Abstract
Nitric oxide (NO) induces airway smooth muscle cell (SMC) relaxation, but the underlying mechanism is not well understood. Consequently, we investigated the effects of NO on airway SMC contraction, Ca2+ signaling, and Ca2+ sensitivity in mouse lung slices with phase-contrast and confocal microscopy. Airways that were contracted in response to the agonist 5-hydroxytryptamine (5-HT) transiently relaxed in response to the NO donor, NOC-5. This NO-induced relaxation was enhanced by zaprinast or vardenafil, two selective inhibitors of cGMP-specific phosphodiesterase-5, but blocked by ODQ, an inhibitor of soluble guanylyl cyclase, and by Rp-8-pCPT-cGMPS, an inhibitor of protein kinase G (PKG). Simultaneous measurements of airway caliber and SMC [Ca2+]i revealed that airway contraction induced by 5-HT correlated with the occurrence of Ca2+ oscillations in the airway SMCs. Airway relaxation induced by NOC-5 was accompanied by a decrease in the frequency of these Ca2+ oscillations. The cGMP analogues and selective PKG activators 8Br-cGMP and 8pCPT-cGMP also induced airway relaxation and decreased the frequency of the Ca2+ oscillations. NOC-5 inhibited the increase of [Ca2+]i and contraction induced by the photolytic release of inositol 1,4,5-trisphosphate (IP3) in airway SMCs. The effect of NO on the Ca2+ sensitivity of the airway SMCs was examined in lung slices permeabilized to Ca2+ by treatment with caffeine and ryanodine. Neither NOC-5 nor 8pCPT-cGMP induced relaxation in agonist-contracted Ca2+-permeabilized airways. Consequently, we conclude that NO, acting via the cGMP–PKG pathway, induced airway SMC relaxation by predominately inhibiting the release of Ca2+ via the IP3 receptor to decrease the frequency of agonist-induced Ca2+ oscillations.
INTRODUCTION
In the airways and lungs, nitric oxide (NO) is produced by epithelial ciliated cells, type II alveolar cells, and neural fibers that innervate the airway smooth muscle cells (SMCs) (Ricciardolo et al., 2004). It has been suggested that the NO released by these cells decreases airway resistance and that NO, released by neural fibers, is a major nonadrenergic, noncholinergic neurotransmitter responsible for airway SMC relaxation (Belvisi et al., 1995). In addition, airway inflammation is associated with a considerable increase in NO synthesis by inflammatory cells, including macrophages, mast cells, and neutrophils. However, in asthma, airway inflammation is accompanied by airway hyperresponsiveness, a behavior characterized by increased airway contraction in response to a variety of stimuli that suggests an impediment of the airways to relax in response to NO and other natural or pharmacological bronchodilators (Ricciardolo et al., 2004). The mechanisms responsible for these asthma-associated changes are still not completely understood.
A first step toward understanding how asthma and other obstructive lung diseases alter airway responsiveness is to elucidate the cellular mechanisms regulating changes in airway resistance induced by agonists and NO in healthy individuals. The small intrapulmonary airways are considered a major site for this regulation; however, the contractile responses of the SMCs of these airways are relatively unexplored because they are difficult to isolate and study with conventional methods used to measure cell signaling and/or force development. Consequently, we have adopted a novel approach that combines the use of mouse lung slices and confocal microscopy to simultaneously evaluate Ca2+ signaling in SMCs and airway contraction. With this approach, we previously emphasized that agonist-induced airway contraction is regulated by two intracellular processes or signals (Sanderson et al., 2008). These are (a) the frequency of agonist-induced oscillations in [Ca2+]i, referred to as Ca2+ oscillations (Perez and Sanderson, 2005) (a Ca2+-dependent mechanism), and (b) the magnitude of an agonist-induced increase in the sensitivity of the contractile machinery to [Ca2+]i, referred to as Ca2+ sensitivity (Bai and Sanderson, 2006b) (a Ca2+-independent mechanism). In addition, the relaxation of airway SMCs induced by the activation of β2 adrenergic receptors with isoproterenol (ISO), albuterol, or formoterol was shown to be accompanied by both a cAMP-dependent decrease in the frequency of Ca2+ oscillations and a decrease in Ca2+ sensitivity (Bai and Sanderson, 2006a; Delmotte and Sanderson, 2008, 2009; Ressmeyer et al., 2009). Here, we extended these studies to investigate the mechanisms responsible for airway relaxation induced by NO.
The signaling cascade by which NO induces SMC relaxation has been mainly studied in vascular SMCs of the systemic circulation. In these blood vessels, NO is synthesized in the endothelial cells and diffuses to the adjacent SMCs, where it activates soluble guanylate cyclase (sGC) to synthesize cGMP. This cGMP acts as a second messenger to activate cGMP-dependent PKG and/or other effector proteins, including ion channels, ion pumps, and phosphodiesterases (PDEs) (Carvajal et al., 2000). The phosphorylation of one or more target molecules by PKG and/or a direct activation/inhibition of ion channels by cGMP are believed to lead to SMC relaxation.
However, the details of the mechanism by which an increase in cGMP results in SMC relaxation are often controversial and can vary considerably, and the precise mechanism occurring in the small pulmonary airways is unknown. For example, the contribution of Ca2+-dependent and Ca2+-independent pathways to SMC relaxation can differ with the location of the blood vessels (Chitaley and Webb, 2002; Kitazawa et al., 2009). Furthermore, many of the regulatory proteins participating in these pathways have been shown to be affected by cGMP or PKG. In addition, cGMP/PKG-independent mechanisms for NO-induced SMC relaxation have been proposed (Janssen et al., 2000; Soloviev et al., 2004).
We hypothesized that, in small airway SMCs, NO activates a cGMP/PKG-dependent pathway to decrease the frequency of agonist-induced Ca2+ oscillations and/or the Ca2+ sensitivity of the contractile machinery to induce airway relaxation. To test this hypothesis, we examined the effects of NO on agonist-induced airway contractility and the associated Ca2+ oscillations and Ca2+ sensitivity of the airway SMCs in mouse lung slices. We found that NO mediated a cGMP-dependent reduction of the frequency of the agonist-induced Ca2+ oscillations, and that this inhibitory effect was the result of a reduced Ca2+ mobilization via the inositol trisphosphate (IP3) receptor (IP3R) from internal Ca2+ stores. However, NO had a minimal effect on the Ca2+ sensitivity of the contractile machinery.
MATERIALS AND METHODS
General reagents were obtained either from Life Technologies or Sigma-Aldrich. 3-[2-hydroxy-1-(1-methylethyl)-2-nitrosohydrazino]-1-propanamine (NOC-5), S-nitroso-N-acetylpenicillamine (SNAP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and zaprinast (ZAP) were obtained from EMD. Ryanodine (free of dehydro-ryanodine) was obtained from Invitrogen. Rp-8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphorothioate·Na (Rp-8-pCPT-cGMPS) and cell-permeant caged IP3 (caged IP3-PM; d-2,3-O-isopropylidene-6-O-(2-nitro-4,5-dimethoxy)benzyl-myo-inositol-1,4,5-trisphosphate-hexakis (propionoxymethyl) ester) was obtained from Enzo Life Sciences, Inc. 8Br-cGMP and 8-(4-chlorophenylthio)guanosine-3′, 5′-cyclic monophosphate (8pCPT-cGMP) were obtained from Sigma-Aldrich. Hanks’ balanced salt solution was supplemented with 20 mM HEPES buffer and adjusted to pH 7.4 (sHBSS). Vardenafil HCl (Levitra) was provided by GlaxoSmithKline and Bayer HealthCare. Stock solutions of vardenafil and ZAP were prepared at 10 mM in DMSO. Vardenafil was prepared on the same day of use, whereas ZAP was stored at −20°C. Vardenafil and ZAP were diluted to the final concentration in sHBSS on the same day of use. NOC-5 was dissolved in 0.1 N NaOH to prepare a 100-mM stock solution. Aliquots of this solution were stored at −20°C for up to 3 mo. SNAP was dissolved in DMSO to prepare a 100-mM stock solution. NO is not released from NOC-5 or SNAP in the stock solution at alkaline pH. NOC-5 was diluted to the final concentration in sHBSS within 15 min of the initiation of each experiment. The release of NO from NOC-5 and SNAP only begins when stock solutions are diluted in sHBSS and the pH is neutralized. The rates of release are different for each donor; the half-life (t1/2, time to release 50% of NO) of NO release from NOC-5 and SNAP at pH 7.4, 22°C is 93 and 600 min, respectively (NOC-5 and SNAP data sheets; EMD). Each molecule of SNAP or NOC-5 releases one or two NO equivalents, respectively. We have calculated that ∼2 µM NO will be dissolved in the sHBSS at 15 min after diluting to 10 µM NOC-5.
