Matrix-bound VEGF elicits more distinct vascular effects than soluble VEGF, including prolonged VEGFR2 activation with altered patterns of tyrosine activation and downstream enhancement of the p38/MAPK pathway.
Abstract
VEGF can be secreted in multiple isoforms with variable affinity for extracellular proteins and different abilities to induce vascular morphogenesis, but the molecular mechanisms behind these effects remain unclear. Here, we show molecular distinctions between signaling initiated from soluble versus matrix-bound VEGF, which mediates a sustained level of VEGFR2 internalization and clustering. Exposure of endothelial cells to matrix-bound VEGF elicits prolonged activation of VEGFR2 with differential phosphorylation of Y1214, and extended activation kinetics of p38. These events require association of VEGFR2 with β1 integrins. Matrix-bound VEGF also promotes reciprocal responses on β1 integrin by inducing its association with focal adhesions; a response that is absent upon exposure to soluble VEGF. Inactivation of β1 integrin blocks the prolonged phosphorylation of Y1214 and consequent activation of p38. Combined, these results indicate that when in the context of extracellular matrix, activation of VEGFR2 is distinct from that of soluble VEGF in terms of recruitment of receptor partners, phosphorylation kinetics, and activation of downstream effectors.
Introduction
Angiogenesis requires the integration of multiple cellular events including sprouting, invasion, lumen formation, and network interconnection. These events are orchestrated by the coordinated effort of a complex array of intracellular signaling pathways, cell–cell and cell–matrix interactions. It is well recognized that among the multiple angiogenic factors, vascular endothelial growth factor A (VEGF-A) is essential for the initiation and overall, regulation of vascular growth and patterning (Gerber et al., 1999; Jośko et al., 2000; Inoue et al., 2002; Ferrara et al., 2003). Notably, the responses of endothelial cells to VEGF are regulated by the nature, frequency, and distribution of other receptors and interacting molecules (Soker et al., 1998; Bazzoni and Dejana, 2004; Reynolds et al., 2004; Ashikari-Hada et al., 2005; Olsson et al., 2006). It is the combinatorial outcome of this input that ultimately dictates the size, type (artery vs. vein), and specialization of blood vessels (Wang et al., 1998; Ruhrberg et al., 2002; Stalmans et al., 2002; Jakobsson et al., 2006).
Two distinct receptor tyrosine kinases have been identified for VEGF-A on endothelial cells; VEGFR1 (flt-1) and VEGFR2 (human KDR/mouse flk-1; Mustonen and Alitalo, 1995; Ferrara et al., 2003; Olsson et al., 2006; Shibuya and Claesson-Welsh, 2006). Their activities and contributions to vascular morphogenesis are distinct and nonoverlapping (Fong et al., 1995; Shalaby et al., 1995). The affinity of VEGF-A for VEGFR1 is ∼10-fold stronger than its affinity for VEGFR2; nonetheless, most VEGF-A–mediated downstream signaling events associated with angiogenesis require VEGFR2 activation (Waltenberger et al., 1994; Zachary, 2005). Binding of VEGFR2 to VEGF induces dimerization and consequent phosphorylation of a subset of intracellular tyrosine residues (Pötgens et al., 1994). A total of 19 tyrosine residues are located in the C-terminal tail of VEGFR2 and at least 7 of these have been identified to be cross-phosphorylated by each monomeric kinase (Dougher-Vermazen et al., 1994; Takahashi et al., 2001; Claesson-Welsh, 2003; Blanes et al., 2007). The functional significance of these sites to the activation of downstream signaling pathways has been under investigation using both cell culture and animal models (Dougher and Terman, 1999; Sakurai et al., 2005). In fact, several phosphatases and adaptor proteins have been found to interact specifically with some, but not other phosphorylation sites assigning function to each tyrosine residue (Guo et al., 1995; Kroll and Waltenberger, 1997; Huang et al., 1999; Wu et al., 2000; Takahashi et al., 2001; Sakurai et al., 2005).
At the cellular level, activation of VEGFR2 results in induction of proliferation and migration, Ca2+ mobilization, prostacyclin (PGI2) production, ERK activation, nitric oxide (NO) production, and phosphatidylinositol-3-kinase (PI3K)/Akt activity (Waltenberger et al., 1994; Kroll and Waltenberger, 1997, 1999; Wheeler-Jones et al., 1997; Gerber et al., 1998; Cunningham et al., 1999). The question of how upon phosphorylation some pathways are preferentially selected has remained unanswered and halts the progress toward a more complete understanding of vascular formation, homeostatic control, and regional differentiation of vessels.
Another interesting aspect of VEGF biology is the large number of isoforms generated by this gene. Alternative splicing of human VEGF mRNA can give rise to at least nine different isoforms (Bates et al., 2002; Takahashi and Shibuya, 2005; Mineur et al., 2007). The most common include VEGF121 (mouse VEGF120), 165 (mouse VEGF164), and 189 (mouse VEGF188; Neufeld et al., 1996; Poltorak et al., 1997). These isoforms differ in their binding to extracellular matrix (ECM) molecules by virtue of the extent of the C-terminal region beyond the receptor-binding domain (Robinson and Stringer, 2001). Notably, the interaction of VEGF with matrix proteins has been considered important for the angiogenic switch facilitating the transition from hyperplastic to malignant tumor formation (Bergers et al., 2000) and for altering the susceptibility of the vasculature to specific chemotherapeutic drugs (Tozer et al., 2008). Recently, our laboratory demonstrated that VEGF isoforms can also be cleaved extracellularly by a subset of matrix metalloproteinases (MMPs) altering the status of matrix-bound isoforms to soluble VEGF (Lee et al., 2005). Interestingly, when mice were injected with tumor cells overexpressing uncleavable or soluble mutant VEGF forms, not only did tumor growth vary significantly, but also distinct vascular patterns were associated with each form. These in vivo results highlighted the notion that VEGF bound to the ECM is bioactive, confirming earlier findings in vitro (Park et al., 1993). Yet, the caliber of vessels, degree of vascular branching, and capillary density were distinct if the process was driven by uncleavable or by soluble VEGF (Lee et al., 2005). These findings were unexpected and required further mechanistic exploration. Here, we demonstrate that matrix-bound VEGF induces prolonged activation kinetics of VEGFR2 with altered patterns of tyrosine activation and subsequent downstream enhancement of the p38/MAPK pathway. These effects require association of VEGFR2 with β1 integrin, a previously unsuspected partner.
Results
VEGF can be retained and remains active within a collagen matrix
Except for VEGF121, all other VEGF isoforms have the ability to bind to multiple proteins and proteoglycans present in the ECM (Ashikari-Hada et al., 2005). To mimic in vitro the likely context of VEGF presentation in vivo, we polymerized VEGF onto gels consisting of collagen and fibrinogen, two major components of ECM, and tested its retention within this matrix (Fig. S1, A and B). This was consistent with our findings from surface plasmon resonance that indicate significant affinity of VEGF for collagen (unpublished data). In contrast, a mutant VEGF form (VEGF113) that lacks the ECM binding domain was rapidly released from the polymerized gel (Fig. S1, C and D). More than 80% of VEGF165 was maintained within the collagen gel after continuous washes, whereas only 20–30% of VEGF113 was left within the gel under the same conditions. The result indicates that VEGF can be retained in collagen gels, but this requires the ECM binding domain.
Next, we tested whether matrix-bound VEGF165 (Vb) remained active by evaluating its ability to phosphorylate VEGFR2 when compared with soluble VEGF165 (Vs) at identical concentrations for 5 min. This time point was selected because it results in maximum activation of VEGFR2 by soluble VEGF165 in vitro. We found that matrix-bound VEGF165 induced phosphorylation of VEGFR2 at equivalent levels as of soluble VEGF165 at 5 min (Fig. 1, A, B, and D). Activation of the receptor required direct contact of cells with matrix, as presence of a nylon membrane that prevented direct interaction no longer resulted in VEGFR2 activation (Fig. 1 C). In contrast, the presence of such membrane did not interfere with activation of VEGFR2 by Vs (Fig. 1 C). Activation of VEGFR2 by matrix-bound VEGF165 was reproduced using multiple primary endothelial cell types and with two commercial sources of VEGF165 (Fig. 1, B and E). Furthermore, lower concentrations of matrix-bound VEGF165 than soluble VEGF165 were sufficient to induce phosphorylation of VEGFR2 (Fig. 1 D). Polymerized collagen in the absence of the growth factor did not elicit activation of VEGFR2 (Fig. 1, A, B, and D).
Figure 1.
