A proteomics approach identifies Drosophila Syd-1 as a Bruchpilot binding partner that controls maturation on both sides of the neuromuscular junction.
Abstract
Active zones (AZs) are presynaptic membrane domains mediating synaptic vesicle fusion opposite postsynaptic densities (PSDs). At the Drosophila neuromuscular junction, the ELKS family member Bruchpilot (BRP) is essential for dense body formation and functional maturation of AZs. Using a proteomics approach, we identified Drosophila Syd-1 (DSyd-1) as a BRP binding partner. In vivo imaging shows that DSyd-1 arrives early at nascent AZs together with DLiprin-α, and both proteins localize to the AZ edge as the AZ matures. Mutants in dsyd-1 form smaller terminals with fewer release sites, and release less neurotransmitter. The remaining AZs are often large and misshapen, and ectopic, electron-dense accumulations of BRP form in boutons and axons. Furthermore, glutamate receptor content at PSDs increases because of excessive DGluRIIA accumulation. The AZ protein DSyd-1 is needed to properly localize DLiprin-α at AZs, and seems to control effective nucleation of newly forming AZs together with DLiprin-α. DSyd-1 also organizes trans-synaptic signaling to control maturation of PSD composition independently of DLiprin-α.
Introduction
Fast chemical synaptic transmission is mediated by precisely regulated neurotransmitter release from synaptic vesicles (SVs) at specialized presynaptic sites. This compartment, called the active zone (AZ), comprises a unique set of proteins (Schoch and Gundelfinger, 2006; Owald and Sigrist, 2009).
Genetic analyses of synapse assembly in Caenorhabditis elegans hermaphrodite-specific motor neuron synapses (HSNLs; Margeta et al., 2008) and in Drosophila neuromuscular junctions (NMJs; Collins and DiAntonio, 2007) have identified several presynaptic proteins important for AZ assembly (Owald and Sigrist, 2009). Syd-2/Liprin-α is needed for AZ formation at C. elegans HSNL synapses (Dai et al., 2006; Patel et al., 2006) and is important for proper AZ morphology in Drosophila (Kaufmann et al., 2002), and ELKS is essential downstream of Syd-2/Liprin-α (Dai et al., 2006). In Drosophila, the ELKS-related protein Bruchpilot (BRP) forms the electron-dense projection at AZs (T bar), and is crucial for AZ maturation (Kittel et al., 2006; Fouquet et al., 2009). Finally, Syd-1 (synapse defective 1), a multidomain RhoGAP-like protein, is required for C. elegans HSNL synapse assembly (Dai et al., 2006; Patel et al., 2006).
Here, a proteomics-based approach identified the Drosophila Syd-1 homologue (DSyd-1) as a BRP binding partner. Using stimulated emission depletion microscopy (STED; Kittel et al., 2006; Fouquet et al., 2009), we show that DSyd-1 specifically localizes to a discrete compartment at the AZ edge, coordinating the BRP-composed T bar at the center of the AZ. Flies lacking DSyd-1 show impaired locomotion and a reduced life span, which is rescued by nervous system expression of the protein. Fewer release sites form at dsyd-1 NMJs, and evoked neurotransmitter release is compromised, likely as a consequence of this. EM and STED results both show that dsyd-1 mutant AZs often “overgrow” their T bars, and that ectopic electron-dense precipitates/BRP accumulations also form distant from AZs. Thus, DSyd-1 inhibits inappropriate localization of BRP and its associated electron density. Both DSyd-1 and DLiprin-α accumulate early during the protracted AZ formation process. Notably, DSyd-1 was needed to properly localize DLiprin-α at AZs, but not vice versa. Thus, one function of the RhoGAP DSyd-1 seems to be to stably target DLiprin-α to maturing AZs, allowing DLiprin-α to execute its AZ assembly function. Independent of DLiprin-α, the presynaptic AZ-localized protein DSyd-1 is also involved in defining the amount and composition of glutamate receptors (GluRs) accumulating at maturing postsynaptic densities (PSDs). DSyd-1 might stall synaptic proteins other than DLiprin-α, e.g., adhesion molecules, to regulate postsynaptic maturation in a trans-synaptic manner.
Results
The AZ protein BRP is an integral part of the electron-dense T bar and is needed for effective Ca2+ channel clustering during synapse maturation (Fouquet et al., 2009). Thus, BRP may be a platform for protein–protein interactions and was well-suited as a starting point for an unbiased proteomics screen for novel Drosophila AZ proteins.
Proteomic identification of Drosophila Syd-1 as a BRP-linked protein
Using the monoclonal antibody Nc82, we immunoprecipitated BRP from adult fly head extracts. Although BRP was strongly enriched in Nc82 precipitates, it was not detected in control eluates as visualized by staining SDS-polyacrylamide gels (Fig. 1 A, arrowhead); this was confirmed by tandem mass spectrometry (MS/MS) using two independent protocols (see Materials and methods). Next, we subjected bands of coimmunoprecipitating proteins to MS/MS analysis. Several peptides (Fig. S1 A) were found to correspond to a conceptual protein annotated at FlyBase (http://flybase.org) as CG1976-PA or RhoGAP100F (for further identified proteins, see Fig. S1 B). Hereupon, we refer to this protein as DSyd-1 because of its striking similarity to C. elegans Syd-1, which has been implicated in AZ assembly (Hallam et al., 2002; Dai et al., 2006; Patel et al., 2006) and has been shown to physically interact with the BRP homologue ELKS (Patel and Shen, 2009). DSyd-1 is predicted to comprise a calcium-sensing/lipid-binding C2 domain, a PDZ protein–protein interaction domain, and a putative RhoGAP domain (Hallam et al., 2002).
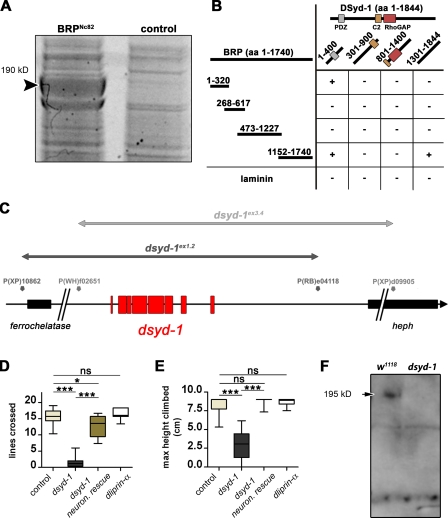
Figure 1.
Proteomics identify DSyd-1 as physical interactor of BRP. (A) Monoclonal BRPNc82 efficiently precipitates BRP (arrowhead), as seen in this SYPRO red–stained SDS-gel. Among other proteins, DSyd-1 was found to coprecipitate with BRP, as confirmed by MS/MS analysis. (B) Matrix showing yeast two-hybrid assay results confirming a direct physical interaction between BRP and DSyd-1. A C-terminal domain of BRP (aa 1,152–1,740) was positive for interaction with a C-terminal region of DSyd-1 (aa 1,301–1,844). Moreover, a bait N-terminal DSyd-1 (aa 1–400) fragment interacted with both the N-terminal fragment of BRP (aa 1–320) and a C-terminal BRP (aa 1,152–1,740) fragment. (C) Genomic location of dsyd-1 on chromosome arm 3R at 100D2-100D3. dsyd-1–deficient animals were constructed using Drosophila lines carrying transposon-mediated flippase recognition target sites (Parks et al., 2004) that neighbored the dsyd-1 locus (black, dsyd-1ex1.2; gray, dsyd-1ex3.4 in gray). We obtained two deficiencies that were confirmed with genomic PCR. In both cases, the entire dsyd-1 locus (red) was excised, whereas in one case (dsyd-1ex1.2, black line), the 5′ ferrochelatase was affected; and in the other case, the 3′ heph (dsyd-1ex3.4, gray line) locus was affected. Taking these deficiencies in trans eliminates both copies of dsyd-1; however, this leaves one intact copy of each heph and ferrochelatase. (D and E) Behavioral tests demonstrate a requirement for DSyd-1 and less stringent requirement for DLiprin-α in the adult CNS. (D) Walking ability (control: 15.69 ± 0.57 lines, n = 15; dsyd-1: 1.62 ± 0.69 lines, n = 8; dsyd-1rescue: 12.86 ± 0.99 lines, n = 10; dliprin-α: 16.19 ± 0.65 lines, n = 7; control × dsyd-1: P = 0.0001; control × dsyd-1rescue: P = 0.02; control × dliprin-α: P = 0.67; dsyd-1 × dsyd-1rescue: P < 0.0001). (E) Negative geotaxis (control: 8.32 ± 0.37 cm; dsyd-1: 2.92 ± 0.60 cm; dsyd-1rescue: 8.833 ± 0.17 cm; dliprin-α: 8.67 ± 0.15 cm; all: n = 10; control × dsyd-1: P < 0.0001; control × dsyd-1rescue: P = 0.32; control × dliprin-α: P = 0.91; dsyd-1 × dsyd-1rescue: P < 0.0001). Impaired locomotive behavior in dsyd-1 flies is rescued by pan-neural (elav-GAL4) reexpression of the dsyd-1 cDNA. Error bars indicate the SEM. *, P < 0.05; ***, P < 0.005; ns, P > 0.05. (F) A polyclonal α-DSyd-1 antibody recognizes a band at the predicted molecular mass of 195 kD on immunoblots of w1118 control fly head lysate (arrow). This band is missing in dsyd-1 head extracts. Statistics: Mann-Whitney test.
To elucidate whether DSyd-1 can bind to BRP directly, subregions of each protein were tested for interaction in a yeast two-hybrid assay. Several interaction sites between both proteins were found (Fig. 1 B). We thus conclude that the physical interaction between Syd-1/DSyd-1 and ELKS/BRP is evolutionarily conserved.
Following the peptide sequence and an existing cDNA clone (Berkeley Drosophila Genome Project; available from GenBank/EMBL/DDBJ under accession no. LD28013; Fig. S1 A), a composite full-length cDNA was assembled, predicting a protein of 195 kD.
DSyd-1–deficient flies suffer from impaired locomotion and decreased lifespan
We generated dsyd-1–deficient animals using Flippase-mediated trans-deletion of flippase recognition target site–containing transposon lines (Parks et al., 2004) flanking the dsyd-1 locus (Fig. 1 C). Two dsyd-1–deficient lines (dsyd-1ex1.2 and dsyd-1ex3.4) were isolated, and deletions were confirmed by genomic PCR (Parks et al., 2004). A combination of both chromosomes results in flies specifically deficient for dsyd-1 (Fig. 1 C). Although dsyd-1 adults appeared morphologically normal and, under optimal culturing conditions, eclosed close to the Mendelian ratio, they rarely survived longer than a week (>80% died within one week, n = 36). In contrast, >80% of control flies (n = 159) lived for at least two weeks. Moreover, the early lethality was completely overcome by elav-GAL4–driven pan-neuronal expression of the composite full-length cDNA (upstream activator sequence [UAS]–dsyd-1cDNA) in the mutant background (n = 42). Notably, dsyd-1 animals showed severely impaired locomotion, as revealed by two independent experimental settings, which was rescued by pan-neuronal expression of UAS–dsyd-1cDNA as well (Fig. 1, D and E).
We raised a polyclonal antibody against a C-terminal peptide of DSyd-1 (DSyd-1 antibody) that identified a band of predicted size (195 kD) in adult head extracts and that was missing in extracts of dsyd-1 mutants (Fig. 1 F).
