Abstract
Objective
Autoantibodies against the chromatin remodeler Mi-2 are found in a distinct subset of patients with dermatomyositis (DM). Previous quantitative immunoblotting experiments demonstrated that Mi-2 protein is up-regulated in DM muscle. We undertook this study to define the population of cells expressing high levels of Mi-2 in DM muscle and to explore the regulation and functional role of Mi-2 during muscle regeneration.
Methods
We analyzed the expression of Mi-2 in human muscle biopsy specimens using immunofluorescence. Then, we used cardiotoxin (CTX) to induce muscle injury and repair in the mouse; Mi-2 expression during muscle regeneration was studied in this model by immunofluorescence and immunoblotting analysis. Finally, we utilized a cell culture system of muscle differentiation to artificially modulate Mi-2 levels during myoblast proliferation and differentiation.
Results
In DM muscle, increased Mi-2 expression is preferentially found in myofibers within fascicles affected by perifascicular atrophy, particularly in the centralized nuclei of small perifascicular muscle fibers expressing markers of regeneration. In the mouse, Mi-2 is dramatically and persistently up-regulated during muscle regeneration in vivo. Premature silencing of Mi-2 with RNAi in vitro resulted in accelerated myoblast differentiation.
Conclusions
Mi-2 expression is markedly up-regulated during muscle regeneration in the mouse model. It is also up-regulated in DM myofibers expressing markers of regeneration. In vitro studies suggest that this protein may play a role in modulating the kinetics of myoblast differentiation. We propose that high levels of Mi-2 expression in DM muscle biopsies reflect the presence of incompletely differentiated muscle cells.
The idiopathic inflammatory myopathies are a group of systemic autoimmune disorders characterized by symmetrical proximal muscle weakness, muscle inflammation, and autoantibodies (1–3). Patients with these diseases, which include dermatomyositis (DM) and polymyositis (PM), frequently produce myositis-specific autoantibodies (MSAs) that are associated with distinct clinical phenotypes. For example, autoantibodies directed against the chromatin remodeling enzyme Mi-2 are found in 10–30% of patients with DM (4–6). These individuals tend to have more severe cutaneous manifestations but a better response to steroid therapy and a diminished incidence of malignancy (7–9).
We recently showed by quantitative immunoblotting that Mi-2 protein levels are low in normal human muscle biopsy specimens, but markedly elevated in muscle biopsies obtained from patients with DM (10). Although several other autoantigens were demonstrated to be expressed at high levels in regenerating muscle cells, similar studies were not performed for Mi-2. Consequently, it has not been established which population of cells express high levels of Mi-2 in DM muscle, nor whether such increased expression has functional implications.
Perivascular inflammation and perifascicular atrophy are the hallmark histopathologic features of DM. DM muscle also often includes regenerating myofibers in perifascicular regions as well as areas of preserved muscle fiber morphology within the central regions of muscle fascicles. Since Mi-2, a subunit of the nucleosome remodeling histone deacetylase (NuRD) complex, regulates developmental processes such as vulval development in C. elegans (11) and formation of the epidermal basal cell layer in mice (12), we hypothesized that this protein may also play a role in the repair of muscles damaged by injury or by myopathic processes such as dermatomyositis.
Here, we utilized immunofluorescence microscopy to define the population of cells in DM muscle expressing high levels of Mi-2. To clarify the kinetics of Mi-2 expression in myofibers during muscle regeneration, we used a mouse model of muscle injury and repair. We then established an in vitro myoblast system to explore the functional role of Mi-2 during myoblast differentiation. The results of these studies suggest that incomplete muscle differentiation may underlie the elevated Mi-2 levels observed in DM muscle. Furthermore, we speculate that persistently high levels of Mi-2 play a role in maintaining myofiber plasticity during the process of sculpting regenerating muscle into a mature tissue.
MATERIALS AND METHODS
Mouse muscle injury
All experiments utilizing mice were approved by the Johns Hopkins Animal Care and Use Committee. Six week old C57BL/6 mice were anesthetized with isoflurane, the right legs cleaned with alcohol and shaved with a disposable razor, and the right tibialis anterior (TA) muscles injected with 0.1 mL of 10 μM cardiotoxin (CTX) in PBS. The contralateral, uninjected muscles served as controls. On days 1, 2, 3, 5, 12, 14, and 28 following muscle injury, mice were euthanized and bilateral TA muscles removed. The muscles were frozen rapidly in dry-ice cooled isopentane and stored at −80°C. For protein analysis, muscle tissue was homogenized in Buffer A (20 mM Tris pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1% NP-40, 2.9 μM pepstatin, 20 μM leupeptin, 16 μM antipain, 20 μM chymostatin, and 1 μM PMSF.) For histochemistry and immunofluorescence, 10 micron frozen sections were cut on a Microm HM550 cryostat; specimens form each time point were mounted together on a single slide for simultaneous processing and analysis under identical conditions.
Cell culture, differentiation, and transfections
Normal human skeletal muscle cells from a single donor (Lonza) were cultured as described previously (13). When the cultures were 80% confluent, the cells were induced to differentiate into myotubes by replacing the growth medium with medium containing DMEM, 2% horse serum, and L-glutamine, and growing the cells for a further 2 weeks without subculturing.
