field settings.26–33 Participants received a pedometer, instructions for wearing the pedometer, and an activity diary at their clinical examination and were asked to wear the pedometer for 7 consecutive days (5 weekdays and 2 weekend days) and to record the number of steps taken daily in an activity diary. There has been some suggestion that spring-lever pedometer accuracy may be compromised in individuals with large BMIs or excess frontal body mass where the pedometer may not remain upright in the vertical plane.34 To ensure that participants wore the pedometer correctly, clinic staff members were trained to instruct participants with large BMIs or excess frontal body mass, which may impede the pedometer from working properly, to wear the monitor on the small of the back on the posterior mid-line of the thigh.35 Moving the pedometer to the back may aid in keeping the pedometer upright therefore reducing reporting errors. This procedure was used only in rare cases when the pedometer failed to meet acceptable standards after a 100 step accuracy test. At the end of the 7-day period, participants were asked to return their pedometer diary to the clinic in a postage paid envelope. The mean number of steps the participant takes per day was calculated by averaging the number of steps recorded each day during the 7-day period. Since previous research has suggested that 3 days of activity can provide a sufficient estimate of weekly physical activity36; participants with 3 or more days of data were included in the study. Steps per day averaged over the week was calculated for any person who had data for 3 or more days, taking the sum of steps per day divided by the number of available days.
Anthropometric measures
Body composition measures were determined using anthropometry. All measurements were taken over a scrub suit or light clothing with the participant’s bladder empty and shoes removed. Body weight was measured using a Tanita BWB-800 5 Adult Digital Scale (Tanita Corp. of America, Arlington Heights, IL) and measures were recorded to the nearest kilogram. Height was recorded using a vertical mounted ruler and measures were recorded to the nearest centimeter. BMI was calculated from height and weight and expressed as kilogram/meters2 and was broken down into categories based on NHLBI cutpoints for overweight and obesity: normal (< 24.9 kg/m2), overweight (25–29.9 kg/m2), obese (30–34.9 kg/m2), obesity I (35 – 39.9 kg/m2 ) and obesity II (≥ 40 kg/m2). Waist measurements were obtained using an anthropometric tape applied at the level of the umbilicus with the patient supine and breathing quietly and recorded to the nearest centimeter.
Statistical Analyses
Descriptive statistics were calculated for the cohort in total and separately by gender and age group. All continuous data were assessed for normality. Depending on normality assessment of the variable, ANOVA or Kruskal-Wallis tests were used to describe and compare differences among age groups (18–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years) separately by gender (male/female). Normally distributed data are reported as mean (SD), non-normal variables as median (25th, 75th percentile). Pedometer data were found to be skewed and natural log transformed prior to analyses. Jonckheere-Terpstra tests were used to estimate the linear trend in physical activity levels across age groups stratified by gender. Pearson partial correlation coefficients adjusted for age were used to evaluate the association of physical activity determined by pedometer steps and anthropometric measures such as BMI, waist and hip circumference, and waist-to-hip ratio for the cohort stratified by gender. Statistical analyses were performed using Statistical Analysis Software, version 8.2. Significance was considered as a P-value <0.05.
To aid in interpretation of the findings, analyses were limited to healthy participants aged ≥18 years who had a minimum of 3 days of pedometer data and BMI data. Healthy participants were considered to be any that did not report a severe chronic disease or disability that may limit their physical activity level. Therefore, participants were eliminated from the analyses if they reported having any of the following conditions that may limit their physical activity: rheumatic heart disease, renal dialysis, kidney failure, cirrhosis of the liver, emphysema, above or below knee amputation, or unable to walk. In addition, those individuals were eliminated whose average number of steps per day was less than 1000 (n=123). Eliminating values this low is not uncommon since they may be considered beyond that expected in people that are physically inactive and may likely reflect not wearing the monitor.
RESULTS
A total of 3665 participants were enrolled in the Strong Heart Family Study. Of these participants, 2604 men and women (71% of the Phase IV Cohort) aged 18–91 years met the inclusion criteria and were included in the analyses. Table 1 presents the descriptive characteristics of the entire sample stratified by gender (male/female) and by age group (18–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years).
Table 1.