Matrix-bound VEGF165 is able to phosphorylate VEGFR2. (A) Confluent PAE-KDR cultures were exposed for 5 min to matrix-bound VEGF165 (Vb), soluble VEGF165 (Vs; 100 ng/ml and 200 ng/ml), vehicle only (−), or polymerized collagen gels. Level of VEGFR2 (210 kD) phosphorylation was determined by Western blot analysis using a pan-phosphotyrosine antibody (4G10). The bottom panels are blots reprobed with antibodies against VEGFR2, as control. (B) Confluent HUVECs, HSVECs, and HAECs were exposed to Vb, Vs, or matrix-collagen gel controls. The blots were stripped and reprobed with antibodies against VEGFR2 as controls. (C) Similar experiment settings as A and B using 200 ng/ml VEGF, but in the presence of a nylon membrane when indicated. (D) VEGFR2 phosphorylation was induced by different concentrations of Vs or Vb, as indicated. (E) VEGF165 (200ng/ml) from two different sources was compared under bound and soluble conditions. BB94 (MMP inhibitor, 2 µM) was incorporated within the collagen gel together with VEGF165. The arrows indicate different phosphorylation patterns of VEGFR2 caused by Vb treatment. The densitometry of D and E were calculated by p-VEGFR2 over VEGFR2 and using vehicle (−) as 1.0. (F) HUVECs exposed to either soluble (200 ng/ml) or matrix-bound VEGF165 and at the indicated times. Phosphorylation levels of VEGFR2 were then determined by Western blot using 4G10 antibody. Note that prolonged phosphorylation of VEGFR2 in bound when compared with soluble VEGF165. (G) Densitometric analysis of same experiments as F (n = 4–5), shown as ratio of Vb over Vs in each time point (*, P < 0.05 for 10- and 20-min time point between Vb and Vs).
Matrix-bound VEGF165 increases kinetics of VEGFR2 activation and mediates clustering of the receptor
Exposure of confluent endothelial cultures to VEGF165 revealed prolonged activation of VEGFR2 only when the growth factor was bound to matrix (Fig. 1, F and G; Fig. S2, A and B). Activation of VEGFR2 did not require the release of VEGF from matrix, as presence of broad-spectrum matrix metalloproteinases and plasmin inhibitors showed identical results (Fig. 1 E; Fig. S2 C), further supporting the conclusion that VEGF165 bound to matrix can induce activation of VEGFR2.
To further explore the effect of matrix-bound VEGF165 on receptor function, we examined the extracellular distribution of receptors upon activation by Vs and Vb. We found that matrix-bound VEGF induced receptor clustering at the cell surface (Fig. 2, A and B). In the absence of VEGF, visualization of the receptor with an extracellular binding domain antibody showed a centrally located pattern of VEGFR2. Exposure of confluent cells to either soluble or matrix-bound VEGF165 induced a broader distribution of VEGFR2 to the periphery of the cell and, in the case of matrix-bound VEGF165, it also resulted in formation of large clusters that were less predominant in cells treated with soluble VEGF165 (Fig. 2, A and B).
Figure 2.
VEGFR2 clustering and internalization induced by soluble and matrix-bound VEGF165. (A) HUVECs were treated with vehicle (−), Vs (200 ng/ml), Vb (200 ng/ml), or collagen (col) for 15 min. Cells were fixed and stained using either rabbit Ig control (top) or antibody against extracellular domain of VEGFR2 in the absence of permeabilization. Computer-generated images (black and white on the right) were used for quantitation of positive staining of VEGFR2 (VEGFR2 distribution) and to identify particle size over 10-fold larger than controls (VEGFR2 clustering). (B) Statistical analysis for VEGFR2 distribution and VEGFR2 clustering are shown. For VEGFR2 clustering, baseline (=1) size was assessed when cells were exposed to vehicle only (*, P < 0.05, between Vb to either −, Vs, or Col). (C) Internalization of VEGFR2 was analyzed in confluent HUVECs treated with Vs (200 ng/ml), Vb (200 ng/ml), and vehicle controls for 15 min. To detect the internalized receptor, cells were treated first with a recombinant single-chain antibody against human VEGFR2 (scFvA7), subsequently exposed to Vs or Vb, followed by acid washes before fixation. Internalized VEGFR2 appears in a vesicular pattern. (D) Same experiments as C but analyzed by Western blots using VEGFR2 or 4G10 antibodies. Total VEGFR2 was measured from the same lysate used for immunoprecipitation used for internalized VEGFR2. (E) Statistics analysis of C. The results (referred to as counts per cell) were reported in the bar graph of three independent experiments. Six random fields were analyzed in each experiment. (F) Densitometric analysis of D. The results were from six independent experiments. Internalized VEGFR2 by Vs and Vb were normalized with total VEGFR2 in the lysate, and then compare normalized Vb over Vs in each experiment. (G) Confluent HUVECs were pretreat with Dynosore or DMSO at 37°C for 15 min. Cells were then treated with Vs, Vb, or vehicles (−) for 5 min. Phosphorylation assays were performed and the cell lysates were then immunoprecipitated with 4G10 and then blotted with total VEGFR2 antibody. Total VEGFR2 was determined by using the same lysate used for immunoprecipitation. In A and C, nuclei stained with TOPRO3 appears in blue. *, P < 0.05; **, P < 0.005.
We next examined kinetics of receptor endocytosis using standard biotinylation assays (Fig. S3 A) and uptake of VEGFR2 antibodies (extracellular epitope) upon exposure to Vs and Vb (Fig. 2, C–F; Fig. S3, B and C). Surprisingly, although Vs and Vb showed no major difference on receptor internalization at early time points (Fig. S3 C), Vb exhibited prolonged internalization kinetics that peaked at 15 min, as shown by both biotinylation and antibody-tagged receptor assays (Fig. 2, C–F; Fig. S3, A and B). Interestingly, the ratio of VEGFR2 internalization between Vs and Vb was similar to the respective phosphorylation levels (Fig. 1, F and G). To further ascertain the relationship between VEGFR2 internalization and phosphorylation, we investigated whether internalized VEGR2 remained phosphorylated using pan-phosphotyrosine antibodies (Fig. 2 D). Under Vb treatment, higher phosphorylation was noted at 15 min in comparison to Vs (Fig. 2 D; see Fig. 9 A). The relationship between phosphorylation and endocytosis was further supported by experiments with Dynosore, a potent dynamin inhibitor. We found that endocytosis was required for VEGFR2 phosphorylation under both bound and soluble conditions (Fig. 2 G).
Figure 9.
β1 integrin is required for clustering and continued internalization of VEGFR2. (A) Internalization of VEGFR2 was analyzed in confluent HUVECs pretreated with β1 inhibitory antibodies or Ig control for 15 min, and then treated with Vs (200 ng/ml), Vb (200 ng/ml), and vehicle controls for another 15 min. To detect the internalized receptor, cells were treated with a recombinant single-chain antibody against human VEGFR2 (scFvA7), subsequently exposed to the indicated treatment followed by acid washes before lysis. Internalized VEGFR2 was analyzed by Western blot using VEGFR2 or 4G10 antibodies. Total VEGFR2 was determined in the same lysates. The numbers indicate densitometric analysis comparing Vs and Vb. (B) HUVECs pretreated with β1 inhibitory antibodies or Ig control for 15 min were exposed to vehicle (−), Vs (200 ng/ml), Vb (200 ng/ml), or collagen (col) for 15 min. Cells were fixed and stained using recombinant single-chain antibody against human VEGFR2 (scFvA7) with E-tag in the absence of permeabilization. FITC-conjugated anti–E-tag was used for detection of positive staining. Nuclei stained with TOPRO3 appears in blue. (C) Schematic model depicting the interaction of soluble and matrix-bound VEGF with VEGFR2. Soluble VEGF (left) results in rapid binding, internalization, and activation of VEGFR2. Matrix-bound VEGF (right) interacts easily with receptors as the cells migrate into the matrix. The affinity for the matrix is two orders of magnitude lower than its receptor. Over time, the progressive recruitment of integrins facilitates clustering of VEGFR2 and results in binding between integrins and VEGFR2. This interaction maintains the phosphorylation status of Y1214.
Matrix-bound VEGF leads to prolonged activation of Y1214 on VEGFR2
Several potential auto-phosphorylation sites have been identified in VEGFR2: Y801 in the juxtamembrane domain, Y951 in the kinase insert, Y1054 and Y1059 in the kinase domain, and Y1175 and Y1214 in the C-terminal tail (Dougher-Vermazen et al., 1994; Takahashi et al., 2001; Claesson-Welsh, 2003). Taking into account the differences in gel mobility (Fig. 1 E and Fig. 3 A) and in timing of activation, we considered that matrix-bound and soluble VEGF165 might elicit differential phosphorylation patterns. Using a cohort of phopho-specific antibodies, we compared levels of phosphorylation induced by either soluble or bound VEGF165. Initially, a pan-phosphotyrosine antibody (4G10) showed overall phosphorylation of VEGFR2 under both conditions (Fig. 3 A) at 5 and 15 min. As shown in Fig. 1, F and G, a prolonged phosphorylation of VEGFR2 was frequently noted in the context of Vb treatment.
