Abstract
Background
The purpose of this study was to evaluate the effect of flexor retinaculum division (simulated carpal tunnel release) on the relative motion of flexor tendon, subsynovial connective tissue, and median nerve in human cadaver specimens.
Methods
Using fluoroscopy, we measured the relative motion of middle finger flexor digitorum superficialis tendon, subsynovial connective tissue, and median nerve in twelve human cadavers with simulated fist motion. Measurements were obtained for three wrist positions: neutral; 60 degrees flexion; and 60 degrees extension. The shear index was defined as the difference in motion between two tissues (tendon, subsynovial connective tissue, or nerve) relative to tendon excursion, expressed as a percentage. After testing with an intact carpal tunnel, the flexor retinaculum was cut and the testing procedure was repeated.
Findings
With an intact flexor retinaculum, the wrist flexion position showed significantly less displacement for the subsynovial connective tissue and median nerve relative to tendon displacement, and thus the highest potential shear strain between subsynovial connective tissue-tendon, and tendon-nerve. The wrist extension position also had a significantly higher potential shear strain for tendon-nerve compared to the neutral position. After division of the flexor retinaculum, the differences in shear index among wrist positions were reduced. For the wrist flexion position, the subsynovial connective tissue and median nerve displacements significantly increased, indicating lower shear index values.
Interpretation
These findings suggest that division of flexor retinaculum reduces the potential shear strain and thus possibly the risk of shear injury to tissues with the carpal tunnel.
Keywords: Carpal Tunnel, Subsynovial Connective Tissue (SSCT), Median Nerve, Fluoroscopy, Human Cadaver, Flexor Tendon
INTRODUCTION
Approximately 250,000 to 300,000 carpal tunnel releases are performed annually in the United States (Keller et al. 1998). Clinical studies of patients with carpal tunnel syndrome (CTS) typically show higher baseline pressures within the carpal tunnel than in normal control subjects (Cobb et al. 1996; Gelberman et al. 1981; Szabo and Chidgey 1989; Werner et al. 1983; Werner et al. 1997). If conservative treatments are ineffective, endoscopic or open surgical release of the flexor retinaculum is commonly selected (Brown, RA, et al. 1993; Nakao et al. 1998; Okutsu et al. 1989). Although release reliably reduces CTS pressure (Okutsu et al. 1989; Schuind 2002), surgery relieves symptoms in only 70–90% of patients (Brown, RA et al. 1993; Hybbinette and Mannerfelt 1975; Kulick et al. 1986; Nagle et al. 1994; Phalen 1972). While in some cases patients’ lack of recovery may be due to the presence of a more severe neuropathy, in many cases patients with similar degrees of neuropathy experience differing degrees of recovery (al-Qattan et al. 1994; Harris et al. 1979; Kulick et al. 1986), suggesting that other factors may be in play. However, other than reducing carpal tunnel pressure (Okutsu et al. 1989; Schuind 2002), the biomechanical effect of carpal tunnel release has received little attention.
Among patients with CTS, the most characteristic histological finding is non-inflammatory fibrosis and thickening of the subsynovial connective tissue (SSCT) (Fuchs et al. 1991; Nakamichi and Tachibana 1998; Neal et al. 1987). The SSCT in the carpal tunnel has a highly specialized function which includes providing a bed for tendon gliding, while serving as a source of tendon nutrition (Ettema et al. 2004; Guimberteau 2001). The mechanical properties and mobility of the SSCT are altered in CTS patients (Ettema et al. 2007; Osamura et al. 2007). Thus, knowledge of the relative motion of SSCT before and after carpal tunnel release may improve our understanding the effectiveness, or lack of effectiveness, with respect to carpal tunnel release in CTS patients. However, while some investigators have studied the difference in dimensions of the carpal arch and changes in excursion of the median nerve after carpal tunnel release (Garcia-Elias et al. 1992; Richman et al. 1989; Viegas et al. 1992), the effect of carpal tunnel release on SSCT motion and shear stresses within the carpal tunnel are unknown.
In this study, in order to assess the biomechanical effects of carpal tunnel release on the shear forces affecting the SSCT and median nerve, we measured the relative motion of flexor tendon, SSCT, and median nerve in different wrist positions before and after releasing the flexor retinaculum in normal human cadaver specimens.