DSyd-1 is an AZ protein
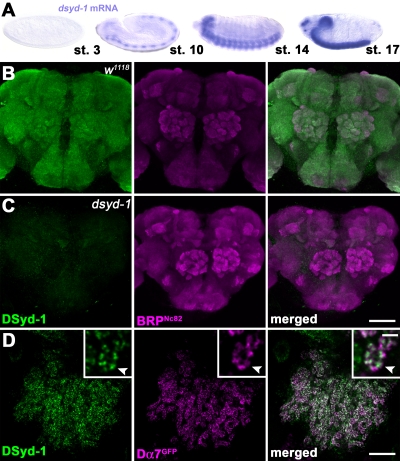
In situ hybridization showed nervous system–specific expression of dsyd-1 (Fig. 2 A), with a similar onset of expression for brp, coincident with postmitotic differentiation (Wagh et al., 2006). The DSyd-1 antibody gave a neuropil-specific staining in larval (not depicted) and adult brains (Fig. 2 B), which was completely absent in dsyd-1 mutant animals (Fig. 2 C) but restored upon pan-neuronal expression of UAS–dsyd-1cDNA (not depicted). Co-labeling revealed a strong overlap with BRPNc82 signals, which suggests that DSyd-1 is an AZ protein. To address this issue more explicitly, we first analyzed synapses within the mushroom body (MB) calyx. Here, postsynaptic specializations were labeled by expressing the GFP-labeled acetylcholine receptor subunit Dα7 within Kenyon cells (Fig. 2 D; Leiss et al., 2009; Raghu et al., 2009). DSyd-1–specific immuno-labeling was found to localize opposite to the Dα7 signal within the presynaptic terminals, which implies that DSyd-1 localizes to AZs (Fig. 2 D).
Figure 2.
DSyd-1 localizes to central synapses. (A) In situ hybridizations show that dsyd-1 is expressed throughout the embryo’s CNS. st., stage. (B) Confocal z projection of adult Drosophila CNS. α-DSyd-1 staining colocalizes with BRPNc82 throughout the brain, but is absent in dsyd-1 animals (C). (D) DSyd-1 localizes opposite to postsynaptic acetylcholine receptors (Dα7GFP) expressed in Kenyon cells at the adult MB calyx. Arrowheads in the inset panels (which show enlarged views) indicate pre- to postsynaptic alignment. Bars: (B and C) 50 µm; (D) 10 µm; (D, insets) 500 nm.
We then turned to the larval NMJ system (Fig. 3). Consistent with our observations in the MB calyx, the DSyd-1 antibody also specifically labeled AZs at NMJs (Fig. 3, A and B).
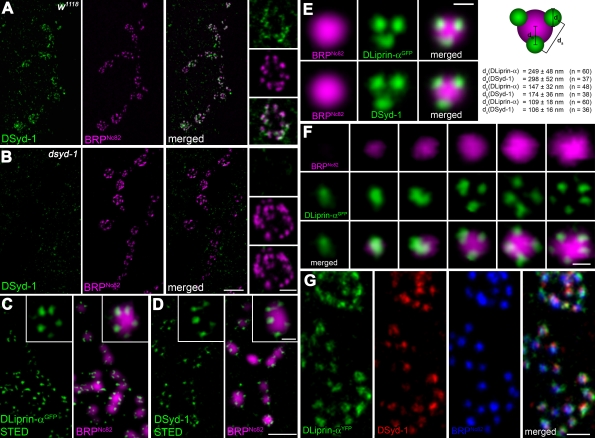
Figure 3.
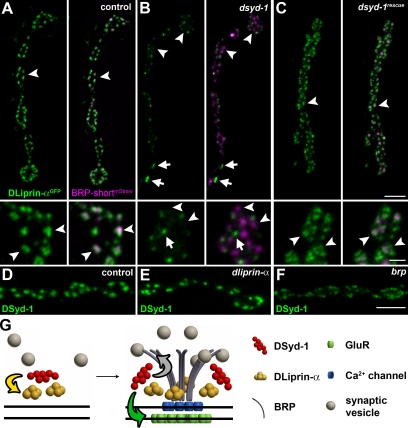
DSyd-1 localizes to a subcompartment surrounding the AZ core. (A) Boutons of larval NMJ innervating muscle 6/7. Most DSyd-1 clusters are found associated with BRPNc82 signal, labeling AZs, as seen in high-magnification images (right). (B) There was no DSyd-1 staining at dsyd-1–deficient NMJs. (C) Single confocal slices of junctions expressing DLiprin-αGFP, as described in Fouquet et al. (2009). STED images of α-GFP labelings show DLiprin-αGFP as discrete spots arranged around the AZ core labeled by BRPNc82. (D) Single confocal slices of NMJs stained for endogenous DSyd-1 (STED) and BRPNc82 (confocal). Distinct separable DSyd-1 spots closely resembling DLiprin-α distribution are arranged around the AZ center. (E) Merged images of several aligned planar imaged AZs of moderate size associated with three DLiprin-α or DSyd-1 clusters. The image shows BRPNc82 in confocal resolution, α-GFP–labeled NMJs (for DLiprin-αGFP), or DSyd-1–labeled NMJs imaged with STED. The arrangement of DSyd-1 clusters resembles that of the DLiprin-α clusters. da, distance between single clusters associated with the AZ; db, distance between AZ associated cluster and AZ center; dc, diameter of clusters associated with AZs. (F) Single confocal slices of junctions expressing DLiprin-αGFP. STED images of α-GFP show DLiprin-αGFP as discrete dots arranged around the AZ core labeled by BRPNc82, ranging from one or two dots at small AZs to four or five dots at mature-sized AZs. (G) Triple labeling for DLiprin-αYFP, DSyd-1, and BRP. Bars: (A and B) 2 µm; (A and B, insets) 500 nm; (C and D), 1 µm; (C and D, insets): 250 nm; (E) 250 nm; (F) 250 nm; (G) 500 nm.
Both DSyd-1 and DLiprin-α localize at the AZ edge
Given that AZ assembly at HSNL synapses in C. elegans (Dai et al., 2006; Patel et al., 2006) involves a tight interplay between Syd-1 and Syd-2/Liprin-α, we reasoned that their homologues might operate together during synaptogenesis in flies. DLiprin-α is known to control proper segregation and shaping of AZs at the developing Drosophila NMJ (Kaufmann et al., 2002; Fouquet et al., 2009). However, we noted that adults lacking DLiprin-α did not display the severe locomotor deficits seen in dsyd-1 mutants, which suggests that both proteins may also have differentiated functions (Fig. 1, D and E).
Using STED microscopy, we recently showed that DLiprin-α forms discrete clusters surrounding the BRP-defined center of AZs (Fig. 3 C; Fouquet et al., 2009). We found DSyd-1 in clusters of similar size and distribution (Fig. 3, D and E) to DLiprin-α clusters. The number of DLiprin-α clusters varied according to the size of AZs (as judged by BRP immunoreactivity) ranging from one cluster at small AZs to four or five clusters at mature-sized AZs (Fig. 3 F).
Correlation analysis of DLiprin-α and DSyd-1 costainings (Fig. 3 G) indicated that both proteins closely colocalize (RDSyd-1::DLiprin-α = 0.81 ± 0.01; n = 12), significantly closer than BRP and DLiprin-α (RBRP::DLiprin-α = 0.66 ± 0.01; P < 0.0001, n = 12; Fig. 3 G). Moreover, the mean distances of individual DLiprin-α and DSyd-1 signals to neighboring spots or to the AZ core were comparable (Fig. 3 E). Thus, DSyd-1 and DLiprin-α together seem to define a common subcompartment surrounding the AZ core.
Reduction of evoked release at dsyd-1 mutant NMJs
To explore whether DSyd-1 was needed for proper synaptic neurotransmitter release at AZs, two electrode voltage clamp recordings of late third-instar larval NMJs were performed. Evoked excitatory junctional currents (eEJCs) were significantly reduced in dsyd-1 mutant larvae compared with controls (Fig. 4 A). These were significantly rescued by presynaptic expression of UAS–dsyd-1cDNA using the motoneuronal driver ok6-GAL4 (Fig. 4 A). For comparison, recordings from mutants in the AZ organizing protein dliprin-α were performed (compare Fig. 4 A with Kaufmann et al., 2002). Interestingly, eEJC amplitudes were decreased to a comparable level in dsyd-1 and dliprin-α. Spontaneous miniature-current amplitudes, in turn, were on average not changed between dsyd-1 and controls (Fig. 4 B, but see “Presynaptic DSyd-1 controls the amount…”).
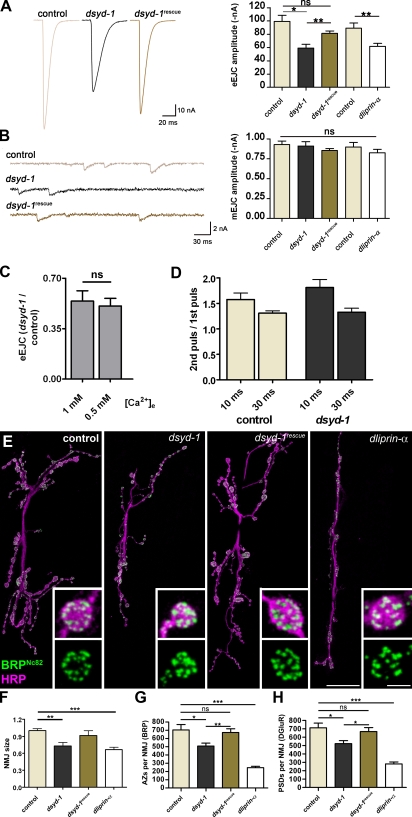
Figure 4.
