Abstract
Objective
Arthritis is a prominent manifestation of Lyme disease, caused upon infection with Borrelia burgdorferi (Bb). Persistent chronic Lyme arthritis, even after antibiotic treatment, is linked to HLA-DRB1*0401 (DR4) and related alleles. On the contrary, Lyme patients who resolve arthritis within 3 months post-infection show an increased frequency of HLA-DRB1*1101 (DR11). The aim of this study was to analyze the underlying mechanism by which HLA-DR alleles confer genetic susceptibility or resistance to antibiotic-refractory Lyme arthritis.
Methods
We generated DR11 transgenic (tg) mice on a murine class II−/− background and compared their immune response to Bb-antigens to that of DR4 tg mice after immunization with Bb outer surface protein (Osp)A or infection with live Bb.
Results
We report that the T cells of OspA-immunized and Bb-infected DR11 tg mice were defective in IFN-γ production compared to those of DR4 mice. On the other hand, DR11 tg mice developed higher titers of anti-OspA and anti-Bb Abs, respectively, than DR4 mice. In accordance with this observation, we found that Bb-infected DR11 tg mice had decreased spirochetal burden compared to DR4 mice, measured by qPCR.
Conclusion
This study provides direct evidence that in the presence of HLA-DR11 the immune response against Bb-antigens is directed towards a protective Ab response. In contrast, an inflammatory Th1 response is induced in the presence of DR4. These observations offer an explanation for the differential genetic susceptibility of DR4+ and DR11+ individuals for the development of chronic Lyme arthritis and eventually the progression to antibiotic-refractory Lyme arthritis.
Lyme disease is a debilitating infection transmitted by the bite of Borrelia burgdorferi (Bb)-infected ticks. One of the most prominent clinical manifestations of Lyme disease is the development of chronic Lyme arthritis. Generally, a 1 to 2-months course of oral doxycycline or a 2 to 4-week course of intravenous (i.v.) ceftriaxone resolve joint inflammation associated with the presence of the spirochetes (1). However, some patients continue to experience persistent joint inflammation, despite antibiotic treatment, a condition termed antibiotic-refractory Lyme arthritis (1–3). This inflammatory response is characterized by proliferative synovitis and may persist for months or even several years.
One of the factors that confer susceptibility to antibiotic-refractory Lyme arthritis is the presence of certain HLA-DR alleles. Indeed, patients presenting with joint inflammation post-antibiotic therapy have a higher frequency of HLA-DRB1*0401 (DR4) and related alleles (4–7). Interestingly, theses alleles, which share a sequence in the third hypervariable region of the HLA-DRB1 chain, have also been associated with susceptibility to rheumatoid arthritis (RA) (8). On the contrary, Lyme patients who are able to resolve arthritis within 3 months post-infection show an increased frequency of the HLA-DRB1*1101 (DR11) allele (6, 7, 9). This HLA-DR linkage prompted the hypothesis that antibiotic-refractory Lyme arthritis represents an autoimmune disease, where the inflammatory response is perpetuated by a self-protein after elimination of the causative agent, Bb (6, 10, 11). This notion is supported by the fact that certain HLA-DR alleles are strongly associated with diseases that have an autoimmune basis, such as RA, multiple sclerosis (MS) and insulin-dependent type 1 diabetes mellitus (12, 13).
HLA alleles affect positive and negative selection of immature T cells in the thymus by presenting a range of self-peptides. In addition, upon exposure to foreign antigen, the various HLA alleles present peptides with different affinities to the peripheral mature T cells, thereby determining the type of cellular immune response that is initiated. By analyzing the crystal structure of disease-associated HLA-DR alleles in complex with peptides, it has been shown that the properties of the peptide-binding groove define the selection of peptides presented and, thus, confer susceptibility to disease (8). Structural comparison of HLA-DR alleles associated with risk for, or protection against, type 1 diabetes, RA and MS has revealed that the properties of the P1, P4, P6 and P9 pockets of the HLA-DRB1 allele, such as volume, hydrophobicity and electrostatic charge, constitute the disease-determining factors (8).
In an effort to elucidate the mechanisms of antibiotic-refractory Lyme arthritis manifestation in humans, we recently developed a mouse model of self-perpetuating arthritis upon Bb-infection of HLA-DR4+CD28−/− mice and subsequent eradication of the spirochetes by antibiotic treatment (14). This model was based on the use of CD28−/− mice, which lack most regulatory T cells and which manifest chronic Lyme arthritis upon Bb-infection (14, 15). Persistent inflammation in the joints of the HLA-DR4+CD28−/− mice post-antibiotic treatment required the previous establishment of chronic joint inflammation in the presence of the HLA-DR4 allele. These data provide direct evidence that the HLA-DR4 allele is indispensable for the development of antibiotic-refractory Lyme arthritis.