MATERIAL AND METHODS
The experimental protocol was reviewed and approved by our Institutional Review Board. A review of available medical records was performed on each potential cadaver donor, to obtain clinical and demographic data. Cadaver specimens were excluded if there was a history of carpal tunnel syndrome or other peripheral nerve disease, as well as conditions potentially associated with peripheral nerve disease or carpal tunnel syndrome, including diabetes or glucose intolerance, thyroid disease, rheumatoid arthritis, osteoarthritis, gout, hemodialysis, sarcoidosis, amyloidosis, or traumatic injuries to the ipsilateral arm. Twelve fresh frozen upper extremity specimens without exclusion criteria (six male, six female, aged 45 to 91, mean 78.9 years) were amputated approximately 15 cm proximal to the wrist joint, and were thawed at room temperature prior to testing.
A custom designed external fixator was used to position the wrist. Two screws were inserted into the index metacarpal bone from the radial side and two screws were inserted into the distal radius. The specimen was mounted in the fixator by clamping the proximal ends of the radius and ulna bones. Each hand was mounted palmar side up. (Fig. 1-A)
Figure 1. Schematic drawing of the experimental setting.
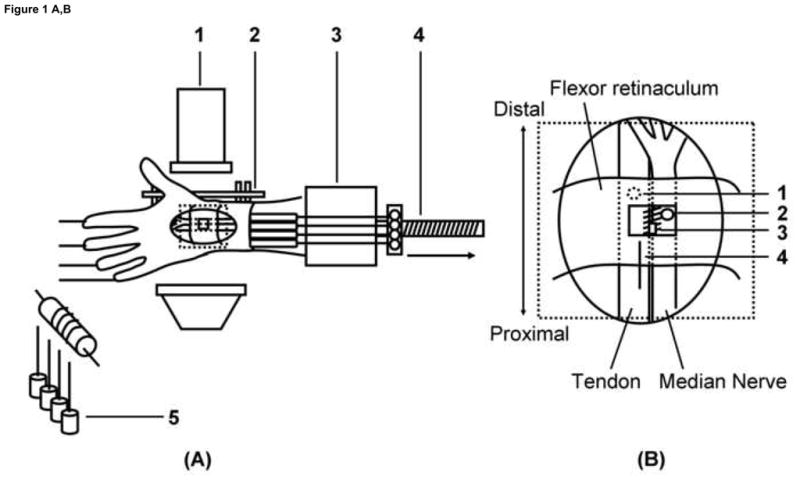
(A) Experimental setting.
1. Fluoroscopy unit, 2. External fixator, 3. Custom fixture, 4. Actuator, 5. Weight.
(B) Schematic drawing for marker placement.
1. Tendon, 2. Median Nerve, 3. SSCT, 4. Ruler.
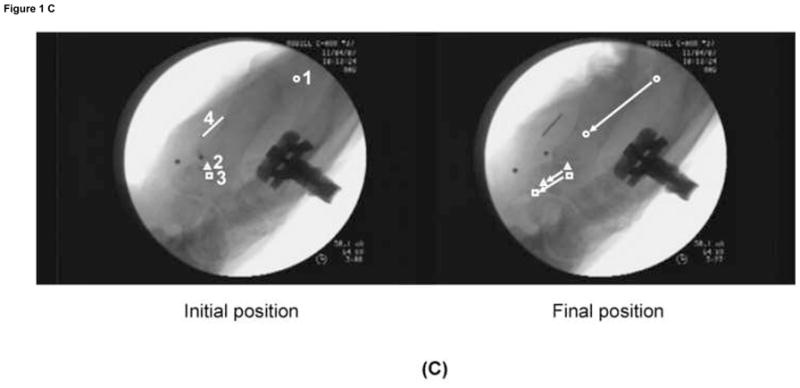
(C) Displacement of each marker for the Flex 60 position.
1. Tendon, 2. Median Nerve, 3. SSCT, 4. Ruler.
A skin incision was made longitudinally to expose the middle finger flexor digitorum superficialis (FDS) tendon from the muscle tendon junction to the proximal end of the finger flexor sheath, with the flexor retinaculum and bursa intact. A small window (5mm diameter) was made in the flexor retinaculum to expose the middle finger FDS tendon, median nerve and SSCT. Two metal markers with a diameter of 1.4–1.6mm (9291K12, McMaster-Carr, Chicago, IL) were completely embedded into the middle finger FDS tendon and median nerve. A third marker was glued on the surface of the SSCT. (Fig. 1-B) The carpal tunnel was otherwise left undisturbed. The proximal ends of the finger FDS tendons were fixed with sutures to a Dacron cord and connected to a mechanical actuator. A 1N weight was attached to each fingertip to maintain tension in the system. The vertical position of the weights was changed according to the wrist position to maintain the finger in a standard position of full extension at the start of testing.