Figure 3.
Prolonged phosphorylation induced by matrix-bound VEGF165 is specific to tyrosine 1214. (A) HUVECs were incubated with Vs or Vb (200 ng/ml) for either 5 or 15 min, as indicated. Phosphorylation assays were performed and 4G10 was used as pan-phosphotyrosine antibody. Densitometric analysis of phospho-VEGFR2 by Vs and Vb were normalized with total VEGFR2 and then compare Vb over Vs as indicated. (B) Phosphorylation assays were performed using phosphotyrosine antibodies specific for the indicated residues on VEGFR2. (C) HEK 293 cell lines expressing Y1214F VEGFR2 mutant were incubated with soluble (Vs; 200 ng/ml) or bound VEGF165 (Vb) for 5 min and 15 min, vehicle (−), or collagen only (col) for 5 min. Equal protein levels were separated by gel electrophoresis and probed with 4G10. The blot was then reprobed with VEGFR2 antibody for normalization. Densitometric analysis of phosphorylation status induced by Vb to Vs is indicated. (D) Similar experiments were performed using HEK 293 stably transfected with native VEGFR2 (293NR).
Antibodies to specific VEGFR2 phosphotyrosine residues were subsequently used in the same lysates analyzed in panel A (Fig. 3 B). At 5 min, Y951 appeared to be preferentially phosphorylated by matrix-bound VEGF165; however, this residue was quickly dephosphorylated (at 15 min) and could not explain the prolonged phosphorylation induced by matrix-anchored VEGF165. By 15 min, while most phosphorylation sites had returned to baseline, Y1214 showed a substantial retention of activation exclusively by matrix-bound VEGF165 and was gradually back to baseline levels only after 30 min (Fig. S3 E). These results indicate that the extended phosphorylation observed by 4G10 was likely mediated by retention in the phosphorylation status of Y1214.
To further ascertain whether the effect was specific, we used HEK 293 cells transfected with VEGFR2 mutant Y1214F (Fig. 3 C), as well as cells transfected with native VEGFR2 (Fig. 3 D) and two additional VEGFR2 mutants, Y951F and Y1130F (Fig. S3 D). Pan-phosphotyrosine antibodies (4G10) were used to detect overall phosphorylation levels in VEGFR2. Only the Y1214 mutation abrogated the increased levels of phosphorylation on VEGFR2 upon exposure to matrix-bound VEGF. These findings further support the concept that matrix-bound VEGF leads to a temporal increase in the phosphorylation of VEGFR2, and that this occurs through Y1214.
Matrix-bound VEGF165 leads to higher activation of the p38/MAPK pathway
The downstream consequences of prolonged activation of Y1214 on VEGFR2 could be predicted based on the fact that this residue has been associated with activation of p38 (Lamalice et al., 2004). Indeed, we found that matrix-bound VEGF165 mediates higher and sustained activation of p38 (Fig. 4 A). Moreover, kinetics of p38 phosphorylation correlated with the activation kinetics of pY1214. This was in contrast to findings using soluble VEGF165-treated cells where p38 peaks rapidly, but drops to basal levels after 5 min (Fig. 4, B and D). To better understand the overall effect of matrix-bound VEGF165, we conducted a protein antibody array analysis on nearly 40 common downstream signaling molecules, including all the major signaling pathways activated downstream of VEGFR2, such as MAPK, p38, PI3K, and Akt (unpublished data). Both matrix-bound and soluble VEGF165 were found to activate several pathways at similar kinetics, except for p38, in agreement with our previous findings. Interestingly, soluble VEGF165 showed one additional difference: higher and prolonged activation kinetics of Akt, in contrast to lower Akt activation by Vb (Fig. 4, C and E). These findings were reproduced with mutant forms of VEGF that were unable to be released from the matrix through intramolecular cleavage (Fig. S4 B) and by using solid phase assay with immobilized VEGF (Fig. S4 A). Overall, these data indicate that presentation of VEGF (i.e., soluble vs. matrix-bound) differentially impacts downstream effectors.
Figure 4.
p38 and Akt pathways are differentially induced by soluble and matrix-bound VEGF165. (A) Confluent HUVEC cultures were incubated in the presence or absence of soluble (Vs) or matrix-bound VEGF165 (Vb; 200 ng/ml) for 5 min. Cell lysates were obtained, resolved by SDS-PAGE, and probed with antibody against phospho-p38 (43 kD). The blot was then stripped and reprobed with total p38 antibodies for normalization. (B) HUVECs were exposed to Vs or Vb (200 ng/ml) at the indicated times. The blot was then stripped and reprobed with total p38 antibody for normalization. (C) Same settings as B, but membrane was immunoblotted with phospho-Akt (S473) and total Akt (60 kD). Densitometry was determined by phospho-p38 over total p38 (n = 6) (D) or phospho-Akt over total Akt (n = 6) (E).
VEGF isoforms that lack matrix-retention domains do not support prolonged activation of VEGFR2
To further test the relevance of matrix anchorage on activation of p38, we tested VEGF121 and VEGF113 (isoforms lacking ECM binding domain) polymerized on collagen gels for their ability to phosphorylate of VEGFR2, Y1214, and p38 (Fig. 5, A and B). Collagen polymerized with VEGF113 or VEGF121 failed to elicit activation of VEGFR2, in contrast to their ability to activate the same receptor when presented in a soluble form. We predict that the difference resided in the inability of these isoforms to bind to the ECM (Fig. S1, C and D).
Figure 5.
Prolonged activation of VEGFR2 requires matrix-binding motifs in VEGF. (A) Various VEGF isoforms (165, 121, 113; 200 ng/ml) were polymerized with collagen as indicated. The gel was prepared and washed as described in Materials and methods and placed on confluent monolayers for 5 min. Phosphorylation assays were performed and total VEGFR2 levels were determined by reprobing the same membrane. (B) Same as A, but treated for 15 min and probed with antibodies against pY1214 (top) or pP38 (bottom). Numbers on the bottom of the Western blot indicate densitometric quantification. (C) Different collagen types were used to anchor VEGF (Vb, 200 ng/ml) as indicated. Exposure to collagen matrices for 15 min resulted in prolonged phosphorylation of Y1214. (D) Dose curve of VEGFR2 activation by VEGF. Detection of phosphorylation was performed using 4G10 antibodies. Numbers below the Western blotndicate densitometric quantification at 15 min using 100 ng/ml VEGF165 treatment as 1.0. (E) 500 ng/ml of VEGF165 was used on cells for 5, 15, and 30 min in the presence or absence of a carrier to stabilize the protein. On the right, 500 ng/ml of VEGF was used at time 0, and an additional fresh aliquot at 500 ng/ml was added at indicated time points for another 5 min. Phosphorylation assay was performed using 4G10 antibody.
Several matrix proteins were able to sustain bioactivity of VEGF165. In addition to type I collagen, we also examined whether mixtures of VEGF165 with type IV, V, and VIII collagen could elicit similar prolonged activation on Y1214 at 15 min (Fig. 5 C). Although activation levels of pY1214 were not as strong as those seen on type I collagen, all these matrices led to higher phosphorylation retention of Y1214 than those detected by soluble VEGF, supporting the concept that multiple matrix proteins can mediate immobilization and possibly result in a similar outcome.
Prolonged activation of VEGFR2 by Vb is not due to local concentration of VEGF165
To control for potential confounding differences in VEGF half-life as result of exposure to different solutes, we analyzed the stability of VEGF165 under both soluble and matrix-bound conditions. VEGF165 in PBS was less stable than when mixed with collagen (Fig. S1, E and F). Nonetheless, it should be stressed that the timing used on the phosphorylation assays was short (5 and 15 min) and minimally affected by the half-life of VEGF in solution (100 min). Therefore, differences of VEGF165 stability would provide a negligible variation, if any, to the experimental outcomes. Yet, to further support the conclusions, we performed an additional assessment of VEGFR2 phosphorylation using soluble VEGF165 at higher concentrations and examined whether these conditions would change the kinetics of receptor activation (Fig. 5 D; Fig. S1 G). VEGF165 concentrations above 150 ng/ml resulted in the same VEGFR2 phosphorylation kinetics, indicating that the receptors were saturated at this concentration. Furthermore, phosphorylation kinetics of VEGFR2 diminished at 15 min regardless of VEGF165 concentration if presented in a soluble form. Therefore, the differences in VEGFR2 phosphorylation kinetics induced by Vb were not the product of changes in VEGF stability. In addition, to compensate for the degradation of Vs at later time points and mimic a “slow release of VEGF” model, we either added the same amount of BSA as a carrier protein to stabilize VEGF or exposed cells to fresh VEGF every 5 min after the initial treatment (Fig. 5 E). Once again, VEGF receptors were saturated and the kinetics of phosphorylation were unaltered, showing a peak at 5 min with a sharp decrease thereafter.