Comparative analysis of NMJ morphology and function in dsyd-1 and dliprin-α mutant animals. (A) Mean traces (left) of eEJCs at 0.2-Hz nerve stimulation recorded from the larval NMJ at 1 mM extracellular calcium (muscle 6) for controls, dsyd-1, and dsyd-1rescue; and mean eEJC amplitudes (right) for dsyd-1 and control group (control: 99.3 ± 9.6 nA; dsyd-1: 59.2 ± 5.9 nA; both: n = 9, P = 0.01), dsyd-1rescue (dsyd-1: 81.4 ± 4.5 nA, n = 9, P = 0.003; control: P = 0.162) as well as for dliprin-α and the control group (control: −89.4 ± 7.7 nA; dliprin-α: −62.0 ± 4.3 nA; both: n = 7, P = 0.007). Both dliprin-α and dsyd-1 show reduced amplitudes compared with controls. This defect is significantly rescued by reexpressing dsyd-1 cDNA in dsyd-1–deficient animals using a motoneuron-specific driver (ok6-GAL4). (B) Sample traces of mEJCs (right) for control, dsyd-1, and dsyd-1rescue animals. Mean mEJC amplitudes (left) for controls (0.93 ± 0.05 nA, n = 7), dsyd-1 (0.91 ± 0.05 nA, n = 8), and dsyd-1rescue (0.86 ± 0.02 nA, n = 9), as well as dliprin-α (0.83 ± 0.05 nA, n = 7) and control (0.90 ± 0.06 nA, n = 7), are comparable (dsyd-1 × control: P = 0.86; dsyd-1 × dsyd-1rescue: P = 0.54; control × dsyd-1rescue: P = 0.14; control × dliprin-α: P = 0.46). (C) dsyd-1 eEJC amplitudes normalized against mean control eEJC amplitude recorded at 1 mM (0.54 ± 0.07, n = 11) or 0.5 mM (0.51 ± 0.05, n = 8; P = 0.48) extracellular calcium, respectively. (D) Paired pulse experiments with a 10-ms (control: 1.58 ± 0.13, n = 11; dsyd-1: 1.81 ± 0.16, n = 13; P = 0.25) or 30-ms (control: 1.31 ± 0.05, n = 12; dsyd-1: 1.33 ± 0.08, n = 12; P = 0.98) interpulse interval recorded at 0.5 mM extracellular calcium. (E) Projection of confocal stacks of muscles 6 and 7 NMJs, labeled with antibodies recognizing BRP (BRPNc82, green) and HRP (magenta). Bars, 10 µm and 1 µm (insets). (F) Morphological size of dliprin-α and dsyd-1 mutant NMJs was reduced compared with controls. The latter was rescued by motoneuron-specific reexpression of dsyd-1cDNA (control: 1.0 ± 0.04, n = 30; dsyd-1: 0.73 ± 0.06, n = 14; dsyd-1rescue: 0.91 ± 0.08, n = 8; dliprin-α: 0.66 ± 0.04, n = 14; control × dsyd-1: P < 0.01; control × dsyd-1rescue: P > 0.05; control × dliprin-α: P < 0.001; dsyd-1 × dsyd-1rescue: P > 0.05; dsyd-1 × dliprin-α: P > 0.05; dsyd-1rescue × dliprin-α: P > 0.05, one-way analysis of variance [ANOVA]). (G) Number of AZs per NMJ counted via α-BRPNc82 labeling. In both dsyd-1 and dliprin-α mutants, AZ numbers were reduced compared with controls. The reduction seen in dsyd-1 mutants was rescued by presynaptic dsyd-1 cDNA expression (control: 704.6 ± 64.94, n = 14; dsyd-1: 508.6 ± 36.07, n = 14; dsyd-1rescue: 673.1 ± 45.30, n = 10; dliprin-α: 247.3 ± 15.81, n = 8; control × dsyd-1: P = 0.020; control × dsyd-1rescue: P = 0.75; control × dliprin-α: P = 0.0002; dsyd-1 × dsyd-1rescue: P = 0.008). (H) Number of PSDs defined by DGluRIID (not depicted). The results were comparable to those in G (control: 712.7 ± 55.24, n = 20; dsyd-1: 523.5 ± 36.35, n = 13; dsyd-1rescue: 667.9 ± 46.85, n = 8; dliprin-α: 281.1 ± 22.83, n = 7; control × dsyd-1: P = 0.025; control × C: P = 0.86; control × dliprin-α: P = 0.0002; dsyd-1 × dsyd-1rescue: P = 0.047). Statistics: Mann-Whitney test. Error bars indicate the SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ns, P > 0.05.
Neurotransmitter release deficits at dsyd-1 mutant NMJs might be explained by a drop in release probability of SVs, e.g., by a reduction of Ca2+ sensitivity of the SVs that are to be released. In this case, a change in short-term plasticity (paired pulse paradigm) or sensitivity to different extracellular Ca2+ concentrations should be observed. However, when we compared evoked release at two different Ca2+ concentrations, the ratio between dsyd-1 mutant and control was unchanged (Fig. 4 C), which argues against a change in Ca2+ sensitivity. Moreover, no clear alteration in paired pulse behavior was observed (Fig. 4 D). Collectively, these data imply that the characteristics of SV release are (if anything) only moderately altered after loss of DSyd-1. Thus, the question arose as to the number of release sites (i.e., an individual PSD + adjunct AZ) forming at dsyd-1 mutant NMJs, and/or whether the number of releasable SVs was reduced.
Reduced numbers of synaptic release sites at dsyd-1 mutant NMJs
To account for SV numbers and distribution, we performed Drosophila vesicular glutamate transporter (DVGlut) immunostainings (Fig. S2 A; Daniels et al., 2004; Mahr and Aberle, 2006). Overall, both dsyd-1 and control NMJs showed comparable immunoreactivity (Fig. S2 B), which indicates that the absolute number of SVs per terminal was not substantially changed. However, the SV signal appeared somewhat uneven between individual boutons at dsyd-1 NMJs when compared with controls (Fig. S2 A). To evaluate whether this distribution would account for the observed release defect at low frequency stimulation (Fig. 4 A), SV distribution closely surrounding the electron-dense projection at AZs was evaluated in electron micrographs (Fig. S2, C and D). Here, the SV size (Fig. S2 E) as well as the number of SVs surrounding the AZs (Fig. S2, D and F) were comparable between control and dsyd-1 mutant animals. We also tested whether mitochondria were properly transported to the NMJ terminal in dsyd-1 mutants, using MitoGFP (Fig. S2 G; Pilling et al., 2006). Here, the mean NMJ signal did not differ significantly between controls and mutants (Fig. S2 H).
To perform quantitative analysis of release sites, NMJs of third-instar larvae were stained (Fig. 4 E). The overall size of individual NMJs (as scored by HRP reactivity) was reduced in both dliprin-α and dsyd-1 mutant animals (Fig. 4, E and F). We scored numbers of release sites by counting (a) BRP spots (for AZs, Fig. 4 E) and (b) DGluRIID spots (for PSDs, not depicted; Qin et al., 2005). In dsyd-1 mutant larvae, a significant reduction of release sites was observed (Fig. 4, G and H). This reduction appeared identical when independently counting either BRP or DGluRIID spots, and was rescued by motoneuron-specific expression of UAS–dsyd-1cDNA (Fig. 4, G and H).
Thus, presynaptic DSyd-1 is needed for developing NMJs to reach full morphological size and adopt a full complement of release sites. Consistent with previous studies, release site numbers were also reduced at dliprin-α mutant NMJs (Kaufmann et al., 2002); however, the phenotype is more pronounced than that observed in dsyd-1 NMJs (Fig. 4, E, G, and H).
Defective AZ assembly and ectopic BRP accumulations at dsyd-1 mutant terminals
Upon scoring BRP signals, we had the impression that atypically large spots formed at dsyd-1 NMJs. To resolve AZ morphology more accurately, we used STED microscopy for the further analysis.
Using this technique, we recently showed that BRP is a direct building block of T bars. The N terminus of BRP localizes close to Ca2+ channels at the AZ membrane, whereas its C-terminus (recognized by the BRPNc82) defines the edge of the distal T bar platform, resulting in a typical donut-shaped appearance at wild-type NMJs (Fig. 5, A and A′, arrowheads; Fouquet et al., 2009).
Figure 5.
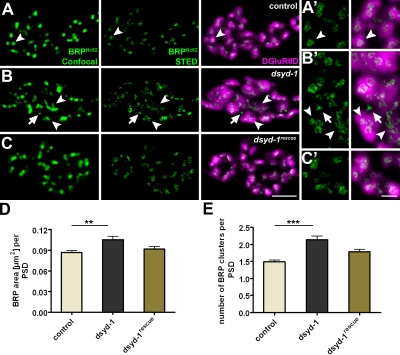
Abnormal BRP clusters in dsyd-1. (A–C) BRP puncta (confocal, left), BRP-donuts (STED, middle), and DGluRIID with BRP donuts (right). (A) The control BRP donut is indicated by arrowheads. (B) AZ size (arrowheads) is affected in dsyd-1. BRP donuts lacking postsynaptic DGluRIID receptors are observed as well (arrows). BRP donuts are frequently interconnected and abnormally shaped (arrowheads). (C) Defects are largely rescued by reexpression of UAS–dsyd-1cDNA. Bar, 1 µm. (A’–C’) Magnified views of A–C. Bar, 250 nm. (D) Quantification shows elevated areas of individual BRPNc82 clusters (control: 0.087 ± 0.002 µm2, n = 298; dsyd-1: 0.105 ± 0.005 µm2, n = 265; dsyd-1rescue: 0.091 ± 0.004 µm2, n = 207; control × dsyd-1: P < 0.01; control × dsyd-1rescue: P > 0.05; dsyd-1 × dsyd-1rescue: P > 0.05). (E) Number of individual BRP clusters per single PSDs (control: 1.49 ± 0.05, n = 297; dsyd-1: 2.14 ± 0.12, n = 265; dsyd-1rescue: 1.79 ± 0.08, n = 207; control × dsyd-1: P < 0.001; control × dsyd-1rescue: P > 0.05; dsyd-1 × dsyd-1rescue: P > 0.05). Statistics: one-way ANOVA. Error bars indicate the SEM. **, P < 0.01; ***, P < 0.005.
At dsyd-1 mutant AZs, this donut-type distribution was compromised (Fig. 5, B and B′, arrowheads) but was partially restored by UAS–dsyd-1cDNA reexpression (Fig. 5, C and C′). In dsyd-1, BRP organization at individual sites often appeared enlarged (Fig. 5, B and B′, arrowheads; and Fig. 5 D) and misshapen. Thus, the STED analysis implied that T bar morphology was affected, with atypical formation of large assemblies (Fig. 5, B and B′).
Individual synaptic release sites (as defined by presynaptic BRP in conjunction with opposing PSDs) showed further abnormalities. Although the size of individual PSDs was enlarged in dsyd-1 mutants (see the following section), individual release sites (defined by the PSD) often comprised several BRP clusters (Fig. 5 E). Furthermore, spacing between individual AZs was irregular, and small BRP assemblies lacking adjacent GluR fields were observed (Fig. 5, B and B′, arrows). These might represent AZ assemblies, which do not progress to maturation properly due to a lack of nucleation assembly.
To address T bar morphology and the nature of increased BRP entities directly, we continued our studies using EM (Fig. 6 A) combined with 3D reconstruction of serial sections. In fact, at dsyd-1 mutant NMJs (Fig. 6, B and B′), T bars often appeared irregular in shape, with pedestals of very high diameter and multiple, atypically prominent filamentous projections in their distal parts (Fig. 6 B, arrowhead). Such misshapen T bars (as in Fig. 6 B) were never observed in controls (Fig. 6 A). Moreover, atypically small T bar–like assemblies were apparent (Fig. 6 B′). These might reflect immature release sites and correspond to the small BRP assemblies observed by STED (Fig. 5, B and B′, arrows).
Figure 6.
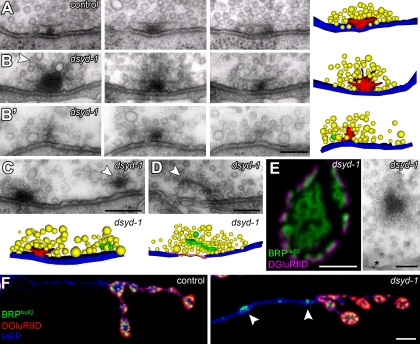
Abnormal organization of T bars and floating electron-dense material in dsyd-1 mutant animals. (A–B’) Serial sections of misshapen T bars at dsyd-1 (B and B’) AZs in comparison to control T bar (A). The arrowhead indicates filaments emerging from an overgrown T bar (B) in dsyd-1. (B’) Immature small T bar in dsyd-1. Reconstruction: red, T bar material; yellow, SVs; membrane blue, AZ. (C) Ectopic electron-dense material can be found at the edge of the AZ membrane (arrowhead, green in reconstruction); electron-dense material (arrowhead, green in reconstruction) associated with SVs is found proximal to AZs (D). (E) Ectopic BRP immunoreactivity (confocal image, left) and ectopic electron-dense material in the center of a dsyd-1 mutant bouton (right). Ectopic electron-dense material (not depicted) and ectopic BRP immunoreactivity (F, arrowheads) are also found in axonal stretches. Bars: (A–B′) 100 nm; (C and D) 150 nm; (E, left) 1 µm; (E, right) 150 nm; (F) 1 µm.