Descriptive characteristics of strong heart family study participants stratified by gender and age group (n=2604)
|
Men n=1022 | |||||||
| Variable | 18–29 n=324 |
30–39 n=236 |
40–49 n=222 |
50–59 n=131 |
60–69 n=79 |
70+ n=30 |
p* |
| Height (m) | 1.76 (0.06) | 1.76 (0.06) | 1.76 (0.06) | 1.75 (0.07) | 1.75 (0.06) | 1.71 (0.05) | 0.0002 |
| Weight (kg) | 97.7 (25.1) | 104.2 (27.5) | 99.5 (25.1) | 99.1 (21.7) | 93.2 (15.3) | 84.1 (18.0) | 0.0006 |
| BMI (kg/m2) | 31.4 (7.5) | 33.7 (8.4) | 32.2 (7.8) | 32.4 (6.6) | 30.5 (4.7) | 28.8(5.5) | 0.004 |
| Waist (cm) | 102.6 (19.6) | 109.3 (19.7) | 106.9 (17.8) | 108.6 (14.6) | 106.4 (11.0) | 102.1 (13.1) | 0.0008 |
|
Women n=1582 | |||||||
| Variable | 18–29 n=422 |
30–39 n=384 |
40–49 n=365 |
50–59 n=198 |
60–69 n=134 |
70+ n=79 |
p* |
| Height (m) | 1.64 (0.07) | 1.63 (0.06) | 1.63 (0.05) | 1.61 (0.06) | 1.60 (0.06) | 1.57 (0.06) | <0.0001 |
| Weight (kg) | 87.2 (23.8) | 90.8 (23.6) | 87.1 (19.0) | 85.1 (18.3) | 82.6 (18.4) | 74.8 (13.7) | <0.0001 |
| BMI (kg/m2) | 32.5 (8.4) | 34.2 (8.8) | 32.9 (7.2) | 32.6 (6.8) | 32.4 (7.0) | 30.3 (5.4) | 0.003 |
| Waist (cm) | 103.6 (37.6) | 105.9 (19.1) | 105.1 (17.8) | 106.4 (15.9) | 105.9 (17.1) | 105.5 (14.3) | 0.04 |
All statistics are M (SD) unless otherwise noted.
p for comparison among age groups.
Participants’ daily pedometer steps ranged from 1001 to 38,755, with a mean number of steps per day for the entire cohort as 4873.6 (95% CI=4758.3, 4991.7). Figure 1 presents mean pedometer step counts of the SHFS cohort stratified by age group and gender. These results suggest that there is a significant linear trend in physical activity across age groups within gender (Jonckheere-Terpstra test for trend, P = 0.002 for men, P <0.0001 for women). Additionally, regardless of gender, younger adults, on average, have higher mean pedometer step counts than older adults. There was no significant difference between reported physical activity levels based on pedometer steps between men and women adjusted for age (4996.6 for men vs 5189.1 for women, p=0.52).
Figure 1.
