Abstract
Objective
We utilized the amyloid imaging ligand Pittsburgh Compound-B (PiB) to determine the presence of AD pathology in different MCI subtypes and to relate elevated PiB binding to other markers of early AD and longitudinal outcome.
Methods
Twenty-six patients with MCI – 13 single domain amnestic-MCI (sd a-MCI), 6 multiple domain amnestic-MCI (md a-MCI), and 7 non-amnestic MCI (na-MCI) – underwent PiB imaging. Twenty-three had clinical follow-up [21.2 ± 16.0 (SD) months] subsequent to their PiB scan.
Results
Using cutoffs established from a control cohort, 14 (54%) had elevated levels of PiB retention and were considered “amyloid-positive.” All subtypes were associated with a significant proportion of amyloid-positive patients (6/13 sd a-MCI, 5/6 md a-MCI, 3/7 na-MCI). There were no obvious differences in the distribution of PiB retention in the na-MCI group despite their atypical early AD phenotype. Predictors of conversion to clinical AD in a-MCI, including poorer episodic memory, increased age, and medial temporal atrophy, were found in the amyloid-positive relative to amyloid-negative a-MCI patients. Longitudinal follow-up revealed 5/13 amyloid-positive patients, but 0/10 amyloid-negative patients, converted to clinical AD. Further, 3/10 amyloid-negative patients “reverted to normal” on follow-up.
Interpretation
These data support the notion that amyloid-positive patients are likely to have early AD and that the use of amyloid imaging may have an important role in determining which patients are likely to benefit from disease-specific therapies. In addition, our data is consistent with longitudinal studies suggesting that a significant percentage of all MCI subtypes will develop clinical AD.
Introduction
Mild Cognitive Impairment (MCI) is an evolving diagnostic construct which is usually defined by the presence of subjective and/or objective cognitive complaints in elderly subjects which are not severe enough to merit the designation of dementia. As such, it is often conceptualized as a transitional stage between healthy aging and dementia.1, 2 In a number of longitudinal studies in specialty clinics this designation carries a high risk for the development of dementia with conversion rates in the range of 5-15%/year.3-7 The majority of these patients develop clinical Alzheimer’s disease (AD). Further, pathologic studies of patients with MCI have reported varying degrees of AD pathology.3, 8-11 Thus, commonly used criteria for MCI produce a population enriched in patients with early clinical AD or “prodromal AD.”
However, not all patients with MCI convert to clinical AD over periods of 5 years or longer.4, 12 Conversion rates vary greatly depending on the criteria applied and the nature of the subject population and clinical setting. For example, epidemiologic studies report much lower rates of development of clinical AD and dementia relative to specialty clinics.13-15 Epidemiologic studies also report relatively frequent reversion to normal cognition on follow-up (>40% in one study 14). This suggests instability in the diagnostic construct. Even in those who do develop dementia, not all have underlying AD pathology at autopsy. For example, Jicha et al. reported a pathological diagnosis of AD in only 71% of 34 MCI cases 16. More importantly, Jicha et al. could identify no demographic variables nor cognitive measures predictive of who would develop the neuropathologic features of AD. Given this heterogeneity it is not surprising that common criteria for MCI have limited specificity in the detection of early AD.17
To enhance specificity, a number of biomarkers have been evaluated as potential predictors of conversion to AD in MCI populations. Characteristics that make patients more “AD-like” have been associated with a higher rate of development of clinical AD. For example, poorer performance on delayed recall memory tasks predicted a higher rate of conversion to AD.18, 19 Other predictors included hippocampal atrophy,20, 21 flourodeoxyglucose (FDG)-positron emission tomography [PET; 22, 23], low cerebrospinal fluid (CSF) amyloid-beta (Aβ) and elevated tau,24 and the presence of the apolipoprotein ε4 (APOE ε4) allele.25 The relative sensitivity and specificity of these predictors have varied across studies and the independent contribution of each to the presence of prodromal AD remains largely unresolved.
The majority of the studies above have involved diagnostic criteria for MCI which include memory impairment as a central feature. More recent formulations have subdivided this construct along the orthogonal dimensions of memory impairment (amnestic vs. non-amnestic) and number of cognitive domains involved [single vs. multiple; 1, 2]. The amnestic-MCI (a-MCI) subtypes, both single and multiple domain, are thought to be the most likely to develop into clinical AD while the non-amnestic-MCI (na-MCI) subtypes are often conceptualized as the prodromal phase for other etiologies of dementia (e.g. primary progressive aphasia for single domain language impairment).1 While this formulation has intuitive appeal, there has been limited work in the longitudinal study of these different classes of MCI to evaluate their etiologic specificity.26-29 The work thus far suggests that a percentage of patients in all subtypes develop clinical AD.