Before testing, the normal FDS tendon excursion of the middle finger was determined by passive full metacarpophalangeal joint (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joint flexion/extension with a weight (5N) attached to the proximal tendon end in the wrist positions described below. This tendon excursion measurement for each position was used to pre set the mechanical actuator excursion for that position.
Testing was randomly performed in three different wrist positions, namely, neutral (0 degrees extension), 60 degrees of flexion (Flex 60), and 60 degrees of extension (Ext 60) for each specimen. The four FDS tendons (index, middle, ring, and little fingers) were pulled together in a proximal direction by the actuator against the weight at a rate of 2.0mm/s until the normal tendon excursion was achieved (simulated fist). This movement of the tendon toward the actuator was regarded as finger flexion. The motion of the three markers was recorded from lateral view fluoroscopy (BV 25, Scopofix MDPM, Philips) using a digital video camera (DCR-TRV350, Sony, Japan) (Fig. 1-C). A ten millimeter ruler was included in the camera field, in the same plane as the specimen, to scale the distance measurement obtained from the image acquisition. Before data collection, the displacement was observed for two conditioning runs, to confirm repeatability. Data was then collected on the third run.
Each of the marker movements (tendon, SSCT, median nerve) was digitized with Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). In addition, the proximal and distal edges of the fixator were digitized as fixed reference points. After testing with an intact carpal tunnel, the flexor retinaculum was cut with a scalpel, to simulate carpal tunnel release. The skin was then closed with several stitches. After the wound was closed, the same testing procedure was repeated for each wrist position.
Digitized coordinate data (X, Y) for the tendon, SSCT, and median nerve were processed using a custom Matlab program (The Mathworks, Inc, Natick, MA). All X and Y coordinate data were converted from pixels to millimeters using the scale factor conversion obtained from the imaged ruler. The coordinates for the tendon, SSCT, and median nerve were normalized relative to the external fixator to correct for any translational or rotational motion of the image or specimen during data collection. Finally, the coordinates of the tendon, SSCT, and median nerve were transformed to a new coordinate system such that the transformed X axis was aligned with the motion direction of the tendon, as defined by a linear polynomial fit to the tendon time series data. Proximal and distal motions were defined as positive and negative motions, respectively. The coordinates at the initial positions for each marker were defined as zero excursion. For the tendon, SSCT, and nerve, the distances along the motion direction of the tendon excursion were calculated. To estimate the potential shear motion occurring at the interface between tendon and nerve, and tendon and SSCT, the relative motions of the tendon, SSCT and nerve were compared using a shear index (SI), defined as the ratio of the difference in motion along the direction of tendon excursion between two tissues divided by tendon excursion, expressed as a percentage. For example, the tendon-SSCT SI would be defined as:
Nomenclature
T = Tendon excursion S = SSCT motion in the direction of tendon excursion
In addition, to estimate the SI during tendon motion, the motion patterns of SSCT and nerve relative to the tendon excursion were calculated. The maximum tendon excursion was defined as 100%, and the percentage of SSCT and median nerve motion relative to tendon excursion were measured at each 10% increment (decile) of tendon excursion.
Statistical Analysis
The effect of different wrist positions and carpal tunnel release were compared for each marker motion. A mixed linear model was used, where each individual specimen was considered as a repeated factor. All results were expressed as mean +/− standard deviation (SD). The wrist position and carpal tunnel release factors were considered statistically significant when the p-value was less than 0.05 in a full model setting. A post hoc pairwise comparison was adapted using Scheffe’s test criteria for the three combinations of wrist position factors (Neutral vs. Flex 60, Neutral vs. Ext 60, Flex 60 vs. Ext 60) controlling for the carpal tunnel release factor. Thus, a p-value less than 0.017 was considered statistically significant for each pairwise comparison. All the analyses were conducted using SAS/STAT version 9.1 software (SAS Institute, Cary, NC).
RESULTS
Summary results for marker motion and SI are shown in Tables 1 and 2. For tendon motion, there was a significant increase with wrist extension and a significant decrease with wrist flexion, compared to the neutral position. For SSCT and median nerve motions, there were significant decreases with 60 degrees of wrist flexion compared to the neutral position in both intact wrists and after division of the flexor retinaculum. For the tendon-SSCT SI, there were significant increases with 60 degree of wrist flexion compared to the neutral in intact wrist. After division of the flexor retinaculum, the differences in shear index among wrist positions were reduced. In comparing the intact and divided flexor retinaculum, only the wrist flexion position showed a significant difference in motion for the SSCT and median nerve.
