Abstract
The serotonergic system is highly plastic, capable of adapting to changing afferent information in diverse mammalian systems. We hypothesized that removing supraspinal and/or peripheral input would play an important role in defining the distribution of one of the most prevalent serotonergic receptors, the 5-HT1A receptor (R), in the spinal cord. We investigated the distribution of this receptor in response to a complete thoracic (T7–T8) spinal cord transection (eliminating supraspinal input), or to spinal cord isolation (eliminating both supraspinal and peripheral input) in adult rats. Using two antibodies raised against either the second extracellular region (ECL2) or the third intracellular region (ICL3) of the 5-HT1AR, we compared the 5-HT1AR levels and distributions in specific laminae of the L3–L5 segments among the control, spinal cord–transected, and spinal cord–isolated groups. Each antibody labeled different populations of 5-HT1AR: ECL2 labeled receptors in the axon hillock, whereas ICL3 labeled receptors predominantly throughout the soma and proximal dendrites. Spinal cord transection increased the number of ECL2-positive cells in the medial region of laminae III–IV and lamina VII, and the mean length of the labeled axon hillocks in lamina IX. The number of ICL3-labeled cells was higher in lamina VII and in both the medial and lateral regions of lamina IX in the spinal cord–transected compared to the control group. In contrast, the length and number of ECL2-immunolabeled processes and ICL3-immunolabeled cells were similar in the spinal cord–isolated and control groups. Combined, these data demonstrate that the upregulation in 5-HT1AR that occurs with spinal cord transection alone is dependent on the presence of sensory input.
Key words: afferent input, 5-HT1A receptor, serotonin, spinal cord isolation, spinal cord transection
Introduction
Descending projections from the raphe magnus, raphe pallidus, and raphe obscuris nuclei, and the ventral parts of the reticular formation in the caudal portion of the medulla are the major sources of 5-HT in the spinal cord (Bowker et al., 1981). These projections synapse on interneurons receiving afferent input in the dorsal horn, interneurons in the intermediate gray area, and motoneurons in the ventral horn of the lumbar spinal cord (Marlier et al., 1991a; Thor et al., 1993), and are able to modulate the locomotor networks at different sites (Schmidt and Jordan, 2000). 5-HT and its agonists increase the likelihood of cells being activated within the locomotor network through the action of multiple 5-HT receptor (R) subtypes associated with a variety of intracellular signaling cascades (Hochman et al., 2001).
The 5-HT1AR is the most plastic and most highly characterized spinal 5-HTR, and modulates a number of different cellular processes in the spinal cord. 5-HT1ARs can increase spinal motoneuron excitability pre-synaptically in neonatal rats by modulating the amount of transmitter released (Wu et al., 2002), while cell excitability can be decreased post-synaptically by inhibiting adenylate cyclase activity through a Gi protein in fetal mouse spinal cord ganglion explants (Makman et al., 1988). Depending on the cell type and intracellular location, this receptor may also couple to other G proteins to affect different second messenger systems (Oleskevich et al., 2005; Malmberg and Strange, 2000). In spinal motoneurons of the adult turtle, 5-HT1AR activation produces a depolarization and an increase in input resistance by inhibiting TASK-1-like K+ channels (Perrier et al., 2003), and also inhibits small conductance Ca2+-activated K+ channels responsible for the medium afterhyperpolarization (Grunnet et al., 2004), both of which increase the excitability of the cell. It is not clear, however, to what extent these effects are mediated through ionic and/or G-protein mechanisms.
After a complete spinal cord transection, the descending serotonergic input from the brainstem raphe nuclei and reticular formation to regions of the cord caudal to the lesion is eliminated. This decreases the 5-HT content to ∼5 –10% of its initial level (Anden et al., 1964), leaving the remaining serotonergic spinal interneurons as the only known source of 5-HT (Kubasak et al., 2008; Newton and Hamill, 1988). Animals transected as adults cannot step spontaneously after a complete spinal cord transection at a mid- to low-thoracic level. Administration of 5-HT or agonists of the 5-HTR family such as 8-OH-DPAT (a 5-HT1A/7R agonist) or quipazine (a general 5-HTR agonist), however, can induce treadmill stepping in the spinal cat (Barbeau and Rossignol, 1990), rat (Antri et al., 2005), and mouse (Cai et al., 2006; Fong et al., 2005).
There is an increase in the level of 5-HT1AR autoradiographic receptor binding in specific laminae of the lumbar segments 15 and 30 days after a complete spinal cord transection in adult cats (Giroux et al., 1999). To begin to understand the underlying causative factors of these changes, we have compared the 5-HT1AR immunoreactivity in the spinal cord after a reduced level of neuromuscular activity (elimination of supraspinal input via a complete mid-thoracic spinal cord transection; Alaimo et al., 1984), and after virtual elimination of neuromuscular activity (elimination of supraspinal and peripheral input via spinal cord isolation; Roy et al., 2007b).
Although a common response to a reduction in the level of an agonist is an increase in the number of receptors for that agonist, we hypothesized that in addition to the availability of its agonist, the activity levels of the neurons would play an important role in the modulation of serotonin receptors. To test this hypothesis we compared how 8 weeks of spinal cord transection or spinal cord isolation affect the cellular and laminar distribution of the 5-HT1AR using two antibodies, each specific for a unique epitope. One antibody identifies an intracellular (ICL3, 3rd loop) and the other an extracellular (ECL2, 2nd loop) epitope sequence on the 5-HT1AR protein in the brain and spinal cord (Azmitia et al., 1992; El Mestikawy et al., 1990). There is a differential topography of 5-HT1AR based on immunolabeling with these antibodies as previously described (Azmitia et al., 1996; Kheck et al., 1995; Kia et al., 1996). Each antibody labeled different populations of 5-HT1AR: ECL2 labeled receptors in the axon hillock, whereas ICL3 labeled receptors predominantly throughout the soma and proximal dendrites. Preliminary data have been published in abstract form (Otoshi et al., 2005).
Materials and Methods
Animal protocol
Eighteen adult female Sprague-Dawley rats (250–300 g body weight) were assigned randomly to one of three groups: control (n = 6), spinal cord–transected (n = 6), or spinal cord–isolated (n = 6). The rats were maintained for 8 weeks after the spinal cord injuries because there are a number of significant adaptations in the neuromuscular system at this time point that could impact receptor function in the spinal cord (Grossman et al., 1998; Roy et al., 2000; Roy et al., 2007b; Talmadge et al., 2002; Talmadge et al. 2004). All procedures were approved by the UCLA Chancellor's Animal Research Committee and followed the American Physiological Society Animal Care Guidelines.