Given the inherent heterogeneity of clinical MCI, more specific biomarkers are needed for detection of underlying AD pathology. Amyloid imaging provides an in vivo quantitative estimate of Aβ amyloid pathology that may serve this role.30-32 The PET ligand N-methyl [11C] 2-(4’-methylaminophenyl)-6-hydroxybenzothiazole, or Pittsburgh Compound-B (PiB), has been the most widely studied and promising agent to date. PiB is a thioflavin-T derivative that binds to fibrillar amyloid.33 PiB is retained in patients with AD in a pattern consistent with pathological descriptions of amyloid plaque distribution.30, 34-36 Recently, Ikonomovic and colleagues reported a high correlation of regional in vivo PiB binding and plaque load in a patient who was autopsied 10 months after PiB PET imaging.33 There have been relatively few studies of MCI with PiB and even fewer that have examined MCI in the context of classification of its different subtypes.37-42
There were three central aims of this study. First, we aimed to extend the current literature on amyloid imaging in MCI with specific attention to the prevalence of elevated PiB retention in both amnestic and non-amnestic subtypes. Only one prior study clearly described PiB imaging in 6 patients with na-MCI and reported that all were in the control range.40 Second, we examined the relationship of psychometric and structural imaging characteristics with PiB binding. We hypothesized that patients who have elevated PiB uptake represent those with MCI who will likely convert to clinical AD. We expected this group to have features predictive of conversion in longitudinal studies. These studies have largely been performed in patients with a-MCI; so we focused on this population for the analysis. Finally, we have followed the majority of patients for an average of two years after their PiB scan and had the opportunity to examine the relationship of PiB binding with outcome (e.g. conversion to dementia).
Methods
Participants
Twenty-six patients with MCI [mean 70.2 ± 8.8 (SD) years; mean education 17.2 ± 3.2 (SD) years; 7 female] were recruited from the University of Pittsburgh’s Alzheimer’s Disease Research Center (ADRC). Note that PiB data for 9 of these patients were previously described.43 As part of their participation in the ADRC, each patient underwent an extensive evaluation, including medical and neurological history and examination, a semi-structured psychiatric evaluation, and psychometric testing. Blood and brain imaging studies were performed for each participant. Clinical diagnosis was made by consensus at a conference attended by neurologists, psychiatrists, and neuropsychologists experienced in the diagnosis of dementia (see 44). The evaluation was repeated annually. ADRC inclusion criteria include age ≥40 years, proficient English speaker, and a reliable caregiver. Exclusions include a lifetime history of schizophrenia, manic-depressive disorder, or schizoaffective disorder, prior electroconvulsive therapy, current, or within 2 years of symptom onset, substance abuse/dependence, and any medical condition that could affect neuropsychological testing.
Diagnosis of MCI was made essentially following the recommendations of Petersen and others 1, 2. Since 2000 the University of Pittsburgh ADRC has classified patients as MCI-amnestic type and MCI-multiple cognitive domain type. The former corresponds to single-domain a-MCI (sd a-MCI). The latter includes all other MCI subtypes. The MCI-multiple cognitive domain cases were reviewed and subdivided into md a-MCI, sd na-MCI, and md na-MCI groups according to the scheme described by Gauthier et al.45 Strict cut-offs on psychometric testing were not used, but generally impairment was below 1.5 SD’s of our University of Pittsburgh ADRC age-adjusted norms. The clinicians were aware of the previous years’ diagnosis at follow-up consensus discussions.
Thirty-five healthy elderly subjects [mean 72.1 ± 7.8 (SD) years; mean education 15.6 ± 2.8 (SD) years; 23 female] served as control group for the structural MRI analyses. This group was recruited from both the community and our population of healthy controls in the ADRC. All subjects underwent a screening process which included psychometric testing and were excluded if they met criteria for dementia or MCI. Additional description of this cohort is presented elsewhere.46 Importantly, the subjects within this reference group were chosen based on their being free of amyloid pathology as detected by PiB. A comparison group of Twenty-Two clinically probable mild AD subjects [mean 70.1 ± 9.6 (SD) years; mean MMSE 22.2 ± 3.3 (SD)] also was included. PiB data for 27 of the controls and 12 of the patients with AD have been described previously.46, 47 All participants and their caregivers (when appropriate) provided informed consent and the study was approved by the University of Pittsburgh’s Institutional Review Board.
Psychometrics
MCI participants underwent the standard psychometric battery of the ADRC assessing attention, memory, language, visual-spatial construction, and executive functions (see 44). The core tests for the ADRC have evolved during the course of the study and some participants had additional psychometric tests available for consensus conference discussion. Some control participants were evaluated outside the ADRC, but had a similar psychometric battery and were adjudicated by neuropsychologists from the ADRC as previously described.46
MRI imaging
MR imaging was performed using a 1.5 Tesla G.E. Signa scanner (University of Pittsburgh MR Research Center). Subjects were positioned in a standard head coil and a brief scout T1-weighted image was obtained. A volumetric spoiled gradient recalled (SPGR) sequence was used to acquire high resolution T1-weighted images optimized for contrast among gray matter, white matter, and CSF (TE/TR = 5/25, flip angle = 40 degree). Images were acquired in the coronal plan, with 1 × 1 mm in plane resolution and 1.5 mm slice thickness/0 mm interslice. A total of 120 continuous slices were acquired.
Full-resolution skull-cropped 1.5T MR images were reoriented along the AC-PC line and the medial longitudinal fissure. These images were normalized to the ICBM 152 template (Montreal Neurological Institute) and tissue priors using the unified segmentation technique of the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/).48 The resulting modulated gray matter images were smoothed with an 8-mm FWHM Gaussian kernel. ICV measurements, in cubic milliliters, were calculated by summing the voxels of the native segmentations with the get_totals script (http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m, Ged Ridgeway).