Table 1.
Displacement of each marker.
| Displacement (mm) | Tendon | SSCT | Nerve |
|---|---|---|---|
| Intact carpal tunnel | |||
| Neutral | 33.4 (1.2) a | 12.0 (2.2) a | 9.0 (2.6) a |
| Flex 60 | 30.3 (1.6) b | 6.4 (2.4) b* | 5.5 (2.3) b* |
| Ext 60 | 36.3 (1.3) c | 11.2 (2.4) a | 8.0 (1.9) a |
| After carpal tunnel release | |||
| Neutral | 33.8 (1.5) a | 11.8 (2.1) a | 9.2 (1.6) a |
| Flex 60 | 30.4 (1.6) b | 8.5 (1.8) b* | 6.8 (2.0) b* |
| Ext 60 | 36.6 (1.0) c | 10.8 (1.6) a | 8.4 (1.8) a |
Different alphabetic letters indicate significant difference within columns (P<0.017).
Significant difference between intact and after releasing carpal tunnel {}.
Table 2.
Shear Index.
| Shear Index (%) | Tendon-SSCT | Tendon-Nerve | SSCT-Nerve |
|---|---|---|---|
| Intact Carpal Tunnel | |||
| Neutral | 64.0 (6.4) a | 73.1 (7.7) a | 9.0 (5.5) a |
| Flex 60 | 78.7 (8.1) b* | 82.0 (7.7) b | 3.2 (6.6) b |
| Ext 60 | 69.1 (6.3) a | 77.9 (5.1) c | 8.8 (7.2) ab |
| After Carpal Tunnel Release | |||
| Neutral | 64.9 (6.9) a | 72.7 (4.7) a | 7.8 (7.1) a |
| Flex 60 | 71.9 (6.3) a* | 77.7 (7.1) b | 5.9 (3.8) a |
| Ext 60 | 70.5 (4.4) a | 77.0 (4.8) ab | 6.4 (5.7) a |
Different alphabetic letters indicate significant difference within columns (P<0.017).
Significant difference between intact and after releasing carpal tunnel (P<0.05).
Summary results of the motion pattern analysis are shown in Figures 2 and 3. For SSCT motion with an intact flexor retinaculum, there were significant decreases in motion at the 7th and 8th deciles of total tendon excursion in the wrist flexion and extension positions compared to neutral position, respectively. After division of the flexor retinaculum, only the wrist extension position remained significantly decreased compared to the neutral position. For median nerve motion with an intact flexor retinaculum, there were significant decreases at the 8th and 6th deciles of tendon excursion in the wrist flexion and extension positions compared to the neutral position, respectively. After carpal tunnel release, there was no significant difference in median nerve motion among wrist positions. In comparison between intact and divided flexor retinaculum states, only the wrist flexion position showed a significant difference for the SSCT and median nerve.
Figure 2. Results of SSCT motion patterns.
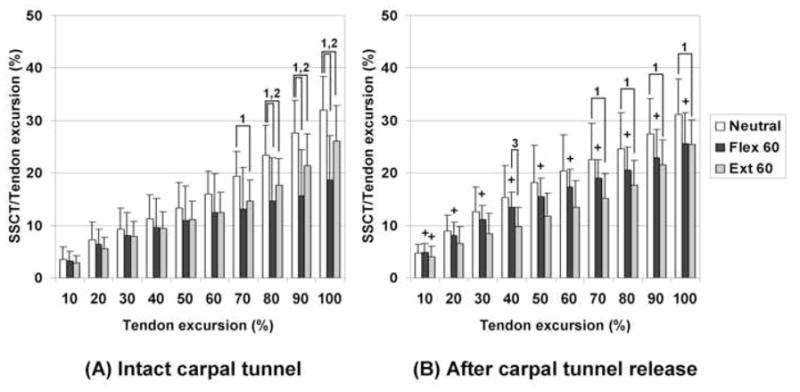
(A) Intact carpal tunnel. (B) After release of carpal tunnel.
1: Significant difference in comparison between Neutral and Ext 60. P<0.017 (0.05/3).
2: Significant difference in comparison between Neutral and Flex 60. P<0.017 (0.05/3).
3: Significant difference in comparison between Ext 60 and Flex 60. P<0.017 (0.05/3).
+ Significant increases after releasing carpal tunnel compared to intact carpal tunnel in same wrist position (P<0.05).
Figure 3. Results of median nerve motion patterns.