Mean pedometer steps by age category in the strong heart family study cohort stratified by gender (n=2604)
* Jonckheere-Terpstra test for trend: p=0.002 for men, p<0.0001 for women; ** Pearson correlations coefficient for age and pedometer steps for both men and women
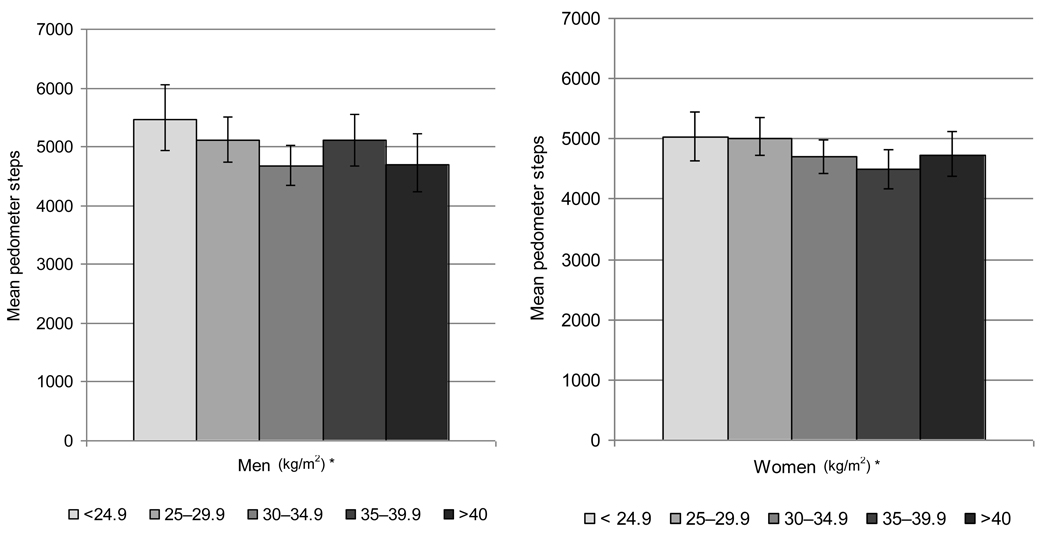
Figure 2 presents mean step counts of the SHFS cohort stratified by BMI group and gender. These data indicate a significant linear trend in mean pedometer steps (Jonckheere-Terpstra test for trend P = 0.05 for men, P = 0.04 for women) with increasing BMI. Similar to the findings within age groups, regardless of gender, those participants with lower BMIs have higher mean step counts compared to those with larger BMIs. In addition, the relationship between average steps per day from the pedometer and BMI and waist circumference was examined separately by gender (n=2604). Results from age-adjusted correlation analyses, indicated significant, albeit weak, inverse associations between average steps per day and BMI (r = –0.07, p= 0.001) and waist circumference (r = −0.08, p = 0.0005) for women. Similar results were noted for men, pedometer steps and BMI (r = −0.07, p = 0.02) and waist circumference (r = −0.06, p = 0.05).
Figure 2.
Age-adjusted mean pedometer steps by NHLBI BMI cutpoints of overweight and obesity in the strong heart family study cohort stratified by gender (n=2604)
* Jonckheere-Terpstra test for trend: p=0.05 for men, p=0.04 for women
DISCUSSION
In this first study to examine physical activity using objective measure, it was found that regardless of gender or age group classification, participants in the SHFS have mean pedometer values well below aggregated reference points.40 In fact, pedometer steps among male participants ranged from an average low of 3111 among men aged >70 years to a high of 5078 among men aged 18– 29 years (Figure 1) and among female participants in the SHFS, pedometer steps ranged from a mean of 3170 among women aged >70 years to 4833 among women aged 18–29 years. These low step counts would suggest that a large proportion of the sample is not meeting the current CDC and American College of Sports Medicine recommendations for physical activity.41
Other studies conducted among racially or ethnically diverse free-living samples with pedometer assessed physical activity have found that minority individuals tend to be less active than the suggested national recommendations. Bennett et al.42 examined pedometer step counts among multiethnic (50% African-American, 42% Hispanic) low income–housing residents aged 18 to >70 years. In this study, mean (SD) pedometer step counts ranged from 6587(4083.6) in participants aged <25 years to 3285(2873.3) in participants aged >70 years. This is in contrast to the findings of the SHFS study in which mean pedometer steps ranged from 5183.0 (95% CI=1548.7, 17,345.6) in participants aged 18–30 years to 3769.8 (95% CI=1036.6, 13710.1) in participants aged >70 years. Additionally, the Cross-Cultural Activity Participation Study38 found median daily step counts of 4783 (3009, 6987) and 4577 (3219, 6385) among 127 American Indian and 135 African American women (mean age 53.8±10.9 years), respectively. In comparison, female participants in the SHFS aged 50–60 years were found to have slightly lower median steps counts of 4568.4 (2620.0, 7066.2), compared to those reported in the Cross-Cultural Activity Participation Study. These findings confirm that the current sample of American Indian adults is at least as inactive as other minority samples.