Hippocampal volumes were calculated using the automated labeling pathway (ALP), an atlas-based segmentation technique using a fully deformable registration approach to measure pre-defined regions of interest.49, 50 ALP and related fully deformable registration techniques for hippocampal measurement have been successfully applied to patients with AD and MCI and yielded high agreement with manual tracings.51 Anatomic regions of interest (ROIs) were from the AAL atlas.52 Hippocampal ROIs defined on the reference brain (MNI colin27), were transformed to fit each individuals anatomic image in a fully deformable manner. Hippocampal ROIs were then segmented into gray, white, and cerebrospinal fluid tissue types and voxel counts of gray matter were automatically obtained.53
PET imaging
Details of PiB PET data acquisition were previously described.43 PET imaging was conducted on a Siemens/CTI ECAT HR+ (3D mode, 15.2 cm field-of-view, 63 planes, reconstructed image resolution ~6 mm). To reduce the contribution of scattered photons, the scanner was equipped with Neuro-insert (CTI PET Systems, Knoxville, TN, USA). Data were reconstructed using filtered back-projection (Fourier rebinning/2D back projection, 3 mm Hann filter) and corrected for photon attenuation (68Ge/68Ga rods), scatter,54 and radioactive decay. The subject’s head was immobilized to minimize head motion during the scan. PiB was injected intravenously (14.3±2.2 mCi, over 20 s, specific activity=1.4 ± 0.8 Ci/μmol), and dynamic PET scanning was performed over 90 min (34 time frames). Scanning was performed within ~6 months of ADRC evaluation [mean: 14.9 ± 7.3 (SD) weeks].
ROI’s were defined on a coregistered MR image as previously described 42; these included frontal, anterior cingulate, precuneus, lateral temporal, parietal, medial temporal, occipital, and sensorimotor cortices, anterior ventral striatum, subcortical white matter, and pons. A cerebellar ROI served as reference region and the pons and subcortical white matter ROIs were measures of non-specific ligand retention. PiB retention was assessed using Logan graphical analysis with the cerebellar ROI as input using 90 minutes of data.43 Distribution volume ratios (DVR=VT/VND) were generated using the cerebellum as reference for each ROI, reflecting the concentration of binding in that volume relative to the concentration in the cerebellum. Two patients with MCI did not complete the entire protocol. For these two participants, standardized uptake values (SUV) were calculated based on the summed regional radioactivity ratio in a late time interval (40-60 minutes). These ROIs were also referenced to the cerebellum to calculate SUVRs. Prior work has demonstrated that SUVRs calculated in this manner produce results similar to analysis with DVRs.43
In addition to the ROI analyses, PiB DVR images were processed with SPM5. After MR segmentation as described above, the images were prepared by applying a positive mask, co-registering to the corresponding MR, and transforming them to template space using the factors created during segmentation. Mean PiB DVR images were determined using the arithmetic mean of each voxel.
Data analysis
We used objective criteria to define amyloid-positivity as previously described.46 These criteria were derived from seven ROIs based on their common association with amyloid deposition in AD. These regions included frontal, anterior cingulate, precuneus, parietal, lateral temporal, and lateral occipital cortices and the occipital pole. To avoid a strict cutoff, we defined an intermediate range 2.5% above and below this cutoff. Any participant who had a PiB DVR value that exceeded the intermediate range in any of these regions was defined as amyloid-positive and those who fell under the intermediate range in all seven ROIs were defined as amyloid-negative. Note that this same process was performed using SUVRs to determine cutoffs for the two MCI patients who did not have DVR values.
After individuals were classified as amyloid-positive or amyloid-negative, additional statistical tests were done to test hypotheses related to the association of PiB binding with demographic, psychometric, and structural imaging characteristics. Wilcoxon Rank Sum tests with exact significance were used for two sample comparisons of psychometric and demographic data. ANCOVA was conducted for comparison of hippocampal volumes. These models included age, gender, and ICV. Similarly, patient and control populations were compared in SPM5 using an ANCOVA model with age, gender, and ICV as covariates.
Results
MCI Subtype Characterization
A total of 19 patients with MCI were classified as a-MCI. Thirteen had sd a-MCI and 6 had md a-MCI. There were 7 patients with na-MCI, 6 of whom had a single domain of involvement (4 language and 2 executive). One patient with na-MCI was best categorized as multiple domains due to both language and executive function impairment. Demographic and psychometric data for the a-MCI and na-MCI subtypes together with a normative sample of healthy ADRC controls are in Table 1. Age-adjusted psychometric test performance from this normative group is used in the adjudication process of the ADRC.
Table 1.