C2C12 cells are a murine-derived myoblast cell line obtained from ATCC (14). Proliferating cells were cultured in growth media (DMEM, 10% fetal calf serum, L-glutamine, and pen/strep.) When the cultures reached ~80% confluence, they were induced to differentiate by replacing growth media with differentiation media (DMEM, 2% horse serum, L-glutamine, and pen/strep).
For transfection experiments, 50,000 C2C12 cells were added to each well of a 6-well plate on day -3 and cultured in growth media overnight. On day -2, growth media was replaced by growth media without antibiotics. On day -1, 200 pmoles per well of Mi-2 siRNA (Dharmacon), 200 pmoles per well of non-targeting siRNA (Dharmacon), 4 μg per well of full-length Mi-2 cDNA in pCMV-SPORT6 (obtained from Open Biosystems), or 4 μg per well of vector alone was transfected using Lipofectamine 2000 (Invitrogen) according the manufacturer’s instruction. On day 0, the media was replaced with differentiation media without antibiotics.
Histochemistry and immunofluorescence
Paraffin sections
The collection and use of human biopsy specimens was approved by the Johns Hopkins Institutional Review Board. Muscle biopsy specimens from seven dermatomyositis patients with symmetric proximal muscle weakness, characteristic skin rashes, and typical muscle biopsy findings were studied. Two of these patients had Jo-1 antibodies and one patient had Mi-2 antibodies. Three normal muscle biopsy specimens were also studied.
Paraffin sections were rehydrated by sequential immersions in xylenes, 100% ethanol, 95% ethanol, and distilled water prior to soaking in target retrieval solution (Dako) at 95 °C for 30 minutes. Paraffin sections were then blocked, incubated with Mi-2 (Novus Biologicals) and NCAM (Santa Cruz Biotechnology) primary antibodies and then with donkey anti-mouse IgG1 Alexa Fluor 594 and donkey anti-mouse IgG2A Alexa Fluor 488 secondary antibodies (Invitrogen). After washing with PBS, a drop of Prolong Gold antifade reagent with DAPI (Invitrogen) was applied and the slides were coverslipped.
Frozen sections
Frozen mouse muscle sections were stained with hematoxylin and eosin using standard techniques (15). For immunofluorescence studies, frozen mouse muscle sections on slides were fixed with ice-cold 100% methanol for 5 minutes and blocked in PBS containing 5% BSA for 30 minutes at room temperature. Sections were incubated in a humidified chamber for 60 minutes at 37°C in PBS containing 1% BSA along with the following primary antibodies: rat anti-laminin (Chemicon) and affinity purified rabbit anti-Mi-2 (raised against the following peptide: RLQMSERNILSRLANRC). The sections were washed and then incubated with donkey anti-rat Alexa Fluor 488 and anti-rabbit Alexa Fluor 594 secondary antibodies (Invitrogen) in PBS with 1% BSA at 37 °C for 90 minutes and visualized as described above. Muscle injury time course experiments were performed twice with similar results on each occasion.
Cultured myoblasts
For immunofluorescence studies on cultured myoblasts, C2C12 cells were permeabilized with 100% methanol at −20°C for 5 minutes. Next, these were blocked and incubated with mouse anti-myosin heavy chain (MF20 from Developmental Studies Hybridoma Bank) as described above. After washing, the cells were incubated with donkey anti-mouse Alexa Fluor 594 secondary antibody (Invitrogen) diluted in PBS with 1% BSA. After washing with PBS, a drop of Vectashield Hard Set mounting media with DAPI (Vector) was applied.
Immunoblotting of antigens from cultured cells and mouse muscle tissue
Biochemical levels of antigens expressed in C2C12 cells and human myoblasts were quantitated by immunoblotting as follows: cells were harvested immediately after changing into differentiation media (day 0) and at days 1, 2, 3, 4, and 5 (C2C12 cells) or days 1,2,3,4,5,6, and 8 (human myoblasts). Culture dishes were washed twice in PBS and the cells were lysed in Buffer A containing protease inhibitors. 5 μg of C2C12 cell lysates and 15 μg of mouse muscle lysates were electrophoresed on 8% SDS-polyacrylamide gels, transferred to nitrocellulose membrane, and immunoblotted with the following primary antibodies: patient sera monospecific for Mi-2 or monoclonal antibodies against myogenin (Santa Cruz), vinculin (Sigma-Aldrich), and myosin heavy chain (MF 20) as previously described (16, 17). RNAi time course experiments were performed four times with similar results for each antigen on every occasion. Immunoblots were quantified by densitometry, and the data were normalized for each antigen relative to vinculin blotted in the same lysate.
