Abstract
Spinal cord injury studies use the presence of serotonin (5-HT)-immunoreactive axons caudal to the injury site as evidence of axonal regeneration. As olfactory ensheathing glia (OEG) transplantation improves hindlimb locomotion in adult rats with complete spinal cord transection, we hypothesized that more 5-HT-positive axons would be found in the caudal stump of OEG- than media-injected rats. Previously we found 5-HT-immunolabeled axons that spanned the transection site only in OEG-injected rats but detected labeled axons just caudal to the lesion in both media- and OEG-injected rats. Now we report that many 5-HT-labeled axons are present throughout the caudal stump of both media- and OEG-injected rats. We found occasional 5-HT-positive interneurons that are one likely source of 5-HT-labeled axons. These results imply that the presence of 5-HT-labeled fibers in the caudal stump is not a reliable indicator of regeneration. We then asked if 5-HT-positive axons appose cholinergic neurons associated with motor functions: central canal cluster and partition cells (active during fictive locomotion) and somatic motor neurons (SMNs). We found more 5-HT-positive varicosities in lamina X adjacent to central canal cluster cells in lumbar and sacral segments of OEG- than media-injected rats. SMNs and partition cells are less frequently apposed. As nonsynaptic release of 5-HT is common in the spinal cord, an increase in 5-HT-positive varicosities along motor-associated cholinergic neurons may contribute to the locomotor improvement observed in OEG-injected spinal rats. Furthermore, serotonin located within the caudal stump may activate lumbosacral locomotor networks. J. Comp. Neurol. 515: 664–676, 2009.
Indexing terms: plasticity, locomotion, OEG, spinal cord injury, 5-HT, ChAT, cholinergic neurons
Adult cats and neonatal rats with completely transected spinal cords (i.e., spinal animals) can be trained to generate fairly coordinated patterns of hindlimb stepping on the treadmill in the absence of supraspinal connections (Lovely et al., 1986; Barbeau et al., 1987; de Leon et al., 1998; Joynes et al., 1999; Jordan and Schmidt, 2002; Edgerton et al., 2004; Kubasak et al., 2005). In contrast, adult spinal rats do not demonstrate coordinated locomotor stepping without therapeutic interventions. Electrical and/or pharmacological interventions, including serotonin (5-HT) receptor agonists that likely activate central pattern generation in the lumbosacral cord, can facilitate bipedal stepping in adult spinal rats (Ichiyama et al., 2005; Lavrov et al., 2006; Gerasimenko et al., 2007). In addition to evidence from in vivo studies, in vitro neonatal models showed that 5-HT is involved in the alternating firing of the contralateral neural networks to elicit bilateral locomotor-like activity (Cazalets et al., 1992; Nishimaru et al., 2000). While the major source of 5-HT in the spinal cord is derived from the brainstem (Steinbusch 1981; Aitken and Tork, 1988; Anderson et al., 1989), the locomotor networks present within the lumbosacral spinal cord retain their ability to respond to 5-HT even in the absence of supraspinal connections (Barbeau and Rossignol, 1990, 1991; Fong et al., 2005; Gerasimenko et al., 2007). For example, embryonic raphe tissue transplanted into the transection site of an adult spinal rat facilitates locomotor recovery (Ribotta et al., 2000). Furthermore, the administration of quipazine, a serotonergic receptor agonist, facilitates the activation of the locomotor networks in spinal mice and results in both short- and long-term improvement of hindlimb stepping on a treadmill (Fong et al., 2005).
Recently we reported that olfactory ensheathing glia (OEG) transplantation combined with long-term treadmill step training improved bipedal locomotor performance of adult spinal rats (Kubasak et al., 2008), but the mechanisms that induced this recovery are not known. One possibility is that the serotonergic axons that crossed the transection site only in OEG-transplanted rats contributed to the functional recovery, an explanation that implicates the contribution of raphespinal pathway regeneration. However, while previous studies interpret the presence of 5-HT-immunoreactive axons in the caudal stump after a complete transection as a marker for raphespinal regeneration (Cheng and Olson, 1995; Chen et al., 1996; Ramon-Cueto et al., 1998, 2000; Lu et al., 2001, 2002; Fouad et al., 2005; Lopez-Vales et al., 2006, 2007), we detected 5-HT-labeled axons immediately caudal to the transection site in both media- and OEG-injected spinal rats (Kubasak et al., 2008).