In the current study, we have analyzed the underlying mechanism by which HLA-DR alleles confer genetic susceptibility or resistance to antibiotic-refractory Lyme arthritis. By generating DR11 transgenic (tg) mice, we were able to directly compare the immune response to Bb-antigens mediated by the DR11 allele, which protects against antibiotic-refractory Lyme arthritis, and the DR4 allele, which predisposes individuals to develop these symptoms. We found that DR11 tg mice mount a vigorous Ab response, but are defective in IFN-γ production. In addition, Bb-infected DR11 tg mice had decreased spirochete burden compared to DR4 tg mice, measured by qPCR of Bb DNA. This is in contrast to DR4 tg mice, which produce an inflammatory response characterized by high level of IFN-γ production, in accordance with our published results (10). Furthermore, the Ab response to Bb-antigens was significantly lower than that of DR11 tg mice, which is consistent with the higher spirochete burden observed in DR4 tg mice after Bb-infection. Thus, our data provide a possible explanation for the differential regulation of the immune response in DR4+ and DR11+ patients upon Bb-infection; namely, HLA-DR4 would predispose individuals to chronic Lyme arthritis by generating an inflammatory milieu to Bb-infection, while HLA-DR11 would exert a protective role through the production of anti-spirochetal Abs.
Material and methods
Mice
DRB1*0401 (DR4) tg mice on a mouse MHC class II−/− B6 background were a gift of T. Forsthuber (Case Western Reserve University, Cleveland) and were bred in our facility. These mice were generated with HLA-DRA-IEα and HLA-DRB1*0401-IEβ chimeric genes (16). The DRB1*1101 (DR11) transgene was generated as follows: the mouse IEβd genomic construct with exon 2 from human DRB1*0401 (a generous gift from Dr. Kouichi Ito (16)), was used as a starting reagent. Exon 2 was then substituted with the intron-flanked exon 2 of DRB1*1101. The DRB1*1101 exon 2 was cloned from PBMC of a DR11 homozygous subject, using a nested PCR strategy. DR11-specific PCR primers, described by Kotsch et al. (17), were used in the first PCR reaction on PBMC genomic DNA template: 5′ DRA: AAT GCC CGG GTA AAG AAA GT, 3′ DRA: GCA GGA AGT GGT GGA GAG AG; 5′ DRB11: CCG GTT AAG GTT CCC AGT G, 3′ DRB11: AAG TCC TTC TGG CTG TTC CA. The second PCR used internal primers, containing an EcoRI site for cloning, and yielded a single product, confirmed to correspond to DRB1*1101 through sequencing. The EcoRI-digested nested PCR product was ligated to the mouse IEβd construct after EcoRI-mediated release of the DRB1*0401 exon. The chimeric IEα/DRA1*0101 (generous gift of Dr. K. Ito) and IEβd/DRB1*1101 constructs were purified with the CsCl method and linearized prior to microinjection into C3H/HeJ embryos at the Tufts Core Transgenic Facility. Positive progeny were screened by chimeric α chain and β chain-specific PCRs and confirmed by immunophenotyping, using anti-DR (L243 clone) mAb. One positive progeny was selected to generate the tg mouse colony, which is kept in heterozygous state. The mice were then backcrossed onto B6×129 mixed MHC class II−/− background for 10 generations and then further backcrossed to pure B6 MHC class II−/− background for another 3 generations. No differences in the immune response against Bb-antigens were detected between mice carrying HLA-DR4 on the B6 or the B6/129 background, implying that the background genes do not contribute to the outcome of the immune response (Supp. Figure 1).
rOspA-immunization of DR11and DR4 tg mice
Six to eight-week-old DR4+/+MHC-II−/− and DR11+/+MHC-II−/− mice were immunized with rOspA (50 μg/mouse), in complete Freund’s adjuvant (CFA) in the footpad. Two-weeks later, the draining popliteal lymph node cells were harvested, and single cell suspensions were prepared at a concentration of 106 cells/ml. Cells were then restimulated in vitro with rOspA (10 μg/ml), as well as with plate-bound anti-CD3 for 72 h, in 96-well tissue culture round bottom plates (Becton Dickinson, Franklin Lakes, NJ). After the incubation period, cells were spun down, and the supernatant was collected and stored at −20° C, until further processing by ELISA.
IFN-γ, IL-17 and IL-4 ELISA
IFN-γ and IL-4 ELISA were performed using a murine IFN-γ and IL-4 ELISA kits (BD Biosciences), per manufacturer’s instructions. To assess IL-17 cytokine production, plates were coated overnight with 3 μg/ml of capture anti-mouse IL-17 Ab (R & D systems, Minneapolis, MN) in PBS and then blocked with 2% BSA, 5% sucrose in PBS at RT for 1h. Recombinant mouse IL-17 (standard curve) and the supernatants from the in vitro restimulation assays were added in duplicates to the ELISA plates and incubated for 45 min at 37° C. Plates were washed and incubated with biotinylated anti-mouse IL-17 (R & D systems) for 1 h at 37° C, followed by another wash and incubation with neutrAvidin-AP for 30 min at RT. Plates were then developed with AP substrate, and were read at 405 nm in a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, CA).
Anti-OspA and anti-Bb ELISA
Flat bottom Immulon 2HB plates (Fisher Scientific, Pittsburgh, PA) were coated overnight with 10 μg/ml of Bb lysate, or 5 μg/ml of rOspA, in coating buffer 0.1M Na2HPO4, pH 9. Uncoated wells served as non-antigen controls. ELISAs were performed as previously described (15).