Lung slices
The methods used to prepare lung slices have been described in detail (Perez and Sanderson, 2005; Perez-Zoghbi and Sanderson, 2007), and only a brief outline is given. Lung slices were prepared from 7–10-wk-old BALB/c mice. The trachea was cannulated, and after opening the chest cavity, the lungs were inflated with ∼1.3 ± 0.1 ml of 2% agarose (low-gelling temperature) in sHBSS, followed by ∼0.2 ml of air to flush the agarose-sHBSS out of the airways and into the distal alveolar space. The agarose was gelled by cooling the lungs with cold sHBSS. A single lung lobe was removed and cut into serial sections of ∼130-µm thick with a vibratome at ∼4°C. Slices were maintained in DMEM at 37°C and 10% CO2. Serum was not used because it contains growth factors (Abdullah et al., 1994). We used lung slices maintained for fewer than 48 h; no significant changes in airway contractility in response to 1 µM 5-hydroxytryptamine (5-HT) were detected during this period.
Measurement of the contractile response of airways
Lung slices were placed on a large cover glass, mounted in a custom-made perfusion chamber, and held in place with a small sheet of nylon mesh. A second cover glass, edged with silicone grease, was placed over the nylon mesh and lung slice. Perfusion of the lung slice within the chamber was maintained at a constant rate during the course of the experiments by a gravity-fed perfusion system. The volume of the chamber was ∼100 µl with a perfusion rate of ∼800 µl/min. The addition and washout of agonists, NO, and drugs were achieved by changing the perfusion solution with a custom-made, computer-controlled valve system. This setup allows multiple challenges of the lung slice with different stimuli without undesired movements of the slice, yet allowing the airways to contract and relax. For phase-contrast microscopy, lung slices were observed with an inverted microscope with a 10× objective, and images were recorded in time-lapse (30 frames/min) using a CCD camera and image acquisition software (Video Savant; IO Industries). The area of the airway lumen was calculated from each image by pixel summing using custom-written software. Experiments were performed at room temperature.
Measurements of intracellular Ca2+
Approximately 10–12 lung slices were incubated in 2 ml of 20 µM Oregon Green 488 BAPTA-1-AM (Invitrogen), 100 µM sulfobromophthalein (an inhibitor temporarily used to prevent dye extrusion through anion exchangers; Sigma-Aldrich), and 0.2% Pluronic F-127 (Invitrogen) for 50 min at 30°C, followed by an additional 50 min at 30°C in sHBSS containing 100 µM sulfobromophthalein. Lung slices were mounted in the perfusion chamber as described. Fluorescence imaging was performed using a video-rate confocal microscope (Sanderson and Parker, 2003). A 488-nm laser supplied the excitation wavelength, and the resultant fluorescence images (>510 nm) were recorded at 15 Hz. Changes in fluorescence intensity were analyzed by selecting regions of interest (ROI) ranging from 5 to 7 pixels2. The average fluorescence intensities of an ROI were obtained, frame by frame, using custom-written software that allowed the tracking of the ROI within an SMC as it moved with contraction. Final fluorescence values (F) were expressed as a fluorescence ratio (F/F0) normalized to the initial fluorescence (F0). Line scan analysis of images was performed by extracting a line of pixels from each image and placing them sequentially to form a time sequence in a single image.
Flash photolysis of caged IP3
Flash photolysis of caged IP3 was used to experimentally increase the [IP3]i. Lung slices were initially loaded with Oregon Green 488 BAPTA-1 AM as described above. Subsequently, slices were incubated at room temperature with 2 µM of caged IP3-PM (for 1 h) in sHBSS containing 0.1% pluronic and 100 µM sulfobromophthalein, followed by de-esterification for 30 min in sHBSS containing 100 µM sulfobromophthalein. The details of the flash photolysis setup have been described (Leybaert and Sanderson, 2001; Bai and Sanderson, 2006a). In brief, a flash of UV light was produced from a mercury arc lamp with a mechanical shutter and focused into the microscope with a biconvex lens (focal distance, 200 mm) through a band-pass filter (330 nm). Flash duration and intensity were controlled by electronic timing and neutral density filters, respectively. The area illuminated was adjusted with an iris diaphragm at a conjugate image plane.
Preparation of Ca2+-permeabilized lung slices
To clamp the [Ca2+]i of airway SMCs, the lung slices were made permeable to external Ca2+ without using detergents or toxins. This approach does not damage the cell membrane and exploits the cell’s inherent Ca2+-permeable ion channels. Lung slices were exposed to 20 mM caffeine with 50 µM ryanodine for 4 min, followed by a washout with sHBSS. This treatment locks the RYRs in the SR of airway SMCs in an open state that depletes the intracellular Ca2+ stores. This, in turn, increases Ca2+ influx via store-operated channels across the plasma membrane. Full details and validation for this technique have been reported (Bai and Sanderson, 2006b). In caffeine-ryanodine–treated lung slices, the [Ca2+]i of airway SMCs is dependent on the extracellular Ca2+ concentration ([Ca2+]e). By exposing the lung slices to sHBSS containing 1.3 mM Ca2+, the [Ca2+]i was clamped at a sustained high level. In all experiments, the [Ca2+]i was examined by confocal microscopy and was confirmed not to change during the addition of experimental compounds.
Statistic values are expressed as mean ± standard error (SE). A Student’s t test was used to test for significant differences between means.
Online supplemental material
Three videos consisting of sequences of phase-contrast or confocal fluorescence images were produced with Video Savant (IO Industries). The playback rate of 30 fps represents an increased time factor of ×15. Videos 1–3 are available at http://www.jgp.org/cgi/content/full/jgp.200910365/DC1.
RESULTS
Airway relaxation induced by NO
To study the effect of NO on airway contraction, we initially stimulated lung slices with the agonist 5-HT at a concentration that produced a submaximal airway contraction (Fig. 1 and Video 1). The addition of 0.1 µM 5-HT induced a fast airway contraction with a reduction in the airway lumen area of 60 ± 9% (mean ± SE; Fig. 1). The subsequent addition of 10 µM NOC-5, an NO donor, induced a biphasic airway relaxation consisting of an initial peak, followed by a partial recontraction to a lower level of sustained relaxation (Fig. 1 B and Table I). The effect of NOC-5 was quickly reversed upon its removal; the airway recontracted to the initial 5-HT–induced contractile state. A similar airway relaxation (Fig. 1 B and Table I) was observed in response to 50 µM SNAP, another NO donor with a different chemical structure and longer lifetime (t1/2 = 600 min for SNAP and t1/2 = 93 min for NOC-5). These results suggest that airway relaxation is induced by NO in a manner that is unrelated to the chemical nature of the donor molecule.
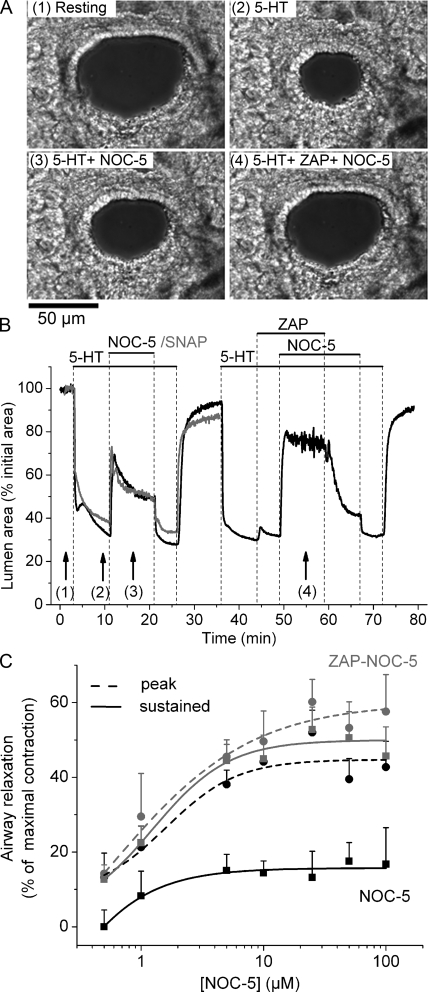
Figure 1.