All surgical procedures were performed under aseptic conditions with the rats initially administered buprenorphine (0.01–0.05 mg/kg IP) 45 min prior to surgery as an analgesic, and then deeply anesthetized with a mixture of ketamine hydrochloride (80 mg/kg IP) and xylazine (10 mg/kg IP). A complete spinal cord transection at the T7–T8 level was performed as described previously (Talmadge et al., 2002). Spinal cord isolation was performed as previously described (Grossman et al., 1998; Roy et al., 2007a,b). Briefly, a partial laminectomy was performed between vertebral levels T7 and ∼S1. After opening the dura, the dorsal roots were cut intra-durally bilaterally from T7–T8 to S1. The dorsal roots were cut as close to the spinal cord as possible, and again as close to their exit from the spinal cord as possible and discarded. After the deafferentation, the spinal cord was gently lifted with a curved glass probe and completely transected at spinal cord level T7–T8 and then at ∼S1 using microdissection scissors. The daily care and management of the spine-injured rats followed the protocols detailed in Roy et al. (1992). Post-surgical care included manual expression of the bladder three times per day for the first 2 weeks, and two times per day for the remainder of the study. Since the hindlimbs are flaccid after spinal cord isolation surgery, the hindlimbs of the spinal cord–isolated rats were manipulated passively through a normal range of motion once per day to maintain joint flexibility. Reflex testing (withdrawal reflex and toe pinch) of the hindlimbs of the spinal cord–isolated rats was also performed once a day. There were no observable reflex responses and the hindlimbs were completely flaccid throughout the study, thus verifying that the hindlimb neuromuscular system was inactive in this preparation. After spinal cord transection, the hindlimbs show spontaneous reflexive activity and thus did not need to be passively manipulated or tested.
Eight weeks after surgery, all animals except for three rats in the SI group were deeply anesthetized with sodium pentobarbital (125 mg/kg; Sigma, St. Louis, MO) and transcardially perfused with 1 mL/g body weight of phosphate buffer solution (PBS at pH = 7.4), followed by 2 mL/g body weight of 4% paraformaldehyde in PBS (Sigma). The spinal cords were removed, post-fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose overnight, and stored at −80°C. Because tissues in some spinal cord–isolated rats were used for other analyses, three of the six spinal cord–isolated rats were decapitated and their spinal cords post-fixed and cryoprotected overnight as described above. To determine whether the two methods of tissue preparation might have affected the results, we compared the ECL2 and ICL3 staining in lamina IX between post-fixed and perfused tissue (L3–L5 spinal cord sections) of spinal cord–isolated rats. No differences were observed in either the mean length of immunopositive axon hillocks or the number of ICL3-positive cell bodies. Therefore, the different procedures used in the immunostaining played no role in defining our experimental results and the data from the six rats were combined for statistical analyses.
Immunohistochemistry
The anti-rabbit 5-HT1AR polyclonal antibody (ECL2 epitope, kindly donated by Dr. Efrain C. Azmitia, New York University, New York, NY) was raised against a synthetic peptide corresponding to amino acids 170–186 of the second extracellular loop of the rat 5-HT1AR. The anti-guinea pig 5-HT1AR polyclonal antibody (ICL3 epitope, Chemicon, Temecula, CA) was raised against a synthetic peptide corresponding to amino acids 242–268 on the third intracellular loop of the receptor protein. This region is known to contain calmodulin binding sites (Turner et al., 2004), putative sites of phosphorylation by protein kinase C (Kemp and Pearson, 1990), sites for G-protein coupled interactions with adenylate cyclase (Okada et al., 1989), phospholipase C (Claustre et al., 1988), protein kinase C (Raymond et al., 1989), potassium channels (Zgombick et al., 1989), extracellular signal-related protein kinase (Garnovskaya et al., 1998), mitogen-activated protein kinase (Garnovskaya et al., 1996), and sodium/hydrogen ion exchange (Garnovskaya et al., 1997). The anti-goat heat shock protein-27 (HSP27)-M20 antibody obtained from Chemicon was used to identify motoneurons.
Immunolabeling experiments using serial dilutions of both ICL3 and ECL2 antibodies from spinal cord segments L3–L5 from control rats that were perfused were conducted before optimum dilutions were selected for the analyses. We used antibody dilutions having strong binding characteristics (ECL2, 1:250 to 1:4000 and ICL3, 1:250 to 1:1400) throughout the entire range, showing similar staining and binding properties as demonstrated previously (El Mestikawy et al., 1990; Azmitia et al., 1992; Kheck et al., 1995; Kia et al., 1996; Zhou et al., 1999; Riad et al., 2000). Although the above studies used various dilutions and incubation times, the two antibodies maintained their respective characteristic patterns of staining.
Comparisons among control, spinal cord–transected, and spinal cord–isolated groups were carried out using single-labeled immunohistochemistry for ECL2 and ICL3 separately. Free-floating transverse sections (30-μm thick for single labeling for ICL3, and 50-μm thick for single labeling for ECL2) from L3–L5 spinal segments were obtained using a cryostat and then washed in PBS (pH = 7.4) for 30 min, blocked in 1% normal goat serum, 0.3% Triton X100, and PBS solution for 30 min, and incubated in either of the two 5-HT1AR antibodies: ECL2 (1:4000 for 48 h at 4°C), or ICL3 (1:700 overnight at room temperature). After a further 30-min wash, the sections were incubated in the following secondary antibodies: ECL2 in biotinylated anti-rabbit IgG, 1:200, 60 min at room temperature (Vector Laboratories, Burlingame, CA) followed by streptavidin indocarbocyanine (Cy3; Sigma), 1:1000, 60 min at room temperature, and ICL3 in anti-guinea pig rhodamine red conjugated IgG 1:200, 60 min at room temperature (Jackson Immunoresearch Laboratories, West Grove, PA), and mounted on slides after an additional 30-min wash.
Control experiments that omitted only the primary antibody, anti-5-HT1AR (ECL2), from the first incubation mixture and included all other steps did not show any specific or non-specific labeling. Incubating spinal cord sections in a solution of 5 μg/mL or 0.5 μg/mL of the antigen peptide and a 1:4000 dilution of the ECL2 anti-5-HT1AR antibody (previously mixed for 6 h at room temperature) for 48 h, and followed by the same protocol as that described above also did not produce any specific or non-specific labeling. Similar control experiments have been performed for the ICL3 primary antibody to demonstrate antibody specificity (Zhang et al., 2004).