RESULTS
Mi-2 expression is increased in small perifascicular myofibers expressing NCAM in DM muscle
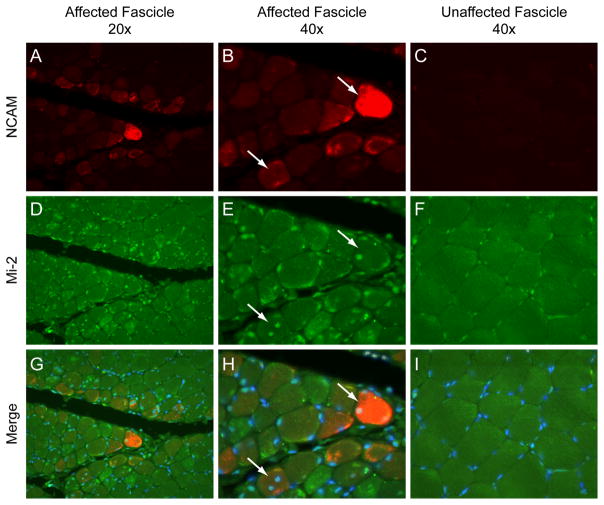
Although Mi-2 levels are quantitatively higher in DM muscle compared to control muscle (10), the population of cells expressing high levels of Mi-2 has not been described. To study Mi-2 expression in individual DM muscle cells, we used immunofluorescence microscopy to stain human muscle biopsy specimens. Sections were co-incubated with anti-NCAM antibodies as a marker of muscle fiber regeneration. As expected from previous immunoblotting experiments, myonuclei from three normal biopsy specimens had low levels of Mi-2 and NCAM expression (data not shown). However, in the seven DM muscle biopsy specimens studied, myofibers within fascicles with perifascicular atrophy frequently contained nuclei with high levels of Mi-2 expression (figure 1). Those fibers with nuclei staining strongly for Mi-2 tended to be small, located at or near the edges of the fascicle, and expressed NCAM. Centralization of nuclei is another characteristic feature of regenerating myofibers and most of these stained intensely for Mi-2 as well. Interestingly, some perifascicular fibers included centralized nuclei with high levels of Mi-2 expression as well as subsarcolemmal nuclei with relatively low levels of Mi-2. In histopathologically normal and intrasfascicular regions from DM muscle biopsies, Mi-2 and NCAM expression was comparatively low and indistinguishable from that seen in normal muscle biopsies (figure 1 and data not shown).
Figure 1. Expression of Mi-2 and NCAM in DM muscle.
Paraffin cross sections from a DM muscle biopsy specimen were analyzed by immunofluorescence. (A, D, and G) A low power (20 X) view of affected fascicles shows the highest expression of cytoplasmic NCAM (red) and nuclear Mi-2 (green) in perifascicular regions. (B, E, and H) A higher power (40X) view of the same region with arrows indicating fibers with centralized nuclei staining strongly for Mi-2 and NCAM. (C, F, and I) Nuclei of histologically normal appearing muscle from the middle of a fascicle from the same specimen express no NCAM and relatively low levels of Mi-2. To insure comparability, images B, C, E, F, H, and I were obtained using identical exposure settings for each channel. Images show NCAM alone (A, B, and C), Mi-2 alone (D, E, and F), and merged NCAM, Mi-2, and DAPI (G, H, I).
Mi-2 is up-regulated during muscle regeneration in vivo
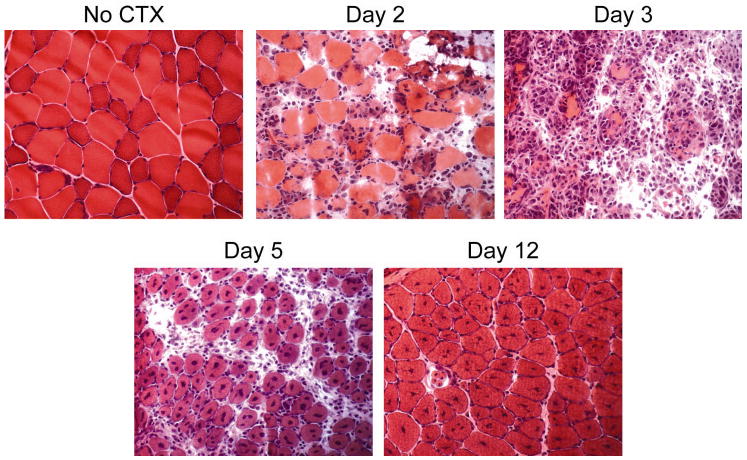
Increased Mi-2 expression in the centralized nuclei of small myofibers expressing high levels of NCAM suggested that high levels of Mi-2 expression might be a feature of regenerating muscle. To define the kinetics of Mi-2 expression during muscle regeneration, we used the cardiotoxin (CTX) model of mouse muscle injury and repair. In this well-characterized model, muscles injected with CTX undergo necrosis followed by virtually complete regeneration (18). We injected the tibialis anterior muscles of mice with CTX and sacrificed the animals 1, 2, 3, 5, 12, and 14 days later. As shown in figure 2, CTX-treated muscle undergoes degeneration on days 2 and 3 characterized by extensive myonecrosis and infiltration of the tissue by inflammatory cells. By day 5, however, the tissue is densely packed with regenerating myofibers containing one or more centralized nuclei. On day 12, the muscle tissue appears relatively normal with the exception that many fibers are moderately atrophic and contain centralized nuclei; these are classic findings of recently regenerated myofibers.
Figure 2. CTX-treated muscle undergoes degeneration and subsequent repair.