At control NMJs, electron-dense material is restricted to the T bar assembly at the center of the AZ (as defined by planar apposition between pre- and postsynaptic membrane). However, ectopic electron-dense material was easily observed at dsyd-1 mutant NMJ terminals. Such material frequently appeared at the edge of AZs (Fig. 6 C), and was only loosely (Fig. 6 D, arrowhead), if at all (Fig. 6 E), associated with the presynaptic plasma membrane. Floating electron-dense material, highly decorated with SVs, was observed in the bouton interior (Fig. 6 E). As BRP seems to be a principal component of the electron-dense T bar (Fouquet et al., 2009), these ectopic electron-dense assemblies in dsyd-1 mutants should contain BRP. Ectopic BRP reactivity at the bouton center and throughout the axon was also consistently detected by light microscopy (Fig. 6, E and F). In agreement with our EM data showing electron-dense material in association with SV-like material, ectopic axonal BRP accumulations colocalized with the SV marker DVGlut (Fig. S3; Daniels et al., 2004; Mahr and Aberle, 2006).
Collectively, fewer full-sized AZs formed in dsyd-1 mutants, most likely because of the failure of some AZs to progress to maturation. However, excessive amounts of BRP were observed at the remaining AZs and within the neighboring plasma membrane and the presynaptic cytoplasm. Thus, DSyd-1 appears to be necessary to distribute AZ material adequately among a sufficient number of forming and maturing AZs. That the NMJ comprises a reduced number of immature AZs in dsyd-1 mutants might contribute to the deposit of excess AZ material at remaining sites, effectively overgrowing them.
Presynaptic DSyd-1 controls the amount and composition of postsynaptic GluRs
At the Drosophila NMJ, ionotropic receptors (assembling as heteromeric tetramers by selecting four from five subunits) mediate the postsynaptic response to glutamate. Three subunits—DGluRIIC, IID, and IIE—are essential for receptor formation and function and are seemingly contained within all GluR complexes (Petersen et al., 1997; Marrus et al., 2004; Qin et al., 2005; Schmid et al., 2008). To assess PSDs in dsyd-1 mutants, we looked into the distribution and signal intensity for different GluR subunits (Fig. 7, A–F).
Figure 7.
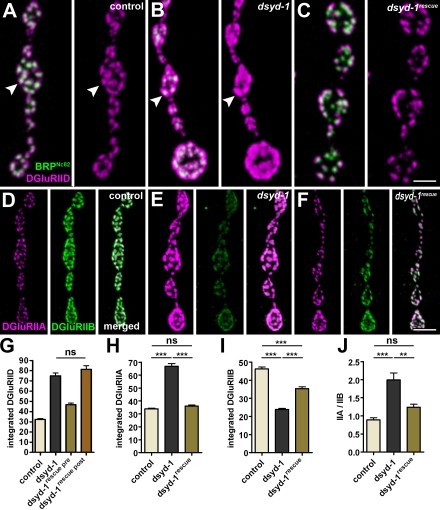
DSyd-1 controls postsynaptic GluR field size and composition. (A–C) Co-labeling of DGluRIID and BRPNc82 for control (A), dsyd-1 mutant (B), and presynaptically rescued (C) NMJs. Individual PSDs are indicated by arrowheads. (D–F) Co-labeling of DGluRIIA and DGluRIIB for control (D), dsyd-1 mutant (E), and presynaptically rescued (F) NMJs. (G) Integrated DGluRIID signal (control: 32.25 ± 0.67 au, n = 1,314; dsyd-1: 74.86 ± 2.98 au, n = 335; dsyd-1rescue pre: 46.71 ± 1.60 au, n = 515; dsyd-1rescue post: 81.25 ± 3.54 au, n = 344; control × dsyd-1: P < 0.001; control × dsyd-1rescue pre: P < 0.001; control × dsyd-1rescue post: P < 0.001; dsyd-1 × dsyd-1rescue pre: P < 0.001; dsyd-1 × dsyd-1rescue post: P > 0.05; dsyd-1rescue pre × dsyd-1rescue post: P < 0.001). (H) Integrated DGluRIIA signal (control: 33.88 ± 0.66 au, n = 1,064; dsyd-1: 66.85 ± 2.09 au, n = 667; dsyd-1rescue: 36.31 ± 0.87 au, n = 830; control × dsyd-1: P < 0.001; control × dsyd-1rescue: P > 0.05; dsyd-1 × dsyd-1rescue: P < 0.001). (I) Integrated DGluRIIB signal (E, control: 46.40 ± 0.99 au, n = 934; dsyd-1: 23.85 ± 0.60 au, n = 783; dsyd-1rescue: 35.46 ± 0.89 au, n = 770; control × dsyd-1: P < 0.001; control × dsyd-1rescue: P < 0.001; dsyd-1 × dsyd-1rescue: P < 0.001) size in dsyd-1 mutants. (J) GluR field composition (control: 0.89 ± 0.06, n = 7; dsyd-1: 1.99 ± 0.19, n = 8; dsyd-1rescue: 1.24 ± 0.08, n = 6; control × dsyd-1: P < 0.001; control × dsyd-1rescue: P > 0.05; dsyd-1 × dsyd-1rescue: P < 0.01). Statistics: one-way ANOVA. Error bars indicate the SEM. **, P < 0.01; ***, P < 0.005; ns, P > 0.05. Bars: (A–C) 1 µm; (D–F) 2 µm.
When we stained dsyd-1 mutants for DGluRIID, we recognized that individual GluR fields (reflecting individual PSDs) were dramatically enlarged at dsyd-1 mutant NMJs (Fig. 5, A and B; and Fig. 7, A–C, arrowheads). This enlargement was rescued after presynaptic (using the motoneuron driver ok6-GAL4; Fig. 7, C and G) but not postsynaptic (using the muscle driver G14-GAL4; Fig. 7 G) expression of UAS–dsyd-1cDNA (DSyd-1 failed to localize to PSDs when expressed in muscles; not depicted). Thus, presynaptic DSyd-1 has a role in defining the size of the postsynaptic GluR population. Each receptor also includes a fourth subunit, either DGluRIIA or DGluRIIB. These two GluR types differ in their single channel properties, and GluR composition also controls the morphological size of the NMJ and the number of individual synaptic contacts (DiAntonio, 2006). DGluRIIA levels were dramatically increased at dsyd-1 mutant NMJs, but were restored to normal levels after presynaptic expression of UAS–dsyd-1cDNA (Fig. 7, D–F and H). However, the levels of DGluRIIB were specifically reduced (Fig. 7, D–F and I), shifting the ratio between the two GluR types (Fig. 7 J).
As DGluRIIA complexes show higher single-channel conductance than DGluRIIB complexes (Schmid et al., 2008), one might expect enlarged miniature excitatory junctional currents (mEJCs) at dsyd-1 NMJs. In Fig. 4 B, we show that mean mEJC amplitudes are not changed between dsyd-1 mutants and controls. However, in histogram plots, a moderate tendency toward elevated frequencies of large mEJCs was observable for dsyd-1 mutants (unpublished data). DGluRIIA-dominated NMJs also show slow decay kinetics (Schmid et al., 2008). Indeed, the mEJC decay τ recorded from dsyd-1 mutant cells was increased by a mean of 12% compared with controls (n = 9 cells each, P = 0.006, Mann-Whitney test), whereas the eEJCs decay τ was ∼48% larger (n = 9 cells each, P = 0.008, Mann-Whitney test; compare with the traces in Fig. 4 A).
Collectively, parallel to its function of blocking overgrowth of presynaptic AZs, DSyd-1 has a specific function in restricting the size and defining the composition of postsynaptic GluR fields.
DLiprin-α is needed for the pre- but not the postsynaptic phenotype of dsyd-1 mutants in embryos
Our behavioral analysis indicated that DSyd-1 shares functions with DLiprin-α, but that DSyd-1 also executes DLiprin-α–independent functions.
If both proteins solely acted in the same pathway, double mutant combinations should show similar phenotypes as single mutants. To test this, dliprin-α; dsyd-1 double mutants were established. Although dsyd-1 and dliprin-α single mutants survived to adulthood, double mutants were embryonic lethal, again indicating that the functions of both proteins do not fully overlap.
We asked whether the embryonic lethality of dsyd-1; dliprin-α might be due to an inability of the double mutant to form AZs and synapses altogether. Thus, ultrastructural analysis of high-pressure frozen/freeze-substituted (Fouquet et al., 2009) embryos was performed. T bars and planar synaptic membrane contacts were found in dsyd-1 and dliprin-α single mutants, as well as in dliprin-α; dsyd-1 double mutant embryos (Fig. 8 A). Hence, synapse formation including T bar assembly can in principle proceed in the absence of both proteins. We therefore consider DSyd-1 and DLiprin-α not to be structurally essential for AZ formation but rather to promote this process (Fig. 8 A).
Figure 8.
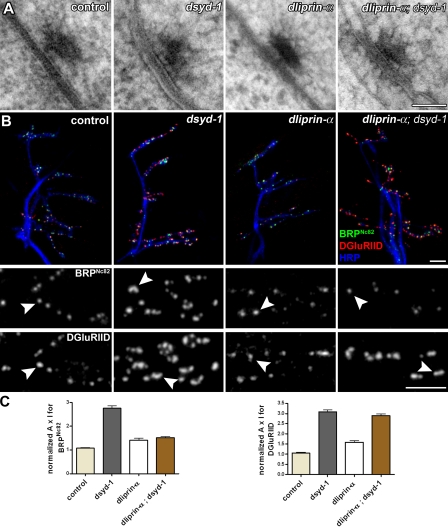
Embryonic dsyd-1 phenotypes. (A) High-pressure freeze/freeze substitution–prepared NMJ synapse of control, dsyd-1, dliprin-α, and dliprin-α; dsyd-1 double mutant embryos. All genotypes still form electron-dense projections (T bars) at the AZ. (B) Immunostaining of a region comprising muscles 6/7, 12/13, and 4 in late embryos of control, dsyd-1, dliprin-α, and dliprin-α; dsyd-1. Staining: HRP, BRP, and DGluRIID. (B, middle and bottom) Magnifications showing single synapses, with arrowheads denoting BRP (middle) and DGluRIID (bottom) puncta in the indicated mutants. (C) Quantification of BRP and DGluRIID signals at embryonic synapses. BRP signal in dsyd-1 single mutants is significantly increased compared with control, dliprin-α, and dliprin-α; dsyd-1 double mutants. DGluRIID is increased to a similar extent in dsyd-1 and dliprin-α; dsyd-1 double mutants compared with control and dliprin-α mutant animals. Statistics for BRP were as follows. Control: 1.07 ± 0.027, n = 735; dsyd-1: 2.75 ± 0.11, n = 457; dliprin-α: 1.41 ± 0.079, n = 183; dliprin-α; dsyd-1: 1.51 ± 0.054, n = 446; control × dsyd-1: P < 0.001; control × dliprin-α: P < 0.05; control × dliprin-α; dsyd-1: P < 0.001; dsyd-1 × dliprin-α: P < 0.001; dsyd-1 × dliprin-α; dsyd-1: P < 0.001; dliprin-α × dliprin-α; dsyd-1: P > 0.05. Statistics for DGluRIID were as follows. Control: 1.06 ± 0.029, n = 765; dsyd-1: 3.08 ± 0.10, n = 541; dliprin-α: 1.59 ± 0.080, n = 218; dliprin-α; dsyd-1: 2.90 ± 0.088, n = 612; control × dsyd-1: P < 0.001; control × dliprin-α: P < 0.001; control × dliprin-α; dsyd-1: P < 0.001; dsyd-1 × dliprin-α: P < 0.001; dsyd-1 × dliprin-α; dsyd-1: P > 0.05; dliprin-α × dliprin-α, dsyd-1: P < 0.001. Statistics: one-way ANOVA. Error bars indicate the SEM. Bars: (A) 70 nm; (B, top) 2 µm; (B, middle and bottom) 1 µm.