To directly visualize the topography of the ECL2 and ICL3 labeling in motoneurons, some sections from control rats were either double-labeled (ECL2 and ICL3) or triple-labeled (ECL2, ICL3, and HSP27) for the following primary antibodies: anti-5-HT1AR (ECL2): 1:4000, anti-5-HT1AR (ICL3): 1:1400, and anti-HSP27(M20): 1:100 dilution, for 48 h at 4°C (Fig. 1). The sections were washed overnight in PBS and incubated in a secondary antibody: 1:200, biotin-SP anti-rabbit IgG (Jackson ImmunoResearch). Sections then were washed for 30 min and blocked in 3% normal horse serum in 0.1% Triton-X100, and PBS solution for 60 min, and incubated with: streptavidin 7-amino-4-methylcoumarin-3-acetic acid (streptavidin AMCA), anti-goat fluorescein isothiocyanate (FITC), and anti-guinea pig rhodamine red secondary antibodies (all at 1:200; Jackson ImmunoResearch) for 60 min, and mounted on slides.
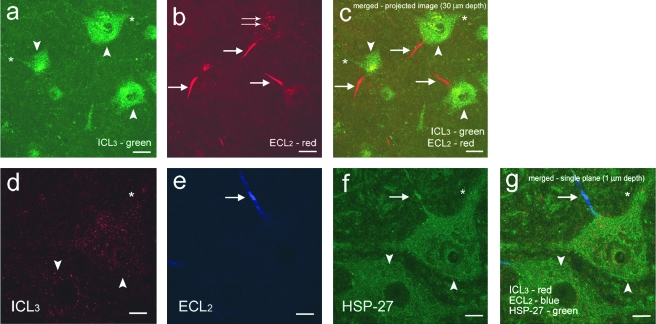
FIG. 1.
Differential topography of 5-HT1AR detected with an intracellular (ICL3) and an extracellular (ECL2) epitope-recognizing antibody. Panels a–c are images projected from 30 single plane 1 μm confocal images of L4 lamina IX neurons in a control rat labeled for both ECL2 and ICL3 antibodies, whereas d–g are single plane (1 μm) images of an L4 control motoneuron. The ECL2 antibody stained primarily the axon hillock and initial segment (arrows in b, c, e, f, and g) of the axon, while the cell body (double arrows in b) showed weak, diffuse staining. The ICL3 antibody detected 5-HT1AR proteins primarily on proximal dendrites (asterisks in a, c, d, f, and g), and on the cell body (arrowheads in a, c, d, f, and g). Motoneurons were identified by HSP27 (f and g) (scale bars for a–c = 20 μm, and d–g = 10 μm). (Color image is available online at www.liebertpub.com/jon.)
Confocal images were taken on a Leica TCS-SP MP Confocal and Multiphoton Microscope (Mannheim, Germany) equipped with argon (488 nm excitation) and krypton (568 nm excitation) and helium-neon (633 nm excitation) lasers and a two photon laser setup consisting of a Spectra-Physics Millenia X 532 nm green diode pump laser and a Tsunami Ti-Sapphire picosecond pulsed infrared laser tuned at 770 nm for UV excitation. An example of a single motoneuron imaged by taking a series of images at intervals of 1 μm on the z plane at a 30-μm depth or until the entire length of the ICL3, HSP27, and ECL2 labeling were scanned, is shown in Figure 1d–g. Images were obtained, overlaid, and projected using Leica Confocal Software (LCS version 2.61 Build 1537).
Quantitation of ECL2 in the lumbar spinal cord
Five regions of the L3–L5 spinal cord segments were selected: the medial and lateral regions of laminae III and IV in the dorsal horn for their association with ascending, descending, afferent, and contralateral projections; the medial cell column in lamina VII for its association with Clarke's nucleus; and the lateral and medial regions of lamina IX in the ventral horn for their association with motor output. For ECL2-labeled sections (4–6 sections), the number of 5-HT1AR-positive processes (axon hillocks) per region was obtained using a Molecular Dynamics confocal laser-scanning microscope (Sarastro 2000 with an argon laser Nikon Optiphot microscope) and a 60 × objective (Molecular Dynamics, Sunnyvale, CA). The confocal laser scanning microscope was set to obtain an image of 174 × 174 μm on the x–y plane and 18 μm in the z plane, taking 10 x–y scans at 2-μm z-plane intervals. These 10 serial images were projected into a single image using maximum intensity of each pixel as the projection parameter to create a two-dimensional image from which the number of 5-HT1A-positive processes in each region was counted. Those hillocks not fully included within the boundaries of the projected image and/or less than 2 μm in length were excluded. The axon hillock lengths from 50 representative neurons from each of the lateral and medial regions of lamina IX from each rat (from 4–6 sections per rat) were measured from confocal projected images as described above.
To quantify the number of ICL3-positive cell bodies in the five regions, images from 4–6 sections at L3–L5 were acquired with an Axiophot microscope (Zeiss, Thornwood, NY) equipped with an KX-15CCD (Apogee Instruments Inc., Roseville, CA) camera under identical magnification (60 ×), and saved as tagged image format files. In the saved images, ICL3-positive neurons in various laminae were counted using the manual tagging feature of Image Pro Plus software (Media Cybernetics Inc., Bethesda, MD). Threshold values were set to discriminate the signal from the background. Data were quantified only for neurons that contained a nucleus.
Statistical analyses
All data are reported as mean ± SEM. A one-way analysis of variance (ANOVA) was used to determine overall group differences with SPSS software (SPSS, Inc., Chicago, IL). The Scheffe test was used for post-hoc comparisons of individual pairs of group means. An alpha level of 0.05, or 0.01 where specified, was set as the minimum criterion for statistical significance.
Results
ECL2 and ICL3 5-HT1AR antibodies show different topographical distributions of 5-HT1AR in spinal neurons in control rats
As previously described for the non-human primate brain (Azmitia et al., 1996) and spinal cord (Kheck et al., 1995), we found that for the same neuron the cellular distributions of the binding sites for ECL2 and ICL3 antibodies differed in the rat spinal cord. The ECL2 antibody predominantly stained a single process of each labeled cell, the initial segment of the axon or axon hillock (arrows in Fig. 1), while the ICL3 antibody labeled receptors predominantly in the cell soma and proximal dendrites in lamina IX neurons (arrowheads in Fig. 1). The ECL2 antibody stained the axon hillock intensely, but weak, diffuse staining also was observed in the cell body (double arrows in Fig. 1b). In contrast, the ICL3 antibody showed intense staining in the cell body (arrowheads in Fig. 1a), and on proximal dendrites (asterisks in Fig. 1a), while showing no staining of the axon hillock. An example of triple immunohistochemistry using the ECL2, ICL3, and HSP27 antibodies followed by single plane confocal light scanning microscopy demonstrates the differential topography of these two receptors within the same motoneuron (Fig. 1d–g).