Uninjured and CTX-injected tibialis anterior muscle specimens were obtained from mice sacrificed at days 2, 3, 5, and 12 following muscle injury. These samples were flash frozen, sectioned, stained with H&E, and visualized by light microscopy. The myofibers underwent extensive destruction followed by regeneration; with the exception of persistent internalization of myonuclei, muscle regeneration is virtually complete by day 12 after muscle injury.
Immunoblotting equal protein amounts of lysates from CTX-injected muscle (figure 3) shows that levels of adult muscle-specific myosin heavy chain (MyHC) are strikingly decreased at day 1 and absent by day 2. As expected, myogenin (a myogenic regulating factor present exclusively in differentiating myoblasts) is absent in control muscle but is transiently up-regulated during muscle regeneration, peaking on day 3 following CTX injection. By day 5, when myofibers have begun to reform, MyHC protein levels return to normal as well.
Figure 3. Mi-2 expression is up-regulated during muscle regeneration.
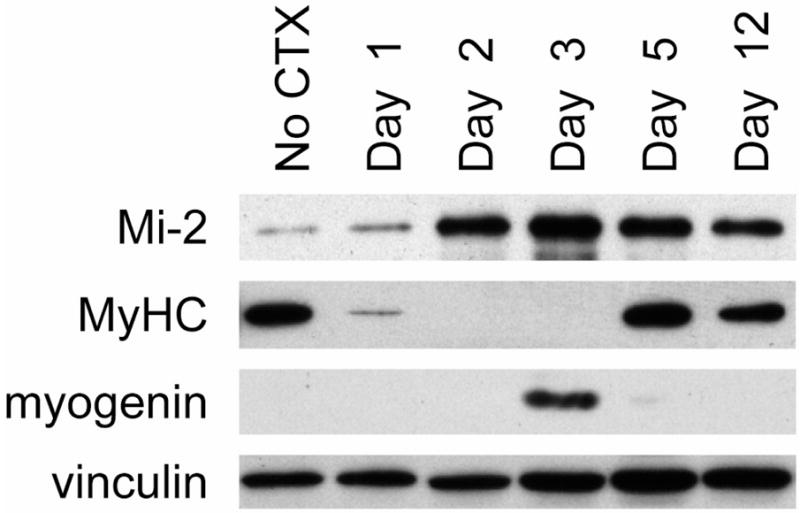
Muscle cell lysates were prepared from uninjured and CTX-injected tibialis anterior muscles at days 1, 2, 3, 5, and 12 following muscle injury. Equal protein amounts were immunoblotted with antibodies against Mi-2, myosin heavy chain (MyHC), myogenin, and vinculin (included as a loading control). Mi-2 expression levels are low in uninjured muscle, peak at day 3 following muscle injury, and remain elevated even 12 days after CTX injection.
In agreement with previous work showing low levels of Mi-2 in normal human muscle biopsies (10), we found uninjured mouse muscle has low levels of Mi-2 protein (figure 3.) Two days following CTX injection, Mi-2 levels began to rise; these levels peaked around day 3 following muscle injury, coinciding with the peak activation of myoblasts. Interestingly, Mi-2 levels remained significantly elevated even 12 days after CTX injection; as shown in figure 2, muscles still have morphological features of regeneration (such as internalized nuclei) at this time.
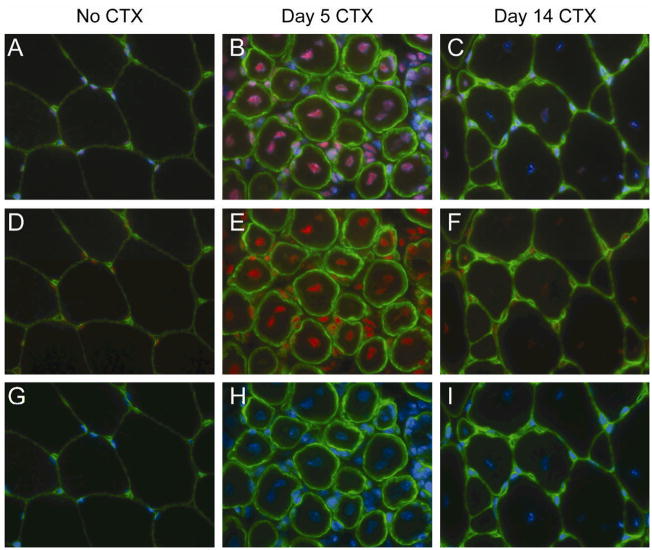
In order to localize Mi-2 expression within normal and regenerating muscle, we performed immunofluorescence staining on muscle sections from uninjured and CTX-treated mouse muscles. As expected, Mi-2 staining is faint and localized to subsarcolemmal nuclei in normal mouse muscle specimens (figure 4). In muscle harvested 5 days after CTX injection, the most intense Mi-2 staining is found within the large centralized nuclei of regenerating myofibers; Mi-2 staining is also observed within the nuclei of myoblasts and some residual inflammatory cells within the endomysium. On day 14 after CTX injection, Mi-2 staining is still more intense than in uninjured muscle and restricted to the centralized nuclei of regenerated myofibers; no endomysial inflammatory cells were observed at this time.
Figure 4. Mi-2 expression is up-regulated in regenerating myonuclei.