Bacterial cultures
Low-passage (passage 2) infectious Bb N40 clone D10E9A1-E (kind gift of Jenifer Coburn) (18, 19) were used for all infections. Bb were cultured in complete Barbour-Stoenner-Kelly medium (Sigma, St. Louis, MO) at 34° C until mid-log phase (5×107 Bb/ml) and were counted by darkfield microscopy.
Determination of Bb burden
DNA was extracted from ear punch and ankle tissue, and the Bb burden was determined by real-time qPCR, as previously described (20).
qRT-PCR analysis of mRNA for IFN-γ, IL-17 and Foxp3
Ankles were harvested from the mice and were immediately frozen in liquid nitrogen. Frozen tissue was pulverized using a mortar and pestle pre-cooled in liquid nitrogen. Popliteal lymph nodes were collected, and single cell suspensions were prepared by disrupting the tissue on a cell strainer with the help of the flat side of a 3 ml syringe plunger. The cells were then washed twice in RPMI (Sigma) 10% fetal bovine albumin (FBS), 2 mM L-glutamine and 100 U/ml penicillin/streptomycin medium (1300 rpm for 5 min, in a Sorvall RT7 plus centrifuge). A minimum of 500,000 cells were frozen in RNA stabilization buffer (Qiagen, Valencia, CA) and were stored in −80° C, until further processing for RNA extraction. RNA from pulverized ankles and popliteal lymph node cells was extracted using the RNAeasy Mini kit (Qiagen, Valencia, CA), per manufacturer’s instructions. qRT-PCR was performed, as previously described (20). Primers and FAM-labeled probes specific for mIL-17 (Mm00439619-m1), mINF-γ (Mm00801778-m1) and mFoxp3 (Mm00475156-m1), as well as VIC-labeled probes specific for murine 18S, were purchased (Applied Biosystems, Foster City, CA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (San Diego, CA). All data were tested for Gaussian distribution, using the Shapiro-Will normality test. Quantitative differences between groups were assessed by a two-tailed Student’s t test for normally distributed data, or the Mann-Whitney test for skewed data. The significance level of each correlation was addressed by the P value. Significance was declared at a two-sided 0.05 level for all statistical analyses.
Results
Generation of DR11 tg mice on a murine class II−/− C57BL/6 (B6) background
In order to understand the mechanism regulating the immune response against Bb in the HLA-DRB1*1101 individuals, we have generated DR11 tg mice on a murine class II−/− B6 background, as described in detail in Materials and Methods. A schematic presentation of the chimeric human/mouse DR11/I-Ed construct that was used for the DR11 tg mouse is shown in Figure 1a. The phenotype of the resulting DR11 tg mice was determined by FACS analysis (Fig. 1b). DR11 tg mice have slightly higher class II expression on their B cells than DR4 tg mice. No difference in the basal antibody or cytokine production levels were detected between DR4 tg and DR11 tg mice. In addition, the size and the absolute number of lymph node cells between the two mice were similar, implying that there is no difference in the lymphoid tissue composition (data not shown).
Figure 1. Generation of DR11 tg mice on a murine class II−/− B6 background.
(A) A schematic representation of the α (HLA-DRA-IEα) and β (HLA-DRB1*1101-IEβ) chain of the chimeric HLA-DR11 molecule. The peptide-binding groove is determined by exon 2 of human DR. (B) Phenotype of the DR11 tg mice. HLA-DR and I-Ab surface expression on the B220+ gated B6 (negative control) DR11 and DR4 tg murine lymphocytes was determined by FACS analysis.
Lymph node cells from outer surface protein (Osp)A-immunized DR11 tg mice produce decreased levels of IFN-γ compared to DR4 tg mice
In order to determine whether the T cell immune response generated against Bb-antigens differs between DR11 and DR4 tg mice, we measured their respective cytokine profiles. For this purpose, mice were immunized with rOspA, and two weeks later popliteal draining lymph node cells were harvested and restimulated with rOspA in vitro. Cell supernatants were tested for the presence of pro-inflammatory cytokines, IFN-γ and IL-17, and anti-inflammatory IL-4 by ELISA. Based on current mouse models for Lyme arthritis and clinical data, there is a strong correlation between Lyme arthritis severity and the presence of the IFN-γ (21–27). As seen in Figure 2a (left panel), T cells from DR11 tg mice produced significantly lower levels of IFN-γ than those from DR4 mice (p=0.003). However, this was not due to an intrinsic defect of IFN-γ production, since no difference was observed when anti-CD3 stimulation was used (Fig. 2a, right panel). These results indicate that the decreased IFN-γ secretion observed in DR11 tg mice is antigen-specific; they are particularly intriguing, since a predominance of Th1/IFN-γ producing cells has been associated with increased arthritis severity and the development of antibiotic-refractory Lyme arthritis (25–27). Since DR11 tg mice were backcrossed to the B6 background for 3 generation, it was possible that the observed difference in IFN-γ production was due to the mixed B6/129 background of the DR11 tg mice. In order to exclude this possibility, we generated DR4 tg mice on a B6/129 MHC class II−/− background and compared their immune response to the DR11 tg mice upon OspA immunization. Lymph node cells from OspA immunized DR11 mice still produced decreased levels of IFN-γ production when compared to the DR4 tg on the mixed B6/129 background, implying that background genes do not contribute to the outcome of this particular immune response (Supp. Figure 1).