We further analyzed embryonic synapse morphology using BRP as a presynaptic marker and DGluRIID as a postsynaptic marker in single and double mutant combinations (Fig. 8, B and C). Compared with controls, BRP reactivity was clearly elevated at dsyd-1, but only very mildly at dliprin-α mutant NMJs (Fig. 8 B). Notably, BRP levels at dliprin-α; dsyd-1 double mutant NMJs were comparable to those at dliprin-α, rather than to those at dsyd-1 mutant NMJs (Fig. 8 B). Thus, loss of DSyd-1 leads to an increase in recruitment of BRP to AZs, which is dependent on the presence of DLiprin-α. Increased levels of BRP were also observed at dsyd-1 mutant larval NMJs (Fig. 5).
Levels of DGluRIID were drastically increased at dsyd-1 and equally at dsyd-1; dliprin-α double mutant NMJs, but only mildly elevated in dliprin-α–deficient synapses (Fig. 8, B and C). Thus, DSyd-1 is involved in regulating GluR field size, independently of DLiprin-α.
DSyd-1 is needed for proper AZ localization of DLiprin-α, but not vice versa
Using extended in vivo imaging of identified release sites, we recently showed that accumulation of DLiprin-α precedes accumulation of DGluRIIA as well as—by hours—the arrival of BRP throughout AZ assembly (Rasse et al., 2005; Schmid et al., 2008; Fouquet et al., 2009).
To place DSyd-1 into the temporal context of AZ assembly, we coexpressed GFPDSyd-1 (which, when pan-neuronally expressed, rescues the sluggish behavior of dsyd-1 mutant adults) and BRP-shortmStraw in motoneurons. As expected from immunostainings (Figs. 2 and 3), presynaptic expression of DSyd-1 labeled AZs (Fig. 9, A and B). Individual NMJs were reimaged after 12 h, and substantial growth of the NMJ along with the addition of new AZs was observed (Fig. 9 A). DSyd-1 clearly and invariably preceded BRP at newly forming release sites (Fig. 9 A, arrowheads).
Figure 9.
DSyd-1 accumulates early during AZ assembly. Confocal stacks of sequentially in vivo imaged NMJs (muscle 26), Δt = 12 h. NMJs coexpressing GFPDSyd-1 and BRP-shortmStraw (A), and DLiprin-αGFP and mStrawDSyd-1 (B). (A) DSyd-1 preceded BRP (arrows and arrowheads) at 65% of the newly forming AZs, and BRP preceded DSyd-1 at 0%. The situation was not resolved at 35% (n = 37). (B) DLiprin-α and DSyd-1 accumulate in close temporal proximity (arrows and arrowheads): DLiprin-α preceded DSyd-1 at 26% of newly forming AZs, and DSyd-1 preceded DLiprin-α at 6%. The situation was not resolved at 68% (n = 35). Bars: (A and B) 4 µm; (A and B, insets) 500 nm.
We went on to co-image DLiprin-αGFP and mStrawDSyd-1. Newly formed AZs were usually decorated with both DLiprin-αGFP and mStrawDSyd-1, suggesting that both proteins arrived at synaptic sites in very close temporal proximity (Fig. 9 B, arrows and arrowheads). Thus, newly forming AZs are characterized by DLiprin-α and DSyd-1–positive clusters from early on.
Genetic analysis in C. elegans has placed the putative RhoGAP DSyd-1 upstream of Syd-2/Liprin-α in the assembly hierarchy (Dai et al., 2006; Patel et al., 2006). We questioned whether both factors would reciprocally influence their distribution and AZ localization (Fig. 10, A–E). As expected (Fouquet et al., 2009), at control NMJs (Fig. 10 A), DLiprin-α and BRP colabeled individual AZs in a regular pattern (Fig. 10 A, arrowheads). Notably, DLiprin-α showed a highly irregular distribution at dsyd-1 mutant terminals (Fig. 10 B), with many AZs (identified via BRP) lacking adequate DLiprin-α labeling (Fig. 10 B, arrowheads). Large DLiprin-α spots distant from BRP spots were often observed, which indicates the presence of ectopic accumulations of DLiprin-α (Fig. 10 B, arrows). After coexpression of DSyd-1 together with DLiprin-α at dsyd-1 NMJs, however, most BRP-positive AZs showed DLiprin-α labeling (Fig. 10 C, arrowheads). In contrast, DSyd-1 targeted normally to AZs in dliprin-α mutants (compare Fig. 10 D with Fig. 10 E). Thus, presynaptic DSyd-1 is needed to properly localize DLiprin-α to AZs, but DLiprin-α is apparently not needed to target DSyd-1.
Figure 10.
Defective DLiprin-α localization in dsyd-1 mutants. (A–C) DLiprin-αGFP/BRP-shortmStraw co-imaging in control (A), dsyd-1 (B), and dsyd-1rescue (C) are shown. The localization of DLiprin-α is changed at dsyd-1 mutant NMJs, but is rescued by reexpression of UAS–dsyd-1cDNA in motoneurons. Bars, 2 µm and 500 nm (insets). Arrowheads indicate AZs marked by BRP and arrows indicate ectopic DLiprin-α in dsyd-1 mutants. (D–F) DSyd-1 localizes to AZs in control (D), dliprin-α (E), and brp (F) animals. (G) Model of AZ assembly. Yellow arrow, DSyd-1 regulates DLiprin-α early in assembly; green arrow, DSyd-1 regulates GluR field size; gray arrow, DSyd-1 binds BRP and regulates BRP supply. Bars: (A, top): 2 µm; (A, bottom) 500 nm; (F) 2 µm.
We also asked whether DSyd-1 would localize to brp mutant terminals. BRP arrives late during synapse assembly and is needed for proper maturation of release sites, as shown for the distribution of calcium channels (Fouquet et al., 2009). Although DSyd-1 targeted to AZs (Fig. 10 F), the distribution of the protein appeared somewhat “smeared,” suggesting that BRP is needed for the proper spacing of DSyd-1 at mature AZs.
Discussion
Mechanisms which regulate assembly and maturation of presynaptic AZs are not well understood (Jin and Garner, 2008). We identified the Drosophila Syd-1 homologue (DSyd-1) as a binding partner of BRP. We found (Fig. 9; Fouquet et al., 2009) that DLiprin-α and DSyd-1 mark presynaptic sites where, subsequently, AZs (and adjunct PSDs) originate and mature, whereas BRP and Ca2+ channels accumulate at later time points than DLiprin-α and DSyd-1. DLiprin-α previously has been shown to be important for proper AZ formation (Kaufmann et al., 2002). Thus, consistent with reduced numbers of AZs forming at NMJs of dsyd-1 and dliprin-α mutants (Fig. 4 G; and Kaufmann et al., 2002) and with both proteins being localized to AZs, the accumulation of DLiprin-α and DSyd-1 at nascent AZs may be instrumental for transforming selected sites into AZs, a process we refer to as “AZ nucleation activity.” However, as the morphological size of dsyd-1 NMJs is reduced, as is the AZ number (Fig. 4 F, G), in principle, other growth processes might also become rate-limiting at dsyd-1 mutant NMJs. In other words, reduced AZ numbers could also be a consequence of a reduction in morphological NMJ growth. Studying the coupling between morphological growth and AZ formation will be important for determining the relevance of morphological size to total AZ number.
Work on en passant synapses of the C. elegans HSNL motor neuron implies that, in genetic terms, Syd-1 operates upstream of Syd-2/Liprin-α. This is based on the fact that a Syd-2/Liprin-α dominant allele can bypass the requirement of syd-1 (Dai et al., 2006), which indicates that the protein’s essential role in AZ assembly at HSNL synapses is mediated via Syd-2/Liprin-α. We here provide evidence that DSyd-1 is required to properly target DLiprin-α to AZs. In the absence of DSyd-1, DLiprin-α distributes unevenly at NMJ terminals, sparing many AZs. Thus, we provide direct evidence that the RhoGAP DSyd-1 operates upstream in AZ assembly in vivo: DSyd-1 seemingly stalls DLiprin-α to developing AZs in order to allow for the AZ nucleation function of DLiprin-α to effectively operate.
DLiprin-α seems to be a direct substrate of DSyd-1 (Fig. 10 G). Our data imply that other presynaptic substrate proteins of DSyd-1 might exist at nascent synapses, a finding that is unexpected based on analysis of AZ formation in C. elegans. Therefore, we deduce from our findings that presynaptic DSyd-1 (but apparently not DLiprin-α) plays an important role in shaping the PSD assembly. Embryos and larvae mutants for dsyd-1, and importantly, dliprin-α; dsyd-1 double mutant embryos (the double mutant is embryonic lethal), showed increased overall amounts of postsynaptic GluRs, whereas dliprin-α single mutant embryos (Fig. 8) and larvae did not (not depicted). These increased amounts of GluRs in dsyd-1 mutants vanished after presynaptic reexpression of UAS–dsyd-1cDNA. It is tempting to speculate that the presynaptic DSyd-1 protein helps the AZ localization of an adhesion protein, which via trans-synaptic interaction might steer the incorporation of postsynaptic GluRs (for a model, see Fig. 10 G). A potential role of the Neurexin–Neuroligin axis should be evaluated in this context (Li et al., 2007; Südhof, 2008).
Drosophila NMJs express two functionally distinct GluR complexes, DGluRIIA and IIB, which influence the number of release sites formed (DiAntonio, 2006). Individual PSDs form distinctly from preexisting ones, and mature over hours, switching from DGluRIIA to IIB incorporation throughout maturation in a manner dependant on presynaptic signaling (Rasse et al., 2005; Schmid et al., 2008). DSyd-1 might mediate such a maturation signal, as dsyd-1 mutants show excessive amounts of DGluRIIA incorporation at PSDs. This regulation is likely not (or only partially) due to compensation for reduced presynaptic glutamate release, as dliprin-α mutants (with similarly reduced transmission levels) do not show this dramatic increase in GluR levels.
Despite enlarged receptor fields and specifically elevated DGluRIIA levels, average miniature event amplitudes were comparable between dsyd-1 animals and controls, which we currently cannot account for. A possible explanation might comprise regulatory processes rendering populations of receptors non-/partially functional. Nonetheless, EJC decay time constants of dsyd-1 mutants resemble those found at dgluRIIB-deficient (and thus GluRIIA dominated) NMJs (Schmid et al., 2008).
Which processes are downstream of the DSyd-1–mediated DLiprin-α activity at nascent AZs? Liprin family proteins steer transport in axons and dendrites (e.g., of AMPA receptors) to support synaptic specializations (Wyszynski et al., 2002; Shin et al., 2003; Wagner et al., 2009). Notably, in dsyd-1 mutants, although many AZs lacked proper amounts of DLiprin-α, large ectopic accumulations of DLiprin-α were observed. At the same time, ectopic accumulations of BRP/electron density were observed in the absence of DSyd-1. It is tempting to speculate that these ectopic pools of DLiprin-α provoke the aberrant accumulation of electron densities in dsyd-1 mutants, which is consistent with the transport function of DLiprin-α (Miller et al., 2005) and the direct interaction of DLiprin-α/Syd-2 and ELKS/BRP (Patel and Shen, 2009). Consistently, large BRP accumulations observed in dsyd-1 embryos were no longer present in dsyd-1; dliprin-α double mutants, which indicates that the presence of DLiprin-α is needed to provoke these overaccumulations of BRP when DSyd-1 is missing.
In the absence of DSyd-1, BRP was inappropriately localized, even within the cytoplasm, forming ectopic electron-dense material (which is consistent with its role as building block for the electron-dense T bars). Such “precipitates” also occurred at and close to non-AZ membranes. Moreover, at dsyd-1 AZs, large malformed T bars formed. Thus, it appears plausible that DSyd-1 keeps BRP “in solution” to organize its proper consumption at AZs. An alternate and not mutually exclusive explanation may be that axonal BRP precipitates also reflect defects in axonal transport due to the absence of DSyd-1. The presence of several binding interfaces between BRP and DSyd-1 may be considered as a basis for regulating their interplay.