Matrix-bound VEGF165 induces association of VEGFR2 with β1 integrin and promotes redistribution of this integrin to focal adhesions
In addition to the two classical receptor tyrosine kinases, VEGFR1 and R2, several other receptors have been shown to modulate VEGF signaling. For instance, VE-cadherin has been shown to antagonize VEGFR2 activation in confluent monolayers (Calera et al., 2004; Lampugnani et al., 2006), whereas neuropilins were found to enhance several responses downstream of VEGFR2 (Becker et al., 2005; Kawamura et al., 2008). We suspected that matrix receptors, particularly integrins, and perhaps other coreceptors might be involved in the distinct pattern of VEGFR2 responses induced when the ligand was bound to matrix. To test for this possibility, we performed coimmunoprecipitation of VEGFR2 and subsequently asked whether other proteins were coprecipitated by using specific antibodies against neuropilin-1, VE-cadherin, VEGFR1, β1 integrin, and β3 integrin. Interestingly, β1 integrin was found to form a stable complex with VEGFR2 exposed to Vb, but not to Vs (Fig. 6 A; Fig. S4 D). From all the other cell surface proteins examined, we noted that association of VEGFR2 with neuropilin-1 was enhanced by soluble VEGF165, as previously shown (Soker et al., 1998; Kawamura et al., 2008). Surprisingly, less neuropilin-1 was found associated to VEGFR2 when cells were exposed to Vb (Fig. S4 C). This was also supported by reverse immunoprecipitation (not depicted).
Figure 6.
VEGFR2 associates with β1 integrin when exposed to matrix-bound but not soluble VEGF165. (A) Confluent HUVEC monolayers were incubated with soluble (200 ng/ml) or bound VEGF165 (200 ng/ml) and collagen for 5, 15, and 30 min. Cultures were then lysed and equal cell lysates were used for immunoprecipitation with VEGFR2 antibodies. The precipitated protein was then resolved on 4–12% SDS-PAGE and probed with a β1 integrin antibody (130 kD). The blot was then reprobed with anti-VEGFR2. (B) Representative images of proximity ligation assays with quantification (C) after treatment with the indicated growth factors. Red dots denote regions of signal amplification consistent with VEGFR2/β1 integrin interactions. Nuclear stain is TOPRO3 (blue) (n = 4; *, P < 0.05 between Vb to either −, Vs, or Col).
Low levels of β1 integrin were found associated with VEGFR2 in the absence of VEGF165 (Fig. 6 A). Interestingly, the association was consistently lost upon exposure to soluble VEGF165, but enhanced when the ligand was bound to matrix (Fig. 6 A). This result was further validated using proximity ligation assays (Fig. 6, B and C). We also found that matrix-bound VEGF165 induces redistribution of β1 integrins to focal adhesions (Fig. 7, A and B). This tropism for focal adhesions did not occur when endothelial cells were exposed to either growth factor–free collagen or to soluble VEGF165.
Figure 7.
β1 integrin redistributes to focal adhesion upon stimulation by matrix-bound VEGF165. HUVECs treated at the conditions indicated for 15 min were fixed and stained for β1 integrin (red), paxillin (green), and TOPRO3 (blue). Arrows (A and B) indicate colocalization of β1 integrin and paxillin, arrowheads in B show focal adhesions without β1 integrin–positive staining. Asterisks in A indicate cells with higher expression of β1 integrin, to indicate that regardless of the diffused expression of β1 integrin only, Vb, but not Vs, resulted in redistribution of the integrin to focal adhesions. (A) Lower magnification; (B) higher magnification of independent experiments.
β1 integrin/VEGFR2 association is required for the increased activation of Y1214 and p38
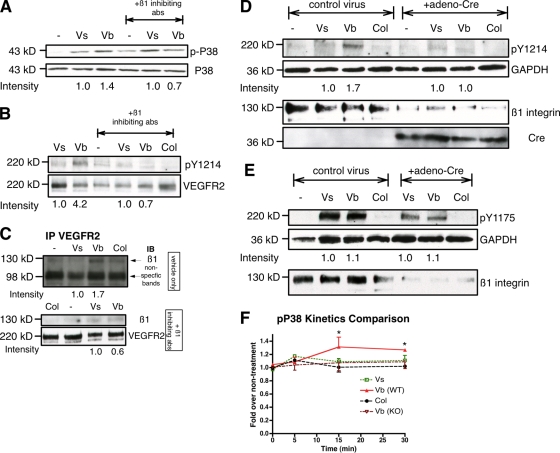
The contribution of β1 integrins to the prolonged responses of p38 and Y1214 under matrix-bound VEGF165 treatment was challenged by β1 integrin inhibitory antibodies. Pretreatment of cultures with inhibitory antibodies blocked prolonged phosphorylation of p38 that now was equivalent to treatment with soluble VEGF165 alone (Fig. 8 A). Furthermore, prolonged phosphorylation of VEGFR2-Y1214 induced by matrix-bound VEGF165 at 15 min was no longer detected if the cells were pretreated with β1 integrin inhibitory antibodies (Fig. 8 B). The association of VEGFR2 and β1 integrin triggered by matrix-bound VEGF was also disrupted when β1 integrins were blocked (Fig. 8 C). It is also important to notice that pretreatment of β1 integrin inhibitory antibodies did not alter the phosphorylation of Y1214 at early time points (Fig. S5 A), consistent with the fact that association with β1 integrin only occurs later (Fig. 6 A).
Figure 8.
Blocking β1 integrin dampens activation of pY1214 and P38 of matrix-bound VEGF165. (A) Confluent HUVEC monolayers were starved for 6 h and pretreated with β1-inhibiting antibodies for 15 min, then treated with the indicated reagents for an additional 15 min. Cell lysates were collected, resolved on 4–12% SDS-PAGE, probed with phopho-p38 antibody, and reprobed with total p38 antibody. (B) Same as A, treated with indicated reagents for 15 min and probed with antibody against pY1214. Membranes were then stripped off and reprobed with total VEGFR2 antibody. (C) HUVECs were treated as in A. Cell lysates were collected and immunoprecipiated with VEGFR2 antibodies. Immunoprecipitated proteins were probed with anti-β1 integrin or anti-VEGFR2. The top panel shows cells without preincubation with β1-inhibiting antibodies. The bottom panel shows cells preincubated with β1-inhibiting antibodies. (D) Mouse liver ECs from β1fl/fl mice were isolated and treated with adeno-Cre virus to induce deletion of the gene. After 3 d, cultures were treated with indicated growth factors for 15 min and cell lysates were probed with pY1214, GAPDH (36 kD), and β1 antibodies. Blots were then stripped and reprobed with anti-Cre (35 kD). (E) Similar experimental setting as D but probed with pY1175. (F) Confluent β1fl/fl or β1ko MLEC monolayers in 96-well plates were starved overnight and exposed to the indicated treatments for 5, 15, or 30 min. p38 activation was determined by cell-based ELISA and the fold change at each time point was calculated by phospho-p38 over total p38 compared with no treatment. The difference between pP38 activation in WT and KO cells is statistically significant at 15 and 30 min. *, P < 0.05 (n = 4).
We further determined the contribution of β1 integrin to the specific phosphorylation of VEGFR2 using genetic loss-of-function experiments. Mouse liver endothelial cells (MLECs) were isolated from β1floxed/floxed (β1fl/fl) mice, and exposed to either adeno-Cre virus or control adenovirus. Evaluation of β1 integrin levels, as well as presence of Cre-recombinase, showed a significant reduction of the integrin in the samples that also expressed Cre (Fig. 8, D and E, bottom two panels). Down-regulation of this integrin did not trigger significant morphological changes when plated on vitronectin (Fig. S5 B). Experimental and control cultures were then treated with soluble or bound VEGF and probed with antibodies against Y1214, Y1175, and GAPDH (Fig. 8, D and E, top panels). In agreement with previous findings, only matrix-bound VEGF165 led to prolonged phosphorylation of Y1214 in wild-type MLECs after 15 min of treatment, whereas both soluble and bound VEGF165 resulted in equivalent activation of Y1175. Once β1 integrin was genetically inactivated, Vb was no longer able to sustain phosphorylation on Y1214, in agreement with the results using β1 integrin inhibitory antibodies. However, we did notice that there was a substantial decrease between β1ECKO cells and control cells on pY1175 levels even though equivalent protein was loaded (as per GAPDH). Further investigation showed that levels of VEGFR2 were decreased in the absence of β1 integrins (β1ECKO cells; Fig. S5 C), a difference that appears to be post-transcriptional (Fig. S5 D). Interestingly, albeit reduced, VEGFR2 was phosphorylated but unable to support prolonged activation of p38 in the absence of β1 integrin (Fig. 8 F).
Together, these findings support the conclusion that association of β1 integrin with VEGFR2 is induced by matrix-bound VEGF and it is critical for the prolonged phosphorylation responses found specifically in Y1214 and downstream activation of the p38 MAPK pathway.