Figure 2. Lymph node cells from OspA-immunized DR11 tg mice produce decreased levels of IFN-γ compared to DR4 tg mice.
DR11 tg (n=15) and DR4 tg mice (n=15) were immunized with rOspA/CFA in the footpad (50 μg/mouse). (A) IFN-γ and (B) IL-17 cytokine production upon in vitro restimulation of popliteal lymph nodes with rOspA (10 μg/ml) (left panels) and anti-CD3 (right panels), as assessed by ELISA. Asterisk indicates significant difference, *p=0.003; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM and include the pooled results of five independent experiments.
In contrast, no difference in IL-17 production was observed between DR11 and DR4 tg mice (Fig. 2b). These data imply that the DR11 allele negatively influences the development of OspA-specific Th1, but not Th17, responses. The production of IL-4 was also investigated in OspA-immunized DR11 and DR4 tg mice. However, this cytokine was not detectable under the experimental conditions used (data not shown).
OspA-immunized DR11 tg mice develop higher titers of anti-OspA Ab than DR4 tg mice
The observation that OspA-specific T cell immunity was influenced by the presence of a particular HLA-DR allele prompted the question of whether the humoral response would be similarly affected. Therefore, we measured the OspA-specific serum Ab level by ELISA in DR11 and DR4 tg mice two weeks post-immunization with rOspA/CFA. Surprisingly, DR11 tg mice showed significantly higher titers of anti-OspA Abs compared to DR4 tg mice (p=0.0005) (Fig. 3a). To further investigate the anti-OspA humoral response in these mice, we determined the isotype of the OspA-specific Abs. We observed that the most prominent isotype in DR11 tg mice was IgG1, followed by IgG2b and IgG2c. There was no difference in the IgM and IgG3 isotypes between DR11 and DR4 tg mice (Fig. 3b).
Figure 3. OspA-immunized DR11tg mice develop higher titers of anti-OspA Ab.
DR11 tg (n=10) and DR4 tg mice (n=10) were immunized with rOspA/CFA in the footpad (50 μg/mouse). (A) Anti-OspA Ab in the serum two weeks post OspA immunization, as assessed by ELISA. (B) Isotypes of anti-OspA Abs. Asterisks indicate significant difference, *p<0.0005; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM and include the pooled results of four independent experiments.
Bb-infected DR11 tg mice had decreased IFN-γ mRNA expression in the lymph nodes and ankles compared to DR4 tg mice
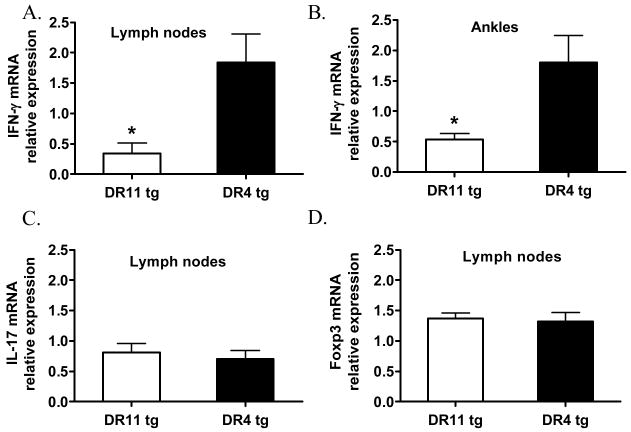
In order to investigate the role of HLA-DR in regulating the immune response against Bb-infection, DR11 and DR4 tg mice were inoculated with 2×104 Bb/mouse. No difference in the development of acute Lyme arthritis was observed in these two strains, as assessed by measures of ankle swelling (data not shown). This was expected, because the acute inflammatory reaction is mainly induced by the innate immune response, and only a minimal adoptive immune response develops in mice of the B6 background. On day 40 post-infection, mice were sacrificed, and RNA was extracted from the draining popliteal lymph nodes and the ankles. mRNA levels of the pro-inflammatory cytokines IFN-γ and IL-17, as well as the Treg-specific transcription factor Foxp3, were assessed by qRT-PCR. As shown in Figure 4a, the popliteal lymph nodes of DR11 tg mice had significantly lower IFN-γ mRNA expression than those of DR4 tg mice (p=0.03). Decreased IFN-γ mRNA expression was also observed in the ankles of Bb-infected DR11 tg mice compared to DR4 tg mice (p=0.04) (Fig. 4b). These results are in accordance with the decrease in IFN-γ production observed in OspA-immunized DR11 tg mice upon OspA-restimulation in vitro, implying that HLA-DR11 regulates the immune response similarly in the Bb-infection system. In order to determine whether the expansion of Th17 cells and Foxp3+ regulatory T cells is differentially influenced by the two HLA-DR alleles in response to Bb-infection, we analyzed IL-17 and Foxp3 mRNA expression in the draining popliteal lymph nodes of these mice. As seen in Figure 4c&d, no differences in IL-17 or Foxp3 transcript levels were observed between the two groups.
Figure 4. Bb-infected DR11 tg mice had decreased IFN-γ mRNA expression in the lymph nodes and ankles.