Frozen sections from uninjured and CTX-treated tibialis anterior muscles (day 5 and day 14) were mounted on a single slide and double labeled with antibodies against Mi-2 (red) and laminin (to reveal the basal lamina of individual myofibers; green). The sections were counterstained with DAPI (blue) to identify nuclei. To insure comparability, images were obtained using identical exposure settings for each section. Merged images show Mi-2, laminin, and DAPI (A–C), Mi-2 and laminin (D–F), or DAPI and laminin (G–I).
Mi-2 is down-regulated during myoblast differentiation in vitro
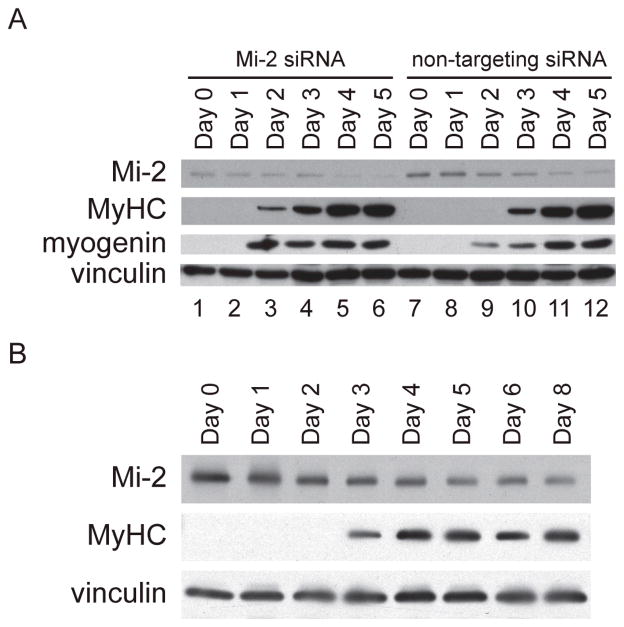
The observation that Mi-2 is expressed at high levels in regenerating myofibers both in DM muscle biopsy specimens and in a mouse model of muscle injury and repair suggests that this chromatin remodeling enzyme may play a functional role during muscle differentiation. In order to study and perturb the expression of Mi-2 during myoblast differentiation, we utilized the mouse-derived C2C12 cell line. These myoblasts proliferate when grown at low density in serum-rich media, but differentiate and fuse to form multinucleated myotubes when allowed to reach confluence in media containing only 2% horse serum (14). Immunoblotting experiments confirmed that proliferating C2C12 myoblasts do not express muscle-specific proteins such as myogenin and MyHC (see figure 5a; day 0, non-targeting siRNA lane). However, these cells begin to express myogenin and MyHC on day 2 and 3, respectively, after placing them in differentiation media. In contrast to the in vivo model of muscle regeneration where high levels of myogenin are found only transiently after muscle injury, myogenin protein levels remained elevated in C2C12 cells for the duration of our experiments. This probably reflects the limited ability of this cell line to fully differentiate and form mature myotubes under routine culturing conditions (19).
Figure 5. Knockdown of Mi-2 accelerates the expression of muscle-specific proteins.
(5a) On day -1, C2C12 cells proliferating in growth media were transfected with Mi-2 or non-targeting siRNA. The next day (day 0), myoblasts were harvested or placed in differentiation media. At days 1, 2, 3, 4, and 5 protein lysates were prepared from differentiating cells. Equal amounts of protein were loaded and immunoblotted with antibodies against Mi-2, MyHC, myogenin, and vinculin. Mi-2 levels are reduced on the first day after transfection with Mi-2 siRNA (day 0). Myogenin and MyHC expression are accelerated by one day in the cultures treated with Mi-2 siRNA. This experiment was performed 4 times with similar results. (5b) Human myoblasts were placed in differentiation media at day 0 and harvested at days 1, 2, 3, 4, 5, 6, and 8. Equal amounts of protein were loaded and immunoblotted with antibodies against Mi-2, MyHC, and vinculin.
We found that C2C12 cells proliferating in growth media express high levels of Mi-2 (figure 5a; non-targeting siRNA; lanes 7–12). This mirrors our finding in CTX-treated muscle, where tissue containing proliferating myoblasts has the highest level of Mi-2 expression. Over the course of a week in differentiation media, C2C12 cells fuse to form multinucleated myotubes and express progressively less Mi-2. This parallels the in vivo muscle regeneration model where mature myofibers expresses the least Mi-2 protein. Thus, the regulated expression of Mi-2 during C2C12 cell differentiation appears to recapitulate some aspects of the expression pattern of Mi-2 during myoblast differentiation in vivo. However, it should be noted that whereas Mi-2 levels decline dramatically during a week of C2C12 cell differentiation, they remain high for at least two weeks in regenerating mouse muscle tissue. This suggests that the differentiation of myofibers in vivo following muscle injury is more complex than seen in the C2C12 system and that the persistently elevated Mi-2 levels within damaged muscle tissue reflects an ongoing regenerative process.
To confirm that the expression of Mi-2 in C2C12 cells mirrors its expression in human cells, we performed a similar study in cultured human myoblasts. As in the mouse cell line, human myoblasts express low levels of MyHC and high levels of Mi-2 when proliferating in serum-rich media. However, as these cells differentiate and begin to express MyHC under conditions of serum-starvation, Mi-2 protein levels are sharply reduced (figure 5b).
