Abstract
Rationale:
Patients on a low salt (LS) diet have increased mortality.
Objective:
To determine whether reduction in NO bioactivity may contribute to the LS-induced cardiac dysfunction and mortality.
Methods and Results:
Adult male mongrel dogs were placed on LS (0.05% sodium chloride) for 2 weeks. Body weight (25.4±0.4 to 23.6±0.4 kg), left ventricular systolic pressure (137.0±3.4 to 124.0±6.7 mm Hg), and mean aortic pressure (111±3.1 to 98±4.3 mm Hg) decreased. Plasma angiotensin II concentration increased (4.4±0.7 to 14.8±3.7 pg/mL). Veratrine-induced (5 μg/kg) NO-mediated vasodilation was inhibited by 44% in LS; however, the simultaneous intravenous infusion of ascorbic acid or apocynin acutely and completely reversed this inhibition. In LS heart tissues, lucigenin chemiluminescence was increased 2.3-fold to angiotensin II (10−8 mol/L), and bradykinin (10−4 mol/L) induced reduction of myocardial oxygen consumption in vitro was decreased (40±1.3% to 16±6.3%) and completely restored by coincubation with tiron, tempol or apocynin. Switching of substrate uptake from free fatty acid to glucose by the heart was observed (free fatty acid: 8.97±1.39 to 4.53±1.12 μmol/min; glucose: 1.31±0.52 to 6.86±1.78 μmol/min). Western blotting indicated an increase in both p47phox (121%) and gp91phox (44%) as did RNA microarray analysis (433 genes changed) showed an increase in p47phox (1.6-fold) and gp91phox (2.0fold) in the LS heart tissue.
Conclusions:
LS diet induces the activation of the renin–angiotensin system, which increases oxidative stress via the NADPH oxidase and attenuates NO bioavailability in the heart.
Keywords: low salt diet, nitric oxide, free radicals, angiotensin II, heart diseases
The role of angiotensin in the control of plasma volume and especially sodium homeostasis is well known.1,2 Historically, restriction of sodium intake has resulted in an increase in plasma renin and angiotensin I and II, an increase in plasma aldosterone,3 and enhanced sodium reabsorption. In lower species, including fishes and amphibians, it is the action of angiotensin II that conserves sodium.4 In addition, it has recently become clear that angiotensin II through its interaction with the angiotensin II type 1 (AT1) receptor activates the NADPH oxidase to increase superoxide production.5 In fact, a portion, perhaps 50%, of the increase in arterial pressure during chronic angiotensin infusion in the rat, is dependent on increased superoxide production,6 as evidenced by the fact that the hypertension is reduced by scavenging superoxide. In tissues from dogs, angiotensin II increases superoxide as measured by lucigenin chemiluminescence in vitro and reduces NO bioactivity both of which are restored to control by addition of agents that scavenge superoxide or by apocynin.7 In hearts from human, primate, dog, hamster, mouse, rat, and frog, in vitro, bradykinin (BK) reduces cardiac oxygen consumption and this is blocked by an NO synthase inhibitor.7-9 In heart from endothelial NO synthase (eNOS) knockout mice, NO-dependent (BK) signaling to mitochondria does not occur, suggesting the importance of eNOS.8 Low salt (LS)/angiotensin II may regulate the development of atherosclerosis and renal hormone production.10 Thus, there is an NO-dependent regulation of mitochondrial function that is important in the control of tissue oxygen metabolism.
Most importantly, the ability of NO to reduce oxygen consumption in vitro in the normal mouse heart is almost abolished during incubation with angiotensin II via an AT1 receptor–dependent mechanism.9 This does not occur in heart from gp91phox knockout mice, suggesting the essential role of assembly of the NADPH oxidase. Thus, during restriction in salt intake, it is already established that there is an increase in plasma angiotensin II.10 Through the actions on the kidney and the adrenal cortex, this results in an increase in sodium reabsorption and a reduction in urine and sodium excretion.
Our studies may shed light on the controversial clinical finding that reduced sodium intake or reduced urine output inversely correlates with cardiac mortality in patients.11-14 With regard to the effect of a LS diet in patients, there is some controversy. Many physicians still recommend LS intake in the treatment of both hypertension and heart failure.11-14 However, an interesting series of clinical studies by Alderman over the past 20 years call into question the wisdom of this approach, lacking a strong mechanistic scientific basis such as reduced NO bioavailability in the heart.15-18 For instance, patients on a LS diet have an increase in coronary events compared to those on normal salt (NS) intake,11,13 leading to the claim that mortality is inversely proportional to salt intake or urine output.12 Patients on a LS diet who increase their salt intake have a reduction in cardiac events,12,13 but not peripheral or cerebral vascular events. Using an almost circular reasoning, it has been shown the AT1 receptor blockers have little effect on arterial pressure in some patients19; however, placing a patient on a LS diet (which increases plasma angiotensin II and perhaps the local renin angiotensin system) increases the “efficacy of angiotensin II receptor blockade.”19,20 That is, if the patient has an increased plasma angiotensin II by restricting sodium intake, the AT1 blocker works better and there is strong evidence for a role for the NADPH oxidase.21,22 The controversy around the potential detrimental effects of a LS diet has been discussed by Aviv,23 proposing the hypothesis that there is a U-shaped function curve governing salt intake. Furthermore, the affect responsible for mortality at both high and LS intake is proposed to be NO-superoxide.
With this in mind, we have designed studies to address the potential mechanisms of LS diet on the heart, suggesting that the scavenging of NO by superoxide anion secondary to angiotensin II activation of the AT1 receptor leads to uncoupling of the control of cardiac cell mitochondrial function by NO, potentially causing myocardial metabolic dysfunction.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Surgical Preparation
Male mongrel dogs (n=10, 25 to 28kg) were anesthetized with sodium pentobarbital (25 mg/kg IV). A thoracotomy was performed, and instruments were implanted for measurement of pressure, flow, and blood sampling as described previously.24-28 All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College.
Hemodynamic Recordings
Hemodynamics were recorded in conscious dogs as we described previously.7,24-29
Measurement of Plasma Angiotensin II
Plasma concentration of Angiotensin II was measured with peptide enzyme immunoassay (Peninsula Laboratories Inc).