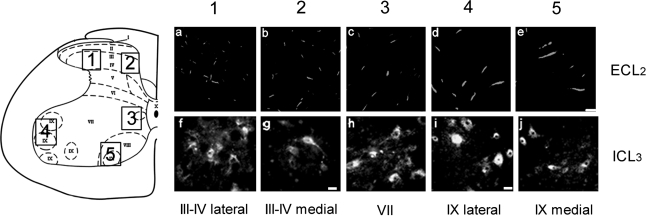
In spinal cord sections from the control group, the numbers of ECL2-labeled 5-HT1AR were about threefold higher in laminae III and IV compared to lamina IX (Figs. 2a, b, d, and e and 3a). In contrast, the numbers of ICL3-labeled cell bodies were similar in the ventral horn and the superficial dorsal horn laminae (Fig. 2f–j and 3b).
FIG. 2.
Laminae distribution of 5-HT1AR immunoreactivity. Regions 1–5 on the schematic spinal cord cross-section show areas of quantification for 5-HT1AR staining: the lateral region of laminae III–IV (1), the medial region of laminae III–IV (2), the medial cell column of lamina VII (3), the lateral region of lamina IX (4), and the medial region of lamina IX (5). Fluorescence microscopy images of ECL2 immunostaining (a–e) and ICL3 immunostaining (f–j) are from a control L4 spinal cord section. Scale bar in e = 20 μm and applies to a–e; scale bar in g = 10 μm and applies to f–h; scale bar in i = 25 μm and applies to i and j.
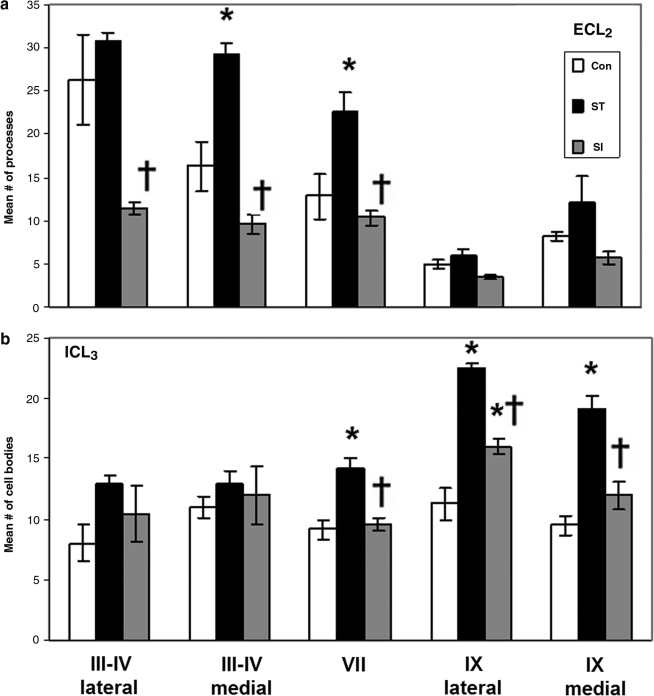
FIG. 3.
The number of 5-HT1AR-positive cell bodies and axon hillocks in spinal cord segments L3–L5 are reduced by the elimination of supraspinal and/or afferent input. Graphs a and b show quantitative changes of ECL2 (a) or ICL3 (b) 5-HT1AR immunoreactivity in the same regions shown in Figure 2 in control (Con; n = 6), spinal cord–transected (ST, supraspinal input eliminated; n = 6), and spinal cord–isolated (SI, supraspinal and afferent inputs eliminated; n = 6) rats. Values are mean ± SEM (*, † indicate significantly different from Con and ST rats, respectively, at p < 0.05).
Spinal cord transection increases the number of 5-HT1AR in the spinal cord, whereas spinal cord isolation has no effect
Laminae III and IV, lateral and medial regions
After 8 weeks of spinal cord transection, but not spinal cord isolation, the number of ECL2-labeled axon hillocks in the medial, but not lateral, area of laminae III–IV was higher than in the control group (Fig. 3a). In contrast, the number of ECL2-labeled axon hillocks in both of these regions in the 8-week spinal cord–isolated group was no different from the control group and lower than that in the spinal cord–transected group. The number of ICL3-labeled cell bodies was not affected by either spinal cord transection or spinal cord isolation.
Lamina VII
There were higher numbers of ECL2-labeled processes (Fig. 3a) and ICL3-labeled cell bodies (Fig. 3b) in lamina VII of the spinal cord–transected than in the control group. Similarly to that observed for laminae III and IV in the dorsal horn, these values in the spinal cord–isolated group were no different from the control group and lower than in the spinal cord–transected group.
Lamina IX, lateral and medial regions
The number of ECL2-labeled processes in both regions of lamina IX was unaffected by either spinal cord transection or spinal cord isolation (Fig. 3a). In contrast, the number of ICL3-labeled cell bodies was higher in both regions of lamina IX in the spinal cord–transected, and in the lateral region in the spinal cord–isolated group than in the control group (Fig. 3b). In addition, these values were lower in both regions of the spinal cord–isolated than the spinal cord–transected group.
The extent of 5-HT1AR distribution along the axon hillock of cells in lamina IX was greater in the spinal cord–transected than the control and spinal cord–isolated groups
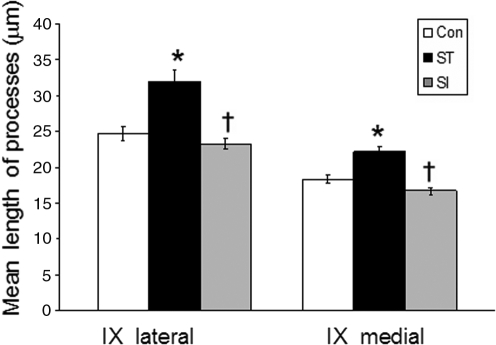
We examined the role of afferent input on the distribution of 5-HT1AR along the axon hillock of lamina IX neurons (Fig. 4). We found that spinal cord transection increased 5-HT1AR distribution along the axon hillock in both the lateral and medial regions of lamina IX, whereas these values were not different in control and spinal cord–isolated rats, and lower in spinal cord–isolated than spinal cord–transected rats.
FIG. 4.
Elimination of supraspinal and afferent inputs affects the distribution of 5-HT1AR proteins in the axon hillock. The mean length of ECL2-positive axon hillocks from confocal projected images in control (Con), spinal cord–transected (ST), and spinal cord–isolated (SI) rats in the lateral and medial regions of lamina IX is shown. The 5-HT1AR protein is distributed over a longer length of axon hillocks in ST compared to Con and SI rats in both regions. Values are mean ± SEM, n = 6 rats/group, using the mean of 50 neurons/rat examined in each region (* and † indicate significantly different from Con and ST, respectively, at p < 0.01).