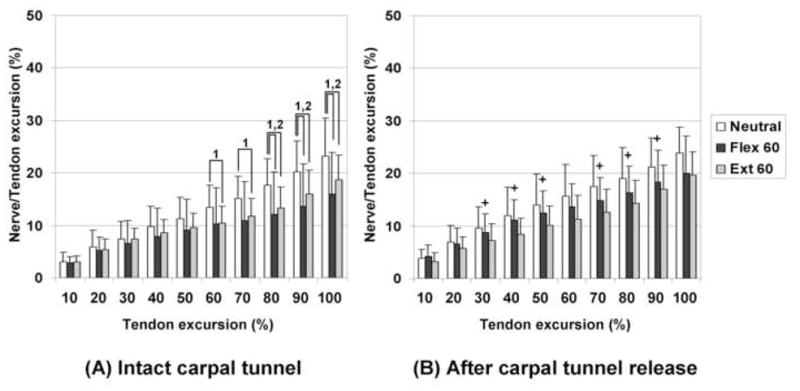
(A) Intact carpal tunnel. (B) After release of carpal tunnel.
1: Significant difference in comparison between Neutral and Ext 60. P<0.017 (0.05/3).
2: Significant difference in comparison between Neutral and Flex 60. P<0.017 (0.05/3).
3: Significant difference in comparison between Ext 60 and Flex 60. P<0.017 (0.05/3).
+ Significant increases after releasing carpal tunnel compared to intact carpal tunnel in same wrist position (P<0.05).
DISCUSSION
This study assessed the relative motions of flexor tendon, SSCT and median nerve in different wrist positions before and after simulated carpal tunnel release. We demonstrated that with an intact flexor retinaculum, the 60 degrees of wrist flexion position showed the least displacement of the SSCT and median nerve with tendon motion, and thus the highest tendon-SSCT and tendon-nerve SI. In addition, the 60 degrees of wrist extension position showed a significantly higher tendon-nerve SI compared to the neutral position. After division of the flexor retinaculum, the SI was reduced significantly in the 60 degrees wrist flexion position. This finding suggests that tendon-SSCT and tendon-nerve SIs are affected both by wrist position and by the integrity of the flexor retinaculum.
There are several methods to estimate shear strain. Wright et al. (1996) introduced a method to measure strain of the median nerve directly with a strain gauge. Goldstein et al. (1987) measured strain with frictional interactions between the flexor tendons and flexor retinaculum for loaded flexor tendons distal to the flexor retinaculum. Bay et al. (1997) used a nerve/tendon ratio to evaluate the effect of wrist positions on nerve strain. This may be the reverse of the SI we quantified in this study. Osamura et al. (2007) measured the force associated with different degrees of shear strain. Here we have measured the strain, but not the force. We do think that it is reasonable to assume that the force associated with similar degrees of strain would be similar in the two circumstances, as both were measured in an intact tendon/SSCT preparation. Indeed, it was the stimulus of the work by Osamura et al. (2007) that prompted this work, to see if shear strains associated with large forces might occur with clinically relevant movements. Given the above caveats, the SI in the 60 degrees of wrist flexion position still suggests that significant shear strains, possibly sufficient to induce SSCT injury, may occur in some wrist and finger positions that might occur physiologically. These strains were significantly reduced after simulated carpal tunnel release.
Before carpal tunnel release, the flexor retinaculum functions as a pulley for the flexor system (Simmons and DeLaCaffiniere 1981; Wehbe 1984). With finger or wrist flexion, the flexor tendons move palmarly (Ugbolue et al. 2005; Zeiss et al. 1989). This suggests that the flexor retinaculum may restrain the SSCT and affect the shear strain between tendon and SSCT in the intact carpal tunnel. After division of the flexor retinaculum, the palmar SSCT displacement increased, especially in wrist flexion. This may be one possible reason for the reduced shear strain that we noted between tendon and SSCT in the wrist flexion position after division of the flexor retinaculum.
Several investigators have reported the median nerve excursion and strain in relation with wrist motion. They have suggested that wrist motion mobilizes the median nerve, and that median nerve strain is maximal in wrist extension (Bay et al. 1997; Coppieters and Alshami 2007; Wright et al. 1996). We have demonstrated that the SI between the tendon and median nerve increased significantly, not only for wrist extension, but also during wrist flexion. This is position associated with carpal tunnel symptoms (Hagberg et al. 1992; Phalen 1972; Szabo and Chidgey 1989).