Furosemide and LS Diet
After performing a control experiment, we gave an intramuscular injection of 100 mg furosemide to dogs to acutely reduce blood volume, and then we put them on a LS diet (0.05% of sodium chloride, approximately 150 mg/d) for 2 weeks to further reduce blood volume.
Cardiac Metabolites
Blood samples from aorta and coronary sinus were collected for the measurements of free fatty acid (FFA), glucose, and lactate as we described previously.26,27
Effects of LS Diet on Activation of Bezold–Jarisch Reflex by Veratrine
We measured the NO-dependent response of cerebral blood flow to veratrine as previously described.24-26
Nitrite Production of Coronary Microvessels
To measure the capacity of the heart to produce NO, nitrite production of coronary microvessels was measured as described previously.30,31
Measurement of O2 Consumption in Cardiac Muscle
Myocardial tissue was isolated from the left ventricular (LV) free wall of hearts. Myocardial oxygen consumption (MVO2) was measured polarographically in vitro using a Clark-type oxygen electrode (YSI-5331, Yellow Springs Instruments, Yellow Springs, Ohio). Cumulative doses (10−7 to 10−4 mol/L) of BK or carbachol were used. The superoxide scavenger tiron (10−3 mol/L), the superoxide dismutase (SOD) mimetic Tempol (10−3 mol/L), or the NADPH oxidase inhibitor apocynin (10−4 mol/L) were used.24,26
Lucigenin Chemiluminescence
The chemiluminescence elicited by O2−· in the presence of lucigenin (5 μmol/L) was measured in cardiac muscle segments from the same dog that was used for measurements of MVO2 as described previously.7,27
Western Blotting Analysis
The preparation of protein samples from myocardial tissues was as described previously.26
RNA Isolation and Microarray Analysis
Total cardiac RNA was extracted from the left ventricle from the control and LS groups (n=4) as described previously.32 All the hybridization data have been submitted to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database (GEO) (http://www.ncbi.nlm.nih.gov/geo) with GEO accession numbers for series GSE17149.
Data Analysis
All data are presented as means and SEM. Statistical significance of differences was determined with Student t test for each peak response, and differences between groups were determined with repeated-measures ANOVA. Changes are considered significant at a value of P<0.05. Statistical significance for changes in gene expression was performed in GeneTraffic using a t test and with variance stabilization. Differences were considered statistically significant at a nominal significance of P≤0.05 and at least a 2.0-fold change in expression.
Results
Plasma Angiotensin II
Plasma concentration of angiotensin II of control dogs was 4.3±0.7 μmol/L (n=10), and 2 weeks of LS diet significantly increased by 340±84% (14.8±3.7 μmol/L).
Hemodynamic Measurements in Dogs
All hemodynamic data and body weight are shown in Table. Body weight significantly decreased (P<0.01, n=10), and both LV systolic pressure (P<0.05, n=10) and mean arterial pressure (P<0.01, n=10) were also significantly decreased. However, LV dP/dt, cerebral blood flow, and heart rate were maintained after 2 weeks of LS. There was a tendency for total peripheral resistance (P<0.12) to increase, but this did not reach statistical significance.
Table.
Effects of LS Diet
| Parameter | Control | 2 Week |
|---|---|---|
| Body weight (kg) | 25.4±0.4 | 23.6±0.4† |
| LVSP (mm Hg) | 137.0±3.4 | 124.0±6.7* |
| MAP (mm Hg) | 110.8±3.1 | 97.6±4.3† |
| CBF (mL/min) | 29.3±4.6 | 29.6±4.4 |
| HR (bpm) | 99.6±6.3 | 106.1±6.3 |
| LVEDD (mm) | 46.0±1.0 | 40.5±1.4† |
| LVPWD (cm) | 1.04±0.05 | 1.05±0.06 |
| IVSWD (cm) | 0.97±0.05 | 0.87±0.01 |
| Cardiac output (L/min) | 3.44±0.19 | 2.10±0.28† |
| LV dP/dt (mm Hg/sec) | 3848.0±238.3 | 3915.3±669.2 |
| TPR (dyn · sec · cm5) | 19.1±2.6 | 26.9±4.4 (P<0.12) |
| EF (%) | 64.8±2.9 | 60.3±4.3 |
Data are means±SEM. CBF indicates coronary blood flow; HR, heart rate; IVSWD, interventricular septal wall diameter; LVEDD, LV end-diastolic diameter; LVPWD, left ventricular end-diastolic posterior wall dimension; LVSP, LV systolic pressure; MAP, mean arterial pressure; TPR, total peripheral vascular resistance.
P<0.05,
P<0.01.
Echocardiographic Measurement
The Table also shows the summary data of echocardiographic analysis of LV function in both baseline and LS. LV end-diastolic diameter was decreased (P<0.01, n=6), which was accompanied by the simultaneous decrease of CO (P<0.01, n=6). However, ejection fraction was unchanged. There was no change in LV posterior wall or interventricular septum wall thickness in diastole, indicating a lack of hypertrophy after 2 weeks on LS.
Effects of Veratrine on the Coronary Circulation
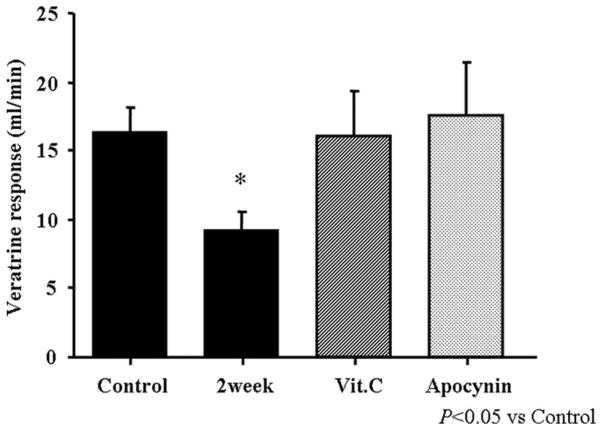
Figure 1 is the summary of veratrine- induced NO dependent coronary vasodilation (Bezold–Jarisch reflex). Veratrine administration at a dose of 5 μg/kg caused significant increases in cerebral blood flow (65±8%, n=10). Two weeks of LS inhibited this response by 44% (P<0,05); the simultaneous infusion of either ascorbic acid or apocynin completely reversed this inhibition (P<0.05, n=6 respectively).