BRP accumulation in the center of the AZ is also in the center of the functional and structural AZ assembly process (Kittel et al., 2006; Wagh et al., 2006; Fouquet et al., 2009). It appears likely that BRP assembly is regulated on multiple levels. Notably, although BRP accumulation is severely compromised in mutants for the kinesin imac (Pack-Chung et al., 2007), it is not fully eliminated. Moreover, the serine/arginine protein kinase SRPK79D was recently shown to associate with BRP and to repress premature “precipitation” of BRP in the axons (Johnson et al., 2009; Nieratschker et al., 2009). Furthermore, mutants for the serine/threonine kinase unc51 have recently been shown to suffer from BRP targeting defects (Wairkar et al., 2009). Phosphorylation of DSyd-1 (e.g., within serine-rich stretches toward the C terminus) might be involved in regulating proper longer-range transport (“blocking precipitation on the way”) as well as proper delivery of BRP at nascent AZ sites.
Recently, the Rab3 GTPase has been shown to be crucial for effective nucleation of BRP at AZs (Graf et al., 2009). In an interesting parallel to dsyd-1 defects, rab3 mutant NMJs showed fewer BRP-positive AZs; however, if present, BRP levels were increased. Nonetheless, instead of overgrown T bars, as observed in dsyd-1 mutants, rab3 mutants rather showed multiple T bar AZs (Graf et al., 2009). It will be interesting to investigate whether these pathways act in parallel or converge, along with their relationships to other synaptogenic signals (Giagtzoglou et al., 2009; Owald and Sigrist, 2009).
Materials and methods
Proteomics
Protein extraction protocols were modified from Luo et al. (1997). Wild-type adult fly heads were mechanically homogenized in deoxycholate buffer (500 mM Tris, pH 9.0, and 1% sodium-deoxycholate containing protease inhibitor cocktail [Roche]) followed by incubation at 36°C for 30 min. 0.1% Triton X-100 was added thereafter, and the lysate was incubated at 4°C for 30 min. After centrifugation for 15 min at 16,000 g, the supernatant was used in immunoprecipitations with the monoclonal antibody BRPNc82 (provided by E. Buchner, Universität Würzburg, Würzburg, Germany) or mouse IgG heavy chain (for control; Dianova) cross-linked to protein A–Sepharose (Bio-Rad Laboratories). After incubation at 4°C for 2 h, beads were washed in deoxycholate/Triton X-100 buffer. In a first approach, proteins were removed en masse from the BRPNc82–Protein A beads with 100 mM glycine, pH 2.0, reduced with dithiothreitol, carboxy-methylated using iodoacetamide, and digested with trypsin (Betschinger et al., 2003). Peptides were extracted with formic acid (FA) and separated by nano–high-performance liquid chromatography (LC) on a PepMap C18 reversed-phase column. Eluting peptides were transferred online to an ion trap mass spectrometer (LTQ; Thermo-Fisher Scientific).
In a second approach, proteins were eluted from the MAB Nc82–Protein A beads with SDS sample buffer. The samples were separated by one-dimensional SDS-PAGE (NuPAGE 4–12% gradient gel; Invitrogen), and protein bands were visualized using SYPRO red (Invitrogen). The elution and control lanes (controls, i.e., immunoprecipitation with mouse IgG) were each cut in 2-mm-thick stripes so that the regions of both lanes aligned with each other.
Each individual stripe was digested in gel with trypsin (sequencing grade; Roche), and peptides were extracted according to Shevchenko et al. (1996). Dried samples from in-gel digests were dissolved in 10% (vol/vol) acetonitrile (CH3CN; LiChrosolve grade; Merck & Co., Inc.), and 0.15% FA (Sigma-Aldrich). The sample volumes were adjusted to the sample amount. The dissolved samples were subjected to a nano-LC coupled electrospray ionization tandem MS using an orthogonal quadruple time-of-flight mass spectrometer (Q-Tof Ultima; Waters). The nano-LC system was equipped with a C18 pepMap100 column (75 µm ID, 3 µm, 100 Å; Dianex) running with a flow rate of 180 nl/min. The buffers used were as follows: buffer A (H2O and 0.1% [vol/vol] FA) and buffer B (80% [vol/vol] acetonitrile and 0.1% [vol/vol] FA). The gradient applied was 90% (vol/vol) buffer A to 55% (vol/vol) buffer A in 60 min, 55% (vol/vol) buffer A to 10% (vol/vol) buffer A in 5 min, and 5 min with 10% (vol/vol) buffer A. Before separation of the peptides by nano-LC, samples were desalted with online coupled precolumns (3 mm) consisting of the same chromatography material. The electrospray was generated with fused-silica 10-µm PicoTip needles (New Objective, Inc.) and was operated at ∼1.8–2.3 kV. Fragment spectra of sequenced peptides were searched against all entries of the nonredundant Database from the National Center for Biotechnology Information using the software search algorithms MASCOT (Matrix Science Ltd.). For the database search, no constraints on molecular weight or biological species were applied.
Both approaches identified DSyd-1 in BRPNc82 immunoprecipitates as physical interactors of BRP; however, DSyd-1 was not detected in control immunoprecipitations.
Yeast two-hybrid
dsyd1 constructs were obtained by PCR on pUASt/dsyd 1 (see the Molecular cloning paragraph) and cloned into pGAD-T7 and pGBK-T7 (both from Takara Bio Inc.). brp constructs have been described previously (Fouquet et al., 2009). In principle, all experiments were conducted as described previously (Fouquet et al., 2009). All cotransformation experiments were conducted according to the yeast two-hybrid protocols of Takara Bio Inc., using the strain AH109. In brief: to ensure the presence of both cotransformed plasmids, the yeast was plated on minimal synthetic defined (SD)/−Leu/−Trp medium plates. After growing for 2–3 d, at least 10 clones each were analyzed on SD/−Ade/−His/−Leu/−Trp/X-α-gal medium plates to select for positive interaction. If >90% of the clones grew (and turned blue in color), this was regarded as positive interaction. As a positive control, pGBKT7-p53 was cotransformed with pGADT7 containing the SV40 large T antigen. Negative controls consisted either of laminin as bait together with the prey to be tested or the corresponding bait together with the empty prey vector (Fouquet et al., 2009).
Genetics
Fly strains were reared under standard laboratory conditions (Sigrist et al., 2003). Either w1 or w1118 strains were used as background for generation of transgenes (BestGene, Inc.). dsyd-1 mutants (dsyd-1ex3.4, eliminating the complete dsyd-1 and partially deleting the 3′ heph locus; and dsyd-1ex1.2, eliminating the complete dsyd-1 locus and partially deleting the 5′ ferrochelatase locus) were constructed and validated by genomic PCR according to Parks et al. (2004). For dliprin-α, dliprin-αEPexR60/dliprin-αF3ex15 (Kaufmann et al., 2002) was used. dliprin-αEPexR60; dsyd-1ex3.4 and dliprin-αF3ex15; dsyd-1ex1.2 were kept using the T(2;3)CyOGFP-TM3GFP compound balancer (Eissenberg et al., 2005).
Genotypes used for in vivo imaging were (all from a w− background): (a) ok6-GAL4, UAS-BRP-shortmStraw/+; UAS–GFPDSyd-1/+; (b) ok6-GAL4, UAS–GFPDLiprin-α /+; UAS–mStrawDSyd-1/+; (c) UAS-MitoGFP/ok6-GAL4 and UAS-MitoGFP/ok6-GAL4; dsyd-1ex1.2/ dsyd-1ex3.4; (d) UAS–GFPDLiprin-α, UAS-BRP-shortmStraw/ok6-GAL4; (e) UAS–GFPDLiprin-α, UAS-BRP-shortmStraw/ok6-GAL4; dsyd-1ex1.2/dsyd-1ex3.4; (f) UAS–GFPDLiprin-α, UAS-BRP-shortmStraw/ok6-GAL4; dsyd-1ex1.2, UAS–DSyd-1/dsyd-1ex3.4; (g) dliprin-αF3ex15/dliprin-αEPexR60; D42-GAL4/ UAS–GFPDSyd-1; and (h) brp69/DfBSC29, ok6-GAL4; UAS–GFPDSyd-1/+.
Genotypes used for DLiprin-α immunostainings were: ok6-GAL4/+; UAS–DLiprin-αGFP/+ and ok6-GAL4, UAS–DLiprin-αYFP/+ (van Roessel et al., 2004). For DSyd-1 immunostainings in the MB calyx, UAS–Dα7EGFP/+; ok107-GAL4/+ was used.
Antibody and Western blotting
A rabbit serum against C-terminal SSGDSKNGSDEYDDIK was produced (Eurogentec). Serum was affinity purified with the same peptide. Drosophila fly head extracts (five heads per lane) were probed with affinity-purified antibody (dilution of 1:500).
In situ hybridization
Whole-mount embryonic in situ hybridizations were performed essentially as described by the Berkeley Drosophila Genome Project (http://www.fruitfly.org/). For the dsyd-1 sense RNA probe (Berkeley Drosophila Genome Project; available from GenBank/EMBL/DDBJ under accession no. LD28013) was cut with XhoI and in vitro transcribed using T7 RNA polymerase. For antisense probes, LD28013 was cut with EcoRI, and SP6 RNA was in vitro transcribed.
Molecular cloning
As the partial clone (GenBank accession no. LD28013) was available, a full-length dsyd1 cDNA was designed according to the exon prediction of FlyBase. For this, the bps 1,183–2,933 not covered by LD28013 were amplified by elongase PCR from adult fly head cDNA using 5′-CCAGTGGGTCCCTCGAGAAGAATG-3′ and 5′-TCCAAATCAGCGCCGAAGAGC-3′. The resulting fragment was StuI digested and ligated with LD28013. This ligation was digested with XhoI, ligated into pBluescript KS (+) (Agilent Technologies), cut out with XhoI–XbaI, and ligated into pUASt (pUASt/dsyd-1, bps 1,183–5,537). Bps 1–1,182 were amplified by elongation of PCR from fly head genomic DNA using 5′-ATGACGGTGCAACCGGCTGAAATG-3′ and 5′-CGTTGACATTCTTCTCGAGGGA-3′. Fragments without introns were amplified via vent PCR. A: (A1) 5′-GAGCGCGGCCGCGATGACG-3′ and (A2) 5′-GAACTGATCTTCCATTTTCCGCCATTTCAGCCGGTTGCAC-3′; B: (B1) 5′-TGCAACCGGCTGAAATGGCGGAAAATGGAAGATCAG-3′ and (B2) 5′-CCGCAAGGATTTCGTCGCCCACCCGCAAGCAGCCG-3′; C: (C1) 5′-CAACAGCGGCTGCTTGCGGGTGGGCGACGAAATCCT-3′ and (C2) 5′-CCGTCATTTCGCGACCATCTCGTGATGAGCGCGGCCTC-3′; and D: (D1) 5′-CCGAGGCCGCGCTCATCACGAGATGGTCGCGAAATGAC-3′ and (D2) 5′-TCCCGTTGACATTCTTCTCG-3′). Fragments A and B were linked via elongation PCR using A1 and B2, and fragments C and D were linked using primers C1 and D2. The resulting fragments were linked using primers A1 and D2. Bps 1–1,182 and pENTER were digested with NotI and XhoI, and ligated. Bps 1,183–5,537 were amplified via PCR from pUASt/dsyd-1 bps 1,183–5,537 using the primers 5′-GTCCGCCAGTGGGTC-3′ and 5′-GTCTATTCTAGACTTGATGTCATCGTACTCAT-3′. pENTER/dsyd-1 (Wagh et al., 2006) bps 1–1,182 and dsyd-1 bps 1,183–5,537 were digested with XhoI and XbaI, and ligated thereafter. All sequences were validated by double strand sequencing. pUASt/dsyd-1 cDNA and pTGW/dsyd-1cDNA constructs were obtained using the Gateway system (Invitrogen).