β1 integrin is required for the prolonged internalization of VEGFR2 and clustering induced by matrix-bound VEGF165
We next investigated whether association with β1 integrin was required for the induction of VEGFR2 clustering and increased internalization. Pretreatment of cultures with inhibitory β1 integrin antibodies blocked clustering and prolonged internalized VEGFR2 at 15 min by Vb, which was now equivalent to treatment with soluble VEGF165 (Fig. 9, A and B).
Based on these findings, we propose a model in which matrix-bound VEGF progressively facilitates proximity and subsequent binding to β1 integrins (Fig. 9 C). This interaction mediates receptor clustering and redistribution of β1 integrins to focal adhesions. Furthermore, the interaction of VEGFR2 to β1 integrin selectively favors retention of the phosphorylation status of one residue (Y1214), resulting in changes in downstream of pP38.
Discussion
VEGF is a master regulator of endothelial differentiation during development (Carmeliet et al., 1996; Ferrara et al., 1996) and it is also required for homeostatic functions of blood vessels in the adult, including permeability and endothelial survival (Lee et al., 2007; Nagy et al., 2008). Nonetheless, it has remained unclear how VEGF elicits such a vast array of cellular responses, which are mostly downstream of VEGFR2. Our results showed that the presentation of VEGF in either soluble or matrix-bound form alters the kinetics of receptor activation, impacts association with other cell surface receptors, and modulates signal transduction pathways downstream VEGFR2 activation. Matrix-bound VEGF induces receptor clustering, association with β1 integrin, and the initiation of a pro-migratory phenotype on endothelial cells that is reminiscent of tip cells (Gerhardt et al., 2003). These effects are dependent on β1 integrin, as pharmacological or genetic inactivation of this molecule suppressed the differential signaling events initiated by matrix-bound VEGF. Overall, these findings demonstrate that VEGFR2 activation can be mediated by VEGF when in association with matrix proteins and further indicate that this activation is likely be relevant to the establishment of the tip cell phenotype during the formation of a vascular sprout.
Activation of VEGF receptors by VEGF in the context of matrix has been previously noted, but not fully characterized (Park et al., 1993). Studies using modified VEGF to make it covalently bound to the matrix have shown that under those conditions, VEGF can lead to increase proliferation of endothelial cells (Zisch et al., 2001; Koch et al., 2006). We have confirmed and extended these experiments, providing molecular explanation for the potential relevance of the distinct VEGF isoforms and a function for the avidity of VEGF for matrix proteins.
The biological meaning of the association of VEGF with the ECM has been studied using transgenic mice by placing the coding region of the three major VEGF isoforms (188, 164, and 120) downstream of the VEGF promoter (Ruhrberg et al., 2002). Mice expressing VEGF188, a form that exhibits significant affinity for matrix proteins, showed enhanced vascular branching and increased capillary density when compared with either wild-type controls or mice with exclusive expression of VEGF164. In contrast, mice expressing VEGF120 (little to no affinity for matrix) displayed lack of filopodia extension, poor branching, formation of vascular tufts, and enlarged vessel diameter (Ruhrberg et al., 2002). Overall, the affinity of VEGF for matrix conveys information with significant consequences to vascular morphogenesis. Although it has been clear that VEGFR2 is the main receptor for these signaling events, our understanding of VEGFR2 activation in the context of matrix is limited.
It has been speculated that association of VEGFR2 with coreceptors or other cell surface proteins was likely responsible for the broad spectrum of responses mediated by VEGF. We considered that integrins might play a role in the case of matrix-bound VEGF, as the presence of matrix proteins would naturally mediate integrin engagement. Furthermore, β1 integrin has long been shown to be involved in angiogenesis. Although binding of VEGFR2 to β3 integrin has been described (Borges et al., 2000; Matsumoto and Claesson-Welsh, 2001), an association between β1 integrins and VEGFR2 has been tested but not found. Similarly to those studies, we were not able to coimmunoprecipitate both receptors in the presence of soluble VEGF (Figs. 6 and 8). Nonetheless, we found that matrix-bound VEGF significantly increased the ratio of β1 integrin associated with activated VEGFR2 in a time-dependent manner, with maximum phosphorylation at 15 min and still clearly detectable by 30 min. This association was biologically significant and necessary for the selective and continued phosphorylation of VEGFR2-Y1214 and for the increase in p38. Furthermore, matrix-bound but not soluble VEGF induced redistribution of β1 integrin to focal adhesions.
Modulation of VEGFR2 responses by other cell surface receptors has been described for VE-cadherin and for neuropilin. In the case of VE-cadherin, it has been noted that clustering of cadherins suppresses VEGFR2 activation by reducing receptor internalization and activation within endocytosed vesicles (Lampugnani et al., 2006). Thus, VE-cadherin appears to be a negative regulator of VEGFR2 function. Consistent with this role, more recently it has been found that VE-cadherin phosphorylates MLC2, leading to suppression of sprouting (Abraham et al., 2009). In contrast, activation of neuropilin appears to mediate VEGFR2 activation, migration, and sprouting (Wang et al., 2003; Becker et al., 2005; Pan et al., 2007; Kawamura et al., 2008; Valdembri et al., 2009). Thus, putting these results together it appears that neuropilins and β1 integrins facilitate and enhance VEGFR2 activation, whereas clustering and engagement of VE-cadherin suppresses VEGFR2 phosphorylation but promotes capillary morphogenesis and vascular maturation.
Another interesting effect found with matrix-bound VEGF was induction of receptor clustering. Both bound and soluble VEGF induced a rapid movement of VEGFR2 receptors from the center to the cell periphery. Notably, although clustering was already seen when cells were exposed to collagen, the size of the clusters and their association to focal adhesion only occurred when nanomolar levels of the growth factor were incorporated in the matrix. The relevance of receptor clustering to signaling events has been well characterized in neurobiology and immunology, where functional synapses correspond to well-orchestrated arrangements of clustered molecules in respond to a stimuli (Cochran et al., 2001; Griffith, 2004). Although organization of clusters has been recognized for other receptor tyrosine kinases (Abu-Ali et al., 2008; Wang et al., 2009), this has not been noted for the VEGF receptor family. Our results indicate that VEGFR2 clustering might provide an important mean to deliver a more robust and prolonged signal needed to sustain a migratory response and within the context of focal adhesions. Given the large array of ECM combinations in distinct tissues, an obvious consideration is whether other integrins might be able to substitute for β1 heterodimers. Although we still do not have the answer to this question, one might speculate that this is likely to be the case and that the matrix molecule, rather than the integrin subtype, might dictate the molecular nature of the cluster.
In a broader perspective, the information unraveled by these studies provides rationale to further investigate the relevance of surface patterning of VEGF in bioactive materials (Anderson et al., 2009). It is likely that the combination of physical and chemical cues presented at a nanoscale level and within an organized array might serve to direct cell migration and further design patterns of vascular morphogenesis.
Overall, based on these findings we would like to introduce the concept that the presentation of VEGF (either soluble or bound) conveys distinct functional responses through the same receptor. Although this work focused on VEGFR2 and β1 integrin, it is likely that other receptors, such as neuropilin, plexin, and Notch are also likely to play an integrative role in the context of bound versus soluble VEGF. Additional efforts should try to investigate how the combinatorial interpretation of matrix-anchored or soluble VEGF in concert with other receptors alters morphogenesis and functional efficacy of blood vessels in a tissue-specific manner.
Materials and methods
Cells and reagents
Human aortic endothelial cells (HAECs) (a gift from Judith Berliner, UCLA, Los Angeles, CA), human umbilical vein endothelial cells (HUVECs; VEC Technologies), and human saphenous vein endothelial cells (HSVECs; VEC Technologies) were cultured in complete MCDB 131 medium (VEC Technologies). Porcine aortic endothelial cells transfected with VEGFR2 (PK cells; a gift from Gera Neufeld, Technicon, Israel) were grown in Ham’s F-12 medium with 10% fetal bovine serum (Omega Scientific). HEK 293 cells transfected with native VEGFR2 or mutants (a gift from Bruce I. Terman) were grown in DME high glucose with FBS (10% vol/vol) and 10 µg/ml puromycin (Sigma-Aldrich). Human VEGF165 was either provided by the NIH Repository, Genentech (San Francisco, CA), or purchased from R&D Systems. mVEGF113 was generated as described previously (Lee et al., 2005). VEGF121 was provided by Gera Neufeld or purchased from R&D Systems. Rabbit anti–human VEGFR 2, phospho-VEGFR 2 (Tyr996 and Tyr1175), p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), Akt, and phospho-Akt (S473) were from Cell Signaling Technology. Antibodies to phosphotyrosine (4G10), mouse anti–human VEGFR2, rabbit anti-phospho VEGFR2 (Tyr951, Tyr1054, Tyr1059), and anti-β1 integrins were from Millipore. Rabbit anti-phospho VEGFR2 (Tyr 1214) was from Invitrogen. Rabbit anti-phospho VEGFR2 (Tyr801) was from ECM Biosciences. Rabbit anti-β1 integrin (C-terminal) was purchased from Abcam. β1 integrin inhibitory antibody was purchased from Covance. Neuropilin-1 antibody was purchased from Santa Cruz Biotechnology, Inc. VEGF antibodies were provided by Donald Senger (Beth Israel Deaconess Medical Center, Boston, MA). For the detection of internalized VEGFR2, anti–human VEGFR2 (single-chain recombinant; clone scFvA7 with E tag; RDI and Fitzgerald) in combination with rabbit anti–E-tag (Abcam) were used for immunofluorescence. Antibodies against the extracellular domain of VEGFR2 were provided by Rolf A. Brekken (UT-Southwestern, Dallas, TX). Collagen matrix (Purecol) was from Advanced Biomatrix. BB94 was a gift from Gerry Weinmaster (UCLA, Los Angeles, CA).