When examining physical activity levels by gender in the SHFS, unlike in previous studies,16,43–45 no significant differences were found between men and women in age-adjusted, pedometer-determined physical activity levels. This finding is likely due to the lack of variability in physical activity levels across the entire SHFS population and the very low levels of activity among both men and women in the SHFS. In contrast, when the relationship between age and physical activity was examined categorically, it was found that physical activity declined with increasing age, which is often shown in population studies. Participants aged ≥70 years were found to accumulate approximately 2000 steps less than participants aged <30 years. These findings are similar to those of another study,42 which showed a 3000-step difference between participants aged >70 years when compared to participants aged <25 years.
In regard to BMI and physical activity, a significant line trend was noted in pedometer steps with increasing BMI in both men and women (P = 0.05 for men, P = 0.04 for women). These data indicate that SHFS participants with lower BMIs have higher step counts compared to those with larger BMIs. These findings are consistent with other studies that have shown decreasing levels of physical activity with increasing BMI.38,42,46 However, given the cross-sectional nature of this study, it was not possible to establish the causality of the association between BMI and daily step counts.
The Strong Heart Family Study provided the unique opportunity to examine physical activity levels in a large cohort of American Indian individuals using an objective measure, more specifically the pedometer. To date, most studies that have examined physical activity levels in American Indian populations have utilized subjective measures such as a questionnaire to assess physical activity in their population of interest. While this method of assessment is relatively reasonable in large population studies, it relies on participant recall and may not provide an adequate assessment of lower intensity, unstructured physical activities like walking and housework. By utilizing a pedometer, it was possible to eliminate some of the problems posed by the use of subjective measures and possibly obtain a truer representation of physical activity levels among American Indian adults.
However, despite the advantages of using the pedometer to capture unstructured and low-intensity physical activity in the SHFS, there are, unfortunately, limitations that need to be considered with its use as an assessment tool. First, the pedometer does not measure activities that are not ambulatory in nature such as resistance training and cycling. Additionally, many pedometers, such as the pedometer used in the current study, lack an internal clock and data storage capability; thus it was necessary to rely on the SHFS participants to accurately record their step counts from the pedometer in their 7-day activity diary. This process may have resulted in reporting errors or lack of data. Further, the pedometer used in this study is unable to discriminate between steps accumulated in walking, running, or stair climbing; therefore, it was not possible to determine intensity of activity. Finally, participant clothing or body habitus may have played a role in the accuracy of the pedometer. In order for a pedometer to accurately assess physical activity, it must be worn snug to the body and kept upright in a vertical plane, perpendicular to the ground. Although every effort was made to ensure that participants were properly instructed on how to wear the pedometer, there was no guarantee that this occurred. Therefore, if the pedometer was not worn in a correct manner, the pedometer may not have worked properly and may have resulted in an underestimation of physical activity levels for those specific individuals.
Other limitations that should be considered when interpreting these findings include the fact that the SHFS is made up of 96 large families selected across three geographic locations and not a random sample of American Indian communities. It has been suggested that physical activity levels may be similar in related individuals or those who share a related environment, therefore reducing inter-individual variation of physical activity levels. Individuals were treated as if they were completely independent of each other, and family structure and environmental influences were not taken into account when evaluating physical activity levels. The estimates of physical activity are believed to be reflective of their low levels of physical activity. However, the variability may be underestimated in this population.
In summary, this study is the first to objectively determine physical activity levels in a large sample of American Indian adults. The findings of this study suggest that based on pedometer steps, a majority of American-Indian participants in the Strong Heart Family Study are not meeting the minimum physical activity public health recommendations. Since physical activity has been shown to reduce the risk of developing many chronic diseases, efforts to increase physical activity levels in this population are warranted.
ACKNOWLEDGEMENTS
The authors acknowledge the assistance and cooperation of the participating tribes and the Indian Health Service facilities that serve those tribes. The authors want to thank the study participants and the SHS/SHFS staff for their important contribution to this effort. KLS wishes to thank the SHS for the opportunity to complete her doctoral dissertation utilizing the SHFS data.
SHS is conducted as a collaborative agreement supported by grants U01 HL41642, U01 Hl41652, and U01 HL4164. MD-000-207-03. Additionally, this research was supported in part by the intramural research program of NIDDK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Indian Health Services.
BVH receives consulting fees from Merck and the Egg Nutrition Council, and research support from Pfizer, Merck, Schering-Plough. No other authors reported financial disclosures.