Figure 1.
Summary data for the veratrine induced NO-dependent coronary vasodilation during LS diet. There was a significant reduction in the coronary vasodilation caused by veratrine (2 weeks), and this was restored by infusion of either ascorbic acid or apocynin. Data are expressed as changes in flow. Values are means±SEM. *P<0.05 for difference from control (n=8).
Nitrite Production of Coronary Microvessels
There was no significant difference in the baseline production of nitrite in coronary microvessels between NS and LS (P=NS, n=10). BK (10−5 mol/L) increased nitrite production in a dose-dependent fashion similarly in both groups (normal: 133±6.7%; versus LS: 138±24.6%; P=NS, n=10), ie, the endogenous NO production was preserved in LS. This effect of BK was completely inhibited by coincubation of NO synthesis inhibitor NG-l-nitro-arginine methyl ester (L-NAME) (10−4 mol/L) in both groups. Similar effects were found using carbachol (not shown).
Effects of LS Diet on MVo2 in Tissue
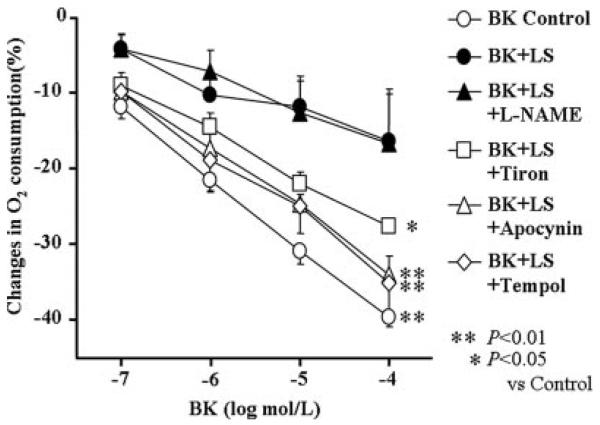
Cumulative doses of BK caused concentration-dependent decreases in MVo2 in control hearts (40±1.3%). BK-induced reduction in MVo2 was significantly attenuated by LS (16±6.3%, P<0.01). The inhibitory effects of LS on MVo2 in response to BK was restored with coincubation with tiron (28±2.5%, P<0.05), tempol (35±3.9%), or apocynin (34±2.5%) (P<0.05). Coincubation with L-NAME in normal tissue also significantly inhibited BK-induced reduction in MVo2 (17±7.1%, P<0.01; Figure 2).
Figure 2.
Changes in oxygen consumption in cardiac muscle segments. BK (from 10−7 to 10−4 mol/L) was used to stimulate endogenous NO release. Coincubation with tiron (10−3 mol/L), tempol (10−3 mol/L), or apocynin (10−4 mol/L) restored the inhibitory effects of LS on oxygen consumption. On the other hand, coincubation with L-NAME (10−4 mol/L) mimicked the inhibitory effect of LS in normal heart tissue. *P<0.05, **P<0.01 for difference from BK+LS (n=6 each).
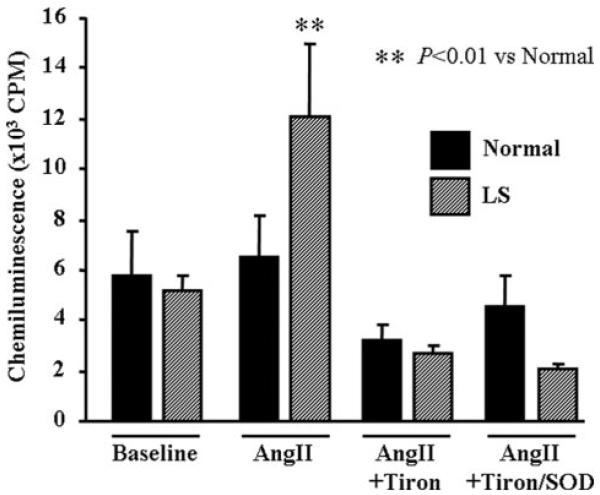
O2−· Production by Angiotensin II
Angiotensin II resulted in significant increase in production of O2−· only in LS. O2−· production was inhibited by coincubation with Tiron or Tiron plus SOD (Figure 3).
Figure 3.
O2−· generation as determined by lucigenin chemiluminescence in LV tissues of LS fed dogs. Data are expressed as means±SE. **P<0.01 (n=7) vs baseline of normal and ##P<0.01 (n=7) vs angiotensin II (AngII) of LS.
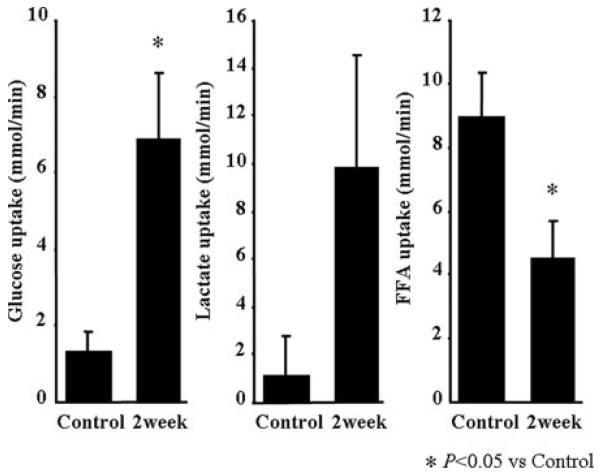
Effects of LS Diet on Energy Metabolism
The LS significantly increased glucose uptake (P<0.05) and also decreased FFA uptake (P<0.05). Lactate uptake tended to increase (P=0.11; Figure 4).
Figure 4.
Summary of the substrate switching from FFA to glucose or lactate (n=6 each) in LS at 2 weeks. Values are means±SEM. *P<0.05 for difference from control.