Szabo et al. (1994) reported the median nerve excursion associated with flexor tendon motion in a cadaver model. Tuzuner et al. (2004) reported the median nerve excursion in the carpal tunnel during wrist motion before and after endoscopic carpal tunnel release in a clinical study. Both studies concluded that there was no significant difference in median nerve excursion when comparing before and after carpal tunnel release. Although our observations showed similar median nerve excursions to their reports in the neutral and wrist extension positions, the displacement of the median nerve increased significantly after division of the flexor retinaculum in the wrist flexion starting from 3rd decile of tendon excursion, and the differences between wrist positions were reduced. This is consistent with our findings for SSCT motion.
In this study, in order to estimate the motion relative to tendon, we considered the direction of tendon excursion as the X axis. Our measurement also captured any tendon bowstringing. Out of plane (transverse) motion can certainly occur, but its magnitude is unlikely to be more than a few mm at most. It would have little effect on overall motion, considering the very large excursion in the longitudinal plane (the direction of tendon motion) and the AP direction (bowstringing), both of which were captured in our 2D measurements.
Nerve strain causes dysfunction in animal peripheral nerves. Although results among studies are not entirely in agreement, in general, strain in excess of 10% of resting length rapidly induces conduction block (Brown, R, et al. 1993; Rydevik et al. 1990; Sunderland 1981). Although we did not measure intraneural strain in this study, we did observe that the differences in SSI between tendon and nerve for the various wrist positions were less than 10% before division of the flexor retinaculum, and further reduced after division of the flexor retinaculum. Of course, we excluded specimens with an antemortem history of carpal tunnel syndrome, and our findings were made in cadaver wrists. CTS patients may show different shear strains, as tethering of median nerve motion is known to occur in CTS patients (Ettema et al. 2007; Hough et al. 2007; Nakamichi and Tachibana 1995). We do believe that our findings suggest that carpal tunnel release, in addition to its effects on reducing carpal tunnel pressure, may also reduce the shear strain between tendon and nerve by increasing the mobility of the median nerve.
The strength of our study is that we simultaneously measured the motion of tendon, SSCT, and nerve in two dimensions within the carpal tunnel before and after a simulated carpal tunnel release. In addition, the measurement procedure that we used in this study made it possible to observe details of SSCT and median nerve motion during finger flexion. This data may be useful to compare with data in CTS patients in future studies.
The limitations of this study should be noted. First, we used human cadaver specimens. Our results, therefore, do not simulate clinical CTS. Disruption of normal hydrostatic relations in a cadaver hand would be expected to alter the shear strain in SSCT and median nerve. However, from the standpoint of a basic science investigation, it is an advantage to isolate the influence of particular factors in a situation where many factors interact. Second, we did not make any attempt to study the effect of isolated FDS tendon motion or different motion speeds in this study. Oh et al. (2007), using color Doppler ultrasound, demonstrated that different tendon speeds induced different relative motion in the SSCT. Thus, the relative motion in SSCT and median nerve may be different with differential finger motion (i.e., two adjacent fingers moving in different directions), or at different speeds. Third, we measured motion within the carpal tunnel. As Goldstein et al. (1987) have shown that median nerve strain differs from proximal to distal to the carpal tunnel, there may be some difference in the shear strain at locations proximal or distal to the carpal tunnel. Fourth, we measured motion only in two dimensions. However, we believe that the motions that we collected were the most relevant ones. We also needed to create a small window on the flexor retinaculum to insert the markers for each tissue. While the markers in the tendon and nerve could be embedded and thus may be unlikely to impeded motion, the marker on the SSCT surface may have impeded SSCT motion, especially in the intact carpal tunnel condition. Fifth, this study was performed using a simulated open carpal tunnel release technique. Results may be different with simulated endoscopic methods. Finally, while hand size would affect the absolute value of tendon excursion when comparing one specimen to another, we focused on within specimen effects, so that each specimen served as its own control. Thus, any variation in hand size would not affect the results.
In conclusion, this study assessed the effects of a simulated open carpal tunnel release on the relative motion of tendon, SSCT, and nerve in different wrist positions. We demonstrated that 60 degrees of wrist flexion maximizes the shear strain in the SSCT and median nerve within an intact carpal tunnel. This strain was significantly reduced after the simulated carpal tunnel release. These findings suggest that the carpal tunnel release, in addition to its effects on reducing carpal tunnel pressure, may also reduce the shear strain between the tendon and SSCT. These data may be useful to the study of the effect of carpal tunnel release on the SSCT and median nerve in CTS patients.