Airway relaxation induced by NO donors and its enhancement by ZAP. (A) Phase-contrast images showing the appearance of an airway in a lung slice (1) at rest, (2) after 8 min of stimulation with 0.1 µM 5-HT, (3) after relaxation induced by 10 µM NOC-5, and (4) after further relaxation induced by NOC-5 in the presence of 10 µM ZAP (image times indicated by arrows in B). (B, black trace) The change in the cross-sectional area of the lumen of an airway with respect to time showing the contraction in response to 5-HT and subsequent relaxation induced by NOC-5 in the absence or presence of ZAP (top bars). The airway contracted in response to 5-HT and relaxed in response to NOC-5. The effect of NOC-5 was biphasic and quickly reversed upon NOC-5 washout. ZAP alone had little effect on airway contraction but increased the relaxing effect of NOC-5. The effect of ZAP was reversed after washout. (B, gray trace) The relaxation of 5-HT–contracted airways induced by 50 µM SNAP. (C) Concentration dependence of airway relaxation induced by NOC-5 in the absence (black traces) or presence (gray traces) of ZAP. Relaxation was measured as the increase in lumen area after 30 s (peak; dashed lines, circles) or after 10 min (sustained; continuous lines, squares) of NOC-5 exposure from experiments shown in B. In control experiments with 5-HT alone (not depicted), we observed a slow but continued increase in airway contraction of 2.1 ± 1.3 and 1.6 ± 1.1% during the time periods of 12–22 min and 48–58 min, which correspond to the exposure times for NOC-5 or NOC-5/ZAP. Consequently, we corrected the measurements of sustained relaxation at these times by adding the corresponding additional contraction. Each point represents the mean ± SE from at least five different slices from at least two mice. A movie of these data is shown in Video 1.
Table I.
Airway relaxation induced by NOC-5 and its modulation by related compounds
| Experimental conditions | Relaxation (% of maximal contraction) | ||
| Peak | Sustained | n | |
| 5-HT–contracted airways | |||
| NOC-5 (10 µM) | 44 ± 6 | 12 ± 3 | 7 |
| SNAP (50 µM) | 49 ± 12 | 17 ± 9 | 3 |
| NOC-5 + ZAP (10 µM) | 49 ± 8 | 46 ± 6b | 7 |
| NOC-5 + ODQ (5 µM) | 4 ± 2a | 2 ± 1b | 6 |
| NOC-5 + Rp-8-pCPT-cGMPS (20 µM) | 5 ± 3a | 3 ± 2b | 5 |
| ACH-contracted airways | |||
| NOC-5 (10 µM) | 40 ± 7 | 11 ± 6 | 3 |
| NOC-5 + ZAP (10 µM) | 46.4 ± 8.7 | 45.7 ± 7.9b | 3 |
| NOC-5 + VAR (1 µM) | 53.1 ± 9.8 | 52.3 ± 9.2b | 5 |
PDE-5 inhibitors: ZAP and vardenifil (VAR); sGC inhibitor (ODQ); PKG inhibitor (Rp-8-pCPT-cGMPS). The peak and sustained relaxation of airways in response to NOC-5 in the absence or presence of ZAP, ODQ, and Rp-8-pCPT-cGMPS calculated from experiments similar to those shown in Figs. 2 and 3.
P < 0.05 compared to peak relaxation induced by NOC-5 alone.
P < 0.05 compared to sustained relaxation induced by NOC-5 alone.
Because NO is believed to act via increases in cGMP, we examined the effect of reducing the rate of cGMP metabolism with the PDE-5–selective inhibitor ZAP on the airway response to NO. The addition of 10 µM ZAP alone to airways contracted with 5-HT had only a small transient relaxing effect (3.2 ± 1.8%; n = 7 of the maximal contraction). However, the presence of ZAP significantly increased the sustained relaxation induced by the subsequent addition of NOC-5 (Fig. 1 B and Table I); ZAP had no significant effect on the peak relaxation induced by NOC-5 (Table I). The removal of ZAP, by washing with sHBSS containing 5-HT and NOC-5, induced a slow airway recontraction, indicating a reversibility of the effects of ZAP. Subsequent removal of NOC-5 resulted in a further airway contraction to reach the original contracted state.
The extent of airway relaxation induced by NOC-5 increased with the concentration of NOC-5 from 0.5 to 10 µM in both the absence and presence of 10 µM ZAP (Fig. 1 C). In the absence of ZAP, the peak relaxation was significantly greater than the sustained airway relaxation at all NOC-5 concentrations. However, in the presence of ZAP, the peak and sustained airway relaxation were similar. These results suggest that PDE-5 activity in airway SMCs normally reduces the cGMP levels to mitigate the effects of NO.
In airways contracted with 1 µM ACh, a similar effect of NOC-5 and ZAP on peak and sustained relaxation was observed (Table I), indicating that the relaxing effect of NOC-5 and ZAP was not specific to 5-HT. In addition, 1 µM vardenafil, a clinically approved PDE-5 inhibitor, had a similar effect as ZAP and enhanced NOC-5–induced sustained airway relaxation from 11.5 ± 3.7% in the absence of vardenafil to 52.3 ± 9.2% in the presence of vardenafil (five experiments from three different slices). These data suggest that ZAP and vardenafil increased NOC-5–induced airway relaxation by inhibiting cGMP-specific PDE activity.
Effect of sGC and PKG inhibitors on NO-induced airway relaxation
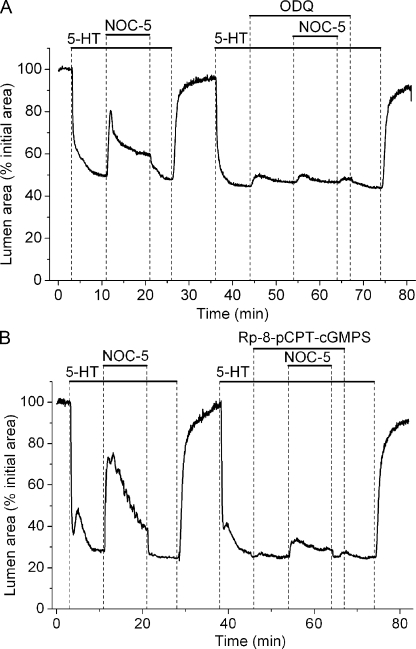
To evaluate if airway relaxation was dependent on cGMP synthesis by NO-activated sGC, we examined the effect of ODQ, a selective inhibitor of sGC, on NO-induced airway relaxation. We found that airway relaxation induced by NOC-5 was significantly inhibited by ODQ (Fig. 2 A and Table I).
Figure 2.
The attenuation of NO-induced airway relaxation by inhibitors of sGC and PKG activity. (A) The effect of 5 µM ODQ, a sGC inhibitor, on airway relaxation induced by NOC-5. The airway, contracted with 5-HT, relaxed in response to NOC-5 in the absence but not in the presence of ODQ. (B) The effect of 20 µM Rp-8-pCPT-cGMPS, a PKG inhibitor, on airway relaxation induced by NOC-5. Airway relaxation induced by NOC-5 was reduced by Rp-8-pCPT-cGMPS. Representative experiments selected from at least five different slices from three mice are shown.
Because these results suggest that NO-induced airway relaxation occurs through a cGMP-dependent mechanism, we further investigated whether NO-induced relaxation requires the activation of PKG by examining the effects of Rp-8-pCPT-cGMPS, a selective PKG inhibitor. At low concentrations (1 µM), Rp-8-pCPT-cGMPS had no significant effect on NOC-5–induced relaxation (five slices from two mice). However, at higher concentrations (20 µM), we found that Rp-8-pCPT-cGMPS strongly inhibited the relaxation induced by NOC-5 (Fig. 2 B and Table I). This concentration of Rp-8-pCPT-cGMPS required to inactivate PKG is similar to the concentration of 8-pCPT-cGMP required to activate PKG (see below). These results suggest that a cGMP-dependent activation of PKG is required for NO-induced airway relaxation.