Western Blotting Analysis
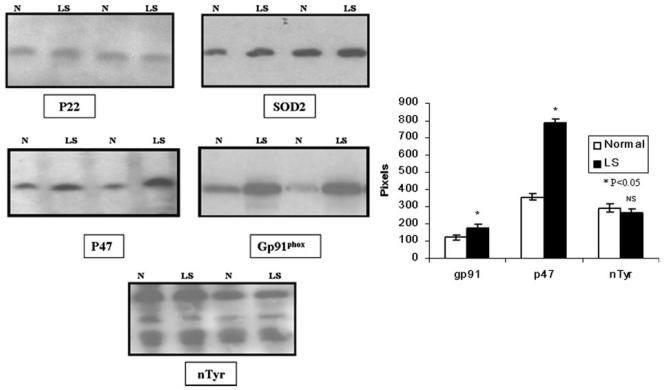
The protein expression of p47phox (121%) and gp91phox (44%) were upregulated in the LV tissues after 2 weeks of LS (Figure 5). There was no change inducible NOS, neuronal NOS, SOD1, SOD2, p67, phospho-p47, p22, Rac-1, nitrotyrosine, or β-actin. For instance, by scanning, there was no change in eNOS or phospho-eNOS (802±16 and 767±33 in normal and 774±9 and 767±26 in LS, respectively). In NS and LS, β-actin was 721±135 and 651±111 (P>0.50), respectively.
Figure 5.
Representative Western blots of p22phox, SOD-2, p47phox, and p67phox from normal dogs and those fed LS. There was a significant increase in p47 and gp91phox and no change in other components of the oxidase or nitrotyrosine.
RNA Microarray Analysis
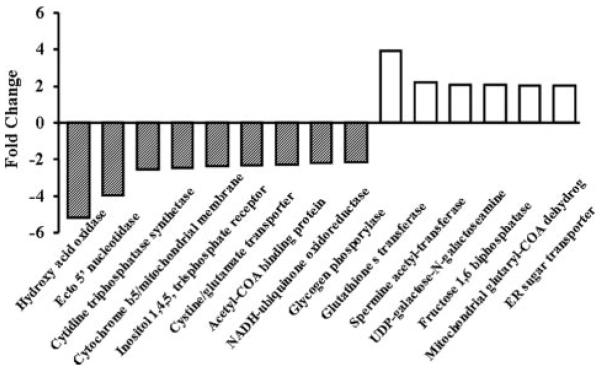
Affymetrix Canine Gene Array 2.0 was used to determine differential gene expression in heart tissues from four LS and four control dogs. There were 443 genes that changed, 110 decreasing and 333 increasing. There was an increase in mRNA for p47phox (1.6-fold), gp91phox (2.0-fold) and in SOD1 (1.7-fold, all P>0.05; Figure 6). In addition to the metabolic genes there was no difference in RNA for p67, p22, p40, rac-1, SOD2, eNOS, inducible NOS, or neuronal NOS. All the genes are listed in Online Table I. eNOS was 16.8±0.6 pixels in normal and 17.4±0.5 pixels in LS (P=NS).
Figure 6.
Fold change of representative genes that are differentially regulated (P<0.05) in LS. Many of the genes are metabolic, and a listing of the other genes affected is provided in the Online Data Supplement.
Discussion
All of our data point toward an important role of NO and the renin–angiotensin–NADPH system as potential mediators of the LS-induced cardiac dysfunction and potentially the mortality associated with salt restriction in patients.11-14,23 Firstly, there was a 340% increase in plasma angiotensin during LS feeding of dogs. There was the expected reduction in body weight, arterial pressure, diastolic dimensions in the heart, along with a reduction in cardiac output with LS. This was accompanied by a reduction in NO-mediated vasodilation in vivo with no change in nitrite production from coronary microvessels in vitro. There was no change in eNOS protein pointing toward a reduction in NO bioactivity during LS, most likely through the scavenging of NO by superoxide. The obtunded NO-mediated coronary vasodilation in vivo was restored by ascorbic acid. The suppression of the ability of BK to regulate MVo2 in cardiac tissue in vitro was restored by tiron and tempol. Most directly, there was an increase in lucigenin-induced chemiluminescence, a direct measure of superoxide, in response to angiotensin, in cardiac tissues from dogs fed LS. The source of superoxide is most likely the NADPH oxidase because: (1) the reduced NO-mediated vasodilation was restored by apocynin in vivo; (2) the ability of BK to control MVo2 was restored by apocynin; (3) there was an increased gene expression of p47phox and gp91phox using microarrays; and (4) there was an increase in p47phox and gp91phox protein by Western blotting. Interestingly and previously unreported, there was a switching of substrate uptake by the heart from fatty acids to glucose, a signature in our previous studies for the involvement of NO. A reduction in NO bioactivity causes the heart to switch from fatty acid uptake and oxidation to oxidation of glucose.33,34 There is also a change in gene expression by microarray analysis toward genes which encode for enzymes of intermediary metabolism, supporting our finding of altered substrate uptake, as yet a new feature of LS consumption. Thus, there are potentially defects in coronary vascular control, in mitochondrial function, in oxygen use, in intermediate metabolism, and in protein and gene expression as a consequence of a LS diet, and all of these may contribute to the development of cardiac disease.
There was, as expected, a reduction in cardiac end-diastolic dimensions and cardiac output along with a reduction in body weight, all characteristic of a reduction in blood volume similar to that in patients with LS diet.11-14 There was also a reduction in LV systolic pressure and phasic and mean arterial pressure as a consequence of LS consumption. Our data did not reach statistical significance (P<0.12); however, they suggest that there is actually an increase in total peripheral resistance, as reported in patients35; another of our studies in mice (unpublished data) indicate a statistically significant increase in total peripheral resistance with LS feeding, which can be interpreted as peripheral vasoconstriction. There was no sign of hypertrophy using measures of anterior wall and septum wall thickness. Caution has to be used when creating ratios of LV/body weight to assess hypertrophy because LS reduced body weight by almost 10% in 2 weeks. Furthermore, the measurement of heart weight by echo is model-dependent and not specific for the canine heart.