DR11 (n= 5) and DR4 (n=8) tg mice were infected with 2×104 Bb/mouse. IFN-γ mRNA expression in the lymph nodes (A) and the ankles (B) of Bb-infected DR11 and DR4 tg mice, 40 days post-infection, as assessed by qRT-PCR. (C) IL-17 and (D) Foxp3 mRNA expression in the lymph nodes of DR11 and DR4 tg mice were also assessed by qRT-PCR. Data were normalized by the signal of the 18S housekeeping gene. Asterisks indicate significant difference, *p<0.04; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM and are representative of five independent experiments.
Bb-infected DR11 tg mice have increased anti-Bb Ab and decreased Bb burden compared to DR4 tg mice
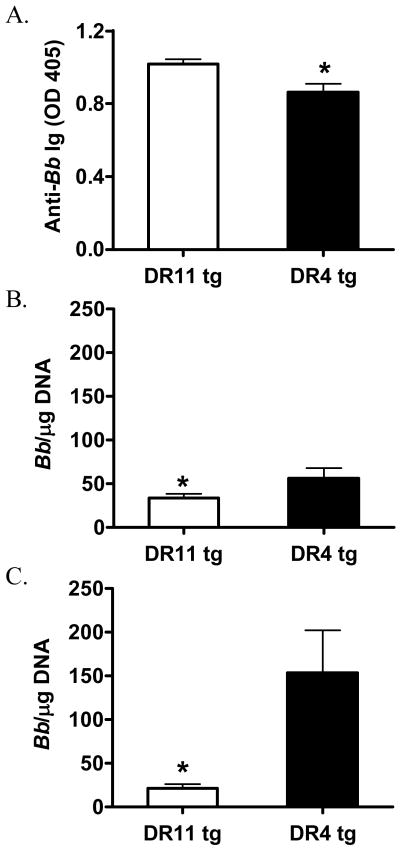
To assess the humoral response in Bb-infected DR11 and DR4 tg mice, we determined anti-Bb Ab levels in serum by ELISA. As shown in Figure 5a, DR11 tg mice had higher anti-Bb Ab titers upon Bb-infection than DR4 tg mice (p=0.01). This observation is in accordance with the increased anti-OspA humoral response observed in the serum of OspA-immunized DR11 tg mice. In order to investigate the effect of HLA-DR4 and DR11 alleles on controlling Bb-infection, we assessed Bb burden. At 40 days post-infection mice were sacrificed, and an ear punch was collected for assessment of Bb systemic levels by qPCR. In addition, we measured the local abundance of Bb DNA in the ankles. As shown in Figure 5b&c, Bb-infected DR11 tg mice had significantly reduced Bb burden, both in the ear punch and in the joints, compared to DR4 tg mice.
Figure 5. Bb-infected DR11 tg mice have increased anti-Bb Ig and decreased Bb burden.
DR11 (n=5) and DR4 (n=8) tg mice were infected with 2×104 Bb/mouse. (A) Anti-OspA Abs in the serum, as measured by ELISA, 40 days post-infection. (B) Ear punch and (C) ankles were collected, DNA was extracted, and the Bb RecA gene was amplified by qPCR. Asterisks indicate significant difference, *p<0.03; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM and are representative of five independent experiments.
Discussion
Numerous studies have established an association of HLA-DR alleles with infectious and autoimmune diseases (12, 13, 28). The various HLA-DR alleles, which are expressed on thymic epithelial cells, as well as on antigen presenting cells, have different peptide-binding requirements. The ability of the HLA-DR alleles to accommodate self- or disease-associated peptides depends on the properties of the respective peptide-binding groove (8). Therefore, the immune response against infectious agents, as well as susceptibility to autoimmune diseases, varies from one individual to another. However, HLA-DR is highly polymorphic, and direct associations with autoimmune diseases have been difficult to make in the presence of other confounding genetic and environmental factors. To circumvent this problem, HLA-DR tg MHC class II−/− mice have been generated, which present an unique opportunity to study how the T-cell repertoire and immune response are affected by a single factor, HLA-DR (29, 30). The use of such in vivo models has been invaluable in understanding the pathogenesis of certain diseases, such as RA, MS and insulin-dependent type 1 diabetes mellitus (30). In this study, we have constructed DR11 tg mice and compared them to DR4 tg mice, in order to identify the mechanism by which HLA-DR determines responsiveness to Bb-antigens and subsequently, disease outcome.
A strong correlation between HLA-DR alleles and disease severity has been documented in Lyme arthritis (4–7). The DR4 allele is the main risk factor for antibiotic-refractory Lyme arthritis, whereas DR11 is associated with antibiotic-responsive Lyme arthritis (6, 7, 9). These clinical observations suggest that CD4+ T cells are directly involved in disease pathogenesis. It has been reported that there is a direct correlation between arthritis severity and the magnitude of the T-cell response against OspA of Bb (31); namely, OspA reactivity is significantly greater in antibiotic-refractory Lyme arthritis patients than in treatment-responsive arthritis patients, and this response is targeted against specific OspA epitopes (31). According to a DR/peptide-binding algorithm designed by Hammer et al., the immunodominant OspA epitope presented by HLA-DR4 is OspA165-173 (32, 33). This was confirmed using DR4 tg mice (10), as well as in in vitro DR/peptide binding assays (9); e.g., the DR4 allele associated with antibiotic-refractory Lyme arthritis binds the OspA165-173 peptide strongly, whereas the DR11 allele, associated with antibiotic-responsiveness, binds weakly to this peptide. The significance of the T-cell response against the OspA165-173 immunodominant epitope in the pathogenesis of chronic Lyme arthritis was accentuated by the observation that OspA165-173-reactive T-cells could be directly isolated from the joint fluid of DR4+ antibiotic treatment-refractory Lyme arthritis patients using MHC-tetramer technology (34).