Effect of NO on SMC Ca2+ signaling
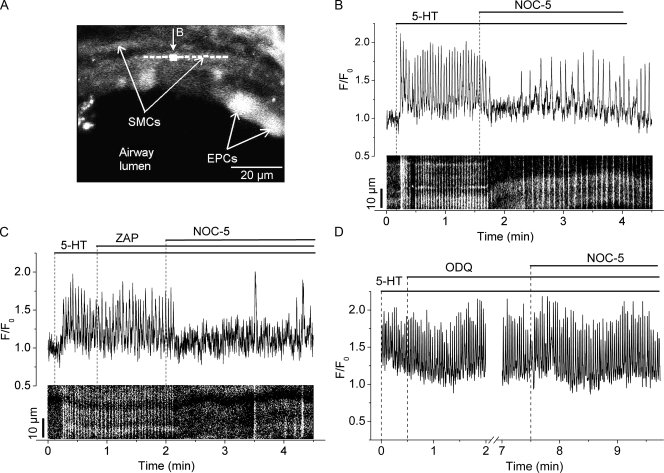
We next examined the effect of NO on 5-HT–induced Ca2+ signaling of airway SMCs. In response to 5-HT, airway SMCs displayed an increase in [Ca2+]i, followed by sustained Ca2+ oscillations (mean frequency, 25.0 ± 6.5 cycles/min; n = 5 SMCs from three slices) that spread throughout the SMCs as intracellular Ca2+ waves (Fig. 3, A and B, and Video 2). The subsequent addition of NOC-5 resulted in a cessation of the Ca2+ oscillations for ∼1 min. This inhibition of the Ca2+ signaling correlated with the relaxation of the airway induced by NOC-5 (Video 2 and Fig. 1). With time, in the continued presence of NOC-5 and 5-HT, the Ca2+ oscillations resumed and slowly increased their frequency to reach a new steady rate, but at a lower frequency (8.0 ± 3.3 cycles/min; n = 5 SMCs from three slices) than the frequency with 5-HT alone (Fig. 3 B). This resumption of the Ca2+ oscillations correlated with the partial recontraction of the airway (Fig. 1).
Figure 3.
The effect of NOC-5, ZAP, and ODQ on the Ca2+ signaling induced by 5-HT in airway SMCs. (A) A fluorescence confocal image of part of an airway in a lung slice showing the epithelial cells (EPCs) lining the airway lumen and the underlying SMCs. (B, top trace) Ca2+ changes in a small region (∼7 × 7 pixels, indicated by the white square in A) within an SMC plotted as a ratio (F/F0) with respect to time showing the effect of 10 µM NOC-5 on the Ca2+ oscillations induced by 0.1 µM 5-HT. In the bottom part of B, a line scan analysis from the longitudinal axis of the SMC (indicated by the dashed line in A) shows the propagation of the Ca2+ oscillations along the SMC as Ca2+ waves (near vertical white lines). The position of both the ROI and the line scan was adjusted to track the same area inside the SMC during airway contraction. NOC-5 transiently blocked the Ca2+ oscillations, which resumed with a lower frequency. (C and D) Ca2+ traces and line scan showing the effect of 10 µM ZAP and 5 µM ODQ on the Ca2+ oscillations induced by 0.1 µM 5-HT and subsequent inhibition by 10 µM NOC-5 in single SMCs. ZAP or ODQ alone had no effect on the Ca2+ oscillations induced by 5-HT. However, in the presence of ZAP, NOC-5 further decreased the frequency and increased the amplitude and duration of the Ca2+ waves (line scan: lines brighter and wider, respectively). In contrast, ODQ blocked the inhibitory action of NOC-5 on the Ca2+ oscillations. Representative data from at least seven different slices from three mice are shown. A movie of the effect of NOC-5 on 5-HT–induced Ca2+ signaling in airway SMCs is shown in Video 2.
Although ZAP alone had little or no effect on the Ca2+ oscillations induced by 5-HT, ZAP significantly increased the inhibitory action of NOC-5 on Ca2+ oscillations (Fig. 3 C). In the presence of ZAP, NOC-5 stopped the Ca2+ oscillations for longer periods (up to 2 min), after which the Ca2+ oscillations reappeared but at much slower frequencies (1.5 ± 0.4 cycles/min; n = 4 SMCs from two slices). These low frequency Ca2+ oscillations continued to form Ca2+ waves, but with a longer duration of elevated Ca2+ (Fig. 3 C, line scan) and an increased amplitude (F/F0 = 0.5 ± 0.1 and 0.8 ± 0.2 before and after NOC-5, respectively) (Fig. 3 C) as compared with those observed in the absence of ZAP. The longer Ca2+ oscillation period may allow for a greater accumulation of Ca2+ in the SR to be available for subsequent release. The greater reduction in the frequency of the Ca2+ oscillations induced by NOC-5 in the presence of ZAP also correlated with a greater relaxation of the airway (Fig. 1 B), suggesting that NO induced airway relaxation by reducing the frequency of the Ca2+ oscillations.
To investigate whether the inhibitory effect of NOC-5 on Ca2+ oscillations was dependent on the activation of sGC by NO, we examined the effects of ODQ on NOC-5 inhibition of 5-HT–induced Ca2+ signaling (Fig. 3 D). ODQ alone had no significant effect on the frequency of Ca2+ oscillations (27.5 ± 1.9 and 26.4 ± 2.3 cycles/min before and 2 min after the addition of ODQ, respectively; n = 5 SMCs from three slices; P > 0.05). However, ODQ completely blocked the ability of NOC-5 to inhibit Ca2+ oscillations; the Ca2+ oscillation frequency was 25.2 ± 2.8 and 27.7 ± 1.5 cycles/min at 1 and 4 min after NOC-5 addition, respectively. These results are consistent with the idea that the effects of NOC-5 are mediated through the NO-induced activation of sGC.
Effect of 8pCPT-cGMP and 8Br-cGMP on airway contraction and Ca2+ signaling
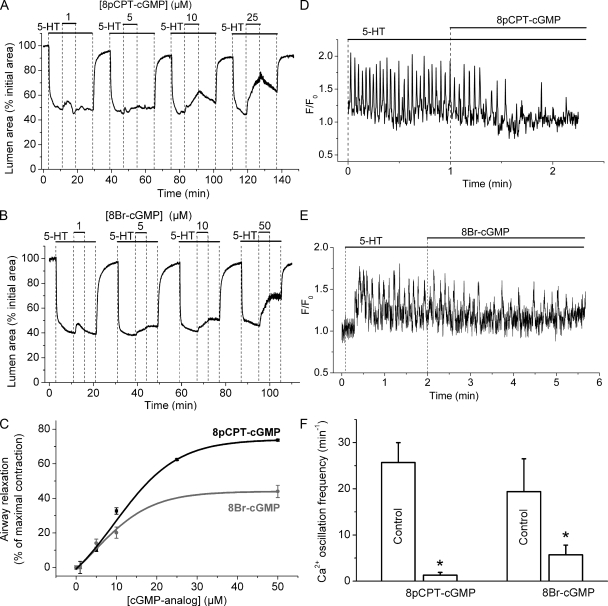
To support the idea that NOC-5 effects were mediated by an increase in cGMP concentration, we studied the effect of the cGMP analogues 8pCPT-cGMP and 8Br-cGMP on the contraction and Ca2+ signaling of airway SMCs (Fig. 4). These cGMP analogues are permeable to cell membranes, resistant to hydrolysis by PDEs, and selective activators of PKG. Increasing concentrations of 8pCPT-cGMP and 8Br-cGMP induced increasing relaxation of 5-HT–contracted airways (Fig. 4, A and B). However, 8pCPT-cGMP induced a greater maximal relaxation than 8Br-cGMP (Fig. 4 C). Compared with NOC-5, the cGMP analogues induced airway relaxation in a slower but more sustained manner that persisted longer after washout of 8pCPT-cGMP or 8Br-cGMP (Figs. 1 B and 4). This slow relaxation kinetics associated with cGMP analogues is consistent with the slow diffusion of the analogues through the plasma membrane and their high resistance to degradation by PDEs.
Figure 4.
Airway relaxation and inhibition of Ca2+ oscillations induced by cGMP analogues. (A and B) Airways contracted with 0.1 µM 5-HT were induced to relax by increasing concentrations (top bars) of (A) 8pCPT-cGMP or (B) 8Br-cGMP. Airways either recontracted slightly or remained relaxed after washout of 8pCPT-cGMP or 8Br-cGMP for 5 min. (C) Concentration–airway relaxation response induced by 8pCPT-cGMP (black line) and 8Br-cGMP (gray line). Relaxation was measured as the increase in the lumen area after 8 min of the addition of cGMP analogues and normalized to the airway contraction before the addition of the cGMP analogue (data points are mean ± SE from four slices from two mice). (D and E) The effect of either 50 µM 8pCPT-cGMP or 8-Br-cGMP on the Ca2+ oscillations induced by 0.1 µM 5-HT. 8pCPT-cGMP and 8Br-cGMP reduced the frequency of the Ca2+ oscillations induced by 5-HT. (F) Summary of the effect of cGMP analogues on the frequency of Ca2+ oscillations measured before (control) and after the addition of cGMP analogues (n = 5 SMCs from different slices from three mice; *, P < 0.05).