Discussion
Differential topographical distribution of 5-HT1AR
Under all experimental conditions, we observed differential topographical distributions of the 5-HT1AR in the spinal cord when using antibodies that recognize epitopes on either extracellular (ECL2) or intracellular (ICL3) loops of the receptor protein as described previously (Kheck et al., 1995; Kia et al., 1996). Using the ECL2 antibody, we observed staining predominantly in the axon hillocks, with some diffuse staining in the cell body. This intense axon hillock labeling by the ECL2 antibody was observed in spinal neurons in both the dorsal and ventral horns. A similar distribution with this antibody was reported for cervical motoneurons (Kheck et al., 1995) and pyramidal neurons of the cerebral cortex, hippocampus, and brainstem (Azmitia et al., 1996) in the macaque. Electron microscopy using this ECL2 antibody showed staining within the cell, identifying receptor molecules in association with the smooth endoplasmic reticulum being transported to distal processes by way of small, spherical organelles (Azmitia et al., 1992).
The ICL3 antibody used in this study recognizes an epitope within the third intracellular loop of the rat and mouse 5-HT1AR protein and shows a somatodendritic staining pattern in the spinal cord corresponding to what has been seen in the brain using the same and other antibodies generated to the same general site on the receptor (Kia et al., 1996; Anthony and Azmitia, 1997; Zhou et al., 1999). Using the same ICL3 antibody that we used in this study, Riad et al. (2000) reported 5-HT1AR immunoreactivity associated with neuronal cell bodies and dendrites in the nucleus raphe dorsalis and pyramidal and granule cells of the hippocampus of adult rats. Electron microscopic examination of the staining showed that the immunolabeling of the 5-HT1AR was extrasynaptic and associated predominantly with the membranes rather than the cytoplasm.
Anthony and Azmitia (1997) observed that different binding properties of the SIA-258 antibody (an epitope of the 3rd intracellular loop) for the 5-HT1AR were dependent on the state of glycosylation of the antibody. Based on these observations, the authors suggested that the differential staining patterns by ECL2 and ICL3 antibodies result from different molecular conformations of the 5-HT1AR. The existence of multiple conformations also could contribute to differences in intracellular coupling of the receptors associated with not only different G proteins (Mannoury la Cour et al., 2006), but also with other types of receptors (Andrade et al., 1986). These studies together point out the potential that the functional effects of the 5-HT1AR protein may be shaped by its intracellular localization, glycosylation state, and its interaction with other types of receptors.
5-HT1AR modulation by supraspinal input
The ability of a complete spinalized rat to recover stepping ability is dependent on the age of the rat when the spinal cord transection is performed. Rats transected at a neonatal stage show a remarkable spontaneous recovery in stepping ability (Weber and Stelzner, 1977; Kubasak et al., 2005), whereas rats receiving a spinal cord transection as adults require rehabilitative interventions (e.g., pharmaceutical agents or epidural stimulation) to regain stepping ability (Edgerton et al., 2006; Edgerton et al., 2008). In the present study, rats were spinalized as adults and consequently did not spontaneously recover the use of their hindlimbs during the 8-weeks post-lesion. During this period, however, a number of changes occurred in the distribution of 5-HT1AR identified with the ECL2 and ICL3 antibodies.
In this study, two of the five regions of focus in the lower lumbar spinal cord showed an increase in the number of ECL2-labeled 5-HT1AR, and two other regions showed an increase in the length of the labeled portion of the axon hillock after spinal cord transection compared to controls (Fig. 5a). As each cell has one 5-HT1A-positive process, the results suggest that removal of the supraspinal pathways is accompanied by a greater number of cells expressing the 5-HT1AR protein in medial laminae III and IV, where some of the large diameter primary afferent fibers terminate, and in lamina VII, an area containing ascending and descending spinal pathways. An increase in ECL2-labeled 5-HT1AR protein was found after spinal cord transection in motoneurons in lamina IX. The ICL3 antibody showed an increase in the number of 5-HT1AR-labeled cell bodies in three of the five regions that were quantified after spinal cord transection. This upregulation suggests that in the gray matter of the spinal cord there is an inverse modulation of the quantity of the 5-HT1AR protein and its endogenous ligand 5-HT. Also an increase in receptor distribution in the dorsal horn after a contusion injury has been associated with allodynia and hyperalgesia (Hains et al., 2003). Although no systematic sensory tests were administered in the present study, there were no overt signs of enhanced sensory excitability in their daily routine care.
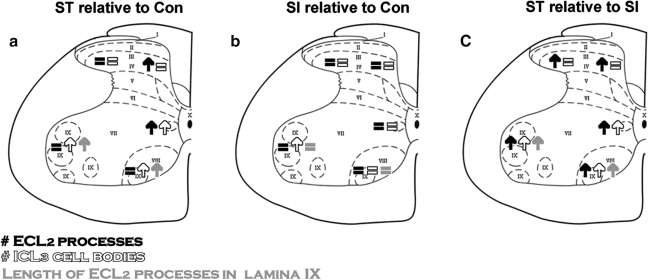
FIG. 5.
Summary of 5-HT1AR distribution in the spinal cord in response to elimination of supraspinal and/or afferent inputs. Significant changes are shown for the number of 5-HT1AR ECL2-positive processes (black), ICL3-positive cell bodies (white) for all five regions, and the length of ECL2-positive processes (gray) for lamina IX. Arrows and equal signs signify significant increases (up arrows), or the absence (=) of significant changes (p < 0.05). Changes of 5-HT1AR immunoreactivity in spinal cord–transected (ST) relative to control (Con) (a), spinal cord–isolated (SI) relative to Con (b), and ST relative to SI (c) are shown schematically on a spinal cord cross-section.
The upregulation of 5-HT1AR that we observed is generally consistent with previous observations. Increases in autoradiographic binding of 3[3 + 1]8-OH-DPAT, reflective of 5-HT1A/7R distribution, were found in laminae II, III, and X in the lumbar segments of spinal cats at 15 and 30 days post-spinal cord transection (Giroux et al., 1999). Similar increases in 5-HT1AR autoradiographic binding were observed in the dorsal horn of rats in which the descending projections were destroyed by 5,7-dihydroxytryptamine injected directly into the cisterna magna (Laporte et al., 1995). 5-HTR levels also have been shown to increase in response to spinal cord transection in rats transected as neonates. For example, 8 weeks after a complete spinal cord transection of rat pups at 2 days of age, the binding of the 5-HT2CR in the lumbar ventral horn (Kim et al., 1999) and the 5-HT2CR immunostaining in both the dorsal and ventral horns (Kao et al., 2006) were increased. These studies, in general, are consistent with the hypothesis that there will be an upregulation of 5-HTR, including the 5-HT1AR, in response to a chronic loss of serotonin in the spinal cord.