Another possible mechanism by which OEG could promote locomotor recovery is through the reorganization of 5-HT-positive axons within the caudal stump. Based on established anatomical (Wu et al., 1993) and functional (Liu and Jordan, 2005) connections between serotonergic axons and somatic motor neurons (SMNs), we asked if the serotonergic axons caudal to a complete spinal cord transection apposed motor-associated cholinergic neurons, i.e., SMNs, central canal cluster cells, and partition cells (Barber et al., 1984; Phelps et al., 1984). Huang et al. (2000) reported that both central canal cluster and partition cells in the lumbar spinal cord upregulate the immediate early gene c-fos in response to stimulation of the mesencephalic locomotor region and suggested that these cholinergic interneurons contribute to central pattern generation. We previously reported that partition cells project their axons into the ipsilateral and contralateral SMN pools (Phelps et al., 1984, 1990) and that cholinergic boutons outline large SMNs (Houser et al., 1983; Barber et al., 1984; Phelps et al., 1984). In addition, Miles et al. (2007) demonstrated that both medial partition cells and central canal cluster cells contact SMNs and can directly modulate their excitability. Furthermore, they reported that 5-HT increased cholinergic interneuron-driven SMN output in vitro. 5-HT is frequently released from varicosities at nonsynaptic sites and diffuses several microns or more within the extrasynaptic space (Ridet et al., 1993; Zhou et al., 1998; Bunin and Wightman, 1999; Zoli et al., 1999; Hentall et al., 2006) and binds to the locomotor-related 5-HT receptor subtypes, 5-HT2A and 5-HT7 expressed by lumbar SMNs (Doly et al., 2004, 2005). Therefore, 5-HT appositions, in either direct or indirect association with SMNs through other cholinergic neurons, could contribute to the improved locomotor ability reported in OEG-injected spinal rats.
The first goal of this study was to compare the density and source of 5-HT axons within the caudal stump of media- and OEG-injected spinal rats. The second goal was to examine the relationships between such serotonergic axons and the motor-associated cholinergic neurons. If more of these axons appose SMNs, medial partition cells and central canal cluster cells of OEG- than media-injected rats, such differences could contribute to the improved locomotor recovery of OEG-transplanted adult rats (Kubasak et al., 2008).
Material and Methods
Animal studies
All procedures followed the National Institutes of Health (NIH) guidelines and were approved by the Chancellor's Animal Research Committee at UCLA. The methods used in the present study were recently reported (Kubasak et al., 2008). Briefly, OEG derived from olfactory bulbs of 8–10-week-old Wistar Hannover rats (Harlan Laboratories, Indianapolis, IN) were immunopurified from the primary culture on day 7–8 using the p75 nerve growth factor receptor antibody (NGFR, 1:5; Chandler et al., 1984) and harvested after 17–19 days in vitro. Female Wistar Hannover rats, 10–12 weeks old, were anesthetized deeply with 2% isoflurane and received a complete spinal cord transection at ≈T9. Media with or without 400,000 OEG were injected ≈1 mm from the transection site into both the rostral and caudal spinal cord stumps with sterile glass needles. During the 7 months after the transection 50% of the rats in both the media- and OEG-injected groups underwent long-term treadmill hindlimb step training. In the current study we analyzed the spinal cord segments below the transection block (lower thoracic to sacral levels) in the cohort of 12 rats analyzed in detail by Kubasak et al. (2008). Three rats in each of the four experimental groups (media-untrained, media-trained, OEG-untrained, and OEG-trained) represented a range of stepping abilities at 8 months post transection (see table 1 in Kubasak et al., 2008).
Tissue preparation
Rats were anesthetized deeply with ketamine (0.9 μL/g) and Anased (0.5 μL/g), perfused with 4% paraformaldehyde, and then postfixed overnight. After a buffer wash the spinal cords were dissected, cryoprotected, frozen on dry ice, and then stored at −80°C. We sectioned the spinal cords in the sagittal plane at 25 μm and mounted the sections on a series of 16 slides, so that each slide contained every 16th section. Every other slide was double-labeled for 5-HT and choline acetyltransferase (ChAT).