Mean demographic and psychometric data for a-MCI and na-MCI groups, collapsed over single- and multiple-domain subtypes. Standard deviations are in parentheses.
| a-MCI (n=19) | na-MCI (n=7) | Norms (n=249) | |
|---|---|---|---|
| Age | 71.6 (8.0) | 69.6 (9.2) | 71.9 (8.1) |
| Education | 16.6(3.3) | 18.7 (2.6) | 15.1 (3.1) |
| Sex (% male) | 84% | 43% | 37% |
| MMSE | 27.2 (2.1) | 27.7 (1.1) | 28.6 (1.4) |
| Boston | 27.4 (2.6) | 23.6 (3.6) | 28.0 (2.1) |
| FAS | 39.3 (8.9) | 35.5 (6.9) | 43.5 (13.1) |
| Category (animals) | 18.1 (4.9) | 14.9 (5.8) | 19.0 (5.1) |
| Rey Copy | 21.2 (2.0) | 21.7 (2.1) | 21.7 (1.9) |
| Rey Immediate | 14.5 (4.5) | 19.1 (3.4) | 19.0 (3.6) |
| Rey Delay | 12.7 (6.7) | 19.6 (2.7) | 18.5 (3.5) |
| Rey Retention | 82.3 % (32.4) | 104.2% (11.9) | 88.1 (14.9) |
| CERAD Immediate | 17.8 (3.4) | 21.3 (3.3) | 22.4 (3.8) |
| CERAD Delay | 2.5 (1.7) | 7.0 (1.3) | 7.4 (2.1) |
| Digit Span Forwards | 6.8 (1.0) | 6.1 (0.9) | 6.7 (1.4) |
| Digit Span Backwards | 4.6 (1.0) | 4.6 (1.0) | 5.2 (1.4) |
| Trails A (sec) | 36.5 (15.7) | 32.7 (9.4) | 40.5 (13.2) |
| Trails B (sec) | 84.8 (33.2) | 81.9 (20.0) | 87.0 (31.7) |
Both MCI groups were highly-educated and of similar age. By definition, patients with a-MCI demonstrated poor performance on verbal (CERAD word list 55) and visuospatial (Rey immediate and delayed recall 56) memory tasks. Importantly, the na-MCI patients exhibited no evidence of memory impairment. Although this is a heterogeneous group, performance was weakest for the na-MCI patients on tests of language [Boston Naming Test 57, category fluency 58, and Controlled Oral Word Association Test (COWAT) 59] due to the fact that the majority had significant language impairment.
PiB results
Levels of PiB retention were calculated for 11 ROIs (see Figure 1 for frontal and subcortical white matter ROIs). Visual inspection of the quantitative data reveals a bimodal distribution of PiB binding for patients with MCI. One group is clearly within the normal range while the other is, for most part, at the level of patients with AD. This is particularly notable for regions known to have early PiB binding, such as frontal cortex.
Figure 1.

PiB retention in controls (red triangles), MCI patients (black circles) and patients with AD (blue squares). Non-amnestic MCI patients are indicated with filled black circles. The white bars represent cutoffs for determining amyloid status in frontal cortex and subcortical white matter. Note that the two MCI patients who only had SUVRs calculated are not included.
Applying our cutoffs, 14 (54%) MCI patients were amyloid-positive and 11 (42%) were amyloid-negative. One patient was in the intermediate range. In the a-MCI group, 11 (58%) were amyloid-positive and the rest were negative. This group was further divided into single- and multiple-domain. Six (46%) patients with sd a-MCI were amyloid-positive while 5 (83%) patients with md a-MCI were amyloid-positive. In the na-MCI group, 3 (43%) were amyloid-positive and 3 were amyloid-negative. One patient was intermediate.
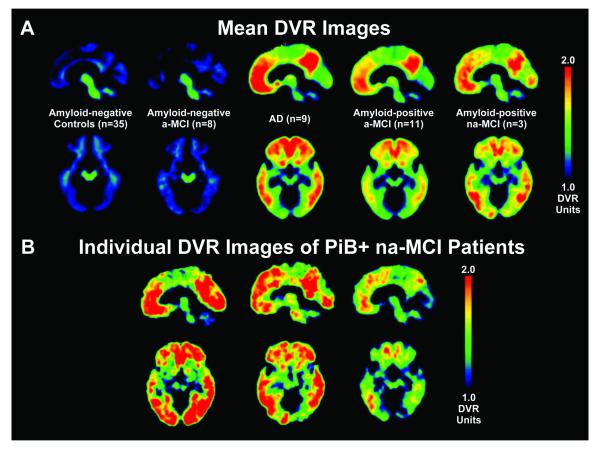
In addition to determination by the quantitative method, both the control group and the patients with a-MCI who were amyloid-negative exhibited no evidence of PiB retention based on mean DVR images (see Figure 2). Note again that the control group was selected on the basis of their amyloid-negative status. The patients with AD and the amyloid-positive patients with a-MCI clearly had increased PiB uptake. In both cases, the distribution of binding was greatest in the frontal, anterior cingulate, precuneus, and lateral temporoparietal cortices, as described in prior work.30, 41 The magnitude of binding also was similar, reflecting the quantitative data (Figure 1). Strikingly, the na-MCI amyloid-positive patients had a distribution of PiB binding similar to that of the a-MCI patients despite their different cognitive phenotype.
Figure 2.
(a) Mean DVR images of amyloid-negative controls, amyloid-negative patients with a-MCI, representative group of patients with AD, amyloid-positive patients with a-MCI, and amyloid-positive patients with na-MCI. (b) Individual DVR images of the three amyloid-positive patients with na-MCI.