Image acquisition
Image acquisition of confocal microscopy was obtained with a confocal microscope (TCS SP5; Leica). STED microscopy was performed with a TCS STED microscope (Leica). Images of fixed and live samples were acquired at room temperature. Confocal imaging of NMJs and whole brains was done using a z step of 0.5 µm. The following objectives were used: 20× 0.7 NA oil immersion for brain scans, 63× 1.4 NA oil immersion for NMJ and calyx confocal imaging, and a 100× 1.4 NA oil immersion STED objective for STED imaging (all from Leica). All images were acquired using the LCS AF software (Leica). For previous descriptions see Fouquet et al. (2009).
Immunostainings of larval and embryonic NMJs
Dissections were performed in HL3 by opening the larvae/embryo dorsally along the midline and removing the innards to grant visual access to the body wall muscles. Dissections were fixated with 4% paraformaldehyde in PBS (pH 7.2) for 10 min. After fixation, the filets were washed with PBS with 0.05% Triton-X 100 (PBT) and blocked for 30 min in 5% normal goat serum (NGS). For the immunostainings, the larvae were incubated with primary antibodies at 4°C overnight and subsequently washed in a 0.05% PBT solution for 12 h at room temperature. For the α-DSyd-1 stainings, the primary antibody was diluted in 0.3% PBT instead of 0.05%. Larvae were then incubated overnight with secondary antibodies at 4°C. Washing procedures were repeated. Immunocytochemistry was equal for both conventional confocal and STED microscopy. Larvae were finally mounted either in Vectashield (Vector Laboratories) or Mowiol (see also Qin et al., 2005). Antibody dilutions were: 1:100–1:200 M-α-Nc82 (provided by E. Buchner); 1:500 Rb-α-DSyd-1 ; 1:500 Rb-α-DGluRIID; 1:100 M-α-DGluRIIA (Developmental Studies Hybridoma Bank); 1:1,000 Rb-α-DGluRIIB (provided by D.E. Featherstone, University of Illinois at Chicago, Chicago, IL; Marrus et al., 2004; Liebl et al., 2005); 1:500 M-α-GFP (Invitrogen); 1:500 Rb-α-GFP (Invitrogen); 1:500 Rb-α-DVGlut (Hermann Aberle, Universität Münster, Münster, Germany); and HRP-Cy5 1:250 (Dianova). All confocal secondary antibodies were diluted 1:500. Secondary antibodies used for STED images (Sheep-α-M-Atto647N and Sheep-α-Rb-Atto647N; Sigma-Aldrich) were diluted 1:100.
Embryos were staged temporally (22–24 h) and morphologically, and stained as described for larvae.
Adult central nervous system (CNS) stainings
Brain stainings were essentially performed as described previously (Wu and Luo, 2006). Brains were dissected in HL3 on ice and immediately fixed in cold 4% PBS for 20 min at RT. The brains were then washed in 0.3% PBT (4× for 15 min) and preincubated in PBT with 10% NGS for 1 h at RT. For primary antibody treatment, samples were incubated in PBT containing 5% NGS and the primary antibodies for 2 d at 4°C. After primary antibody incubation, brains were washed in PBT for 4× for 20 min at RT, then overnight at 4°C. All samples were then incubated in PBT with 5% NGS containing the secondary antibodies (1:500) for 3 d at 4°C. Brains were finally washed for 4× for 20 min at RT, then stored overnight at 4°C, and transferred in Vectashield onto slides (Vector Laboratories).
Live imaging
Intact living Drosophila larvae were covered with Voltalef H10S oil (Arkema, Inc.) and placed into an airtight imaging chamber. During image acquisition, the larvae were shortly (10 to 20 min) anaesthetized by introducing a desflurane (Baxter) air mixture into the imaging chamber. Selected NMJs were exclusively located in abdominal segments A2 and A3 on muscles 26 and 27.
Also see Rasse et al. (2005) and Schmid et al. (2008). During incubation time, the imaged larvae were maintained at 25°C, which corresponded to our normal rearing temperature.
Image processing
Confocal imaging.
Confocal stacks were processed with ImageJ software (http://rsbweb.nih.gov/ij/). Deconvolutions were used for single slices and confocal stacks. The ImageJ plug-ins used were iterative deconvolution and iterative deconvolution 3D, respectively (OptiNav, Inc.).
STED imaging.
STED images were processed using linear deconvolution software integrated into the Imspector Software bundle (Max Planck Innovation GmbH). For visualization, images for figures were enhanced using the brightness/contrast function of ImageJ and edited in Photoshop (Adobe).
Quantifications of AZ/PSD number, size, and intensity
All images for synapse quantification from fixed samples were acquired using the same microscope settings. Control and mutant dissections were stained in the same vial.
To measure the number of synapses per NMJ, first, the original stack was scaled up twofold. A Gaussian filter with a radius of two pixels was applied. The contrast of the maximum projection of an image stack was adjusted in such way that the intensity maximum of the picture was set to 255 (min/max contrast function in ImageJ). Afterward, a threshold was set excluding all pixels with a value <51. The segmentation of single synapses was done by hand with the pencil tool and a line thickness of 2 pixels. The processed picture was then transformed into a binary picture; all pixels with a value <51 received the value “0” and all pixels with a value ≥51 were reassigned to a value of “255.” This binary mask was then projected onto the original unmodified image using the “min” operation from the ImageJ image calculator. The synapses of the resulting images were counted with the help of the “analyze particle” function with the threshold set to 1.
The STED images were quantified using ImageJ. BRPNc82 size quantification was performed as described in proceeding paragraph, whereas the “analyze particles” tool was applied within a predefined region of interest surrounding single PSDs. The sum of the area of all BRPNc82 particles was measured for each PSD, and the number of particles was subsequently counted. No Gaussian blur was applied here.
To define the DVGlut and MitoGFP signal intensity of NMJs, a region of interest was applied by surrounding the 1b innervations (based on the HRP signal), and the mean pixel intensity was measured. To compare several experiments, the mean signal was subsequently normalized to the corresponding HRP signal.
To compare different time points in live imaging experiments, all images were normalized by adjusting the brightest pixel composing the NMJ to 255 arbitrary units (au).
Two-electrode voltage clamp recordings
Two-electrode voltage clamp recordings were essentially performed as described by Fouquet et al. (2009). In brief: for dsyd-1 (ok6-GAL4/+; dsyd-1), dsyd-1 rescue (ok6-GAL4/+; dsyd-1, UAS–dsyd-1cDNA/dsyd-1), and controls (w1118 ; ok6-GAL4/+), as well as dliprin-α and controls (w1118), recordings were made from late third-instar larvae (muscle 6, segments A2 and A3; experimental groups consisted of either males or females only). For all experiments, the recording solution consisted of HL3: 70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 115 mM sucrose, 5 mM Hepes, and 1 or 0.5 mM CaCl2, pH adjusted to 7.2. The cells from which recordings were made had an input resistance ≥ 4 MΩ. Intracellular electrodes were filled with 3 M KCl, and resistances ranged from 10 to 25 MΩ. Stimulation artifacts of eEJCs were removed for clarity. Paired pulse stimulation protocols and analyses were essentially performed as in Kittel et al. (2006). Paired pulse intervals were either 10 ms or 30 ms, and experiments were performed in 0.5 mM extracellular calcium. For determination of the base line of the second pulse at the 10-ms interpulse interval, the decay of the first pulse was extrapolated.
Transmission EM
For high-pressure freezing, ∼2–10 (22–24 h) staged Drosophila embryos were placed in aluminum specimen carrier 200 µm deep (type A; Leica), filled with yeast paste, and covered with a lid (specimen carrier type). The samples were frozen immediately in a high-pressure freezing machine (HPM010; Bal-Tec) and rapidly transferred to liquid nitrogen for storage.
Freeze substitution and embedding were performed in acetone in a freeze-substitution device (EM AFS; Leica). In brief, freeze substitution was performed in acetone with 0.1% tannic acid at –90°C for 4 d, followed by acetone with 2% osmium during the last 7 h. The samples were warmed (5°C/h) to –20°C and incubated for 16 additional hours before being warmed (10°C/h) to 4°C. At 4°C, the samples were washed in acetone and warmed to room temperature. They were then embedded in epon (see Rostaing et al., 2006; Siksou et al., 2007).
Subsequently, 55–65-nm (gray-silver) sections were cut using an ultramicrotome (EM Ultracut 6; Leica). Sections were collected on formvar-coated 100-mesh grids. Sections were dried and post-stained with uranyl acetate and lead citrate as described previously (Schmid et al., 2006). Micrographs were taken with a 1024 × 1024 charge-coupled device (CCD) detector (Proscan CCD HSS 512/1024; Proscan Electronic Systems GmbH) in a transmission EM (EM 902A; Carl Zeiss, Inc.) operated in bright field mode.
Conventional RT embedding was essentially performed as described previously (Wagh et al., 2006). Images were obtained from dissected preparations of third-instar larvae (NMJ 6/7, segments A2/A3). Instead of 1 h of fixation in 1% osmium tetroxide, the fixation was performed in 1% osmium tetroxide and 0.8% KFeCn in 0.1 M cacodylate buffer. After infiltration in epon resin, muscles were cut out (six animals for each genotype) and embedded in a single block.
Quantifications
The number of vesicles at the AZ was evaluated within three shells (each shell was 50-nm thick) surrounding the T bar (n w1 = 26; n dsyd-1 = 26 AZs). For the vesicle diameter, all vesicles in a radius of 150 nm surrounding the T bar were taken and the diameter was measured with the ImageJ software.
Reconstructions.
For 3D-reconstructions of larval T bars (w1 vs. dsyd-1), 3–5 serial 60-nm sections were reconstructed with the free software Reconstruct (Fiala, 2005).
Behavioral analysis
Female animals were tested within 48 h after eclosure and at least one night at 18°C. Before testing, flies were anesthetized on ice and wings were clipped. Experiments were performed under a red light, and animals were allowed to adapt to darkness for at least 1 h before testing. To test walking ability, flies were placed on a flat surface with a 2 × 2-cm grid and allowed to walk freely for 10 s. The number of lines crossed was counted. Negative geotaxis was measured with flies placed on the bottom of an empty, scaled food vial, and the maximum height (max = 9 cm) reached within 30 s was recorded.
Statistics
Data were analyzed with Prism (GraphPad Software). Asterisks are used to denote significance (*, P < 0.05; **, P < 0.01; ***, P < 0.005; ns, P > 0.05).
Online supplemental material
Fig. S1 shows the amino acid sequence of DSyd-1 with peptides identified via MS highlighted in red. Fig. S2 deals with the distribution of SVs and mitochondria in dsyd-1 mutants. Fig. S3 shows that axonal BRP and DVGlut colocalize. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200908055/DC1.
Acknowledgments
We would like to thank Franziska Zehe for excellent technical assistance and Ulrich Thomas for comments on the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to S.J. Sigrist (Exc 257, SI849/2-1 and 2-2, TP A16/SFB 551, TP B23/SFB581).