In keeping with the reduction of the Ca2+ oscillation frequency by NOC-5, these cGMP analogues gradually reduced the frequency of the 5-HT–induced Ca2+ oscillations until they almost completely stopped (with 8pCPT-cGMP) or attained a new slower steady frequency (with 8Br-cGMP) (Fig. 4 D, E and F). These results suggest that increases in cGMP concentration induce airway relaxation by reducing the frequency of agonist-induced Ca2+ oscillations.
Effect of NO on intracellular Ca2+ release
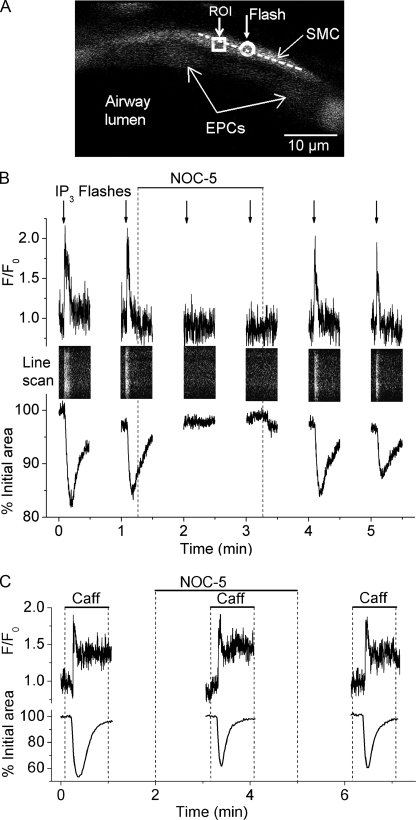
To investigate the mechanism by which NO inhibits Ca2+ oscillations, we studied the effect of NOC-5 on the release of Ca2+ from internal Ca2+ stores via the IP3R by the photolytic release of IP3 from caged IP3 (Fig. 5). In the absence of NOC-5, the photolysis of caged IP3 in a limited area within the SMC (Fig. 5 A, circle) triggered an instantaneous increase in [Ca2+]i in the illuminated area that resulted in a propagating increase in [Ca2+]i along the longitudinal axes of the SMC and a local airway contraction (Video 3). The increase in [Ca2+]i was observed in a selected ROI adjacent to the flash area (Fig. 5 B, top trace) and throughout the length of the SMC by a line scan analysis (Fig. 5 B). Because the flash was restricted to the center of the cell, the increase in fluorescence at the ROI and along the whole cell (bright white line) indicates the Ca2+ increase spread out as a wave from the point of illumination. This is illustrated by Video 3. This Ca2+ wave was accompanied by a transient contraction of the SMC and reduction of the airway lumen area (Fig. 5 B, bottom trace). A second flash exposure a minute later to release IP3 again in the same area stimulated a similar Ca2+ and contractile response. However, in the presence of NOC-5, a third and fourth flash photolysis of caged IP3 triggered neither a Ca2+ nor contractile response. The subsequent removal of NOC-5 by washing with sHBSS alone restored the ability of flash photolysis to trigger a Ca2+ and contractile response (Fig. 5 B). The flash exposure of SMCs not loaded with caged IP3 had no effect on Ca2+ release or contraction, suggesting that the SMC responses to UV flashes described above were dependent on the uncaging of IP3 and not an artifact of the UV flash. In other control experiments, the SMCs responded similarly to six sequential flash exposures, confirming that the quantity of caged IP3 was not a limiting factor. A longer UV flash induced Ca2+ oscillations (Bai et al., 2009) but depleted the caged IP3 to prevent multiple responses.
Figure 5.
The effect of NOC-5 on the release of Ca2+ through IP3Rs. (A) Fluorescence image of part of an airway indicating the area (white circle) within an SMC exposed to light to uncage IP3, the ROI (white square) used to determine the changes in [Ca2+]i with respect to time and the row of pixels (dashed line) used for line scan analysis plotted in B. The position of both the ROI and the line scan were adjusted to track the same area inside the SMC during airway contraction. (B) Simultaneous changes in Ca2+ signaling (top traces and line scans) and contraction (bottom traces) triggered by uncaging IP3 with UV flashes (arrows) in the absence or presence of 10 µM NOC-5 (top bar). The airway was allowed to relax completely between challenges with UV flashes to assure that the same area within the SMC was illuminated. In the absence of NOC-5, each UV flash triggered a transient increase in [Ca2+]i (trace) that was initiated in the flash area and propagated along the SMC as a Ca2+ wave (white vertical line in line scan). The Ca2+ wave was accompanied by a transient airway contraction (bottom). Perfusion with NOC-5 (top bar) blocked the Ca2+ transient and the contraction triggered by releasing IP3. Representative data from seven different slices from two mice are shown. (C) Ca2+ signaling in airway SMCs and airway contraction stimulated by 20 mM caffeine in the absence or presence of 10 µM NOC-5 (top bars). NOC-5 had no apparent effect on the Ca2+ signal and contraction induced by caffeine. Representative data from four different slices from two mice are shown. A movie showing the effect of NOC-5 on the Ca2+ waves triggered by uncaging IP3 in an SMC is shown in Video 3.
These results indicate that NO inhibited IP3-induced release of Ca2+ from the SR via the IP3R. However, these results do not rule out the possibility that the internal Ca2+ store was depleted by NO or that NO may inhibit Ca2+ release by blocking RYRs. To address these issues, we investigated the effect of NOC-5 on caffeine-induced Ca2+ release that represents Ca2+ release via the RYR. Caffeine triggered a biphasic transient increase in Ca2+ that was accompanied by a transient airway contraction (Fig. 5 C). NOC-5 had no effect on this caffeine-induced Ca2+ signaling and contraction, suggesting that NOC-5 did not block release of Ca2+ through the RYR. In addition, the ability of caffeine to stimulate a similar Ca2+ response after exposure to and removal of NOC-5 suggests that the intracellular Ca2+ stores are not altered by NO.
Effect of NO on Ca2+ sensitivity
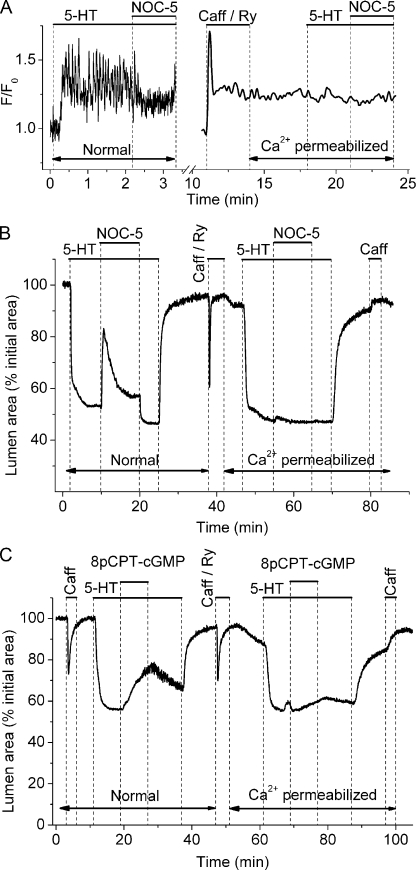
A second major mechanism regulating the contractility of airway SMCs is the sensitivity of the contractile apparatus to Ca2+ or “Ca2+ sensitivity” (Somlyo and Somlyo, 2003). To investigate if NO relaxed airway SMCs by reducing their Ca2+ sensitivity, we compared Ca2+ signaling and airway relaxation induced by NOC-5 or 8pCPT-cGMP before (normal) and after Ca2+ permeabilization of lung slices by the treatment with caffeine and ryanodine (Fig. 6). This Ca2+ permeabilization protocol (see Materials and methods and Bai and Sanderson, 2006b) is based on the combined ability of caffeine to activate and ryanodine to irreversibly lock RYRs in an open state. As a result, the internal Ca2+ stores empty and activate store-operated channels to increase the permeability of the plasma membrane to Ca2+.