Comparison of MCI groups by PiB binding
Wilcoxon Rank sum tests were used to analyze the demographic and psychometric data of the MCI groups (see Table 2). When collapsed across single- and multiple-domain subtypes, there are several important differences between the amyloid-positive and amyloid-negative a-MCI patients. Amyloid-positive patients were older (p < 0.01) and more educated (p < 0.05). Verbal delayed recall was significantly worse in the amyloid-positive group (p < 0.01). Other tests that approached significance included the visual delayed recall task (p = 0.079), Trails A (p = 0.065), and Trails B (p = 0.051). In each case, the amyloid-positive group performed more poorly than the amyloid-negative group.
Table 2.
Mean demographic and psychometric data for a-MCI and na-MCI groups, collapsed over single- and multiple-domain subtypes, based on amyloid status (amyloid-positive vs. amyloid-negative). Standard deviations are in parantheses.
| a-MCI amyloid-pos. (n=11) |
a-MCI amyloid-neg. (n=8) |
na-MCI amyloid-pos. (n=3) |
na-MCI amyloid-neg. (n=3) |
|
|---|---|---|---|---|
| Age | 74.7 (6.9) | 62.6 (7.3) * | 71.7 (4.9) | 70.0 (12.5) |
| Education | 17.9 (3.3) | 14.8 (2.6) * | 17.3 (1.5) | 20.0 (3.5) |
| Sex | 1 F; 10 M | 2 F; 6 M | 3 F; 0 M | 1 F; 2 M |
| MMSE | 26.7 (2.6) | 27.9 (1.0) | 27.3 (0.6) | 27.7 (1.5) |
| APOE ε4 Carriers (%) | 9 (90%) | 4 (50%) | 0 (0%) | 1 (33%) |
| Boston | 27.1 (2.8) | 27.8 (2.5) | 22.7 (1.2) | 22.7 (4.6) |
| FAS | 37.0 (7.5) | 42.4 (10.1) | 36.0 (9.8) | 35.0 (4.6) |
| Category (animals) | 16.7 (4.9) | 19.9 (4.6) | 15.7 (6.5) | 15.0 (7.2) |
| Rey Copy | 21.6 (2.0) | 20.8 (1.9) | 21.3 (2.3) | 23.0 (1.0) |
| Rey Immediate | 13.3 (4.6) | 16.1 (4.1) | 19.2 (2.4) | 20.7 (3.5) |
| Rey Delay | 10.5 (7.1) | 15.8 (4.9) | 18.8 (1.9) | 21.0 (3.6) |
| Rey Retention | 70.7% (35.8) | 98.2% (19.1) | 98.7 (8.1) | 101.6 (2.7) |
| CERAD Immediate | 17.4 (3.5) | 18.5 (3.4) | 22.3 (3.5) | 20.3 (4.0) |
| CERAD Delay | 1.6 (1.4) | 3.6 (1.4) * | 7.7 (1.2) | 7.0 (1.0) |
| CERAD Disc | 7.5 (2.0) | 8.1 (2.0) | 10.0 (0.0) | 10.0 (0.0) |
| Digit Span Forwards | 6.7 (0.9) | 7.0 (1.1) | 6.0 (1.0) | 6.7 (0.6) |
| Digit Span Backwards | 4.8 (0.8) | 4.3 (1.2) | 4.0 (1.0) | 5.0 (1.0) |
| Trails A (sec) | 42.3 (17.7) | 28.6 (7.7) | 3.3 (14.0) | 35.3 (3.1) |
| Trails B (sec) | 97.3 (37.3) | 67.8 (16.4) | 68.0 (21.7) | 91.3 (15.0) |
Note: = p < 0.05
Given the small numbers of patients in the na-MCI groups (3 each), inference from the psychometric data is limited. In general, there were no obvious differences based on PiB retention. While by definition patients with na-MCI should not have impairment of memory, it is possible that those who were amyloid-positive may be on the lower end of the normal range of memory function. This was not observed and both groups performed essentially equivalently on tests of memory function and exhibited no evidence of even subtle impairment.
We also examined whether the duration of cognitive symptoms was related to amyloid status. There was no difference in duration of symptoms for amyloid-positive versus amyloid-negative MCI patients (4.5 vs. 3.9 years, respectively; t(23) < 1, p > .5). The same result was found when comparing positive and negative a-MCI patients (4.4 vs. 4.5 years, respectively; t(17) < 1, p > 0.9).
Relationship of Structural Imaging to PiB binding
Structural imaging markers have been found to be predictive of conversion to AD in a-MCI populations. In a regression model including age, ICV, and gender as covariates, right [F(1,41) = 18.0, p < 0.001] and left hippocampal [F(1,41) = 13.7, p < 0.01] volumes were smaller in the amyloid-positive a-MCI patients than controls (see Figure 3). The amyloid-negative a-MCI patients did not statistically differ from the controls although the right hippocampal volume approached significance [F(1,38) =3.4, p = 0.07]. Although hippocampal volumes were smaller in absolute terms for the amyloid-positive relative to amyloid-negative a-MCI patients, this difference only approached statistical significance on the right [F(1,14) = 3.1, p = 0.10].
Figure 3.