Footnotes
Abbreviations used in this paper:
- au
- arbitrary units
- ANOVA
- analysis of variance
- AZ
- active zone
- BRP
- Bruchpilot
- CNS
- central nervous system
- DVGlut
- Drosophila vesicular glutamate transporter
- eEJC
- evoked excitatory junctional current
- FA
- formic acid
- GluR
- glutamate receptor
- HSNL
- hermaphrodite-specific motor neuron synapse
- LC
- liquid chromatography
- MB
- mushroom body
- mEJC
- miniature excitatory junctional current
- MS
- mass spectrometry
- NGS
- normal goat serum
- NMJ
- neuromuscular junction
- PSD
- postsynaptic density
- STED
- stimulated emission depletion microscopy
- SV
- synaptic vesicle
- UAS
- upstream activator sequence
References
- Betschinger J., Mechtler K., Knoblich J.A. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 422:326–330 10.1038/nature01486 [DOI] [PubMed] [Google Scholar]
- Collins C.A., DiAntonio A. 2007. Synaptic development: insights from Drosophila. Curr. Opin. Neurobiol. 17:35–42 10.1016/j.conb.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Dai Y., Taru H., Deken S.L., Grill B., Ackley B., Nonet M.L., Jin Y. 2006. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 9:1479–1487 10.1038/nn1808 [DOI] [PubMed] [Google Scholar]
- Daniels R.W., Collins C.A., Gelfand M.V., Dant J., Brooks E.S., Krantz D.E., DiAntonio A. 2004. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J. Neurosci. 24:10466–10474 10.1523/JNEUROSCI.3001-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A. 2006. Glutamate receptors at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75:165–179 10.1016/S0074-7742(06)75008-5 [DOI] [PubMed] [Google Scholar]
- Eissenberg J.C., Wong M., Chrivia J.C. 2005. Human SRCAP and Drosophila melanogaster DOM are homologs that function in the notch signaling pathway. Mol. Cell. Biol. 25:6559–6569 10.1128/MCB.25.15.6559-6569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala J.C. 2005. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218:52–61 10.1111/j.1365-2818.2005.01466.x [DOI] [PubMed] [Google Scholar]
- Fouquet W., Owald D., Wichmann C., Mertel S., Depner H., Dyba M., Hallermann S., Kittel R.J., Eimer S., Sigrist S.J. 2009. Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol. 186:129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N., Mahoney T., Yao C.K., Bellen H.J. 2009. Rab3 GTPase lands Bruchpilot. Neuron. 64:595–597 10.1016/j.neuron.2009.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.R., Daniels R.W., Burgess R.W., Schwarz T.L., DiAntonio A. 2009. Rab3 dynamically controls protein composition at active zones. Neuron. 64:663–677 10.1016/j.neuron.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam S.J., Goncharov A., McEwen J., Baran R., Jin Y. 2002. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat. Neurosci. 5:1137–1146 10.1038/nn959 [DOI] [PubMed] [Google Scholar]
- Jin Y., Garner C.C. 2008. Molecular mechanisms of presynaptic differentiation. Annu. Rev. Cell Dev. Biol. 24:237–262 10.1146/annurev.cellbio.23.090506.123417 [DOI] [PubMed] [Google Scholar]
- Johnson E.L., III, Fetter R.D., Davis G.W. 2009. Negative regulation of active zone assembly by a newly identified SR protein kinase. PLoS Biol. 7:e1000193 10.1371/journal.pbio.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann N., DeProto J., Ranjan R., Wan H., Van Vactor D. 2002. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 34:27–38 10.1016/S0896-6273(02)00643-8 [DOI] [PubMed] [Google Scholar]
- Kittel R.J., Wichmann C., Rasse T.M., Fouquet W., Schmidt M., Schmid A., Wagh D.A., Pawlu C., Kellner R.R., Willig K.I., et al. 2006. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 312:1051–1054 10.1126/science.1126308 [DOI] [PubMed] [Google Scholar]
- Leiss F., Koper E., Hein I., Fouquet W., Lindner J., Sigrist S., Tavosanis G. 2009. Characterization of dendritic spines in the Drosophila central nervous system. Dev. Neurobiol. 69:221–234 10.1002/dneu.20699 [DOI] [PubMed] [Google Scholar]
- Li J., Ashley J., Budnik V., Bhat M.A. 2007. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 55:741–755 10.1016/j.neuron.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl F.L., Chen K., Karr J., Sheng Q., Featherstone D.E. 2005. Increased synaptic microtubules and altered synapse development in Drosophila sec8 mutants. BMC Biol. 3:27 10.1186/1741-7007-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Lee T., Tsai L., Tang G., Jan L.Y., Jan Y.N. 1997. Genghis Khan (Gek) as a putative effector for Drosophila Cdc42 and regulator of actin polymerization. Proc. Natl. Acad. Sci. USA. 94:12963–12968 10.1073/pnas.94.24.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Aberle H. 2006. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns. 6:299–309 10.1016/j.modgep.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Margeta M.A., Shen K., Grill B. 2008. Building a synapse: lessons on synaptic specificity and presynaptic assembly from the nematode C. elegans. Curr. Opin. Neurobiol. 18:69–76 10.1016/j.conb.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus S.B., Portman S.L., Allen M.J., Moffat K.G., DiAntonio A. 2004. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24:1406–1415 10.1523/JNEUROSCI.1575-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.E., DeProto J., Kaufmann N., Patel B.N., Duckworth A., Van Vactor D. 2005. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr. Biol. 15:684–689 10.1016/j.cub.2005.02.061 [DOI] [PubMed] [Google Scholar]
- Nieratschker V., Schubert A., Jauch M., Bock N., Bucher D., Dippacher S., Krohne G., Asan E., Buchner S., Buchner E. 2009. Bruchpilot in ribbon-like axonal agglomerates, behavioral defects, and early death in SRPK79D kinase mutants of Drosophila. PLoS Genet. 5:e1000700 10.1371/journal.pgen.1000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D., Sigrist S.J. 2009. Assembling the presynaptic active zone. Curr. Opin. Neurobiol. 19:311–318 10.1016/j.conb.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Pack-Chung E., Kurshan P.T., Dickman D.K., Schwarz T.L. 2007. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat. Neurosci. 10:980–989 10.1038/nn1936 [DOI] [PubMed] [Google Scholar]
- Parks A.L., Cook K.R., Belvin M., Dompe N.A., Fawcett R., Huppert K., Tan L.R., Winter C.G., Bogart K.P., Deal J.E., et al. 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36:288–292 10.1038/ng1312 [DOI] [PubMed] [Google Scholar]
- Patel M.R., Shen K. 2009. RSY-1 is a local inhibitor of presynaptic assembly in C. elegans. Science. 323:1500–1503 10.1126/science.1169025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M.R., Lehrman E.K., Poon V.Y., Crump J.G., Zhen M., Bargmann C.I., Shen K. 2006. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 9:1488–1498 10.1038/nn1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.A., Fetter R.D., Noordermeer J.N., Goodman C.S., DiAntonio A. 1997. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 19:1237–1248 10.1016/S0896-6273(00)80415-8 [DOI] [PubMed] [Google Scholar]
- Pilling A.D., Horiuchi D., Lively C.M., Saxton W.M. 2006. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 17:2057–2068 10.1091/mbc.E05-06-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Schwarz T., Kittel R.J., Schmid A., Rasse T.M., Kappei D., Ponimaskin E., Heckmann M., Sigrist S.J. 2005. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 25:3209–3218 10.1523/JNEUROSCI.4194-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu S.V., Joesch M., Sigrist S.J., Borst A., Reiff D.F. 2009. Synaptic organization of lobula plate tangential cells in Drosophila: Dalpha7 cholinergic receptors. J. Neurogenet. 23:200–209 10.1080/01677060802471684 [DOI] [PubMed] [Google Scholar]
- Rasse T.M., Fouquet W., Schmid A., Kittel R.J., Mertel S., Sigrist C.B., Schmidt M., Guzman A., Merino C., Qin G., et al. 2005. Glutamate receptor dynamics organizing synapse formation in vivo. Nat. Neurosci. 8:898–905 [DOI] [PubMed] [Google Scholar]
- Rostaing P., Real E., Siksou L., Lechaire J.P., Boudier T., Boeckers T.M., Gertler F., Gundelfinger E.D., Triller A., Marty S. 2006. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24:3463–3474 10.1111/j.1460-9568.2006.05234.x [DOI] [PubMed] [Google Scholar]
- Schmid A., Qin G., Wichmann C., Kittel R.J., Mertel S., Fouquet W., Schmidt M., Heckmann M., Sigrist S.J. 2006. Non-NMDA-type glutamate receptors are essential for maturation but not for initial assembly of synapses at Drosophila neuromuscular junctions. J. Neurosci. 26:11267–11277 10.1523/JNEUROSCI.2722-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Hallermann S., Kittel R.J., Khorramshahi O., Frölich A.M., Quentin C., Rasse T.M., Mertel S., Heckmann M., Sigrist S.J. 2008. Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat. Neurosci. 11:659–666 10.1038/nn.2122 [DOI] [PubMed] [Google Scholar]
- Schoch S., Gundelfinger E.D. 2006. Molecular organization of the presynaptic active zone. Cell Tissue Res. 326:379–391 10.1007/s00441-006-0244-y [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850–858 10.1021/ac950914h [DOI] [PubMed] [Google Scholar]
- Shin H., Wyszynski M., Huh K.H., Valtschanoff J.G., Lee J.R., Ko J., Streuli M., Weinberg R.J., Sheng M., Kim E. 2003. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J. Biol. Chem. 278:11393–11401 10.1074/jbc.M211874200 [DOI] [PubMed] [Google Scholar]
- Sigrist S.J., Reiff D.F., Thiel P.R., Steinert J.R., Schuster C.M. 2003. Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci. 23:6546–6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L., Rostaing P., Lechaire J.P., Boudier T., Ohtsuka T., Fejtová A., Kao H.T., Greengard P., Gundelfinger E.D., Triller A., Marty S. 2007. Three-dimensional architecture of presynaptic terminal cytomatrix. J. Neurosci. 27:6868–6877 10.1523/JNEUROSCI.1773-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. 2008. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 455:903–911 10.1038/nature07456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roessel P., Elliott D.A., Robinson I.M., Prokop A., Brand A.H. 2004. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 119:707–718 10.1016/j.cell.2004.11.028 [DOI] [PubMed] [Google Scholar]
- Wagh D.A., Rasse T.M., Asan E., Hofbauer A., Schwenkert I., Dürrbeck H., Buchner S., Dabauvalle M.C., Schmidt M., Qin G., et al. 2006. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 49:833–844 10.1016/j.neuron.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Wagner O.I., Esposito A., Köhler B., Chen C.W., Shen C.P., Wu G.H., Butkevich E., Mandalapu S., Wenzel D., Wouters F.S., Klopfenstein D.R. 2009. Synaptic scaffolding protein SYD-2 clusters and activates kinesin-3 UNC-104 in C. elegans. Proc. Natl. Acad. Sci. USA. 106:19605–19610 10.1073/pnas.0902949106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wairkar Y.P., Toda H., Mochizuki H., Furukubo-Tokunaga K., Tomoda T., Diantonio A. 2009. Unc-51 controls active zone density and protein composition by downregulating ERK signaling. J. Neurosci. 29:517–528 10.1523/JNEUROSCI.3848-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.S., Luo L. 2006. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 1:2110–2115 10.1038/nprot.2006.336 [DOI] [PubMed] [Google Scholar]
- Wyszynski M., Kim E., Dunah A.W., Passafaro M., Valtschanoff J.G., Serra-Pagès C., Streuli M., Weinberg R.J., Sheng M. 2002. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 34:39–52 10.1016/S0896-6273(02)00640-2 [DOI] [PubMed] [Google Scholar]