5-HT1AR modulation by afferent input
In contrast to the observed increases in 5-HT1AR immunoreactivity in the axon hillocks in the dorsal horn (laminae III and IV) after removal of supraspinal pathways by spinal cord transection, the removal of both the supraspinal serotonergic pathways and of the primary afferent fibers by spinal cord isolation resulted in little or no change in the immunoreactivity in these regions (Fig. 5b and c). This was unexpected based on the decrease in autoradiographic binding of [3H]8-OH-DPAT in the superficial layers of the ipsilateral dorsal horn after unilateral sectioning of the dorsal roots (C4–T2) in the rat (Laporte et al., 1995; Daval et al., 1987). Deafferentation by p-chloroamphetamine in membrane preparations from the rat cerebral cortex and hippocampus also resulted in a loss of the low affinity [3H]8-OH-DPAT binding sites (Nenonene et al., 1994).
The lower number of 5-HT1AR processes in spinal cord–isolated compared to spinal cord–transected rats may have resulted from several factors, including reduced neuromuscular activity and/or reduced neurotrophic support in the absence of primary afferent activity (Chalmers et al., 1992; Kossel et al., 1995; Marty et al., 1997; Friedman et al., 1995, Sauer et al., 1995; Gomez-Pinilla et al., 2004; Marlier et al., 1990, 1991b,c). For example, spinal cord isolation has been shown to decrease BDNF mRNA and protein levels in the isolated region of the spinal cord (Gomez-Pinilla et al., 2004). Although spinal cord isolation almost totally eliminates motoneuron activity based on repeated 24-h EMG analyses made in several hindlimb muscles over a 1-month period after spinal cord isolation (Roy et al., 2007b), the size and succinate dehydrogenase activity of the lumbar motoneurons are unaffected (Roy et al., 2007a), suggesting that there is little metabolic compensation in the absence of supraspinal and afferent activity (Chalmers et al., 1992). Although the upregulation of 5-HT1AR may have been a response to reduced agonist availability, such an effect was completely neutralized by the additional perturbation of eliminating afferent input. The present results, therefore, would suggest that afferent input plays a potentially greater role than agonist availability in determining the expression levels of 5-HT1AR in the rat lumbar spinal cord.
The thoracic lesion used in the present study would eliminate long propriospinal neurons that are known to interconnect lumbosacral and cervical regions of the spinal cord (Dutton et al., 2006; Courtine et al., 2008; Yakovenko et al., 2007). However, we have not distinguished input from the shorter projecting propriospinal neurons within the lumbosacral segments versus other lumbosacral spinal neurons in this study. In regard to the issue of the role of shorter ascending and descending propriospinal neurons (Courtine et al., 2008; Kiehn, 2006; Yakovenko et al., 2007), there is little doubt that the spinal circuitry to which we are referring is inclusive of these neurons. Propriospinal neurons are known to project within and across multiple segments and to contribute to the neural control of locomotion. Based on our concept of separating supraspinal and peripheral influences, we are referring to the spinal circuitry as an entity within the spinal cord that might be affected by the elimination of either one of these inputs. Thus, we are assuming that the propriospinal neurons reflect some unknown part of the spinal circuitry that is being impacted.
Conclusions
The novel findings of the present study are as follows: (1) a marked upregulation of 5-HT1A based on both ECL2 and ICL3 immunolabeling in response to a complete spinal cord transection, and (2) a complete neutralization of this effect when the spinal cord was transected and completely deafferented. These data demonstrate that the upregulation in 5-HT1AR that occurs with spinal cord transection alone is dependent on the presence of sensory input. In addition, these results suggest that agonist availability does not play a dominant role in modulating the levels of 5-HT1AR under these conditions. The physiological implications of the high concentration of 5-HT1AR at the axon hillock, as well as the general upregulation of 5-HT1AR immunostaining after a complete spinal cord lesion are unclear.
Acknowledgments
The authors thank Maynor Herrera for his expert assistance and guidance involving the care and handling of the animals, and Dr. Matthew Schibler of the UCLA Carol Moss Spivak Microscope Facility for critical assistance and instruction in the techniques of confocal microscopy.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Alaimo M. Smith J.L. Roy R.R. Edgerton V.R. EMG activity of slow and fast ankle extensors following spinal cord transection. J. Appl. Physiol. 1984;56:1608–1613. doi: 10.1152/jappl.1984.56.6.1608. [DOI] [PubMed] [Google Scholar]
- Anden N.E. Haeggendal J. Magnusson T. Rosengren E. The time course of the disappearance of noradrenaline and 5-hydroxytryptamine in the spinal cord after transection. Acta Physiol. Scand. 1964;62:115–118. doi: 10.1111/j.1748-1716.1964.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Andrade R. Malenka R.C. Nicoll R.A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Anthony T.E. Azmitia E.C. Molecular characterization of antipeptide antibodies against the 5-HT1A receptor: evidence for state-dependent antibody binding. Brain Res. Mol. Brain Res. 1997;50:277–284. doi: 10.1016/s0169-328x(97)00201-5. [DOI] [PubMed] [Google Scholar]
- Antri M. Barthe J.Y. Mouffle C. Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OH-DPAT and quipazine. Neurosci. Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Azmitia E.C. Gannon P.J. Kheck N.M. Whitaker-Azmitia P.M. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Azmitia E.C. Yu I. Akbari H.M. Kheck N. Whitaker-Azmitia P.M. Marshak D.R. Antipeptide antibodies against the 5-HT1A receptor. J. Chem. Neuroanat. 1992;5:289–298. doi: 10.1016/0891-0618(92)90016-j. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Bowker R.M. Westlund K.N. Coulter J.D. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res. 1981;226:187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Cai L.L. Fong A.J. Otoshi C.K. Liang Y. Burdick J.W. Roy R.R. Edgerton V.R. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 2006;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers G.R. Roy R.R. Edgerton V.R. Adaptability of the oxidative capacity of motoneurons. Brain Res. 1992;570:1–10. doi: 10.1016/0006-8993(92)90556-o. [DOI] [PubMed] [Google Scholar]