Acknowledgments
The project described was supported by Grant Number NIAMS AR49823 from NIH. The authors would like to thank Mr. Stephen Cha for help with the statistical analysis.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no financial or personal relationships with other persons or organizations which may lead to a conflict of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Qattan MM, Bowen V, Manktelow RT. Factors associated with poor outcome following primary carpal tunnel release in non-diabetic patients. J Hand Surg Br. 1994;19:622–625. doi: 10.1016/0266-7681(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Bay BK, Sharkey NA, Szabo RM. Displacement and strain of the median nerve at the wrist. J Hand Surg Am. 1997;22:621–627. doi: 10.1016/S0363-5023(97)80118-9. [DOI] [PubMed] [Google Scholar]
- Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J, Kwan M, Garfin SR. Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Relat Res. 1993;296:288–294. [PubMed] [Google Scholar]
- Brown RA, Gelberman RH, Seiler JG, 3rd, Abrahamsson SO, Weiland AJ, Urbaniak JR, Schoenfeld DA, Furcolo D. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75:1265–1275. doi: 10.2106/00004623-199309000-00002. [DOI] [PubMed] [Google Scholar]
- Cobb TK, Cooney WP, An KN. Aetiology of work-related carpal tunnel syndrome: the role of lumbrical muscles and tool size on carpal tunnel pressures. Ergonomics. 1996;39:103–107. doi: 10.1080/00140139608964437. [DOI] [PubMed] [Google Scholar]
- Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25:972–980. doi: 10.1002/jor.20310. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Zhao C, Amadio PC, O’Byrne MM, An KN. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: a pilot study. Clin Anat. 2007;20:292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16:753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias M, Sanchez-Freijo JM, Salo JM, Lluch AL. Dynamic changes of the transverse carpal arch during flexion-extension of the wrist: effects of sectioning the transverse carpal ligament. J Hand Surg Am. 1992;17:1017–1019. doi: 10.1016/s0363-5023(09)91049-8. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–383. [PubMed] [Google Scholar]
- Goldstein SA, Armstrong TJ, Chaffin DB, Matthews LS. Analysis of cumulative strain in tendons and tendon sheaths. J Biomech. 1987;20:1–6. doi: 10.1016/0021-9290(87)90261-2. [DOI] [PubMed] [Google Scholar]
- Guimberteau JC. New ideas in hand flexor tendon surgery. Institut Aquitain De La Main; 2001. The sliding system. Vascularized flexor tendon transfers. [Google Scholar]
- Hagberg M, Morgenatern H, Kelsh M. Impact of occupations and job tasks on the prevalence of carpal tunnel syndrome. Scand J Work Environ Health. 1992;18:337–345. doi: 10.5271/sjweh.1564. [DOI] [PubMed] [Google Scholar]
- Harris CM, Tanner E, Goldstein MN, Pettee DS. The surgical treatment of the carpal-tunnel syndrome correlated with preoperative nerve-conduction studies. J Bone Joint Surg Am. 1979;61:93–98. [PubMed] [Google Scholar]
- Hough AD, Moore AP, Jones MP. Reduced Longitudinal Excursion of the Median Nerve in Carpal Tunnel Syndrome. Arch Phys Med Rehabil. 2007;88:569–576. doi: 10.1016/j.apmr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Hybbinette CH, Mannerfelt L. The carpal tunnel syndrome. A retrospective study of 400 operated patients. Acta Orthop Scand. 1975;46:610–620. doi: 10.3109/17453677508989243. [DOI] [PubMed] [Google Scholar]
- Keller RB, Largay AM, Soule DN, Katz JN. Maine Carpal Tunnel Study: small area variations. J Hand Surg Am. 1998;23:692–696. doi: 10.1016/S0363-5023(98)80057-9. [DOI] [PubMed] [Google Scholar]
- Kulick MI, Gordillo G, Javidi T, Kilgore ES, Jr, Newmayer WL., 3rd Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11:59–66. doi: 10.1016/s0363-5023(86)80104-6. [DOI] [PubMed] [Google Scholar]
- Nagle D, Harris G, Foley M. Prospective review of 278 endoscopic carpal tunnel releases using the modified chow technique. Arthroscopy. 1994;10:259–265. doi: 10.1016/s0749-8063(05)80108-2. [DOI] [PubMed] [Google Scholar]