The Bezold–Jarisch induced increase in coronary blood flow following injection of veratrine is entirely NO-dependent, because it is abolished by L-NAME.24-26 It is also reduced during the development of dilated cardiomyopathy36 or insulin dependent diabetes mellitus24 conditions in which NO production is reduced and during hyperhomocysteinemia29 and endothelial stunning7 in which NO bioactivity is reduced because of superoxide anion. In contrast, with pregnancy or exercise training, the reflex dilation is largely attributable to upregulation of eNOS.25,26 During LS feeding, the reflex dilation is also reduced but it is restored by infusion of ascorbic acid, indicating a reduction in NO bioavailability. Previous studies by us showed that vagal stimulation in the anesthetized dog, to directly control efferent activity, resulted in a entirely NO dependent coronary vasodilation.28 Furthermore, the bradycardia following veratrine injection was unaffected by NO synthesis inhibition.36 Thus, it is unlikely that the reduction in the reflex dilation was caused by an antioxidant effect on the ganglion or nuclei in the brain stem.
We have shown previously that BK through an NO-dependent mechanism regulates cardiac oxygen consumption in vitro.15-18 These data support the concept that NO, through a competition with oxygen for cytochrome oxidase, regulates oxygen consumption in all types of cells37,38 from cardiac muscle to endothelial cells.15-17,37-40 This effect occurs in the wild-type mouse heart but not in the eNOS−/− heart and not in the presence of L-NAME. In our studies, BK reduced cardiac tissue oxygen consumption; this was blocked by L-NAME, indicating that it was NO dependent and was reduced to a similar degree in hearts from dogs fed a LS diet. Although this was not attributable to a reduction in NO production, because there was no decrease in nitrite production by coronary microvessels, it was restored by superoxide scavengers. We have previously used these techniques in a model of “endothelial stunning” in the dog heart,7 in which there was no change in NO or nitrite production but a reduction in NO ability to regulate oxygen consumption caused by a reduction in the half life of NO. Similar results were found in both the rat and dog heart after methionine feeding, leading to hyperhomocysteinemia.28 Thus, the measurement of cardiac tissue oxygen consumption is a sensitive index of NO bioactivity and also addresses a mechanism perhaps contributing to cardiac dysfunction.
There was an increase in lucigenin chemiluminescence in vitro to angiotensin II only in the heart of dogs fed a LS diet; this was confirmed using tiron and SOD. The enzymatic source of superoxide was the NADPH oxidase, because the components were upregulated and the biological effects were restored by apocynin both in vivo and in vitro. We have shown previously that apocynin in vivo blocks endothelial stunning7 and restores the NO-dependent coronary vasodilation during hyperhomocysteinemia.28 However, there is some question in the literature regarding whether apocynin not only prevents the assembly of the NADPH oxidase but also has other effects.41 When given chronically in rats, apocynin downregulated components of the NADPH oxidase; however, in our studies, apocynin was given for 1 hour. It is possible that apocynin, particularly the monomeric form, acts as an antioxidant42 and not to inhibit assembly of the oxidase. Myeloperoxidase is needed to form the apocynin dimer and in tissues lacking myeloperoxidase the active dimer does not form. All of our studies in vivo and those using lucigenin or measuring oxygen consumption were performed in mixed tissues containing white cells in which myeloperoxidase allows for dimerization of apocynin, conferring its activity as an assembly inhibitor. If in ours studies apocynin only acts as an antioxidant then our studies support finding using SOD, tiron, tempol, and ascorbic acid. If apocynin also acts as an NADPH oxidase inhibitor, it supports our studies using western blotting and the gene array. Thus, experiments using apocynin confirm the role of superoxide and the upregulation of p47phox and gp91phox support the involvement of the NADPH oxidase.
Not to our surprise, hearts from dogs fed a LS diet switched substrate use from predominantly FFAs to primarily glucose. In a number of studies, we have shown that reduction in cardiac NO production or bioactivity resulting from heart failure,43,44 administration of L-NAME,31 during endothelial stunning,7 and hyperhomocysteinemia29 all result in a shift in substrate uptake by the heart. This seems to be in contrast to a healthy or unstressed heart, as for instance in pregnancy and exercise, conditions in which fatty acids take on a larger role as substrate to support increased cardiac mechanical function.26 The first studies to suggest that NO might regulate glucose uptake by the heart by Depre and colleagues45,46 indicated that this was a cGMP-mediated action in contrast to the chemical action of NO through cytochrome oxidase to control MVo2.47,48 Tada et al7 confirmed the studies by Depre et al44 using eNOS−/− mouse heart and a Langendorff heart preparation. In hearts from eNOS−/− mouse BK did not alter glucose uptake whereas in eNOS+/+ heart inhibition of guanylate cyclase with ODQ increases glucose uptake via and 8-Br cGMP, a cGMP mimetic, inhibits glucose uptake. The role of NO in the control of cardiac FFA uptake and oxidation is more controversial. Firstly, this is best studied using live animals, such as the conscious dog, because fatty acids are carried bound to plasma proteins and are normally consumed by the heart. In the isolated heart, fatty acids are difficult to use as a substrate because they are detergents. However, fatty acid uptake by the heart is dependent on the plasma concentration; at arterial concentrations less than 0.2 to 0.3 mmol/L, there is little uptake.33 In our studies, the arterial plasma FFA concentrations were 0.79±0.08 and 0.71±0.22 mmol/L (P=NS) during control and LS, respectively. Nonetheless, our studies indicate a reduction in fatty acid uptake and increase in glucose uptake, both attributable to a reduction in NO bioactivity.
Although consistent with our previous studies but still surprising, there was a shift in the expression of 433 genes, 110 increasing and 323 decreasing, in dogs fed a LS diet which is also indicative of the dramatic effects of a LS diet on the heart. Although this observation has not been reported previously, it should be noted that the tissues used in this analysis were sections of heart containing many different cell types. There were 6 genes upregulated more than 2-fold and 9 downregulated more than 2-fold. These genes include some related to mitochondrial function such as cytochrome b5; some related to fatty acid transport and metabolism such as acetyl-coenzyme A binding protein; and some related to glucose metabolism, such as fructose 1,5-bisphosphatase and glycogen phosphorylase. The exact impact of these changes in gene expression is difficult to evaluate, but the data indicate the profound effect that a LS diet has on the heart. Furthermore, these data support the conclusion that a LS diet has an unexpected impact on the heart when hereto for the major consequences of a LS diet were thought to be vascular, resulting in superoxide generation, removal of NO and vasoconstriction.