Hippocampal volumes for controls and the amyloid-positive and amyloid-negative a-MCI groups calculated with ALP. * represents p < 0.05 after correction for ICV, age, and gender. Error bars represent one SEM. black = control; gray = amyloid-positive a-MCI; white = amyloid-negative a-MCI.
For a more global survey of differences in grey matter volume and to confirm the above results, we performed an SPM analysis comparing the amyloid-positive a-MCI patients to the control group corrected for multiple comparisons [false discovery rate (FDR)], thresholded at p < 0.05, and with an extent threshold of 25 voxels. Most prominently, this analysis revealed grey matter loss in the medial temporal lobes bilaterally for the a-MCI patients, including involvement of the hippocampi (see Figure 4). Other areas of atrophy included a region in the left superior temporal gyrus, left insula, and left middle frontal gyrus. There were no significant differences between the amyloid-negative a-MCI patients and the control group or the amyloid-positive a-MCI patients at these thresholds. To reduce the number of voxel comparisons and enhance statistical power, a voxel mask for the statistically significant regions from the amyloid-positive a-MCI versus control analysis was applied. Using this mask, all regions had significantly reduced grey matter volume with FDR correction in the amyloid-positive relative to amyloid-negative a-MCI patients with the exception of the left middle frontal gyrus.
Figure 4.

Amyloid-positive a-MCI patients versus amyloid-negative controls VBM of MRI grey matter density. The map is corrected for multiple comparisons (FDR) and thresholded at p < 0.05. The color bar represents the value of the T-statistic.
Longitudinal clinical data
Twenty-three of the 26 patients had at least one annual follow-up examination after their PiB scan. Overall mean follow-up was 21.2 ± 16.0 (SD) months. There was no difference in follow-up between the amyloid-positive and amyloid-negative groups [21.9 vs. 22.3 months, respectively; t(23) < .1, p > 0.1]. Of the 13 amyloid-positive patients who had at least one follow-up examination in the ADRC, 5 patients (38%) “converted” to Alzheimer’s disease (see Figure 5). The rest of this group continued to hold the diagnosis of MCI. There were longitudinal data on 10 of the amyloid-negative patients; none converted to clinical AD. Seven patients (70%) retained a diagnosis of MCI whereas three (30%) no longer qualified for this designation (i.e. “reversion to normal”). Difference in the rate of conversion to clinical AD was statistically significant (p = 0.046, Fisher’s Exact Test, 2-tailed).
Figure 5.

Outcomes of patients with MCI based on amyloid status. Amnestic, single-domain (A-SD) and Amnestic, multiple-domain (A-MD) MCI patients are indicated with black boxes. Non-amnestic patients (all single-domain) are indicated with white boxes that also indicate the non-memory area of cognitive impairment (language or executive). The numbers beside the middle column represent the MMSE scores. The bars at the far right indicate the duration of follow-up from initial diagnosis. Note that the MCI patient with an MMSE of 21 had an isolated amnestic deficit on focused testing and was felt by consensus to qualify for an MCI designation.
Discussion
The current work explored the use of PiB imaging in a cohort of patients with MCI. One of our central goals was to extend the limited literature of amyloid imaging in this population and, in particular, focus on the relationship of PiB binding in different clinical subtypes of MCI. We found that both amnestic and non-amnestic subtypes were associated with a significant proportion of amyloid-positive scans. Our finding of na-MCI patients with elevated PiB binding is the first reported in the literature. Additionally, we examined psychometric and imaging characteristics of patients with elevated levels of PiB binding with the expectation that those who were amyloid-positive would show features that were predictive of “conversion” to clinical AD in longitudinal studies of MCI. Indeed, we found that our amyloid-positive a-MCI patients were older, had poorer memory, and greater medial temporal lobe atrophy than the amyloid-negative patients – all characteristics which have been associated with a higher rate of conversion to AD.12, 18, 19, 21 This hypothesis was driven by the notion that those with increased PiB uptake are the patients most likely to convert. Our longitudinal data support this notion, as only patients who were amyloid-positive developed clinical AD. We will discuss each of these findings in more detail below.
A dichotomous criteria for being amyloid-positive or -negative was established.46 Using these cut-offs, we found that 54% of our MCI patients had elevated PiB uptake. This proportion is quite similar to other reports using a variety of different operationalizations for being “amyloid-positive.” 37, 38, 40, 41 Although the number of patients limits formal comparisons between subtypes of MCI, we found elevated levels of PiB binding in patients with both a-MCI and na-MCI. The md a-MCI group had the highest proportion of amyloid-positive patients. While some have suggested that md a-MCI is more heterogeneous in etiology than sd a-MCI, the higher rate of increased amyloid burden in this population (83% versus 46%, respectively) is inconsistent with this notion. This is not surprising given that the criteria for md a-MCI are closer to the criteria for clinical AD than those for any other MCI subgroup.
Despite na-MCI often being conceptualized as the prodromal state for non-AD etiologies of dementia, three of the seven patients with this designation were amyloid-positive. Only one other study specifically reported on PiB binding in na-MCI and found none of their 6 patients had binding above the control range.40 Importantly, none of our patients had any evidence of memory impairment. Thus, these patients appear to have an atypical phenotype of early AD although one can not exclude the possibility that they have additional non-AD pathologies. Further clinical follow up is necessary to distinguish between these possibilities.