Figure 6.
The effect of NOC-5 on Ca2+ sensitivity of airway SMCs. (A) The effect of 10 µM NOC-5 on Ca2+ signaling induced by 0.1 µM 5-HT in the same SMC before (Normal) and after Ca2+ permeabilization with 20 mM caffeine and 50 µM ryanodine in sHBSS ([Ca2+] = 1.3 mM). Before Ca2+ permeabilization, Ca2+ oscillations were induced by 5-HT and inhibited by NOC-5. After Ca2+ permeabilization, [Ca2+]i was constantly elevated, and neither 5-HT nor NOC-5 induced further changes in [Ca2+]i. (B and C) The response of airways contracted with 0.1 µM 5-HT to (B) 10 µM NOC-5 or (C) 10 µM 8pCPT-cGMP in lung slices before and after Ca2+ permeabilization. Both NOC-5 and 8pCPT-cGMP relaxed airways before Ca2+ permeabilization, but neither had much effect on airways after Ca2+ permeabilization. Contraction data are representative of at least seven different slices from three mice. Ca2+ data are representative of three different slices from one mouse.
The effect of NOC-5 on 5-HT–induced Ca2+ signaling before and after Ca2+ permeabilization with caffeine and ryanodine is shown in Fig. 6 A. As reported earlier here, 5-HT stimulated and NOC-5 inhibited Ca2+ oscillations in the normal SMCs. This change in the Ca2+ signaling of the SMCs correlated with the expected increase and decrease in airway contraction (Fig. 6 B). After Ca2+ permeabilization, the [Ca2+]i of the same airway SMCs was elevated by 1.3 mM of extracellular Ca2+ (in sHBSS) and remained constant regardless of the subsequent expose to either 5-HT and NOC-5 (Fig. 6 A). This result confirms our previous results showing no changes in [Ca2+]i in response to stimulation with other agonists (methacholine and ISO) and forskolin in Ca2+-permeabilized lung slices (Bai and Sanderson, 2006b; Perez-Zoghbi and Sanderson, 2007).
Before continuing to address our current results, it is extremely important to understand a specific behavior of mouse airways. Regardless of the fact that the [Ca2+]i is elevated in Ca2+-permeabilized mouse SMCs (Fig. 6 A), the mouse airways are, as observed here, almost completely relaxed (Fig. 6, B and C) in the absence of a contractile agonist. This result, although counterintuitive, is consistently observed and has been extensively investigated in our previous work (Bai and Sanderson, 2006b; Perez-Zoghbi and Sanderson, 2007; Wang et al., 2008). Our hypothesis explaining this behavior is that the elevated [Ca2+]i has reduced the Ca2+ sensitivity of the SMCs to almost 0 because of an increase in myosin light chain phosphatase (MLCP) activity. This MLCP activity counteracts the predicted increased myosin light chain kinase (MLCK) activity that is stimulated by the elevated [Ca2+]i (Wang et al., 2008).
Returning to the current results, the addition of the contractile agonist 5-HT to the Ca2+-permeabilized slices invoked a contractile response that was similar to that of the normal airway (Fig. 6, B and C), but in this case, it occurred without any change in [Ca2+]i (Fig. 6 A). The implication of this result is that 5-HT has substantially increased the Ca2+ sensitivity of airway SMCs to bring about contraction. However, the subsequent exposure to 10 µM NOC-5 or 10 µM 8pCPT-cGMP had very little effect in terms of relaxation in Ca2+-permeabilized airways (Fig. 6, B and C; 4.4 ± 1.1%, n = 6; 6.3 ± 3.2%, n = 3, respectively). The lack of a contractile response to caffeine at the end of the experiment indicated that the SMCs remained permeabilized to Ca2+, and the Ca2+ stores were empty (Fig. 6, B and C). However, if the NOC-5 concentration was more than doubled to 25 and 50 µM, some airway relaxation was observed (10.8 ± 2.4% and 10.9 ± 5.6%, respectively; n = 3). Similarly, in Ca2+-permeabilized airways, the relaxation induced by 25 µM NOC-5 in the presence of 10 µM ZAP or 1 µM vardenafil (27.7 ± 5.7 and 23.0 ± 4.2%, respectively; n = 3) was appreciable, although small as compared with the relaxation observed in normal airways. ZAP alone had little effect on the airway relaxation in Ca2+-permeabilized airways (5.2 ± 0.5%; n = 3). These results suggest that NO and cGMP at concentrations shown to relax airways do not exercise their action via significant changes in SMC Ca2+ sensitivity.
Changes in Ca2+ sensitivity induced by cAMP-elevating agonists
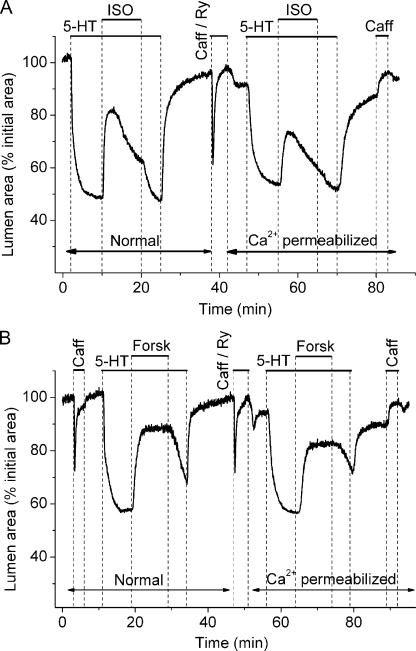
To confirm the ineffectiveness of NO/cGMP to induce airway relaxation via a decrease in Ca2+ sensitivity, we examined and compared the effect of two cAMP-elevating agents—ISO, a β2 adrenergic receptor agonist, and forskolin, an activator of adenylyl cyclase—on 5-HT–contracted airways in normal and Ca2+-permeabilized slices (Fig. 7). Like NO-induced airway relaxation, the relaxation induced by ISO in normal slices was biphasic (Fig. 7 A); a fast peak relaxation (53 ± 7.1%; n = 6) was followed by recontraction to a lower sustained relaxation level (23 ± 4.1%), and the removal of ISO resulted in a fast airway recontraction to reach the contraction level before ISO exposure. By comparison, forskolin induced a higher and sustained (73.6 ± 4.8%; n = 4) airway relaxation than ISO and NOC-5. These results are consistent with our previous results that demonstrated that ISO, forskolin, and cAMP slowed SMC Ca2+ oscillations, and the idea that ISO and forskolin stimulate synthesis of cAMP to induce airway relaxation (Bai and Sanderson, 2006a). The recontraction after ISO exposure is believed to result from a decrease in cAMP concentration because of a combination of receptor desensitization and PDE-4 activity.
Figure 7.
The effect of ISO and forskolin on Ca2+ sensitivity in airway SMCs. The relaxation of airways, contracted with 0.1 µM 5-HT, induced by (A) 1 µM ISO or (B) 1 µM forskolin in lung slices before and after Ca2+ permeabilization with 20 mM caffeine and 50 µM ryanodine. ISO and forskolin strongly relaxed airways before and after Ca2+ permeabilization. Data are representative of at least three different slices from two mice.
However, in Ca2+-permeabilized slices, airway relaxation induced by the cAMP agonists was significantly larger (44.9 ± 6.9%; n = 4 for forskolin) than the relaxation induced by NOC-5 and the cGMP analogues. These results demonstrate that Ca2+-permeabilized SMCs show a substantial decrease in Ca2+ sensitivity in response to cAMP analogues, which, in turn, emphasizes the inability of NO/cGMP to induce a similar action to bring about airway relaxation.
DISCUSSION
We investigated the mechanism of NO-induced SMC relaxation in intrapulmonary airways by studying the effects of NO donors on agonist-induced airway contraction and Ca2+ signaling of airway SMCs in lung slices. In general, NO-induced SMC relaxation or other biological responses are mediated by two main mechanisms: (1) a cGMP-dependent mechanism in which NO binds to and activates sGC to generate cGMP, or (2) a cGMP-independent mechanism in which a functional alteration of protein (i.e., activation) occurs via nitrosylation of thiol groups (Gaston et al., 2006; Saraiva and Hare, 2006). Both cGMP-dependent and cGMP-independent mechanisms have been implicated in mediating NO-induced vascular (Saraiva and Hare, 2006) and tracheal (Janssen et al., 2000; White et al., 2002) SMC relaxation. In addition, smooth muscle relaxation induced by NO has been proposed to be mediated by both a reduction of intracellular Ca2+ and a decrease in Ca2+ sensitivity (Carvajal et al., 2000; Kitazawa et al., 2009). Consequently, we studied here the contribution of these mechanisms to the relaxation of small airways.