There is a remarkable congruence between the gene array and Western blotting. By either method, there was no change in p67phox, p22phox, rac-1, SOD2, eNOS, inducible NOS, or neuronal NOS. By both methods, there was a significant increase in p47phox and gp91phox. There was an increase in SOD1 by gene array but not in the protein. This was not attributable to our inability to measure SOD1 by Western blotting because we have recently done so in the heart from pregnant dogs.26,27 There was no change in nitrotyrosine. Because nitrite production did not change in coronary microvessels and eNOS or phosphorylated eNOS proteins were not changed; the production of NO limited the amount of nitrotyrosine. Because the interaction of NO and O2−· to form peroxynitrite is diffusion limited, and the stoichiometry of NO and superoxide is 1:1, the amount of peroxynitrite formed is determined by the lowest concentration of either NO or superoxide. Although tyrosine nitration could originate from the scavenging of NO by superoxide, other processes may dominate (eg, the breakdown of nitrated proteins). Because nitrite levels were similar in both animal groups and the biological activity of NO was restored by antioxidants (tiron, tempol, SOD, and ascorbic acid), we conclude that NO production was not different between LS and NS fed dogs but rather that the scavenging by superoxide was increased and biological half life of NO was reduced resulting in aberrant control of cardiac metabolism and coronary vasodilation.
Our studies add a new perspective on the effects of LS diet in the treatment of both hypertension and heart failure. The early studies by Alderman11-14 suggested an increased mortality of cardiac origin in the face of a reduced mean arterial pressure in patients with LS diet. They concluded that the mortality was attributable to other than hemodynamic causes, perhaps caused by the reduction in cardiac NO bioactivity. In the case of heart failure, where eNOS is downregulated, the reduction in blood volume resulting from the use of diuretics and salt restriction may further increase the already elevated renin-angiotensin levels, competing with the effect of the AT1 blocker or angiotensin-converting enzyme inhibitor, scavenging NO and having effects on cardiac oxygen consumption and contractile efficiency. Characteristically, oxygen consumption is elevated in the failing heart not only because of the increased hemodynamic load, including diastolic wall stress in dilated myopathies, but, we think, also because of a reduction in NO.43,44 In the conscious dog, during graded treadmill exercise, oxygen consumption is increased, independent of load, by 10% at each level of exercise during inhibition of NO synthesis.49,50 Thus, the effect is small but leads to a reduced cardiac efficiency when calculated as work/oxygen consumed. The same conclusion pertains to the failing heart where NO production is reduced and oxygen consumption is elevated. There is an error in the calculation of oxygen consumption, which assumes that 100% of the measured oxygen consumed is used to support the generation of ATP, although some of the oxygen consumed is wasted because it forms superoxide anion and not ATP. When NO is present and competing with oxygen for the binding site on cytochrome oxidase, the amount of oxygen consumed may fall, leading to an increase in intracellular oxygen to maintain the diffusion gradient or to saturate myoglobin in cardiac cells.40 Hemodynamic measurement identified no reduction in contractile indices and no change in wall thickness, therefore 2 weeks of LS did not result in overt pathology.
In summary, our studies have defined a potential mechanism, the reduction in NO bioactivity, which may contribute to the detrimental effects of LS in patients. A report of a recent clinical trial NHANES III, by Cohen, Hailpern, and Alderman,51 suggests a “modest and mostly not statistically significant” correlation of LS diet and mortality and no direct correlation with elevated salt. The clinical relevance of our study still remains to be determined.
Significance and Novelty
A low salt diet is used in the treatment of both congestive heart failure and hypertension and yet the exact mechanism for the benefit it provides is poorly understood. In fact, there is substantial evidence that a low salt diet can have detrimental effects resulting in an increase in cardiac mortality. Thus, it is important to determine the mechanism of action of a low salt diet and the potential cause of adverse cardiovascular affects. Our studies use chronically instrumented animals placed on a low salt diet for up to 2 weeks. We found that during this period of time, there was an increase in plasma angiotensin levels. Plasma angiotensin is known to activate the NADPH oxidase and to subsequently produce superoxide. We documented this using a number of different approaches from measurements of coronary blood flow in vivo to measurements of oxygen consumption and substrate use by the heart. We also used a gene array approach to screen for potential genes that would be affected by a low salt diet. The novel ideas that these studies contribute include a focus on the regulation of the production of superoxide during a low salt diet and its subsequent impact on nitric oxide bioactivity. These studies are important in that they provide a translational basis for explaining the adverse cardiovascular effects of a low salt diet and its implication for medicine.
What is Known
A low salt diet is used in the treatment of heart failure and hypertension.
A low salt diet is thought to have beneficial effects in most patients except that in a subgroup of patients a low salt diet actually increases mortality.
Angiotensin drives the production of superoxide from NADPH oxidase and the increase in superoxide production is thought to be part of many disease processes.
What This Article Contributes
This article documents the reduction in nitric oxide bioavailability in a high superoxide state attributable to the rise in angiotensin during low salt feeding.
Thus, an upregulation of the components of the NADPH oxidase make an important contribution to the development of disease.
Our article contributes to the better understanding of the mechanism of action of the detrimental effects of a low salt diet, including activation of the renin angiotensin system and the generation of superoxide.
Acknowledgments
Sources of Funding
Supported by NIH grants P0-1 HL 43023, HL 50142, HL 31069, and P0-1 HL 74137. F.A.R. is an Established Investigator of the American Heart Association.
Non-standard Abbreviations and Acronyms
- AT1
angiotensin II type 1
- BK
bradykinin
- eNOS
endothelial NO synthase
- FFA
free fatty acid
- L-NAME
NG-l-nitro-arginine methyl ester
- LS
low salt
- LV
left ventricular
- MVo2
myocardial oxygen consumption
- NS
normal salt
- SOD
superoxide dismutase
Footnotes
Disclosures
None.