- Claustre Y. Benavides J. Scatton B. 5-HT1A receptor agonists inhibit carbachol-induced stimulation of phosphoinositide turnover in the rat hippocampus. Eur. J. Pharmacol. 1988;149:149–153. doi: 10.1016/0014-2999(88)90054-4. [DOI] [PubMed] [Google Scholar]
- Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.M. Ao Y. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping mediated by indirect propriospinal relay connections after severe spinal cord injury. Nature Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daval G. Verge D. Basbaum A.I. Bourgoin S. Hamon M. Autoradiographic evidence of serotonin 1 binding sites on primary afferent fibers in the dorsal horn of the rat spinal cord. Neurosci. Lett. 1987;83:71–76. doi: 10.1016/0304-3940(87)90218-7. [DOI] [PubMed] [Google Scholar]
- Dutton R.C. Carsten M.I. Antognini J.F. Carsten E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res. 2006;1119:76–85. doi: 10.1016/j.brainres.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Edgerton V.R. Courtine G. Gerasimenko Y.P. Lavrov I. Ichiyama R.M. Fong A.J. Cai L.L. Otoshi C.K. Tillakaratne N.J. Burdick J.W. Roy R.R. Training locomotor networks. Brain Res. Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V.R. Kim S.J. Ichiyama R.M. Gerasimenko Y.P. Roy R.R. Rehabilitative therapies after spinal cord injury. J. Neurotrauma. 2006;23:560–570. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S. Riad M. Laporte A.M. Verge D. Daval G. Gozlan H. Hamon M. Production of specific anti-rat 5-HT1A receptor antibodies in rabbits injected with a synthetic peptide. Neurosci. Lett. 1990;118:189–192. doi: 10.1016/0304-3940(90)90623-h. [DOI] [PubMed] [Google Scholar]
- Fong A.J. Cai L.L. Otoshi C.K. Reinkensmeyer D.J. Burdick J.W. Roy R.R. Edgerton V.R. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. Kleinfeld D. Ip N.Y. Verge V.M. Moulton R. Boland P. Zlotchenko E. Lindsay R.M. Liu L. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J. Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya M.N. Gettys T.W. van Biesen T. Prpic V. Chuprun J.K. Raymond J.R. 5-HT1A receptor activates Na+/H+ exchange in CHO-K1 cells through Gialpha2 and Gialpha3. J. Biol. Chem. 1997;272:7770–7776. doi: 10.1074/jbc.272.12.7770. [DOI] [PubMed] [Google Scholar]
- Garnovskaya M.N. Mukhin Y. Raymond J.R. Rapid activation of sodium-proton exchange and extracellular signal-regulated protein kinase in fibroblasts by G-protein-coupled 5-HT1A receptor involves distinct signaling cascades. Biochem. J. 1998;330:489–495. doi: 10.1042/bj3300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya M.N. van Biesen T. Hawe B. Casanas Ramos S. Lefkowitz R.J. Raymond J.R. Ras-dependent activation of fibroblast mitogen-activated protein kinase by 5-HT1A receptor via a G protein beta gamma-subunit-initiated pathway. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- Giroux N. Rossignol S. Reader T.A. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J. Comp. Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Gomez-Pinilla F. Ying Z. Roy R.R. Hodgson J.A. Edgerton V.R. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J. Neurophysiol. 2004;92:3423–3432. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- Grossman E.J. Roy R.R. Talmadge R.J. Zhong H. Edgerton V.R. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve. 1998;21:375–389. doi: 10.1002/(sici)1097-4598(199803)21:3<375::aid-mus12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Grunnet M. Jespersen T. Perrier J.F. 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J. Neurosci. Res. 2004;78:845–854. doi: 10.1002/jnr.20318. [DOI] [PubMed] [Google Scholar]
- Hains B.C. Willis W.D. Hulsebosch C.E. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp. Brain Res. 2003;149:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- Hochman S. Garraway S.M. Machacek D.W. Shay B.L. 5-HT receptors and the neuromodulatory control of spinal cord function. In: T.C. Cope., editor. Motor Neurobiology of the Spinal Cord. CRC Press; Boca Raton, FL: 2001. pp. 47–87. [Google Scholar]
- Kao T. Shumsky J.S. Jacob-Vadakot S. Himes B.T. Murray M. Moxon K.A. Role of the 5-HT2C receptor in improving weight-supported stepping in adult rats spinalized as neonates. Brain Res. 2006;1112:159–168. doi: 10.1016/j.brainres.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Kemp B.E. Pearson R.B. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kheck N.M. Gannon P.J. Azmitia E.C. 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J. Comp. Neurol. 1995;355:211–220. doi: 10.1002/cne.903550205. [DOI] [PubMed] [Google Scholar]
- Kia H.K. Miquel M.C. Brisorgueil M.J. Daval G. Riad M. El Mestikawy S. Hamon M. Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kim D. Adipudi V. Shibayama M. Giszter S. Tessler A. Murray M. Simansky K.J. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J. Neurosci. 1999;19:6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossel A. Lowel S. Bolz J. Relationships between dendritic fields and functional architecture in striate cortex of normal and visually deprived cats. J. Neurosci. 1995;15:3913–3926. doi: 10.1523/JNEUROSCI.15-05-03913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasak M.D. Hedlund E. Roy R.R. Carpenter E.M. Edgerton V.R. Phelps P.E. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp. Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kubasak M.D. Jindrich D.L. Zhong H. Takeoka A. McFarland K.C. Minoz-Quiles C. Roy R.R. Edgerton V.R. Ramon-Cueto A. Phelps P.E. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte A.M. Fattaccini C.M. Lombard M.C. Chauveau J. Hamon M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J. Neural. Transm. Gen. Sect. 1995;100:207–223. doi: 10.1007/BF01276459. [DOI] [PubMed] [Google Scholar]
- Makman M.H. Dvorkin B. Crain S.M. Modulation of adenylate cyclase activity of mouse spinal cord-ganglion explants by opioids, serotonin and pertussis toxin. Brain Res. 1988;445:303–313. doi: 10.1016/0006-8993(88)91193-6. [DOI] [PubMed] [Google Scholar]
- Malmberg A. Strange P.G. Site-directed mutations in the third intracellular loop of the serotonin 5-HT(1A) receptor alter G protein coupling from G(i) to G(s) in a ligand-dependent manner. J. Neurochem. 2000;75:1283–1293. doi: 10.1046/j.1471-4159.2000.751283.x. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C. El Mestikawy S. Hanoun N. Hamon M. Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol. Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- Marlier L. Poulat P. Rajaofetra N. Privat A. Modification of serotonergic immunoreactive pattern in the dorsal horn of the rat spinal cord following dorsal root rhizotomy. Neurosci. Lett. 1991b;128:9–12. doi: 10.1016/0304-3940(91)90748-i. [DOI] [PubMed] [Google Scholar]