The data gained from the current study provide a possible mechanism for the differential association of DR4 and DR11 with treatment-refractory Lyme arthritis. We show that, upon OspA-immunization and in vitro restimulation, CD4+ T cells from DR11 tg mice produce decreased levels of IFN-γ compared to DR4 tg mice. Similarly, decreased IFN-γ mRNA expression was observed in the lymph nodes and ankles of DR11 tg mice upon Bb-infection. Therefore, in the presence of HLA-DR11 the CD4+ T cell immune response to Bb-antigens is less inflammatory than in the presence of DR4. It has been reported that Th1 cells are expanded in the synovial fluid of antibiotic-refractory Lyme arthritis patients, which directly correlates with arthritis severity (25). In addition, IFN-γ production in the synovial tissue has been associated with antibiotic-refractory Lyme arthritis (25–27). Using cytometric bead array and flow cytometry techniques, it has recently been shown that, during the period of antibiotic treatment-refractory Lyme arthritis, the synovial fluid of patients had higher levels of Th1 chemoattractants CXCL9 and CXCL10 and pro-inflammatory cytokines IFN-γ, TNF-α and IL-1β, compared to patients who had antibiotic-responsive arthritis (26). This is indicative of a strong pro-inflammatory response that takes place during antibiotic-treatment refractory Lyme arthritis. At a median time of 9 months post-antibiotic therapy, antibiotic-treatment refractory Lyme arthritis patients continued to demonstrated a Th1 pro-inflammatory phenotype, compared to patients that cleared arthritis (26). In addition, in a murine model of antibiotic-refractory Lyme arthritis that we have recently established we observed increased levels of IFN-γ mRNA expression in the joints of the mice with persistent arthritis post-antibiotic treatment, implying that IFN-γ contributes to disease pathogenesis (20).
When the humoral response was compared in DR4 and DR11 tg mice upon OspA-immunization or Bb-infection, we observed that DR11 tg mice developed higher titers of anti-OspA and anti-Bb Abs, respectively. The strong humoral response against Bb-antigens in DR11 tg mice is particularly intriguing in light of the decreased Th1 response observed in these mice. Thus, this finding might provide an explanation why DR11+ Lyme arthritis patients do not present with antibiotic-refractory Lyme arthritis; namely, it is possible that Abs generated in these individuals have strong borreliacidal activity that efficiently controls Bb-infection. It has been reported that IFN-γ can suppress the production of borreliacidal antibody in an in vitro Bb culture system (35). Thus, decreased levels of IFN-γ may favor increased borreliacidal activity in DR11 tg mice, controlling Bb-infection. Interestingly, when we assessed Bb-burden after infection, DR11 tg mice had reduced spirochete levels, both systemically and locally in the joints, compared to Bb-infected DR4 tg mice.
Based on these data, the cellular and humoral immune response against Bb-antigens is differentially regulated by DR4 and DR11 alleles. This pattern has been observed for other inflammatory and autoimmune diseases; e.g., an increased frequency of HLA-DR11 has been documented in thymoma patients with myasthenia gravis, whereas DR4 is negatively associated with the disease (36). In addition, peptides associated with systemic lupus erythematosus bind strongly to HLA-DR4 that is positively associated with the disease, compared to HLA-DR11 (37). Furthermore, in the case of thyroid carcinoma, the DR11 allele is positively associated with disease development, as opposed to the DR4 allele, which is negatively associated (38). Finally, HLA-DR4 confers susceptibility to RA, MS, type 1 diabetes and Crohn’s disease (39), while no such associations with HLA-DR11 has been documented thus far.
The fact that DR4 and DR11 alleles are not genetically linked to the same diseases is partially due to their differential peptide-binding groove. Each allele has different peptide binding specificity, which determines the array of the presented peptides and subsequently, the immune response that will take place. Indeed, when HLA-DR4 and DR11 are compared, they differ in two amino acids at position 67 and 71 of the HLA-DRB1 chain, which contributes to the P4 pocket. The DR4 allele has Leu67B1 and Lys71B1, whereas DR11 has Phe67B1 and Arg71B1 (8, 40, 41). The binding properties of the P4 pocket of DR4 have been specifically associated with susceptibility to RA, and even a single Lys to Glu exchange at position 71 of the HLA-DRB1 chain alters the peptide binding specificity and subsequently, the disease association (41). In addition, it has been reported that amino acid substitution at position 67, 71 and 86 of the DR11 chain alters its peptide binding specificity and influences the DR-peptide interactions (40). Thus, we would like to propose that the differential peptide-binding properties of the P4 pocket in HLA-DR4 and DR11 account for the differential susceptibility to antibiotic-refractory Lyme arthritis.