Premature down-regulation of Mi-2 accelerates the differentiation of C2C12 cells
Since Mi-2 is down-regulated as myoblasts differentiate, we hypothesized that high levels of Mi-2 may function to restrain full myoblast differentiation. To test this, we used Mi-2 RNAi to prematurely down-regulate Mi-2 during myoblast differentiation. In these experiments, proliferating C2C12 cells at approximately 40% confluence were transfected in growth media with Mi-2 RNAi or non-targeting RNAi. Sixteen hours later (at the “day 0” timepoint) transfected cells were either collected for protein analysis or placed in differentiation media. Western blot analysis showed that at day 0, Mi-2 levels were approximately 70% reduced in those cells transfected with Mi-2 RNAi (Figure 5; lanes 1–6) relative to cells transfected with control RNAi (Figure 5; lanes 7–12). We were unable to fully silence Mi-2 in these experiments, perhaps because of its long half-life prior to the initiation of differentiation.
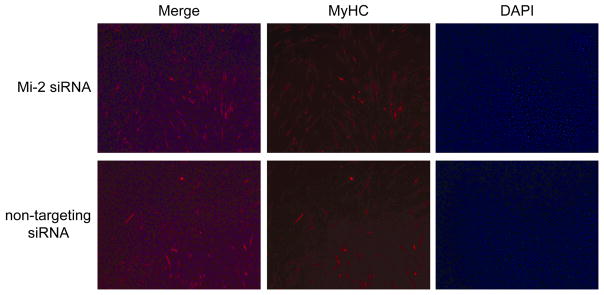
Interestingly, we found that premature down-regulation of Mi-2 with RNAi consistently resulted in the accelerated expression of myogenin and myosin heavy chain (Fig 5; lanes 1–6 compared to lanes 7–12). To determine whether premature reduction of Mi-2 protein levels in differentiating myoblasts also accelerates morphological features of differentiation, we performed immunofluorescence on C2C12 cells transfected with Mi-2 RNAi or non-targeting RNAi. After 48 hours in differentiation media, we found no difference in the number of DAPI positive nuclei in the two sets of cultures (figure 6). However, we found that the number of MyHC expressing myotubes was significantly increased in the cultures transfected with Mi-2 RNAi (48.3 +/− 11.7 tubes/20X field) compared to those transfected with control RNAi (27 +/− 6.5 tubes/20X field). This suggests that high levels of Mi-2 present in myoblasts may inhibit the formation of myotubes, a critical step in the myogenic differentiation pathway.
Figure 6. Knockdown of Mi-2 accelerates the formation of MyHC expressing myotubes.
48 hours after transfection with Mi-2 or non-targeting siRNA, cultures of C2C12 cells were fixed with methanol and stained with MyHC antibodies (MF20) and DAPI. The number of MHC positive tubes and DAPI staining nuclei in four random 20X fields were counted for each condition. The density of nuclei is similar in each condition. However, the cells treated with Mi-2 siRNA have a marked increase in the number of myotubes expressing MyHC (48.3 +/− 11.7 vs. 27 +/− 6.5 tubes/20X field; confidence interval 98.09%).
Since myoblast differentiation was accelerated by premature down-regulation of Mi-2, we considered the possibility that maintaining high levels of Mi-2 expression might disrupt myoblast differentiation. To explore this, we transfected proliferating C2C12 cells with either an expression vector encoding the full-length Mi-2 protein or vector alone. Sixteen hours after the transfections (at the “day 0” time point), cells were either collected for protein analysis or placed in differentiation media. Immunoblotting revealed that Mi-2 levels were several fold higher at day 0 in the cells transfected with vector encoding Mi-2 (data not shown). However, this effect was transient; by day 2, Mi-2 levels were equivalent in cells transfected with Mi-2-expressing constructs and those transfected with vector alone (data now shown.) We speculate that the induction of efficient proteolytic pathways during initiation of differentiation may degrade Mi-2 even in the face of its increased expression during these experiments. Consequently, we have not yet been able to determine how prolonging high-level expression of Mi-2 in myoblasts affects their ability to differentiate.
DISCUSSION
Mi-2 is a member of the SNF2 superfamily of chromatin remodeling enzymes and was first identified as an autoantigen in patients with DM (6, 20, 21). Through its association with other members of the NuRD complex, Mi-2 regulates gene expression by modifying chromatin accessibility (22).
Emerging evidence indicates that Mi-2 participates in regulating developmental programs. In mice, for example, Mi-2 is expressed at high levels in the embryonic ectoderm and developing hair follicle where it plays a critical role in the development of the epidermis and its associated structures (12). Selective inactivation of Mi-2 in the basal epidermis has a variety of phenotypic effects depending upon when during development Mi-2 levels are reduced. For example, early loss of Mi-2 leads to depletion of the basal and superficial layers by preventing the development of epidermal stem cells; hair follicles are also absent. When Mi-2 is depleted later during the developmental process, epidermal basal cells are established and are still capable of differentiating into the epidermis. However, these Mi-2-deficient basal cells cannot initiate follicle formation, suggesting that Mi-2 sustains the plasticity of basal cells to assume a follicular fate. Other studies have shown that Mi-2 suppresses vulval development genes in C. elegans (11) and participates in the repression of HOX gene expression in Drosophila (23). However, the expression of Mi-2 during muscle regeneration has not been explored.