References
- 1.Braunwald E, editor. Heart Disease. WB Saunders; Philadelphia, Pa: 1980. pp. 1836–1837. [Google Scholar]
- 2.Berne RM, Levy MN, editors. Cardiovascular Physiology. 4th ed. Mosby Inc; St Louis, Mo: 1998. pp. 729–734. [Google Scholar]
- 3.Luchner A, Stevens TL, Borgeson DD, Redfield MM, Bailey JE, Sandberg SM, Heublein DM, Burnett JC., Jr Angiotensin II in the evolution of experimental heart failure. Hypertension. 1996;28:472–477. doi: 10.1161/01.hyp.28.3.472. [DOI] [PubMed] [Google Scholar]
- 4.Connel EM, Kaley G. Evidence for the presence of renin in kidneys of marine fish and amphibia. Biol Bull. 1964;127:366–368. [Google Scholar]
- 5.Griendling K, Sorescu D, Ushio-Fukai M. NADPH oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 6.Laursen JB, Rajagopalan S, Galis S, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:557–559. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 7.Kinugawa S, Post H, Kaminski PM, Zhang XP, Xu XB, Huang H, Recchia FA, Ochoa M, Wolin MS, Kaley G, Hintze TH. Coronary microvascular stunning after acute pressure overload in conscious dogs is caused by oxidant processes: the role of angiotensin II type 1 receptor and NAD(P)H oxidase. Circulation. 2003;108:2934–2940. doi: 10.1161/01.CIR.0000096488.78151.97. [DOI] [PubMed] [Google Scholar]
- 8.Tada H, Thompson CI, Recchia FA, Loke KE, Ochoa M, Smith CJ, Shesely EG, Kaley G, Hintze TH. Myocardial glucose uptake is regulated by nitric oxide via endothelial nitric oxide synthase in Langendorff Mouse heart. Circ Res. 2000;86:270–274. doi: 10.1161/01.res.86.3.270. [DOI] [PubMed] [Google Scholar]
- 9.Kinugawa S, Zhang J, Messina E, Walsh E, Huang H, Kaminiski PM, Wolin MS, Hintze TH. Gp91phox-containing NADPH oxidase medicates attenuation of nitric oxide–dependent control of myocardial oxygen consumption by Angiotensin II. Am J Physiol. 2005;289:H862–H867. doi: 10.1152/ajpheart.00076.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ivanoski O, Szumilak D, Nguyen-Khoa T, Dechaux M, Massy ZA, Phan O, Mothu N, Lacour B, Drueke TB, Muntzel M. Dietary salt intake accelerates atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2005;180:271–276. doi: 10.1016/j.atherosclerosis.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Alderman MH, Cohen K, Madhavan S. Dietary sodium intake and mortality: National Health and Nutrition Examination Survey (NHANES) Lancet. 1998;351:1508–1509. doi: 10.1016/S0140-6736(97)09092-2. 352: 987–988. [DOI] [PubMed] [Google Scholar]
- 12.Alderman MH, Madhavan S, Cohen H, Sealy JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;27:1153–1154. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 13.Alderman MH. Dietary sodium and cardiovascular health in hypertensive patients: the case against sodium restriction. J Am Soc Nephrol. 2004;15(suppl 1):S47–S50. doi: 10.1097/01.asn.0000093236.74397.f3. [DOI] [PubMed] [Google Scholar]
- 14.Alderman MH, Cohen HW. Impact of dietary sodium on cardiovascular disease mortality and morbidity. Curr Hypertens Rep. 2002;4:453–457. doi: 10.1007/s11906-002-0025-2. [DOI] [PubMed] [Google Scholar]
- 15.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAP(P)H oxidase generated superoxide anion accounts for reduced control of myocardial oxygen consumption by NO in aged Fischer 344 rats. Am J Physiol. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 16.Mital S, Loke KE, Addonizio L, Oz M, Hintze TH. Left ventricular assist device preserves nitric oxide dependent control of mitochondrial respiration in failing human hearts. J Am Coll Cardiol. 2000;36:1897–1902. doi: 10.1016/s0735-1097(00)00948-7. [DOI] [PubMed] [Google Scholar]
- 17.Forfia PR, Zhang X, Knight DR, Smith AH, Doe CPA, Wolfgang EA, Flynn DM, Wolin MS, Hintze TH. NO modulates myocardial O2 consumption in the nonhuman primate: an additional mechanism of action of amlodipine. Am J Physiol. 1999;276:H2069–H2075. doi: 10.1152/ajpheart.1999.276.6.H2069. [DOI] [PubMed] [Google Scholar]
- 18.Loke KE, Messina E, Mital S, Hintze TH. Impaired nitric oxide modulation of myocardial oxygen consumption in genetically cardiomyopathic hamster. J Molec Cell Cardiol. 2000;32:2299–2306. doi: 10.1006/jmcc.2000.1258. [DOI] [PubMed] [Google Scholar]
- 19.Chen HH, Redfield MM, Nordstrom LJ, Cataliotti A, Burnett JC. Angiotensin II AT1 receptor antagonism prevents the detrimental renal actions of acute diuretic therapy in human heart failure. Am J Physiol. 2003;284:F1115–F1119. doi: 10.1152/ajprenal.00337.2002. [DOI] [PubMed] [Google Scholar]
- 20.Greenburg A. Diuretic complications. Am J Med Sci. 2000;319:10–24. [PubMed] [Google Scholar]
- 21.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 22.Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res. 1999;85:23–28. doi: 10.1161/01.res.85.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Aviv A. Salt consumption, reactive oxygen species and cardiovascular ageing: a hypothetical link. J Hyperten. 2002;20:555–559. doi: 10.1097/00004872-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Zhang X, Smith CJ, Xu X, Ochoa M, Greenhouse D, Vogel T, Curran C, Hintze TH. Reduced coronary NO production in conscious dogs after the development of alloxan-induced diabetes. Am J Physiol. 1999;277:H268–H278. doi: 10.1152/ajpheart.1999.277.1.H268. [DOI] [PubMed] [Google Scholar]
- 25.Zhao G, Zhang X, Xu X, Ochoa M, Hintze TH. Exercise training enhances reflex cholinergic, NO dependent coronary vasodilation in conscious dogs. Circ Res. 1997;80:868–876. doi: 10.1161/01.res.80.6.868. [DOI] [PubMed] [Google Scholar]