In this study, we have shown in a murine model that in the presence of DR11 the immune response against Bb-antigens is directed towards a protective Ab response that is reflected in lower Bb burden after infection with the spirochete. On the contrary, in the presence of DR4, Th1 cells dominate the immune response against Bb-antigens, providing a pro-inflammatory environment, but not affecting the spirochete burden. Thus, our data may unravel a mechanism for the positive and negative association of HLA-DR4 and DR11, respectively, with antibiotic-refractory Lyme arthritis. It is likely that the excessive inflammation induced in the synovium of DR4+ patients upon Bb-infection predisposes to autoimmune phenomena, which can be attributed to either cross-reactivity due to molecular mimicry and/or bystander activation of autoreactive cells. Indeed, we have demonstrated that chronic joint inflammation constitutes a prerequisite for the development of antibiotic-refractory Lyme arthritis (20). The data from our recently established murine model for antibiotic-refractory Lyme arthritis directly indicate that the DR4 allele is indispensable for the development of this disease. Furthermore, our preliminary data indicate that Bb-infected DR11+CD28−/− B6 do not develop antibiotic-refractory Lyme arthritis, in agreement with our working hypothesis (Supp. Figure 2). Therefore, our animal model complements the clinical data demonstrating that there is genetic predisposition to the development of antibiotic-refractory Lyme arthritis and identifies, for the first time, the underlying mechanism.
Supplementary Material
Lymph node cells from OspA-immunized DR11 tg mice produce decreased levels of IFN-γ compared to DR4 tg mice on a B6/129 background. DR4 tg mice on a B6/129 mixed MHC class II−/− background were generated, and their INF-γ response was compared to that of DR11 tg mice upon OspA immunization. DR11tg (n=3) and DR4tg mice on the B6/129 background (n=3) were immunized with rOspA/CFA in the footpad (50 μg/mouse). IFN-γ and production upon in vitro restimulation of popliteal lymph nodes with rOspA (10 μg/ml) (A) and anti-CD3 (B), as assessed by ELISA. Asterisk indicates significant difference, *p=0.01; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM.
Bb-infected DR11+CD28−/− mice do not develop antibiotic-refractory Lyme arthritis. DR11+CD28−/− mice (n=5) and CD28−/− mice (n=5) were infected with 2×104 Bb/mouse, and arthritis was assessed by measuring the ankles. On day 75 post-infection mice were treated with antibiotics and were monitored for 50 days post-antibiotic therapy. Both DR11+CD28−/− and CD28−/− mice resolved inflammation post-antibiotic treatment. Asterisks indicate significant difference, in ankle swelling compared to the mock-infected mice, *p<0.05; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM.
Acknowledgments
We are grateful to Dr. Jenifer Coburn for the gift of B. burgdorferi. We would like to thank Lin Miao for technical help. The authors have no conflicting financial interests. This work was funded by NIH RO1 grant AR045386.
References
- 1.Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH, 3rd, Liu NY, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–88. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 2.Carlson D, Hernandez J, Bloom BJ, Coburn J, Aversa JM, Steere AC. Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 1999;42:2705–9. doi: 10.1002/1529-0131(199912)42:12<2705::AID-ANR29>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 4.Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–9. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalish RA, Leong JM, Steere AC. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–35. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–52. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–71. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones EY, Fugger L, Strominger JL, Siebold C. MHC class II proteins and disease: a structural perspective. Nat Rev Immunol. 2006;6:271–82. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- 9.Steere AC, Falk B, Drouin EE, Baxter-Lowe LA, Hammer J, Nepom GT. Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 2003;48:534–40. doi: 10.1002/art.10772. [DOI] [PubMed] [Google Scholar]
- 10.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–6. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 11.Guerau-de-Arellano M, Huber BT. Development of autoimmunity in Lyme arthritis. Curr Opin Rheumatol. 2002;14:388–93. doi: 10.1097/00002281-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 13.Ridgway WM, Fathman CG. MHC structure and autoimmune T cell repertoire development. Curr Opin Immunol. 1999;11:638–42. doi: 10.1016/s0952-7915(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Iliopoulou BP, Huber BT. Emergence of Chronic Lyme Arthritis: Putting the Breaks on CD28 Costimulation. Immunopharmacol Immunotoxicol. 2008:1–12. doi: 10.1080/08923970802391459. [DOI] [PubMed] [Google Scholar]
- 15.Iliopoulou BP, Alroy J, Huber BT. CD28 deficiency exacerbates joint inflammation upon Borrelia burgdorferi infection, resulting in the development of chronic Lyme arthritis. J Immunol. 2007 doi: 10.4049/jimmunol.179.12.8076. In press. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med. 1996;183:2635–44. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotsch K, Wehling J, Blasczyk R. Sequencing of HLA class II genes based on the conserved diversity of the non-coding regions: sequencing based typing of HLA-DRB genes. Tissue Antigens. 1999;53:486–97. doi: 10.1034/j.1399-0039.1999.530505.x. [DOI] [PubMed] [Google Scholar]