Antibody characterization
For serotonin immunolabeling, we used a goat antiserum (1:20,000, Immunostar, Hudson, WI, Cat. No. 20079; Kubasak et al., 2008) generated against 5-HT that was conjugated to bovine serum albumin (BSA) with paraformaldehyde. Staining is completely eliminated by pretreatment of the diluted antibody with 100 μg of serotonin/BSA conjugate (manufacturer's datasheet) and the pattern of serotonin labeling was not altered by BSA preabsorption (unpubl. data). For ChAT immunolabeling, either monoclonal (3F12, 1.5–2.5 μg/μL; Houser et al., 1983) or polyclonal anti-ChAT antiserum (AB144P, 1:500, Chemicon, Temecula, CA, Cat. No. 28329; Phelps et al., 2002) raised against purified rat brain and human placental ChAT, respectively, was used. Crawford et al. (1982) characterized binding properties of the 3F12 antibody to ChAT using HPLC analyses. The molecular weight of the immune complex formed was consistent, with each antibody binding two ChAT molecules of ≈68 kD. The polyclonal ChAT antiserum recognized a single band of 69 kD molecular weight on Western blot of homogenized rat brain (manufacturer's datasheet). The pattern of cellular distribution of both ChAT antibodies was identical to previous reports (Barber et al., 1984; Phelps et al., 1984).
Immunohistochemical procedures
We performed double-labeling experiments to identify the interactions between 5-HT-labeled axons and cholinergic neurons. The 5-HT protocol reported in Kubasak et al. (2008) was followed, except that we added 0.3% Triton to 0.1M Tris buffered saline (TBS) containing 1.4% NaCl and 0.1% BSA throughout the procedure. Sections were rinsed in 0.1M acetate buffer, developed with 0.06% 3,3-diaminobenzidine (DAB) intensified with nickel glucose oxidase, producing a black product. To identify the cholinergic neurons we used a TBS rinse followed by serum and avidin-biotin blocking steps before incubating with an anti-ChAT antibody overnight at room temperature. Sections were incubated with Vector standard or rabbit antigoat IgG (1:200, Vectastain Elite standard or Goat IgG kit, Vector Laboratories, Burlingame, CA) and ABC (1:100) for 1 hour each for signal amplification. We then used 0.02M imidazole-DAB to identify distinct amber brown cholinergic somata and to differentiate them from the black serotonin-positive axons. All data were recorded with a Zeiss AxioCam digital camera and Openlab 5.1 software. Openlab files were imported to Adobe Photoshop 7.0 (San Jose, CA) to assemble figures and minor modifications in color balance, brightness, and contrast were made if necessary.
Quantification
In our previous study (Kubasak et al., 2008) we histologically confirmed a complete transection of all spinal cords analyzed in the present study and, therefore, the 5-HT axons found in the caudal stump did not arise from spared raphespinal axons. We identified and counted 5-HT immunopositive axons longer than 25 μm in all sections processed for 5-HT (alternate sections, 50% of the lower thoracic to sacral spinal cord), a method similar that used by Fouad et al. (2005) and Kubasak et al. (2008). We initially traced and counted the longest single fiber. Other fibers in the area with no obvious continuity with the longest fiber then were counted. Finally, we measured the volume of all sections processed for 5-HT immunoreactivity using Openlab (Improvision, Lexington, MA) software and calculated the 5-HT fiber density. To quantify the number of 5-HT-positive varicosities along the cholinergic neurons, we counted the 5-HT-positive axons in the area immediately adjacent (estimate of 1–2 μm) to cholinergic somata or dendrites at the light microscope level and normalized these counts per unit volume (mm3). The criteria used to identify 5-HT-labeled appositions to cholinergic neurons were an obvious varicose structure along the axons and no visible space between the varicosity and the neuron when viewed at high magnification with the light microscope. Our results do not distinguish between serotonergic synaptic contacts and nonsynaptic associations that utilize volume transmission (Ridet et al., 1993; Bunin and Wightman, 1999; Zoli et al., 1999). Serotonergic varicosities adjacent to cholinergic neurons were quantified with a technique similar to that used by Miller and Salvatierra (1998) and Mullner et al. (2008), whose studies are also based on the assumption that 5-HT utilizes nonsynaptic transmission. Since Kubasak et al. (2008) did not find a training effect on the number of plantar steps during treadmill locomotion for either OEG- or media-injected rats, our analyses primarily focused on differences between the OEG and media transplantations.