- 26.Williams JG, Rancon-Skinner T, Sun D, Wang Z, Zhang S, Zhang X, Hintze TH. Role of nitric oxide in the coupling of myocardial oxygen consumption and coronary vascular dynamics during pregnancy in the dog. Am J Physiol. 2007;293:H2479–H2486. doi: 10.1152/ajpheart.00036.2006. [DOI] [PubMed] [Google Scholar]
- 27.Williams JG, Ojaimi C, Qanud K, Zhang S, Xu X, Recchia FA, Hintze TH. Coronary nitric oxide production controls cardiac substrate metabolism during pregnancy in the dog. Am J Physiol. 2008;294:H2516–H2423. doi: 10.1152/ajpheart.01196.2007. [DOI] [PubMed] [Google Scholar]
- 28.Shen W, Ochoa M, Xu X, Wang J, Hintze TH. Role of EDRF/NO in parasympathetic coronary vasodilation following carotid chemoreflex activation in conscious dogs. Am J Physiol. 1994;267:H605–H613. doi: 10.1152/ajpheart.1994.267.2.H605. [DOI] [PubMed] [Google Scholar]
- 29.Suematsu N, Ojaimi C, Kinugawa S, Wang Z, Xu X, Koller A, Recchia FA, Hintze TH. Hyperhomocysteinemia alters cardiac substrate metabolism by impairing nitric oxide bioavailability through oxidative stress. Circulation. 2007;115:255–262. doi: 10.1161/CIRCULATIONAHA.106.652693. [DOI] [PubMed] [Google Scholar]
- 30.Gerritsen M, Printz M. Sites of prostaglandin synthesis in the bovine heart and isolated coronary microvessels. Circ Res. 1981;49:1152–1163. doi: 10.1161/01.res.49.5.1152. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels- an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–580. doi: 10.1161/01.cir.97.6.576. [DOI] [PubMed] [Google Scholar]
- 32.Ojaimi C, Qanud K, Hintze TH, Recchia FA. Altered expression of a limited number of genes contributes to cardiac decompensation during chronic ventricular tachypacing in dogs. Physiol Genomics. 2006;29:76–83. doi: 10.1152/physiolgenomics.00159.2006. [DOI] [PubMed] [Google Scholar]
- 33.Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopashuk GD, Hintze TH, Stanley WC. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol. 2002;282:E197–E206. doi: 10.1152/ajpendo.2002.282.1.E197. [DOI] [PubMed] [Google Scholar]
- 34.Lei B, Lionetti V, Young ME, Chandler MP, d'Agnostino C, Kang E, Altarejos M, Matsuo K, Stanley WC, Hintze TH, Recchia FA. Paradoxical downregulation of glucose oxidation pathway despite enhanced flux in heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Omvik P, Lund-Johansen P. Is sodium restriction effective treatment of borderline and mild essential hypertension? A long-term hemodynamic study at rest and during exercise. J Hypertens. 1986;4:535–541. doi: 10.1097/00004872-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zhao G, Shen W, Xu X, Ochoa M, Bernstein R, Hintze TH. Selective impairment of vagally mediated, nitric oxide-dependent coronary vasodilation in conscious dogs after pacing-induced heart failure. Circulation. 1995;91:2655–2663. doi: 10.1161/01.cir.91.10.2655. [DOI] [PubMed] [Google Scholar]
- 37.Hibbs JB, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophy Res Commun. 1998;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 38.Granger DL, Lehninger AL. Sites of inhibition of mitochondrial electron-transport in macrophage injured neoplastic cells. J Cell Biol. 1982;85:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clementi E, Brown GC, Foxwell N, Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc Natl Acad Sci U S A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor VM, Nunez C, D'Ocon P, Taylor CT, Esplugues JV, Moncada S. Regulaton of oxygen distribution in tissues by endothelial nitric oxide. Circ Res. 2009;104:1178–1183. doi: 10.1161/CIRCRESAHA.109.197228. [DOI] [PubMed] [Google Scholar]
- 41.Pechanova O, Jendekovi L, Vrankova S. Effect of chronic apocynin treatment on nitric oxide and reactive oxygen species production in borderline and spontaneous hypertension. Pharmacol Rep. 2009;61:116–122. doi: 10.1016/s1734-1140(09)70013-1. [DOI] [PubMed] [Google Scholar]
- 42.Huemuller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidase but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 43.Recchia FA. Role of nitric oxide in the regulation of substrate metabolism in heart failure. Heart Fail Rev. 2002;7:141–148. doi: 10.1023/a:1015324508556. [DOI] [PubMed] [Google Scholar]
- 44.Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ Res. 1998;83:969–979. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- 45.Depre C, Hue L. Cyclic GMP in the perfused rat heart: effects of ischemia, anoxia and nitric oxide synthase inhibitor. FEBS Lett. 1994;345:241–245. doi: 10.1016/0014-5793(94)00459-5. [DOI] [PubMed] [Google Scholar]
- 46.Depre C, Vanoverscheltd JLJ, Goudemart JF, Mottet I, Hue L. Protection against ischemic injury by non-selective nitric oxide synthase inhibitors in the perfused rabbit heart. Circulation. 1995;92:1911–1918. doi: 10.1161/01.cir.92.7.1911. [DOI] [PubMed] [Google Scholar]
- 47.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neuro-degenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 48.Depre C, Gaussin V, Ponchaut N, Fisher Y, Vanoverschelde JLJ, Hue L. Inhibition of myocardial glucose uptake by cGMP. Am J Physiol. 1998;274:H1443–H1449. doi: 10.1152/ajpheart.1998.274.5.H1443. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein RW, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, Hintze TH. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res. 1996;79:840–848. doi: 10.1161/01.res.79.4.840. [DOI] [PubMed] [Google Scholar]
- 50.Trochu J, Bouhour J, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism. Circ Res. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 51.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III) J Gen Med. 2008;23:1537–1538. doi: 10.1007/s11606-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]