This finding in na-MCI is not unexpected given the fact that atypical presentations of autopsy confirmed AD have been described frequently in the literature.44, 60 Indeed, these cases underscore the utility of amyloid imaging for potential disease specific therapies. Further, longitudinal studies of na-MCI have also largely not supported the notion that these patients purely represent the prodromal phase of non-AD dementias.26-29 These studies have generally found a somewhat lower, but significant, rate of conversion to AD for patients with na-MCI than a-MCI. For example, Fischer and colleagues reported that 26.8% of patients in a community-based cohort with na-MCI converted to clinical AD in 30 months, relative to 48.7% in patients with a-MCI. Thus, our finding that 3/7 na-MCI patients were amyloid-positive is consistent with the literature and suggests that the division of MCI into subtypes provides limited etiologic specificity.
One particularly interesting finding was that the distribution of PiB binding in patients with na-MCI did not appear to differ in any obvious way from those with memory impairment. One might have expected a different distribution of amyloid pathology in these patients given their alternative phenotype. While some studies have found correlations between Aβ burden and cognition 61-63, this relationship is controversial and often considered weaker than that of neurofibrillary tangle pathology or synapse loss 64-66. It is likely that other aspects of AD-related pathology are driving the differences in clinical presentation between the amnestic and non-amnestic groups despite the similar amyloid plaque distribution.
We found several predictors of conversion to AD in prior longitudinal studies of the amnestic subtype of MCI to be associated with being amyloid-positive. Poorer performance on delayed recall tasks has been a consistent predictor of conversion in longitudinal studies 18, 19 and was poorer in the amyloid-positive patients. This result is consistent with two other recent studies demonstrating a relationship between higher PiB binding in MCI and reduced episodic memory performance 38, 40, although not all studies have found this relationship and FGD-PET may correlate to a greater extent.41, 67 Amyloid-positive patients also were older reflecting studies which have found age to be a risk factor for conversion to AD.12, 19 Finally, we found significant atrophy in the medial temporal lobes of amyloid-positive a-MCI patients, consistent with numerous studies reporting medial temporal volumes as a predictor of conversion to clinical AD in a-MCI populations.20, 21, 68
It is worth noting that the only other study to examine the structural MRI correlates of PiB binding in a-MCI found no obvious difference in hippocampal volume between their amyloid-positive and -negative patients – both groups displayed hippocampal atrophy.41 While a number of methodological issues could have contributed to this difference, it is possible that the amyloid-negative groups differed in etiology of their memory impairment. If we assume that the amyloid-negative patients have non-AD causes of their MCI phenotype, a variety of other conditions may affect episodic memory performance. While some of these may result in hippocampal atrophy (e.g. hippocampal sclerosis, Frontotemporal Dementia), others may not (e.g. cerebrovascular disease, aging, depression, Dementia with Lewy Bodies).69 The amyloid-negative group is likely to be heterogeneous and differences in its composition may impact structural imaging findings. This composition may further vary by the subtype of MCI. For example, a patient with sd a-MCI may more likely to have hippocampal sclerosis while a patient with executive na-MCI may be more likely to have Frontotemporal Dementia.
Perhaps even more impressive than the amyloid-positive group being associated with predictors of AD is the finding from the longitudinal data that 38% of these patients converted to clinical AD over approximately 2 years of follow up. In contrast, none of the patients who were amyloid-negative qualified for a diagnosis of AD in this time frame. In fact, 30% of these patients were judged at subsequent visits to no longer merit the designation of MCI (i.e. “reversion to normal”). Population-based studies of MCI have frequently demonstrated high rates of reversion to normal in their samples suggesting instability in the diagnostic construct.13-15 As MCI is being diagnosed more frequently outside of specialty memory clinics, it is likely that this phenomenon will not be uncommon and amyloid imaging may be of particular benefit in this context. The finding that PiB binding is associated with risk of conversion corroborates and extends the only other report to describe longitudinal data in MCI (2 to 16 months of follow-up).38 Forsberg and colleagues found higher PiB uptake in converters than non-converters. It should be noted that variability in the duration of follow-up in the current work is a limitation and that further longitudinal data from our center and others will determine the specificity of PiB PET in MCI for development of clinical AD.
One major issue is the use of cutoffs for designation of whether a patient is amyloid-positive or -negative. The deposition of fibrillar Aβ is a continuous variable; however, there is logic to the notion that its presence is abnormal and that the cutoffs generated from the controls represent the upper limit of the noise of the methodology for the dichotomous determination of the presence or absence of amyloid pathology. Further, when patients with MCI have elevated PiB uptake it is most frequently within the AD range (Figure 1). This is in contradistinction from the 20-25% of clinically unimpaired amyloid-positive elderly controls who generally have PiB uptake intermediate between amyloid-negative controls and AD patients.40, 46, 70 The degree of PiB retention in amyloid-positive elderly controls from these studies, with a few exceptions, is clearly distinguishable from that in AD patients, but spans a broad range from amyloid-negative to AD levels. 46 In contrast, the amyloid-positive MCI patients are typically indistinguishable from AD patients in terms of the degree and pattern of PiB retention. This suggests that amyloid deposition occurs during an asymptomatic phase and, in general, does not produce clinical cognitive impairment until considerable (i.e. AD level) deposition has occurred and/or neurofibrillary tangle burden has reached a critical level.