REFERENCES
- 1.Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, et al. Cadiovascular health in childhood: a statement for health professionals from the committee on atherosclerosis, hypertension, and obesity in young (AHOY) of the Council on Cardiovascular Disease in teh Youth, American Heart Association. Circulation. 2002;106:143–160. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]
- 2.Strauss RS, Rodzilsky D, Burack G, Colin M. Psychosocial correlates of physical activity in Psychosocial correlates of physical activity in. Arch Ped Adolesc Med. 2001;155:897–902. doi: 10.1001/archpedi.155.8.897. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HWr, Barlow CE, Paffenbarger RSJ, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(13):1093–1098. [PubMed] [Google Scholar]
- 4.Hu G, Eriksson J, Barengo NC, Lakka TA, Valle TT, Nissinen A, et al. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation. 2004;110:666–673. doi: 10.1161/01.CIR.0000138102.23783.94. [DOI] [PubMed] [Google Scholar]
- 5.Richardson CR, Kriska AM, Lantz PM, Hayward RA. Physical activity and mortality across cardiovascular disease risk groups. Med Sci Sports Exerc. 2004;36:1923–1929. doi: 10.1249/01.mss.0000145443.02568.7a. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease. Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 7.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erikkson KF. F. L. Prevention of type 2 (non-insulin dependent) diabetes mellitus by diet and physical activity. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 9.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 10.Hagberg JM. Exercise, fitness, and hypertension. In: Shephard RJ, Stephens T, Sutton JR, editors. Exercise, fitness, and health. Champaign, IL: Human Kinetics; 1990. pp. 455–466. [Google Scholar]
- 11.Hagberg JM, Brown MD. Does exercise training play a role in the treatment of essential hypertension? J Cardiovasc Risk. 1995;2(4):296–302. [PubMed] [Google Scholar]
- 12.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousliahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 13.Wing RR, Hill JO. Successful weight loss maintenance. Ann Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 14.Breslow RA, Ballard-Barbash R, Munoz K, Graubard BI. Long-term recreational physical activity and breast cancer in the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer Epidemiol Biomarkers Prev. 2001;10(7):805–808. [PubMed] [Google Scholar]
- 15.Slattery ML, Potter JD. Physical activity and colon cancer: confounding or interaction? Med Sci Sports Exerc. 2002;34:913–919. doi: 10.1097/00005768-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 16.USDHHS. Leisure-time physical activity among adults: U.S., 1997–98. USDHHS, CDC, National Center for Health Statistics; 2002. [Google Scholar]
- 17.Schoenborn CA, Adams PF, Barnes PM, Vickerie JL, Schiller JS. Health behaviors of adults: U.S., 1999–2001. Vital Health Stat. 2004;10(219):1–79. [PubMed] [Google Scholar]
- 18.Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, et al. Cardiovascular disease risk factors among American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142(3):269–287. doi: 10.1093/oxfordjournals.aje.a117633. [DOI] [PubMed] [Google Scholar]
- 19.Fischer ID, Brown DR, Blanton CJ, Casper ML, Croft JB, Brownson RC. Physical activity patterns of Chippewa and Menominee Indians: the Inter-Tribal Heart Project. Am J Prev Med. 1999;17(3):189–197. doi: 10.1016/s0749-3797(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Levin S, Jacobs DR, Jr, Ainsworth BE, Richardson MT, Leon AS. Intra-individual variation and estimates of usual physical activity. Ann Epidemiol. 1999;9(8):481–488. doi: 10.1016/s1047-2797(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 21.Stolarczyk LM, Gilliland SS, Lium DJ, Owen CL, Perez GE, Kriska AM, et al. Knowledge, attitudes and behaviors related to physical activity among Native Americans with diabetes. Ethn Dis. 1999;9(1):59–69. [PubMed] [Google Scholar]