Statistical analyses
We used the bootstrapping method (Efron and Tibshirani 1991) to determine the mean differences for all statistical analyses. This resampling method uses the computational simulation of the null hypothesis with minimal assumptions about the data distribution, sample size, or means, and therefore is suitable for small samples. Customized scripts were written using the Resampling stats in MatLab software package (Resampling Stats, Arlington, VA; MatLab 7.0, Math-Works, Natick, MA). Statistical significance was set at P < 0.05.
Results
Serotonergic axons are present throughout the caudal stump after a complete spinal cord transection in adult rats
The descending raphespinal tract originates mainly from the raphe pallidus (B1), obscurus (B2), and magnus (B3) and in the intact spinal cord gives rise to an extensive network of 5-HT-positive axons concentrated in laminae VII, VIII, and X (Fig. 1a) (Steinbusch, 1981; Aitken and Tork, 1988; Anderson et al., 1989). After a complete spinal cord transection, 5-HT-positive axons derived from the descending raphespinal pathways degenerate within several weeks (Cheng and Olson, 1995). Surprisingly, we initially found that both media- and OEG-injected rats contained 5-HT-positive axons directly below the transection site (Kubasak et al., 2008). After examining the entire caudal stump of these same spinal rats, we now report that all spinal rats contained some 5-HT-immunoreactive axons between the lower thoracic and sacral segments (Fig. 1b,c), regardless of their transplantation status. We found many of these 5-HT-positive fibers in lamina X (Fig. 1d). Other 5-HT-immunoreactive axons associated with the sympathetic preganglionic neurons in the intermediate gray matter (see below) or coursed within the dorsal horn (data not shown). A majority of the 5-HT axons detected caudal to the transection site coursed within the gray matter, although a few axons traveled at the border between the gray and white matter (Fig. 1f) or just within the white matter (Fig. 1g). There were no significant differences in the density of 5-HT-labeled axons (>25 μm in length) in the lower thoracic (T12), middle lumbar (L3), or upper sacral (S1) segments between OEG- and media-injected rats (Fig. 1h; thoracic: P = 0.45, lumbar: P = 0.44, sacral: P = 0.18). These results are consistent with, and expand upon, the observations made in the same cohort of spinal rats just below the transection site (Kubasak et al., 2008).
Figure 1.
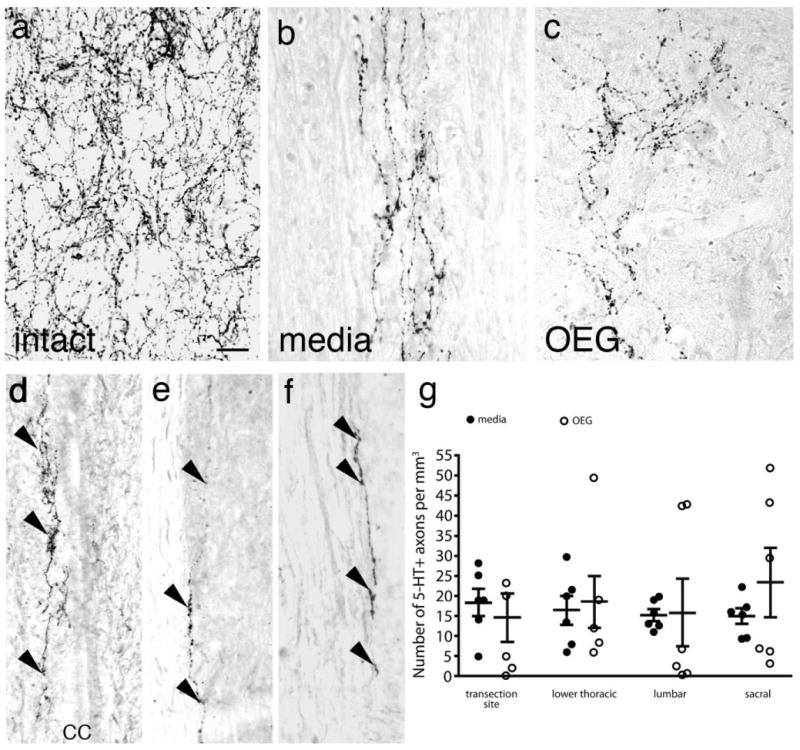
Serotonergic (5-HT) axons in intact, media- and OEG-injected complete spinal cord transected rats caudal to the injury site. a–c: Sagittal sections processed for 5-HT immunoreactivity show a high density of immunopositive axons in the lower thoracic cord of an intact rat (a) and occasional 5-HT immunopositive axons in media- (b) and OEG-injected (c) spinal rats. d: 5-HT-positive axons (arrowheads) frequently course along the central canal (cc) of spinal rats. e,f: 5-HT-positive axons (arrowheads) are found at the border between the gray and white matter (e) or within the white matter (f). g: The density of 5-HT-labeled fibers greater than 25 μm in length is not significantly different between media-injected and OEG-injected rats at the transection site (data from Kubasak et al., 2008), lower thoracic, lumbar, or sacral levels. Bars in (g), mean ± SEM for six media- and six OEG-injected rats. Scale bar = 50 μm in a (applies to b–f).