- 18.Coburn J, Barthold SW, Leong JM. Diverse Lyme disease spirochetes bind integrin alpha IIb beta 3 on human platelets. Infect Immun. 1994;62:5559–67. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn J, Leong JM, Erban JK. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci U S A. 1993;90:7059–63. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliopoulou BP, Alroy J, Huber BT. Persistent arthritis in Borrelia burgdorferi-infected HLA-DR4-positive CD28-negative mice post-antibiotic treatment. Arthritis Rheum. 2008;58:3892–901. doi: 10.1002/art.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keane-Myers A, Nickell SP. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–8. [PubMed] [Google Scholar]
- 22.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matyniak JE, Reiner SL. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–4. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crandall H, Dunn DM, Ma Y, Wooten RM, Zachary JF, Weis JH, et al. Gene expression profiling reveals unique pathways associated with differential severity of lyme arthritis. J Immunol. 2006;177:7930–42. doi: 10.4049/jimmunol.177.11.7930. [DOI] [PubMed] [Google Scholar]
- 25.Gross DM, Steere AC, Huber BT. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–8. [PubMed] [Google Scholar]
- 26.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 27.Yssel H, Shanafelt MC, Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves C, Souza T, Meyer I, Toralles MB, Brites C. Immunogenetics and infectious diseases: special reference to the mayor histocompatibility complex. Braz J Infect Dis. 2006;10:122–31. doi: 10.1590/s1413-86702006000200010. [DOI] [PubMed] [Google Scholar]
- 29.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory diseases. Adv Immunol. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 30.Abraham RS, David CS. Identification of HLA-class-II-restricted epitopes of autoantigens in transgenic mice. Curr Opin Immunol. 2000;12:122–9. doi: 10.1016/s0952-7915(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, Steere AC. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–22. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Hammer J, Bono E, Gallazzi F, Belunis C, Nagy Z, Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J Exp Med. 1994;180:2353–8. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Searching for borrelial T cell epitopes associated with antibiotic-refractory Lyme arthritis. Mol Immunol. 2008;45:2323–32. doi: 10.1016/j.molimm.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer AL, Trollmo C, Crawford F, Marrack P, Steere AC, Huber BT, et al. Direct enumeration of Borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proc Natl Acad Sci U S A. 2000;97:11433–8. doi: 10.1073/pnas.190335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munson EL, Du Chateau BK, Jensen JR, Callister SM, DeCoster DJ, Schell RF. Gamma interferon inhibits production of Anti-OspA borreliacidal antibody in vitro. Clin Diagn Lab Immunol. 2002;9:1095–101. doi: 10.1128/CDLI.9.5.1095-1101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Ramos G, Tellez-Zenteno JF, Zapata-Zuniga M, Yamamoto-Furusho JK, Ruiz-Morales JA, Villarreal-Garza C, et al. HLA class II genotypes in Mexican Mestizo patients with myasthenia gravis. Eur J Neurol. 2003;10:707–10. doi: 10.1046/j.1468-1331.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 37.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur J Immunol. 2003;33:287–96. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 38.Juhasz F, Kozma L, Stenszky V, Gyory F, Luckas G, Farid NR. Well differentiated thyroid carcinoma is associated with human lymphocyte antigen D-related 11 in Eastern Hungarians: a case of changing circumstances. Cancer. 2005;104:1603–8. doi: 10.1002/cncr.21382. [DOI] [PubMed] [Google Scholar]
- 39.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4:e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeliszewski D, Golvano JJ, Gaudebout P, Dorval I, Freidel C, Gebuhrer L, et al. Implication of HLA-DR residues at positions 67, 71, and 86 in interaction between HLA-DR11 and peptide HA306-320. J Immunol. 1993;151:6237–47. [PubMed] [Google Scholar]
- 41.Hammer J, Gallazzi F, Bono E, Karr RW, Guenot J, Valsasnini P, et al. Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med. 1995;181:1847–55. doi: 10.1084/jem.181.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lymph node cells from OspA-immunized DR11 tg mice produce decreased levels of IFN-γ compared to DR4 tg mice on a B6/129 background. DR4 tg mice on a B6/129 mixed MHC class II−/− background were generated, and their INF-γ response was compared to that of DR11 tg mice upon OspA immunization. DR11tg (n=3) and DR4tg mice on the B6/129 background (n=3) were immunized with rOspA/CFA in the footpad (50 μg/mouse). IFN-γ and production upon in vitro restimulation of popliteal lymph nodes with rOspA (10 μg/ml) (A) and anti-CD3 (B), as assessed by ELISA. Asterisk indicates significant difference, *p=0.01; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM.
Bb-infected DR11+CD28−/− mice do not develop antibiotic-refractory Lyme arthritis. DR11+CD28−/− mice (n=5) and CD28−/− mice (n=5) were infected with 2×104 Bb/mouse, and arthritis was assessed by measuring the ankles. On day 75 post-infection mice were treated with antibiotics and were monitored for 50 days post-antibiotic therapy. Both DR11+CD28−/− and CD28−/− mice resolved inflammation post-antibiotic treatment. Asterisks indicate significant difference, in ankle swelling compared to the mock-infected mice, *p<0.05; Student’s two-tailed, unpaired t test. Data are shown as mean ± SEM.