Here, we present several experimental results suggesting that airway relaxation induced by NO is a cGMP- and PKG-dependent mechanism. First, the effects of NO (i.e., relaxation of agonist-induced airway contraction and inhibition of agonist-induced Ca2+ signaling) were quickly reversible upon the withdrawal of NO. This short-lived response is more compatible with the relatively fast antagonistic activities of PDEs and phosphatases, rather than the slower kinetics associated with the non-enzymatic breakage of covalent bonds between NO and thiol groups in nitrosylated proteins. Second, NO-induced relaxation was transitory when NO was added alone, but sustained when initiated in the presence of ZAP or vardenafil, two selective inhibitors of the cGMP-specific PDE-5. These results support the hypothesis that NO activates the synthesis of cGMP that, in turn, activates PDE-5 (Carvajal et al., 2000). Increased activity of PDE-5 would result in cGMP degradation and thereby self-limit the effects of NO. The sustained relaxation of the airway in the presence of PDE-5 inhibitors is consistent with a continued elevation of cGMP. Third, the effects of NO on Ca2+ signaling and contraction were blocked by ODQ, a specific inhibitor of sGC that synthesizes cGMP and is activated by NO. Fourth, the membrane-permeable cGMP analogues, 8pCPT-cGMP and 8Br-cGMP, which are both selective activators of cGMP-dependent PKG, induced airway relaxation and inhibited the Ca2+ oscillations induced by agonists. Finally, Rp-8-pCPT-cGMPS, a specific inhibitor of PKG activity, abolished NO-induced airway relaxation.
To further characterize the mechanisms of NO-induced airway relaxation, we studied the effects of NO on Ca2+ signaling in airway SMCs. Previously, we observed that agonist-induced Ca2+ signaling in airway SMCs consisted of Ca2+ oscillations and waves that persist at a sustained frequency during the presence of agonist (Perez and Sanderson, 2005). Importantly, the sustained frequency correlated with the magnitude of airway contraction, suggesting that airway contraction is partially regulated by the frequency of Ca2+ oscillations (Sanderson et al., 2008). Here, we observed that NO, as well as cGMP analogues, decreased the frequency of these Ca2+ oscillations, and this was accompanied by airway relaxation. Furthermore, in response to NO, the Ca2+ oscillations were initially abolished, but resumed with a steady, but greatly reduced frequency. This biphasic change in the frequency of Ca2+ oscillations correlates with the initially strong airway relaxation followed by airway recontraction to a steady level of reduced airway contraction. When NO was added in the presence of PDE-5 inhibitors, the frequency of Ca2+ oscillations was reduced further (after a longer phase of complete inhibition), and this correlated with a larger airway relaxation than that observed with NO alone. A similar NO-induced inhibition of Ca2+ oscillations has also been observed in isolated porcine tracheal (Prakash et al., 1997) and vascular SMCs (Kasai et al., 1997). Consequently, our results support the hypothesis that the reduction in the frequency of Ca2+ oscillations is a mechanism responsible for the early and late phases of the NO-induced airway relaxation and the more general idea that airway contraction is a function of the frequency of Ca2+ oscillations (Sanderson et al., 2008).
To investigate how NO inhibited agonist-induced Ca2+ oscillations, we examined the effects of NO on Ca2+ signaling triggered by the photolytic release of IP3 in single SMCs in the absence of agonists. Ca2+ oscillations of increasing frequency are induced in airway SMCs by increasing amounts of IP3 release (Bai et al., 2009). However, by reducing the exposure time, we released a very small amount of IP3 in the SMC that only initiated a Ca2+ transient and a brief contraction. NO reversibly blocked this IP3-triggered Ca2+ signaling and contraction, suggesting that NO inhibited the release of Ca2+ through IP3R. Alternatively, the NO-induced inhibition of Ca2+ oscillations could occur because of a decrease in the Ca2+ content of intracellular stores. For example, in isolated tracheal SMCs, Ca2+ release induced by caffeine (an agonist of the RYR) was completely inhibited by NO (Prakash et al., 1997). However, this result may be interpreted to mean that either the intracellular Ca2+ stores were emptied by NO or that the RYR was inhibited by NO. In contrast, in our experiments, NO did not influence the magnitude of the Ca2+ release induced by caffeine. This result indicates that in mouse airway SMCs, the capacity or content of the Ca2+ stores was not altered significantly and that the RYR remained unaffected by NO. Collectively, these results suggest that NO, and the resulting increase in cGMP, decreased the frequency of agonist-induced Ca2+ oscillations by reducing the activity of the IP3R. The molecular mechanism by which NO/cGMP/PKG inhibited the IP3R was not investigated here, but an IP3R-associated cGMP kinase substrate protein called IRAG has been identified in trachea SMC (Schlossmann et al., 2000; Ammendola et al., 2001). This protein is phosphorylated by PKG, associates with the IP3R, and blocks IP3R activation by IP3 and Ca2+. Our results with cGMP analogues that either activate or inactivate PKG, and that mimic or inhibit NO-induced effects, support the hypothesis that PKG-mediated phosphorylation is involved.
A concurrent mechanism regulating SMC contraction is the Ca2+ sensitivity of the SMC—a Ca2+-independent change in SMC contraction (Somlyo and Somlyo, 2003). Previously, we found that agonists strongly increase Ca2+ sensitivity in airways and blood vessels in lung slices (Bai and Sanderson, 2006b; Perez-Zoghbi and Sanderson, 2007). NO-induced relaxation is mostly attributed to a decrease in Ca2+ sensitivity in vascular SMCs (Carvajal et al., 2000). Here, we investigated the contribution of Ca2+ sensitivity in NO-induced airway relaxation and found, with Ca2+ permeabilized slices (caffeine-ryanodine treated), that NO and cGMP analogues did not substantially decrease agonist-induced Ca2+ sensitivity. However, using the same experimental approach, we found that airway relaxation induced by ISO and forskolin, agents that increase cAMP, was mediated by a pronounced decrease in Ca2+ sensitivity. A similar decrease in Ca2+ sensitivity has also been observed with other cAMP-elevating agents, including albuterol (a short acting β2 bronchodilator) (Delmotte and Sanderson, 2008) and 8Br-cAMP (Bai and Sanderson, 2006b). Thus, these results indicate that although decreased Ca2+ sensitivity contributes to airway relaxation induced by cAMP, this process is relatively unimportant in airway relaxation induced by cGMP. Because cAMP and cGMP appear to act via PKA and PKG, respectively, an implication of this difference between ISO- and NO-induced relaxation is that airway SMC Ca2+ sensitivity is regulated by a phosphorylation-specific event. In contrast, decreased Ca2+ sensitivity has been reported to be a central mechanism for the NO-induced relaxation of vascular SMCs of systemic arteries (Somlyo and Somlyo, 2003; Kitazawa et al., 2009). These differences emphasize that the intracellular pathways and mechanisms regulating contraction in various SMC types can be different.
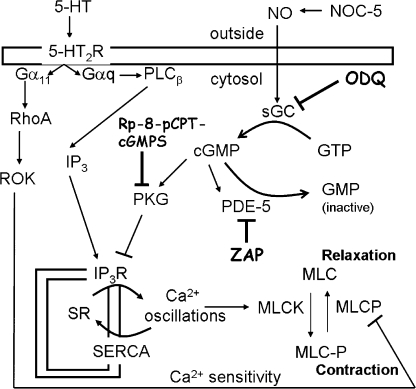
In conclusion, our experiments indicate that NO induces a transient airway SMC relaxation via activation of sGC to increase cGMP that, in turn, activates PDE-5 and PKG (Fig. 8). Activation of PDE-5 results in degradation of cGMP, explaining the transient nature of the NO-induced airway relaxation. Activation of PKG results in the inhibition of IP3R and Ca2+ release from the SR. This inhibition results in a decrease in the frequency of agonist-induced Ca2+ oscillations. Because NO appears to have little effect on Ca2+ sensitivity, the decrease in the frequency of the Ca2+ oscillations is predominately responsible for airway relaxation.
Figure 8.