Matrix-bound (Vb) and soluble (Vs) VEGF165 preparation
To generate matrix-bound VEGF (Vb), indicated concentrations of the growth factor (different isoforms as indicated) were mixed with neutralized Purecol collagen gel (3.0 mg/ml; Advanced Biomatrix) and allowed to polymerize at 37°C for 1 h on plastic wrap. The solidified gel was then immersed in 4 ml of 1x PBS for 10 min. The process was repeated five additional times to remove majority of unbound VEGF. The gel was then placed on top of confluent endothelial monolayers. Control gels were done without any VEGF165 added. Soluble VEGF165 was diluted into 1x PBS into same concentration as matrix-bound VEGF165 and used as is.
To challenge direct contact of cells with the matrix, nylon membranes were used in some experiments. In such experiments, the gel was then transferred on top of a 40-µm cell strainer (Millipore) as a “holder” with the bottom of the strainer covered by an 8-µm nylon membrane. The device loaded with collagen gel containing growth factor was then transferred onto a confluent monolayer of HUVECs with serum-free media for 5 min. The gel was covered by the media but did not touch cells directly. The cells were then collected and phosphorylation of VEGFR2 was determined as described below. Under this condition, soluble VEGF165 could diffuse, but bound VEGF165 was unable to diffuse from the gel. When specified we also used VEGF113 and VEGF121; the incorporation of these into matrix was done in a similar manner as described for VEGF165.
VEGF release and retention assays
To determine the release of VEGF from polymerized matrix gels, 2 ml of collagen-bound VEGF was allowed to polymerize in a 50-ml falcon tube. 50 ml of 1x PBS was then added to the tube and incubated under gentle agitation. At indicated times, a 200-µl aliquot was removed and the level of VEGF released was determined by ELISA (R&D Systems).
To indirectly assess the amount of VEGF retained in the collagen gel, 2 ml of collagen-bound VEGF was polymerized in one 35-mm well, washed with 4 ml of 1x PBS, and the concentration of VEGF in each wash was measured by ELISA. Retained VEGF was estimated considering the initial input and the levels released by each wash.
To directly assess matrix-retained VEGF and its half-life, collagen-bound and soluble VEGF were made as described in the previous subheading. Then both types of VEGF (Vb and Vs) were incubated at 37°C at indicated times. To measure the levels of VEGF bound to matrix, gels were placed on ice for 30 min to depolymerize and then either mixed with Laemmli buffer and boiled for 10 min for Western blot or diluted 50 times into sample buffer for ELISA analysis.
Immunoblotting and immunoprecipitation
Endothelial cells were lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycolate, 1 mM EDTA, 1 mM sodium vanadate, 10 mM β-glycerophosphate, and protease inhibitors [1 mM phenylmethanesulfonylfluoride (PMSF), 20 µg/ml leupeptin, and 20 µg/ml aprotinin]). Cell lysates were further incubated under agitation at 4°C for 30 min, and then centrifuged at 20,000 g for 30 min at 4°C. Equivalent levels (500 µg) of protein, determined using the DC protein assay reagent (Bio-Rad Laboratories), were precleared by TrueBlot anti–rabbit Ig IP beads (eBioscience) and immunoprecipitated overnight at 4°C with the antibody of interest. Immune complexes were retrieved using the TrueBlot anti–rabbit Ig IP beads. Immunoprecipitates were washed three times with the same lysis buffer and then separated by SDS-PAGE. Proteins were then transferred to nylon membranes (GE Healthcare), probed with indicated antibodies, and detected by an enhanced chemiluminescence technique (Thermo Fisher Scientific). After detection, the results were quantified by densitometry using ImageJ (National Institutes of Health, Bethesda, MD). Protein phosphorylation was determined by immunoblotting as described previously (Luque et al., 2003).
VEGFR2 internalization assay
Cells were precooled for 30 min on ice and subsequently incubated with 10 µg/ml of monoclonal anti–VEGFR2 antibodies (RDI and Fitzgerald) for an additional 30 min under gentle agitation. The VEGFR2 monoclonal antibodies recognize the extracellular domain of human VEGFR2, lack biological activity, and do not interfere with VEGF-mediated activation of the receptor. These antibodies were used previously in internalization experiments (Lampugnani et al., 2006). After exposure to the antibodies and before stimulation with VEGF, cells were washed with ice-cold 1% BSA in MCDB 131 to remove unbound antibody, and fresh medium was added. Cells were stimulated with either Vs, Vb, Col, or vehicle at 37°C for indicated times and finally washed (three washes with ice-cold 50 mM glycine in Ca2+/Mg2+ HBSS, pH 2.5, followed by two washes with Ca2+/Mg2+ HBSS, pH 7.5) to remove any residual antibody/growth factor from the cell surface.
To detect internalized VEGFR2 by immunofluorescence studies, cells were fixed in 1% PFA in 2.5 mM triethanolamine, pH 7.5, containing 0.1% Triton X-100 and 0.1% NP-40 for 25 min at room temperature. Before staining, 0.5% Triton X-100 in PBS was added for 10 min at room temperature (Lallemand et al., 2003). The distribution of the primary antibody was revealed with rabbit anti–E-tag (Abcam) followed by FITC-conjugated donkey anti–rabbit. To detect internalized VEGFR2 by Western blots, cell lysates were collected as previously described. Lysates were immunoprecipitated with rabbit anti–E-tag (Abcam) and separated by SDS-PAGE. Internalized VEGFR2 was then revealed by anti-VEGFR2.
Proximity ligation assay
HUVECs grown on Labtek chamberslides (Thermo Fisher Scientific) were starved for 6 h and incubated in the presence or absence of soluble VEGF (200 ng/ml), bound VEGF, or collagen alone for 20 min. Cells were washed with chilled PBS and fixed with 4% paraformaldehyde for 15 min, blocked, permeabilized, and incubated overnight with mouse anti–VEGF-R2 (Millipore) and rabbit anti-β1 integrin (Abcam) as indicated. Proximity ligation was performed according to the manufacturer’s protocol using the Duolink Detection Kit with PLA PLUS and MINUS Probes for mouse and rabbit (Olink Bioscience; Söderberg et al., 2006; Jarvius et al., 2007). TOPRO3 nucleus stain (Invitrogen) was added to the mounting media (Olink Bioscience) at 1:1,000 and examined with a confocal microscope (LSM 510 Meta; Carl Zeiss, Inc.) under a 60x oil objective. Texas red (or Cy3) signal amplification was scanned in only the red channel and counted by Blob Finder V3.2 (Uppsala University, Sweden). Four fields at 600x were randomly chosen for analysis and averaged per condition, examining four independent preparations individually.
Immunocytochemistry and confocal microscopy
Endothelial cells were grown on Labtek chamberslides (Thermo Fisher Scientific) and treated as indicated. Cultures were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.1% Triton X-100 in 1x PBS. F-actin was detected using Texas red–conjugated phalloidin (Invitrogen) diluted 1:40 in 1% BSA/PBS. TOPRO3 nucleus stain (Invitrogen) was added in the mounting media (90% glycerol + 10x PBS) at 1:1,000; antibody against the extracellular domain of VEGFR2 was diluted 1:100 in 1% BSA/PBS. Antibodies against paxillin (Abcam) and β1 integrin (BD) were also used 1:100 in 1% BSA/PBS. Slides were then examined under a confocal microscope (LSM 510 Meta; Carl Zeiss, Inc.) under a 60x oil objective.
Quantitation of VEGFR2 clustering
Images were collected randomly (10 images from each treatment; 3 individual experiments were evaluated), and the calculations were determined with the “analyze particles” function of ImageJ (NIH) by using all 10 random images from each treatment, with particle threshold size over 10 for positive VEGFR2 distribution and over 100 for positive VEGFR2 clustering.