- Marlier L. Poulat P. Rajaofetra N. Privat A. Modifications of serotonin-, substance P- and calcitonin gene-related peptide-like immunoreactivities in the dorsal horn of the spinal cord of arthritic rats: a quantitative immunocytochemical study. Exp. Brain Res. 1991c;85:482–490. doi: 10.1007/BF00231731. [DOI] [PubMed] [Google Scholar]
- Marlier L. Rajaofetra N. Poulat P. Privat A. Modification of serotonergic innervation of the rat spinal cord dorsal horn after neonatal capsaicin treatment. J. Neurosci. Res. 1990;25:112–118. doi: 10.1002/jnr.490250114. [DOI] [PubMed] [Google Scholar]
- Marlier L. Teilhac J.R. Cerruti C. Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991a;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Marty S. Berzaghi Mda P. Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- Nenonene E.K. Radja F. Carli M. Grondin L. Reader T.A. Heterogeneity of cortical and hippocampal 5-HT1A receptors: a reappraisal of homogenate binding with 8-[3H]hydroxydipropylaminotetralin. J. Neurochem. 1994;62:1822–1834. doi: 10.1046/j.1471-4159.1994.62051822.x. [DOI] [PubMed] [Google Scholar]
- Newton B.W. Hamill R.W. The morphology and distribution of rat serotonergic intraspinal neurons: an immunohistochemical study. Brain Res. Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Okada F. Tokumitsu Y. Nomura Y. Pertussis toxin attenuates 5-hydroxytryptamine1A receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in rat hippocampal membranes. J. Neurochem. 1989;52:1566–1569. doi: 10.1111/j.1471-4159.1989.tb09209.x. [DOI] [PubMed] [Google Scholar]
- Oleskevich S. Leck K.J. Matthaei K. Hendry I.A. Enhanced serotonin response in the hippocampus of Galphaz protein knock-out mice. Neuroreport. 2005;16:921–925. doi: 10.1097/00001756-200506210-00009. [DOI] [PubMed] [Google Scholar]
- Otoshi C.K. Fong A.J. Cai L.L. Zhong H. Roy R.R. Tillakaratne N.J.K. Edgerton V.R. Society for Neuroscience 34th Annual Meeting; Oct. 23–27. San Diego, CA: 2004. Changes in 5-HT1A receptor distribution following spinal cord transection are dependent upon spinal 5-HT content and peripheral sensory input in adult rats. 418.11. [Google Scholar]
- Perrier J.F. Alaburda A. Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J. Physiol. 2003;548:485–492. doi: 10.1113/jphysiol.2002.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.R. Fargin A. Middleton J.P. Graff J.M. Haupt D.M. Caron M.G. Lefkowitz R.J. Dennis V.W. The human 5-HT1A receptor expressed in HeLa cells stimulates sodium-dependent phosphate uptake via protein kinase C. J. Biol. Chem. 1989;264:21943–21950. [PubMed] [Google Scholar]
- Riad M. Garcia S. Watkins K.C. Jodoin N. Doucet E. Langlois X. el Mestikawy S. Hamon M. Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Roy R.R. Hodgson J.A. Lauretz S.D. Pierotti D.J. Gayek R.J. Edgerton V.R. Chronic spinal cord-injured cats: surgical procedures and management. Lab. Anim. Sci. 1992;42:335–343. [PubMed] [Google Scholar]
- Roy R.R. Kim J.A. Grossman E.J. Bekmezian A. Talmadge R.J. Zhong H. Edgerton V.R. Persistence of myosin heavy chain-based fiber types in innervated but silenced rat fast muscle. Muscle Nerve. 2000;23:735–747. doi: 10.1002/(sici)1097-4598(200005)23:5<735::aid-mus11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Roy R.R. Matsumoto A. Zhong H. Ishihara A. Edgerton V.R. Rat α- and γ-motoneuron soma size and succinate dehydrogenase activity are independent of neuromuscular activity level. Muscle Nerve. 2007a;36:234–241. doi: 10.1002/mus.20810. [DOI] [PubMed] [Google Scholar]
- Roy R.R. Zhong H. Khalili N. Kim S.J. Higuchi N. Monti R.J. Grossman E. Hodgson J.A. Edgerton V.R. Is spinal cord isolation a good model of muscle disuse? Muscle Nerve. 2007b;35:312–321. doi: 10.1002/mus.20706. [DOI] [PubMed] [Google Scholar]
- Sauer H. Wong V. Bjorklund A. Brain-derived neurotrophic factor and neurotrophin-4/5 modify neurotransmitter-related gene expression in the 6-hydroxydopamine-lesioned rat striatum. Neuroscience. 1995;65:927–933. doi: 10.1016/0306-4522(95)00019-f. [DOI] [PubMed] [Google Scholar]
- Schmidt B.J. Jordan L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Talmadge R.J. Garcia N.D. Roy R.R. Edgerton V.R. Myosin heavy chain isoform mRNA and protein levels after long-term paralysis. Biochem. Biophys. Res. Commun. 2004;325:296–301. doi: 10.1016/j.bbrc.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Talmadge R.J. Roy R.R. Caiozzo V.J. Edgerton V.R. Mechanical properties of rat soleus after long-term spinal cord transection. J. Appl. Physiol. 2002;93:1487–1497. doi: 10.1152/japplphysiol.00053.2002. [DOI] [PubMed] [Google Scholar]
- Thor K.B. Nickolaus S. Helke C.J. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- Turner J.H. Gelasco A.K. Raymond J.R. Calmodulin interacts with the third intracellular loop of the serotonin 5-hydroxytryptamine1A receptor at two distinct sites: putative role in receptor phosphorylation by protein kinase C. J. Biol. Chem. 2004;279:17027–17037. doi: 10.1074/jbc.M313919200. [DOI] [PubMed] [Google Scholar]
- Weber E.D. Stelzner D.J. Behavioral effects of spinal cord transection in the developing rat. Brain Res. 1977;125:241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Wu X. Kushwaha N. Albert P.R. Penington N.J. A critical protein kinase C phosphorylation site on the 5-HT(1A) receptor controlling coupling to N-type calcium channels. J. Physiol. 2002;538:41–51. doi: 10.1113/jphysiol.2001.012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S. Kowalczewski J. Prochazka A. Intraspinal stimulation caudal to spinal cord transections in rats. Testing the propriospinal hypothesis. J. Neurophysiol. 2007;97:2570–2574. doi: 10.1152/jn.00814.2006. [DOI] [PubMed] [Google Scholar]
- Zgombick J.M. Beck S.G. Mahle C.D. Craddock-Royal B. Maayani S. Pertussis toxin-sensitive guanine nucleotide-binding protein(S) couple adenosine A1 and 5-hydroxytryptamine1A receptors to the same effector systems in rat hippocampus: biochemical and electrophysiological studies. Mol. Pharmacol. 1989;35:484–494. [PubMed] [Google Scholar]
- Zhang Y. Gray T.S. D'Souza D.N. Carrasco G.A. Darnjanoska K.J. Dudas B. Garcia F. Zainelli G.M. Sullivan Hanley N.R. Battaglia G. Muma N.A. Van de Kar L.D. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exp. Ther. 2004;310:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]
- Zhou F.C. Patel T.D. Swartz D. Xu Y. Kelley M.R. Production and characterization of an anti-serotonin 1A receptor antibody which detects functional 5-HT1A binding sites. Brain Res. Mol. Brain Res. 1999;69:186–201. doi: 10.1016/s0169-328x(99)00101-1. [DOI] [PubMed] [Google Scholar]