Signaling pathway of NO-induced SMC relaxation in mouse small airways. Agonists, such as 5-HT, stimulate airway contraction by binding to their specific G protein–coupled membrane receptors (5-HT2R) to stimulate the dissociation of their α subunit (Gqα). This Gqα activates phospholipase C β (PLCβ) to synthesize IP3 that, in turn, activates SR IP3Rs to release Ca2+ from the SR. The resulting high [Ca2+]i inactivates the IP3R, and the Ca2+ is pumped back into the SR by the SR/ER Ca2+ ATPase (SERCA), resulting in a decrease in [Ca2+]i and reactivation of IP3R. The cyclic release from and reuptake of Ca2+ into the SR results in Ca2+ oscillations. Ca2+ activates, via calmodulin, myosin light chain (MLC) kinase (MLCK) to phosphorylate myosin (MLC-P) and initiate SMC contraction. The simultaneous inactivation of MLC phosphatase (MLCP) by agonists, i.e., via Rho kinase (ROK), enhances MLC phosphorylation and SMC contraction. The relative activities of MLCK and MLCP determine the contractile state of the SMC. NO induces airway relaxation by the activation of sGC to synthesize cGMP from GTP. ODQ specifically inhibits sGC activity. The elevation of cGMP activates PKG, which inhibits the IP3R, resulting in a lowering of the frequency of Ca2+ oscillations, deactivation of MLCK, and airway relaxation. The cGMP analogue Rp-8-pCPT-cGMPS specifically inhibits PKG activity. In addition, cGMP activates PDE-5 to convert cGMP to inactive GMP. ZAP inhibits PDE-5 to maintain high cGMP concentrations.
Acknowledgments
We thank Paurvi Shinde and Seema Mukherjee for their contribution to this study.
This work was supported by the National Institutes of Health grants HL71930 and HL87401 (to M.J. Sanderson).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- 5-HT
- 5-hydroxytryptamine
- IP3
- inositol trisphosphate
- IP3R
- IP3 receptor
- ISO
- isoproterenol
- MLCK
- myosin light chain kinase
- MLCP
- myosin light chain phosphatase
- NO
- nitric oxide
- PDE
- phosphodiesterase
- ROI
- region(s) of interest
- SE
- standard error
- sGC
- soluble guanylate cyclase
- SMC
- smooth muscle cell
- ZAP
- zaprinast
References
- Abdullah N.A., Hirata M., Matsumoto K., Aizawa H., Inoue R., Hamano S., Ikeda S., Xie Z., Hara N., Ito Y. 1994. Contraction and depolarization induced by fetal bovine serum in airway smooth muscle. Am. J. Physiol. 266:L528–L535 [DOI] [PubMed] [Google Scholar]
- Ammendola A., Geiselhöringer A., Hofmann F., Schlossmann J. 2001. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. J. Biol. Chem. 276:24153–24159 10.1074/jbc.M101530200 [DOI] [PubMed] [Google Scholar]
- Bai Y., Sanderson M.J. 2006a. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir. Res. 7:34 10.1186/1465-9921-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Sanderson M.J. 2006b. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L208–L221 10.1152/ajplung.00494.2005 [DOI] [PubMed] [Google Scholar]
- Bai Y., Edelmann M., Sanderson M.J. 2009. The contribution of inositol 1,4,5-trisphosphate and ryanodine receptors to agonist-induced Ca(2+) signaling of airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L347–L361 10.1152/ajplung.90559.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi M.G., Ward J.K., Mitchell J.A., Barnes P.J. 1995. Nitric oxide as a neurotransmitter in human airways. Arch. Int. Pharmacodyn. Ther. 329:97–110 [PubMed] [Google Scholar]
- Carvajal J.A., Germain A.M., Huidobro-Toro J.P., Weiner C.P. 2000. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 184:409–420 [DOI] [PubMed] [Google Scholar]
- Chitaley K., Webb R.C. 2002. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension. 39:438–442 10.1161/hy02t2.102960 [DOI] [PubMed] [Google Scholar]
- Delmotte P., Sanderson M.J. 2008. Effects of albuterol isomers on the contraction and Ca2+ signaling of small airways in mouse lung slices. Am. J. Respir. Cell Mol. Biol. 38:524–531 10.1165/rcmb.2007-0214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Sanderson M.J. 2009. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. In press 10.1165/rcmb.2008-0403OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B., Singel D., Doctor A., Stamler J.S. 2006. S-nitrosothiol signaling in respiratory biology. Am. J. Respir. Crit. Care Med. 173:1186–1193 10.1164/rccm.200510-1584PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L.J., Premji M., Lu-Chao H., Cox G., Keshavjee S. 2000. NO(+) but not NO radical relaxes airway smooth muscle via cGMP-independent release of internal Ca(2+). Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L899–L905 [DOI] [PubMed] [Google Scholar]
- Kasai Y., Yamazawa T., Sakurai T., Taketani Y., Iino M. 1997. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. J. Physiol. 504:349–357 10.1111/j.1469-7793.1997.349be.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Semba S., Huh Y.H., Kitazawa K., Eto M. 2009. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J. Physiol. 587:3587–3603 10.1113/jphysiol.2009.172189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L., Sanderson M.J. 2001. Intercellular calcium signaling and flash photolysis of caged compounds. A sensitive method to evaluate gap junctional coupling. Methods Mol. Biol. 154:407–430 [DOI] [PubMed] [Google Scholar]
- Perez J.F., Sanderson M.J. 2005. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J. Gen. Physiol. 125:535–553 10.1085/jgp.200409216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zoghbi J.F., Sanderson M.J. 2007. Endothelin-induced contraction of bronchiole and pulmonary arteriole smooth muscle cells is regulated by intracellular Ca2+ oscillations and Ca2+ sensitization. Am. J. Physiol. Lung Cell. Mol. Physiol. 293:L1000–L1011 10.1152/ajplung.00184.2007 [DOI] [PubMed] [Google Scholar]
- Prakash Y.S., Kannan M.S., Sieck G.C. 1997. Nitric oxide inhibits ACh-induced intracellular calcium oscillations in porcine tracheal smooth muscle. Am. J. Physiol. 272:L588–L596 [DOI] [PubMed] [Google Scholar]
- Ressmeyer A.R., Bai Y., Delmotte P., Uy K.F., Thistlethwaite P., Fraire A., Sato O., Ikebe M., Sanderson M.J. 2009. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am. J. Respir. Cell Mol. Biol. In press 10.1165/rcmb.2009-0222OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardolo F.L., Sterk P.J., Gaston B., Folkerts G. 2004. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 84:731–765 10.1152/physrev.00034.2003 [DOI] [PubMed] [Google Scholar]
- Sanderson M.J., Parker I. 2003. Video-rate confocal microscopy. Methods Enzymol. 360:447–481 10.1016/S0076-6879(03)60123-0 [DOI] [PubMed] [Google Scholar]
- Sanderson M.J., Delmotte P., Bai Y., Perez-Zogbhi J.F. 2008. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc. Am. Thorac. Soc. 5:23–31 10.1513/pats.200704-050VS [DOI] [PubMed] [Google Scholar]
- Saraiva R.M., Hare J.M. 2006. Nitric oxide signaling in the cardiovascular system: implications for heart failure. Curr. Opin. Cardiol. 21:221–228 10.1097/01.hco.0000221584.56372.dc [DOI] [PubMed] [Google Scholar]
- Schlossmann J., Ammendola A., Ashman K., Zong X., Huber A., Neubauer G., Wang G.X., Allescher H.D., Korth M., Wilm M., et al. 2000. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 404:197–201 10.1038/35004606 [DOI] [PubMed] [Google Scholar]
- Soloviev A., Lehen’kyi V., Zelensky S., Hellstrand P. 2004. Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell Calcium. 36:165–173 10.1016/j.ceca.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Somlyo A.P., Somlyo A.V. 2003. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83:1325–1358 [DOI] [PubMed] [Google Scholar]
- Wang I., Politi A.Z., Tania N., Bai Y., Sanderson M.J., Sneyd J. 2008. A mathematical model of airway and pulmonary arteriole smooth muscle. Biophys. J. 94:2053–2064 10.1529/biophysj.107.113977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.A., Walseth T.F., Kannan M.S. 2002. Nitric oxide inhibits ADP-ribosyl cyclase through a cGMP-independent pathway in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1065–L1071 [DOI] [PubMed] [Google Scholar]