Statistical methods
Data and graph were evaluated using a Student’s two-tailed t test or ANOVA with GraphPad Prism 4 software. In all analyses, P < 0.05 was taken to be statistically significant.
Isolation of endothelial cells from β1fl/fl mice
Transgenic mice with loxP sites flanking the β1 integrin locus (β1fl/fl) have been previously described (Raghavan et al., 2000). Mice were perfused with 1x PBS and collagenase (500 µg/ml in DME; Sigma-Aldrich). Livers were harvested and incubated in collagenase (500 µg/ml) for another 30 min. Supernatants were mixed with equal volume of DME containing 20% FBS. Cells were collected after centrifugation at 2,000 rpm for 5 min, resuspended, and filtered through a 40-µm pore size top filter (BD). Filtered cells were plated onto fibronectin/vitronectin-coated tissue culture dishes and washed after 2–3 h to remove cell debris and remaining blood cells.
To inactivate the β1 integrin gene, the isolated endothelial cells were incubated with 1:1,000 adeno-Cre virus stock for 1 h with serum-free media or with adenoviral vector alone. After initial 1 h, complete media (20% FBS) was added and cells were incubated overnight at 37°C. Cells were cultured for an additional 3 d before experimentation.
Examination of p38 activation on β1KO cells
To assess the p38 activation on β1KO mouse endothelial cells, cells (30,000 cells/well) were plated onto 96-well plates and cultured for 2 d until confluency. Cells were starved overnight in serum-free complete MCDB 131 (VEC Technologies). Cells were then treated with either VEGF, collagen VEGF, or collagen only treatment in triplicate for the indicated length of time. The level of p38 phosphorylation was determined with the cell-based phospho-p38 ELISA kit (RayBiotech).
Online supplemental material
Fig. S1 shows that VEGF is greatly retained in a polymerized collagen matrix and that this retention requires the C-terminal binding motif. It also compares the half-life of soluble and matrix-bound VEGF. Fig. S2 demonstrates densitometric analysis of VEGFR2 phosphorylation induced by soluble and matrix-bound VEGF. It also shows an ELISA VEGFR2 detection system that provides additional independent support to the fact that matrix-bound VEGF can activate VEGFR2. The figure further illustrates that presence of protease inhibitors does not prevent phosphorylation of VEGFR2 by matrix-bound VEGF. Fig. S3 compares levels of VEGFR2 internalization and demonstrates differences between soluble and bound VEGF. It also shows phosphorylation level of VEGFR2 displayed by two additional VEGFR2 tyrosine mutants. Kinetics of Y1214 activation by soluble and bound is also included here. Fig. S4 shows presentation of VEGF using a solid phase assay and demonstrates that matrix metalloproteinase–resistant VEGF mutants also induce activation of VEGFR2 and p38. In addition, it also showed VEGFR2 association with β1 integrin under Vb, but not with neuropilin-1. Fig. S5 illustrates the effect of β1 inhibitory antibodies at early time points and the morphology of mouse endothelial cells isolated from floxed β1 integrin mice and further exposed to adenovirus (Cre-recombinase and control), and demonstrates that protein levels of VEGFR2 are reduced when β1 integrin is not present in endothelial cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200906044/DC1.
Acknowledgments
We thank members of the Arispe laboratory for stimulating discussions. Also, we truly appreciate the generosity of several colleagues for their valuable reagents; without their contribution we could not have executed the work as presented. In particular: Dr. Bruce Terman (in memorian) for VEGFR2 mutant cells and Dr. Rolf Brekken for the VEGFR2 extracellular domain antibody.
Funding for this research was obtained from the National Institutes of Health, National Cancer Institute (grant RO1CA126935) to M.L. Iruela-Areispe. Tom Chen was funded by a pre-doctoral fellowship from the Vascular Biology Training Grant at UCLA (T32 HL69766).
Footnotes
Abbreviations used in this paper:
- HUVEC
- human umbilical vein endothelial cells
- Vb
- matrix-bound VEGF
- VEGFR
- VEGF receptor
- Vs
- soluble VEGF
References
- Abraham S., Yeo M., Montero-Balaguer M., Paterson H., Dejana E., Marshall C.J., Mavria G. 2009. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr. Biol. 19:668–674 10.1016/j.cub.2009.02.057 [DOI] [PubMed] [Google Scholar]
- Abu-Ali S., Fotovati A., Shirasuna K. 2008. Tyrosine-kinase inhibition results in EGFR clustering at focal adhesions and consequent exocytosis in uPAR down-regulated cells of head and neck cancers. Mol. Cancer. 7:47 10.1186/1476-4598-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.M., Chen T.T., Iruela-Arispe M.L., Segura T. 2009. The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials. 30:4618–4628 10.1016/j.biomaterials.2009.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari-Hada S., Habuchi H., Kariya Y., Kimata K. 2005. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J. Biol. Chem. 280:31508–31515 10.1074/jbc.M414581200 [DOI] [PubMed] [Google Scholar]
- Bates D.O., Cui T.G., Doughty J.M., Winkler M., Sugiono M., Shields J.D., Peat D., Gillatt D., Harper S.J. 2002. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 62:4123–4131 [PubMed] [Google Scholar]
- Bazzoni G., Dejana E. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84:869–901 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- Becker P.M., Waltenberger J., Yachechko R., Mirzapoiazova T., Sham J.S., Lee C.G., Elias J.A., Verin A.D. 2005. Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circ. Res. 96:1257–1265 10.1161/01.RES.0000171756.13554.49 [DOI] [PubMed] [Google Scholar]
- Bergers G., Brekken R., McMahon G., Vu T.H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. 2000. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2:737–744 10.1038/35036374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanes M.G., Oubaha M., Rautureau Y., Gratton J.P. 2007. Phosphorylation of tyrosine 801 of vascular endothelial growth factor receptor-2 is necessary for Akt-dependent endothelial nitric-oxide synthase activation and nitric oxide release from endothelial cells. J. Biol. Chem. 282:10660–10669 10.1074/jbc.M609048200 [DOI] [PubMed] [Google Scholar]
- Borges E., Jan Y., Ruoslahti E. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867–39873 10.1074/jbc.M007040200 [DOI] [PubMed] [Google Scholar]
- Calera M.R., Venkatakrishnan A., Kazlauskas A. 2004. VE-cadherin increases the half-life of VEGF receptor 2. Exp. Cell Res. 300:248–256 10.1016/j.yexcr.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L. 2003. Signal transduction by vascular endothelial growth factor receptors. Biochem. Soc. Trans. 31:20–24 10.1042/BST0310020 [DOI] [PubMed] [Google Scholar]
- Cochran J.R., Aivazian D., Cameron T.O., Stern L.J. 2001. Receptor clustering and transmembrane signaling in T cells. Trends Biochem. Sci. 26:304–310 10.1016/S0968-0004(01)01815-1 [DOI] [PubMed] [Google Scholar]
- Cunningham S.A., Tran T.M., Arrate M.P., Bjercke R., Brock T.A. 1999. KDR activation is crucial for VEGF165-mediated Ca2+ mobilization in human umbilical vein endothelial cells. Am. J. Physiol. 276:C176–C181 [DOI] [PubMed] [Google Scholar]
- Dougher M., Terman B.I. 1999. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 18:1619–1627 10.1038/sj.onc.1202478 [DOI] [PubMed] [Google Scholar]
- Dougher-Vermazen M., Hulmes J.D., Böhlen P., Terman B.I. 1994. Biological activity and phosphorylation sites of the bacterially expressed cytosolic domain of the KDR VEGF-receptor. Biochem. Biophys. Res. Commun. 205:728–738 10.1006/bbrc.1994.2726 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O’Shea K.S., Powell-Braxton L., Hillan K.J., Moore M.W. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380:439–442 10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H.P., LeCouter J. 2003. The biology of VEGF and its receptors. Nat. Med. 9:669–676 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 376:66–70 10.1038/376066a0 [DOI] [PubMed] [Google Scholar]
- Gerber H.P., McMurtrey A., Kowalski J., Yan M., Keyt B.A., Dixit V., Ferrara N. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 273:30336–30343 10.1074/jbc.273.46.30336 [DOI] [PubMed] [Google Scholar]
- Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. 1999. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5:623–628 10.1038/9467 [DOI] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161:1163–1177 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L.C. 2004. Receptor clustering: nothing succeeds like success. Curr. Biol. 14:R413–R415 10.1016/j.cub.2004.05.031 [DOI] [PubMed] [Google Scholar]
- Guo D., Jia Q., Song H.Y., Warren R.S., Donner D.B. 1995. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem. 270:6729–6733 10.1074/jbc.270.12.6729 [DOI] [PubMed] [Google Scholar]
- Huang L., Sankar S., Lin C., Kontos C.D., Schroff A.D., Cha E.H., Feng S.M., Li S.F., Yu Z., Van Etten R.L., et al. 1999. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J. Biol. Chem. 274:38183–38188 10.1074/jbc.274.53.38183 [DOI] [PubMed] [Google Scholar]
- Inoue M., Hager J.H., Ferrara N., Gerber H.P., Hanahan D. 2002. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 1:193–202 10.1016/S1535-6108(02)00031-4 [DOI] [PubMed] [Google Scholar]