- 22.Brownson RC, Eyler AA, King AC, Brown DR, Shyu YL, Sallis JF. Patterns and correlates of physical activity among U.S. women 40 years and older. Am J Public Health. 2000;90(2):264–270. doi: 10.2105/ajph.90.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coble JD, Rhodes RE. Physical activity and Native Americans: a review. Am J Prev Med. 2006;31(1):36–46. doi: 10.1016/j.amepre.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.NHLBI. Strong Heart Study Data Book: a report to American Indian communities. Bethesda, MD: National Heart, Lung and Blood Institure, Division of Epidemiology and Clinical Applications; 2001. Nov, [Google Scholar]
- 25.Kriska AM, Caspersen CJ. Introduction to the Collection of Physical Activity Questionnaires. Medicine and Science in Sports and Exercise. 1997;29 Supplement:S5–S9. [Google Scholar]
- 26.Bassett DR, Jr, Ainsworth BE, Leggett SR, Mathien CA, Main JA, Hunter DC, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28(8):1071–1077. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O'Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32(9 Suppl):S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 28.Bassett DR, Jr, Cureton AL, Ainsworth BE. Measurement of daily walking distance-questionnaire versus pedometer. Med Sci Sports Exerc. 2000;32(5):1018–1023. doi: 10.1097/00005768-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Crouter SE, Schneider PL, Karabulut M, Bassett DR., Jr Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35(8):1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 30.Schneider PL, Crouter SE, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36(2):331–335. doi: 10.1249/01.MSS.0000113486.60548.E9. [DOI] [PubMed] [Google Scholar]
- 31.Schneider PL, Crouter SE, Lukajic O, Bassett DR., Jr Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc. 2003;35(10):1779–1784. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 32.Nelson TE, Leenders NYJM, Sherman WM. Comparison of activity monitors worn during treadmill walking. Med Sci Sports Exerc. 1998;30:S11. (Abstract) [Google Scholar]
- 33.Leenders NY, Nelson TE, Sherman WM. Ability of different physical activity monitors to detect movement during treadmill walking. Int J Sports Med. 2003;24(1):43–50. doi: 10.1055/s-2003-37196. [DOI] [PubMed] [Google Scholar]
- 34.Crouter SE, Schneider PL, Bassett DR., Jr Spring-levered versus piezo-electric pedometer accuracy in overweight and obese adults. Med Sci Sports Exerc. 2005;37(10):1673–1679. doi: 10.1249/01.mss.0000181677.36658.a8. [DOI] [PubMed] [Google Scholar]
- 35.Swartz AM, Bassett DR, Jr, Moore JB, Thompson DL, Strath SJ. Effects of body mass index on the accuracy of an electronic pedometer. Int J Sports Med. 2003;24(8):588–592. doi: 10.1055/s-2003-43272. [DOI] [PubMed] [Google Scholar]
- 36.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40(3):293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 38.Whitt MC, DuBose KD, Ainsworth BE, Tudor-Locke C. Walking patterns in a sample of African American, Native American, and Caucasian women: the cross-cultural activity participation study. Health Educ Behav. 2004;31(4 Suppl):45S–56S. doi: 10.1177/1090198104266034. [DOI] [PubMed] [Google Scholar]
- 39.Tudor-Locke CE, Myers AM. Methodological considerations for researchers and practitioners using pedometers to measure physical (ambulatory) activity. Res Q Exerc Sport. 2001;72(1):1–12. doi: 10.1080/02701367.2001.10608926. [DOI] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34(12):2045–2051. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 41.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 42.Bennett GG, Wolin KY, Puleo E, Emmons KM. Pedometer-determined physical activity among multiethnic low-income housing residents. Med Sci Sports Exerc. 2006;38(4):768–773. doi: 10.1249/01.mss.0000210200.87328.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sequeira MM, Rickenbach M, Wietlisbach V, Tullen B, Schutz Y. Physical activity assessment using a pedometer and its comparison with a questionnaire in a large population survey. Am J Epidemiol. 1995;142(9):989–999. doi: 10.1093/oxfordjournals.aje.a117748. [DOI] [PubMed] [Google Scholar]
- 44.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Tudor-Locke C, Ham SA, Macera CA, Ainsworth BE, Kirtland KA, Reis JP, et al. Descriptive epidemiology of pedometer-determined physical activity. Med Sci Sports Exerc. 2004;36(9):1567–1573. doi: 10.1249/01.mss.0000139806.53824.2e. [DOI] [PubMed] [Google Scholar]
- 46.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]