In a prior study, we performed quantitative immunoblotting which showed that Mi-2 is highly expressed in DM muscle compared to control muscle. Although DM muscle may contain proliferating myoblasts, newly-formed myofibers, and mature muscle cells, we did not identify which cells expressed high levels of Mi-2. Here, we used immunofluorescence to study the expression of Mi-2 in cells from human muscle biopsy specimens. Whereas the nuclei of normal muscle fibers had low levels of Mi-2 protein, the nuclei of many myofibers from DM patients had high levels of Mi-2. These myofibers were typically small, located in perifascicular regions, and expressed NCAM, a marker of muscle regeneration. Perifascicular myofibers often included centralized nuclei, another feature of muscle regeneration, and these nuclei frequently stained very strongly for Mi-2. In contrast, myofibers in unaffected fascicles and those located near the center of mildly affected fascicles expressed low levels of NCAM and nuclear Mi-2. Taken together, these observations suggested that Mi-2 is expressed at high levels preferentially in myofibers with characteristic features of regeneration.
Given the scarcity of biopsies obtained from Mi-2 positive patients, we cannot address whether there is any difference in Mi-2 expression levels in specimens from Mi-2 autoantibody positive versus Mi-2 autoantibody negative patients. However, our previous quantitative immunoblotting studies showed that at least 60% of muscle biopsies from DM patients had elevated Mi-2 protein levels compared to controls (10). Since only 20% of DM patients have Mi-2 autoantibodies, it is very unlikely that all patients expressing high levels of Mi-2 autoantigen are Mi-2 autoantibody positive. The Mi-2 immune response likely requires additional contributions, including from the MHC complex.
To define the kinetics of Mi-2 expression in regenerating muscle, we used the CTX model of mouse muscle injury and repair. We found that, as in normal human muscle, undamaged mouse muscle expresses very low levels of Mi-2. However, following muscle injury, nuclear Mi-2 expression is dramatically up-regulated and peak levels coincide with the expression of myogenin, a marker of myoblast differentiation. As newly regenerated myofibers replace the damaged tissue, Mi-2 levels slowly decline, but remain substantially elevated over a period of weeks.
Since Mi-2 participates in other differentiation processes, we hypothesized that Mi-2 might play a role in regulating myoblast differentiation during muscle repair. To study the functional role of Mi-2 in a system amenable to manipulation, we used C2C12 cells as an in vitro model of myoblast proliferation and differentiation. As suspected based on the in vivo results, proliferating myoblasts express high levels of Mi-2 which are down-regulated as the cells differentiate and fuse to form myotubes. We attempted to maintain high levels of Mi-2 expression in myoblasts by transfecting them with a Mi-2 expression vector. Although these cells transiently over-expressed Mi-2, high levels of Mi-2 were not maintained for more than 48 hours and so we could not assess the impact of persistently high Mi-2 levels on myoblast differentiation. However, when Mi-2 expression was prematurely decreased using RNAi, myoblast differentiation was accelerated as measured by both biochemical and morphological indices. These results suggest that in vitro, high levels of Mi-2 are associated with proliferating myoblasts and that down-regulation accelerates their differentiation.
Although the studies using C2C12 cells are intriguing, it should be emphasized that these cells have major limitations in reflecting muscle tissue development and regeneration. Furthermore, neither the mouse model of muscle injury and repair nor in vitro models of myoblast differentiation reflect the systemic processes encountered by muscle during a disease process such as DM. Since these models provide, at best, only an approximation of how muscle regeneration might occur in DM, the functional role of high levels of Mi-2 in regenerating DM muscle fibers remains an open but important question.
Although many critical steps of muscle repair take place within a week of muscle injury, certain characteristic histological features of regeneration, such as myofiber atrophy and centralized nuclei, persist for many months (24). Furthermore, Matsuura and colleagues have shown that biochemical differentiation continues for weeks after muscle injury (25). Their study shows that normal soleus muscle includes ~ 53% fast twitch fibers, ~ 35% slow twitch fibers, and ~ 12% hybrid-type fibers. Two weeks after injury, there were ~ 29% fast fibers, ~ 38% slow fibers, and ~33% hybrid fibers; after 8 weeks, there were ~15% fast fibers, ~80% slow fibers, and 5% hybrid fibers. These findings indicate that regenerating muscle remains functionally plastic for up to 2 months.
The current study reveals that Mi-2 levels remain elevated for at least two weeks following muscle injury in the mouse, suggesting that Mi-2 plays a role in regulating long-term aspects of muscle regeneration. Since our in vitro studies support a role for Mi-2 in restraining myoblast differentiation, we speculate that high levels of Mi-2 might function to maintain regenerating muscle in a plastic state during the later stages of muscle re-sculpting. We plan to test this hypothesis in future experiments using mutant mice to modulate Mi-2 expression during muscle regeneration in vivo.