- Jakobsson L., Kreuger J., Holmborn K., Lundin L., Eriksson I., Kjellén L., Claesson-Welsh L. 2006. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell. 10:625–634 10.1016/j.devcel.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Jarvius M., Paulsson J., Weibrecht I., Leuchowius K.J., Andersson A.C., Wählby C., Gullberg M., Botling J., Sjöblom T., Markova B., et al. 2007. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol. Cell. Proteomics. 6:1500–1509 10.1074/mcp.M700166-MCP200 [DOI] [PubMed] [Google Scholar]
- Jośko J., Gwóźdź B., Jedrzejowska-Szypułka H., Hendryk S. 2000. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med. Sci. Monit. 6:1047–1052 [PubMed] [Google Scholar]
- Kawamura H., Li X., Goishi K., van Meeteren L.A., Jakobsson L., Cébe-Suarez S., Shimizu A., Edholm D., Ballmer-Hofer K., Kjellén L., et al. 2008. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 112:3638–3649 10.1182/blood-2007-12-125856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., Yao Ch., Grieb G., Prével P., Noah E.M., Steffens G.C. 2006. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J. Mater. Sci. Mater. Med. 17:735–741 10.1007/s10856-006-9684-x [DOI] [PubMed] [Google Scholar]
- Kroll J., Waltenberger J. 1997. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J. Biol. Chem. 272:32521–32527 10.1074/jbc.272.51.32521 [DOI] [PubMed] [Google Scholar]
- Kroll J., Waltenberger J. 1999. A novel function of VEGF receptor-2 (KDR): rapid release of nitric oxide in response to VEGF-A stimulation in endothelial cells. Biochem. Biophys. Res. Commun. 265:636–639 10.1006/bbrc.1999.1729 [DOI] [PubMed] [Google Scholar]
- Lallemand D., Curto M., Saotome I., Giovannini M., McClatchey A.I. 2003. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 17:1090–1100 10.1101/gad.1054603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamalice L., Houle F., Jourdan G., Huot J. 2004. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 23:434–445 10.1038/sj.onc.1207034 [DOI] [PubMed] [Google Scholar]
- Lampugnani M.G., Orsenigo F., Gagliani M.C., Tacchetti C., Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174:593–604 10.1083/jcb.200602080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Jilani S.M., Nikolova G.V., Carpizo D., Iruela-Arispe M.L. 2005. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 169:681–691 10.1083/jcb.200409115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chen T.T., Barber C.L., Jordan M.C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K.P., Iruela-Arispe M.L. 2007. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 130:691–703 10.1016/j.cell.2007.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A., Carpizo D.R., Iruela-Arispe M.L. 2003. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 278:23656–23665 10.1074/jbc.M212964200 [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Claesson-Welsh L. 2001. VEGF receptor signal transduction. Sci. STKE. 2001:re21 10.1126/stke.2001.112.re21 [DOI] [PubMed] [Google Scholar]
- Mineur P., Colige A.C., Deroanne C.F., Dubail J., Kesteloot F., Habraken Y., Noël A., Vöö S., Waltenberger J., Lapière C.M., et al. 2007. Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. J. Cell Biol. 179:1261–1273 10.1083/jcb.200703052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen T., Alitalo K. 1995. Endothelial receptor tyrosine kinases involved in angiogenesis. J. Cell Biol. 129:895–898 10.1083/jcb.129.4.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J.A., Benjamin L., Zeng H., Dvorak A.M., Dvorak H.F. 2008. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 11:109–119 10.1007/s10456-008-9099-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gitay-Goren H., Poltorak Z., Tessler S., Sharon R., Gengrinovitch S., Levi B.Z. 1996. Similarities and differences between the vascular endothelial growth factor (VEGF) splice variants. Cancer Metastasis Rev. 15:153–158 10.1007/BF00437467 [DOI] [PubMed] [Google Scholar]
- Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. 2006. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7:359–371 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- Pan Q., Chathery Y., Wu Y., Rathore N., Tong R.K., Peale F., Bagri A., Tessier-Lavigne M., Koch A.W., Watts R.J. 2007. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J. Biol. Chem. 282:24049–24056 10.1074/jbc.M703554200 [DOI] [PubMed] [Google Scholar]
- Park J.E., Keller G.A., Ferrara N. 1993. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell. 4:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak Z., Cohen T., Sivan R., Kandelis Y., Spira G., Vlodavsky I., Keshet E., Neufeld G. 1997. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 272:7151–7158 10.1074/jbc.272.11.7151 [DOI] [PubMed] [Google Scholar]
- Pötgens A.J., Lubsen N.H., van Altena M.C., Vermeulen R., Bakker A., Schoenmakers J.G., Ruiter D.J., de Waal R.M. 1994. Covalent dimerization of vascular permeability factor/vascular endothelial growth factor is essential for its biological activity. Evidence from Cys to Ser mutations. J. Biol. Chem. 269:32879–32885 [PubMed] [Google Scholar]
- Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. 2000. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150:1149–1160 10.1083/jcb.150.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.R., Reynolds L.E., Nagel T.E., Lively J.C., Robinson S.D., Hicklin D.J., Bodary S.C., Hodivala-Dilke K.M. 2004. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 64:8643–8650 10.1158/0008-5472.CAN-04-2760 [DOI] [PubMed] [Google Scholar]
- Robinson C.J., Stringer S.E. 2001. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 114:853–865 [DOI] [PubMed] [Google Scholar]
- Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H., Betsholtz C., Shima D.T. 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16:2684–2698 10.1101/gad.242002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Ohgimoto K., Kataoka Y., Yoshida N., Shibuya M. 2005. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA. 102:1076–1081 10.1073/pnas.0404984102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., Schuh A.C. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 376:62–66 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- Shibuya M., Claesson-Welsh L. 2006. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 312:549–560 10.1016/j.yexcr.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K.J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L.G., Landegren U. 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 3:995–1000 10.1038/nmeth947 [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. 1998. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 92:735–745 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- Stalmans I., Ng Y.S., Rohan R., Fruttiger M., Bouché A., Yuce A., Fujisawa H., Hermans B., Shani M., Jansen S., et al. 2002. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 109:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Shibuya M. 2005. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. (Lond.). 109:227–241 10.1042/CS20040370 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yamaguchi S., Chida K., Shibuya M. 2001. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768–2778 10.1093/emboj/20.11.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer G.M., Akerman S., Cross N.A., Barber P.R., Björndahl M.A., Greco O., Harris S., Hill S.A., Honess D.J., Ireson C.R., et al. 2008. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res. 68:2301–2311 10.1158/0008-5472.CAN-07-2011 [DOI] [PubMed] [Google Scholar]
- Valdembri D., Caswell P.T., Anderson K.I., Schwarz J.P., König I., Astanina E., Caccavari F., Norman J.C., Humphries M.J., Bussolino F., Serini G. 2009. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 7:e25 10.1371/journal.pbio.1000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C.H. 1994. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 269:26988–26995 [PubMed] [Google Scholar]
- Wang H.U., Chen Z.F., Anderson D.J. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 93:741–753 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- Wang L., Zeng H., Wang P., Soker S., Mukhopadhyay D. 2003. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J. Biol. Chem. 278:48848–48860 10.1074/jbc.M310047200 [DOI] [PubMed] [Google Scholar]
- Wang S.E., Xiang B., Zent R., Quaranta V., Pozzi A., Arteaga C.L. 2009. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 69:475–482 10.1158/0008-5472.CAN-08-2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Jones C., Abu-Ghazaleh R., Cospedal R., Houliston R.A., Martin J., Zachary I. 1997. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 420:28–32 10.1016/S0014-5793(97)01481-6 [DOI] [PubMed] [Google Scholar]
- Wu L.W., Mayo L.D., Dunbar J.D., Kessler K.M., Ozes O.N., Warren R.S., Donner D.B. 2000. VRAP is an adaptor protein that binds KDR, a receptor for vascular endothelial cell growth factor. J. Biol. Chem. 275:6059–6062 10.1074/jbc.275.9.6059 [DOI] [PubMed] [Google Scholar]
- Zachary I. 2005. Signal transduction in angiogenesis. EXS. 2005:267–300 [DOI] [PubMed] [Google Scholar]
- Zisch A.H., Schenk U., Schense J.C., Sakiyama-Elbert S.E., Hubbell J.A. 2001. Covalently conjugated VEGF—fibrin matrices for endothelialization. J. Control. Release. 72:101–113 10.1016/S0168-3659(01)00266-8 [DOI] [PubMed] [Google Scholar]