- Nakamichi K, Tachibana S. Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg Br. 1995;20:460–464. doi: 10.1016/s0266-7681(05)80153-6. [DOI] [PubMed] [Google Scholar]
- Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998;23:1015–1024. doi: 10.1016/s0363-5023(98)80009-9. [DOI] [PubMed] [Google Scholar]
- Nakao E, Short WH, Werner FW, Fortino MD, Palmer AK. Changes in carpal tunnel pressures following endoscopic carpal tunnel release: a cadaveric study. J Hand Surg Am. 1998;23:43–47. doi: 10.1016/S0363-5023(98)80087-7. [DOI] [PubMed] [Google Scholar]
- Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg Br. 1987;12:229–232. doi: 10.1016/0266-7681_87_90020-9. [DOI] [PubMed] [Google Scholar]
- Oh S, Belohlavek M, Zhao C, Osamura N, Zobitz ME, An KN, Amadio PC. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color Doppler sonographic imaging. J Ultrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- Okutsu I, Ninomiya S, Hamanaka I, Kuroshima N, Inanami H. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg Am. 1989;71:679–683. [PubMed] [Google Scholar]
- Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech. 2007;22:999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen GS. The carpal-tunnel syndrome. Clinical evaluation of 598 hands. Clin Orthop Relat Res. 1972;83:29–40. doi: 10.1097/00003086-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Richman JA, Gelberman RH, Rydevik B, Hajek PC, Braun RM, Gylys-Morin VM, Berthoty D. Carpal tunnel syndrome: morphologic changes after release of the transverse carpal ligament. J Hand Surg Am. 1989;14:852–857. doi: 10.1016/s0363-5023(89)80089-9. [DOI] [PubMed] [Google Scholar]
- Rydevik BL, Kwan MK, Myers RR, Brown RA, Triggs KJ, Woo SL, Garfin SR. An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res. 1990;8:694–701. doi: 10.1002/jor.1100080511. [DOI] [PubMed] [Google Scholar]
- Schuind F. Canal pressures before, during, and after endoscopic release for idiopathic carpal tunnel syndrome. J Hand Surg Am. 2002;27:1019–1025. doi: 10.1053/jhsu.2002.36541. [DOI] [PubMed] [Google Scholar]
- Simmons BP, DeLaCaffiniere JY. The hand. Philadelphia: WB Saunders; 1981. Physiology of flexion of the fingers; pp. 377–388. [Google Scholar]
- Sunderland S. Stretch-compression neuropathy. Clin Exp Neurol. 1981;18:1–13. [PubMed] [Google Scholar]
- Szabo RM, Bay BK, Sharkey NA, Gaut C. Median nerve displacement through the carpal canal. J Hand Surg Am. 1994;19:901–906. doi: 10.1016/0363-5023(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg Am. 1989;14:624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- Tuzuner S, Ozkaynak S, Acikbas C, Yildirim A. Median nerve excursion during endoscopic carpal tunnel release. Neurosurgery. 2004;54:1155–1160. doi: 10.1227/01.neu.0000119232.57668.98. [DOI] [PubMed] [Google Scholar]
- Ugbolue UC, Hsu WH, Goitz RJ, Li ZM. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech. 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Viegas SF, Pollard A, Kaminksi K. Carpal arch alteration and related clinical status after endoscopic carpal tunnel release. J Hand Surg Am. 1992;17:1012–1016. doi: 10.1016/s0363-5023(09)91048-6. [DOI] [PubMed] [Google Scholar]
- Wehbe MA. Differential tendon gliding in the hand. J Hand Surg Am. 1984;9:596. doi: 10.1016/s0363-5023(85)80086-1. [DOI] [PubMed] [Google Scholar]
- Werner CO, Elmqvist D, Ohlin P. Pressure and nerve lesion in the carpal tunnel. Acta Orthop Scand. 1983;54:312–316. doi: 10.3109/17453678308996576. [DOI] [PubMed] [Google Scholar]
- Werner R, Armstong TJ, Bir C, Aylard MK. Intracarpal canal pressures: the role of finger, hand, wrist and forearm position. Clin Biomech. 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Wright TW, Glowczewskie F, Wheeler D, Miller G, Cowin D. Excursion and strain of the median nerve. J Bone Joint Surg Am. 1996;78:1897–1903. doi: 10.2106/00004623-199612000-00013. [DOI] [PubMed] [Google Scholar]
- Zeiss J, Skie M, Ebraheim N, Jackson WT. Anatomic relations between the median nerve and flexor tendons in the carpal tunnel: MR evaluation in normal volunteers. AJR Am J Roentgenol. 1989;153:533–6. doi: 10.2214/ajr.153.3.533. [DOI] [PubMed] [Google Scholar]