In conclusion, our data strengthen the evidence that amyloid imaging will be an important tool in the diagnosis and prognosis of patients with MCI. Both amnestic and non-amnestic subtypes of MCI are heterogeneous populations with prodromal AD represented in both groups. Understanding the role of the distribution of amyloid deposition in the different phenotypic expressions of AD will be valuable in enhancing our understanding of the pathophysiologic process of the disease. While our current cohort is modest in number, limiting statistical inference, elevated uptake is associated strongly with predictors of conversion to AD and eventual clinical progression. Nonetheless, further longitudinal study will be needed to confirm that the presence of amyloid in this and similar cohorts signify early clinical AD. If effective anti-amyloid therapy becomes available for the treatment of MCI, it will be critical to distinguish those patients likely to benefit from those who would bear the cost and adverse events with no prospect of improvement. Amyloid imaging with PiB may be one way to make this important distinction.
Acknowledgements
Supported by The National Institutes of Health: R01 AG018402, P50 AG005133, K02 AG001039, R01 AG020226, R01 MH070729, K01MH001976, R37 AG025516, P01 AG025204, K23 AG028018, R01 AG20098, R01 MH070729; The Alzheimer’s Association: TLL-01-3381; The U.S. Department of Energy DE-FD02-03 ER63590; and the Dana Foundation.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment. Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Feldman H, Scheltens P, Scarpini E, et al. Behavioral symptoms in mild cognitive impairment. Neurology. 2004;62:1199–1201. doi: 10.1212/01.wnl.0000118301.92105.ee. [DOI] [PubMed] [Google Scholar]
- 6.Daly E, Zaitchik D, Copeland M, et al. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 7.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 8.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 9.Markesbery WR, Schmitt FA, Kryscio RJ, et al. Neuropathologic substrate of mild cognitive impairment. Archives of Neurology. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early alzheimer’s disease. Annals of Neurology. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 12.Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67:1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- 13.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 17.Visser PJ, Scheltens P, Verhey FR. Do MCI criteria in drug trials accurately identify subjects with predementia Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2005;76:1348–1354. doi: 10.1136/jnnp.2004.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney MC, Black SE, Szalai JP, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Archives of Neurology. 2001;58:1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- 19.Fleisher AS, Sowell BB, Taylor C, et al. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 20.de Toledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiology of Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 23.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 24.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal NT, Wilson RS, Beck TL, et al. The apolipoprotein E epsilon4 allele and incident Alzheimer’s disease in persons with mild cognitive impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 26.Busse A, Hensel A, Guhne U, et al. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 27.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 28.Rountree SD, Waring SC, Chan WC, et al. Importance of subtle amnestic and nonamnestic deficits in mild cognitive impairment: prognosis and conversion to dementia. Dement Geriatr Cogn Disord. 2007;24:476–482. doi: 10.1159/000110800. [DOI] [PubMed] [Google Scholar]
- 29.Lopez OL, Kuller LH, Becker JT, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 30.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 31.Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 32.Verhoeff NP, Wilson AA, Takeshita S, et al. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 33.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008 doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 35.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 36.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 37.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 38.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 40.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 41.Jack CR, Jr., Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 43.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 44.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 46.Aizenstein HJ, Nebes RD, Saxton JA, et al. Amyloid deposition is frequent and often not associated with significant cognitive impairment in the elderly. Archives of Neurology. 2008 doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Wu M, Carmichael O, Lopez-Garcia P, et al. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27:747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreescu C, Butters MA, Begley A, et al. Gray Matter Changes in Late Life Depression-a Structural MRI Analysis. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmichael OT, Aizenstein HA, Davis SW, et al. Atlas-based hippocampus segmentation in Alzheimer’s disease and mild cognitive impairment. Neuroimage. 2005;27:979–990. doi: 10.1016/j.neuroimage.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 54.Watson CC. New, faster, image-based scatter correction for 3D-PET. IEEE Transactions of Nuclear Science. 2000;47:1587–1594. [Google Scholar]
- 55.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 56.Saxton JA, Becker JT, Wisniewski S. The ROCF and dementia. In: Knight JA, editor. The handbook of Rey-Osterrieth complex figure usage: clinical and research applications. Psychological Assessment Resources Inc.; Lutz: 2003. pp. 569–582. [Google Scholar]
- 57.Saxton J, Ratcliff G, Newman A, et al. Cognitive test performance and presence of subclinical cardiovascular disease in the cardiovascular health study. Neuroepidemiology. 2000;19:312–319. doi: 10.1159/000026270. [DOI] [PubMed] [Google Scholar]
- 58.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentatory. 2nd ed Oxford University Press; New York: 1998. [Google Scholar]
- 59.Ivnik RJ, Malec JF, Smith GE, et al. Neuropsychological tests’ normsabove age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- 60.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 61.Cummings BJ, Pike CJ, Shankle R, Cotman CW. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiol Aging. 1996;17:921–933. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 62.Prohovnik I, Perl DP, Davis KL, et al. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- 63.Naslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 64.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 65.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 66.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 67.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 68.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 69.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 70.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]