The presence of NCAM-positive myofibers in DM muscle biopsies with nuclei expressing high levels of Mi-2 suggests that such fibers are regenerating, but remain incompletely differentiated. Although these studies are consistent with our theory that regenerating myofibers are the most likely source of autoantigen driving the Mi-2 autoantibody response, it is not yet known whether Mi-2 over-expression changes the immunological properties of these cells. Future studies will be directed towards answering this question as well as identifying differentiation pathways regulated by Mi-2 during muscle regeneration; we predict these pathways may be important in coordinating muscle remodeling after injury and could be up-regulated in DM muscle by persistently high levels of Mi-2.
Supplementary Material
Acknowledgments
Dr. Mammen’s work was supported by the NIH (grant K08-AR-054783) and a Passano Physician Scientist award. Dr. Casciola-Rosen’s work was supported by the NIH (R01-AR-044684). Dr. Christopher-Stine’s work is supported by the NIH (grant K23-AR-053197). Dr. Rosen’s work is supported by the NIH (grantR37-DE-12354). These studies were also supported by the Stabler Foundation. Dr. Rosen is a Cosner Scholar in Translational Research.
References
- 1.Suber TL, Casciola-Rosen L, Rosen A. Mechanisms of disease: autoantigens as clues to the pathogenesis of myositis. Nat Clin Pract Rheumatol. 2008 Apr;4(4):201–9. doi: 10.1038/ncprheum0760. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003 Sep 20;362(9388):971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 3.Christopher-Stine L, Plotz PH. Adult inflammatory myopathies. Best Pract Res Clin Rheumatol. 2004 Jun;18(3):331–44. doi: 10.1016/j.berh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo R, Gambari PF, et al. Anti-Mi-2 antibodies. Autoimmunity. 2005 Feb;38(1):79–83. doi: 10.1080/08916930400022681. [DOI] [PubMed] [Google Scholar]
- 5.Targoff IN, Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985 Jul;28(7):796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- 6.Reichlin M, Mattioli M. Description of a serological reaction characteristic of polymyositis. Clin Immunol Immunopathol. 1976 Jan;5(1):12–20. doi: 10.1016/0090-1229(76)90145-8. [DOI] [PubMed] [Google Scholar]
- 7.Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991 Nov;70(6):360–74. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hengstman GJ, Vree Egberts WT, Seelig HP, Lundberg IE, Moutsopoulos HM, Doria A, et al. Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2 beta antigen. Ann Rheum Dis. 2006 Feb;65(2):242–5. doi: 10.1136/ard.2005.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targoff IN. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002 Nov;28(4):859–90. viii. doi: 10.1016/s0889-857x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 10.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005 Feb 21;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solari F, Ahringer J. NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr Biol. 2000 Feb 24;10(4):223–6. doi: 10.1016/s0960-9822(00)00343-2. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi M, Morgan BA, Georgopoulos K. The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development. 2007 Apr;134(8):1571–82. doi: 10.1242/dev.001750. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraju K, Casciola-Rosen L, Rosen A, Thompson C, Loeffler L, Parker T, et al. The inhibition of apoptosis in myositis and in normal muscle cells. J Immunol. 2000 May 15;164(10):5459–65. doi: 10.4049/jimmunol.164.10.5459. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22–29;270(5639):725–7. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 15.http://neuromuscular.wustl.edu/pathol/histol/h&e.htm
- 16.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995 Dec 1;182(6):1625–34. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003 Dec 15;42(8):933–45. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Cooper ST, Maxwell AL, Kizana E, Ghoddusi M, Hardeman EC, Alexander IE, et al. C2C12 co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil Cytoskeleton. 2004 Jul;58(3):200–11. doi: 10.1002/cm.20010. [DOI] [PubMed] [Google Scholar]
- 20.Seelig HP, Moosbrugger I, Ehrfeld H, Fink T, Renz M, Genth E. The major dermatomyositis-specific Mi-2 autoantigen is a presumed helicase involved in transcriptional activation. Arthritis Rheum. 1995 Oct;38(10):1389–99. doi: 10.1002/art.1780381006. [DOI] [PubMed] [Google Scholar]
- 21.Seelig HP, Renz M, Targoff IN, Ge Q, Frank MB. Two forms of the major antigenic protein of the dermatomyositis-specific Mi-2 autoantigen. Arthritis Rheum. 1996 Oct;39(10):1769–71. doi: 10.1002/art.1780391029. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998 Oct 16;95(2):279–89. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 23.Kehle J, Beuchle D, Treuheit S, Christen B, Kennison JA, Bienz M, et al. dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science. 1998 Dec 4;282(5395):1897–900. doi: 10.1126/science.282.5395.1897. [DOI] [PubMed] [Google Scholar]
- 24.Salvini TF, Morini CC, Selistre de Araujo HS, Ownby CL. Long-term regeneration of fast and slow murine skeletal muscles after induced injury by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (broad-banded copperhead) venom. Anat Rec. 1999 Apr 1;254(4):521–33. doi: 10.1002/(SICI)1097-0185(19990401)254:4<521::AID-AR7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura T, Li Y, Giacobino JP, Fu FH, Huard J. Skeletal muscle fiber type conversion during the repair of mouse soleus: potential implications for muscle healing after injury. J Orthop Res. 2007 Nov;25(11):1534–40. doi: 10.1002/jor.20451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.