As 5-HT is implicated as an important factor for locomotor recovery after a complete spinal cord transection (Barbeau and Rossignol, 1990; Feraboli-Lohnherr et al., 1999; Ribotta et al., 2000; Fong et al., 2005), we asked if differences in the density of 5-HT axons in the caudal stump reflect the improvement in hindlimb stepping that we observed in the OEG-trained group in our previous study (Kubasak et al., 2008). We compared the density of 5-HT axons in the OEG-trained group to each of the other experimental groups and found no significant differences at the lower thoracic, lumbar, or sacral levels (thoracic: P = 0.26, lumbar: P = 0.28, sacral: P = 0.33). We then asked if step training altered the density of 5-HT axons present in the caudal stump. Neither training status (OEG- and media-trained vs. OEG- and media-untrained; P = 0.064) nor transplantation status (OEG-trained vs. OEG-untrained or media-trained vs. media-untrained; P = 0.46 and P = 0.064) significantly influenced the 5-HT density in the caudal stump. Thus, the overall density of 5-HT-positive axons in the caudal stump did not correlate with the level of functional recovery.
5-HT-positive interneurons are present in the caudal stump of adult spinal rats
Newton et al. (1986, 1988) first reported the presence of serotonergic interneurons in completely transected adult rats, but there are no further reports of serotonergic interneurons in the adult rat spinal cord until our study (Kubasak et al., 2008). Based on our frequent observation of 5-HT-positive axons in the caudal stump of media- and OEG-injected rats, we searched for and found a limited number of serotonergic interneurons (Fig. 2a–d). These 5-HT-positive cell bodies are round or oval in shape, 10–25 μm in diameter, and have bipolar or multipolar dendritic processes, as reported previously (Newton et al., 1986; Newton and Hamill, 1988). We found a high density of 5-HT-positive axonal processes in the area surrounding the serotonergic cell bodies, consistent with an interpretation that these processes originate from interneurons. We processed 50% of the entire caudal spinal stump (every other 25 μm sagittal section) for 5-HT immunoreactivity and detected 0–3 serotonergic somata per rat within lamina X. These interneurons were immediately dorsal or dorsolateral to the central canal, and primarily within the lower thoracic and upper lumbar segments, with only a few cells found at the sacral levels. These interneurons are one likely source of the 5-HT-positive axons detected in the spinal cord caudal to the transection. In addition, we found several 5-HT-positive interneurons above the transection site, i.e., in the intact thoracic spinal cord (Fig. 2e). Combined, these observations indicate that the expression of 5-HT interneurons persists in adult rats with or without supraspinal serotonergic innervation.
Figure 2.
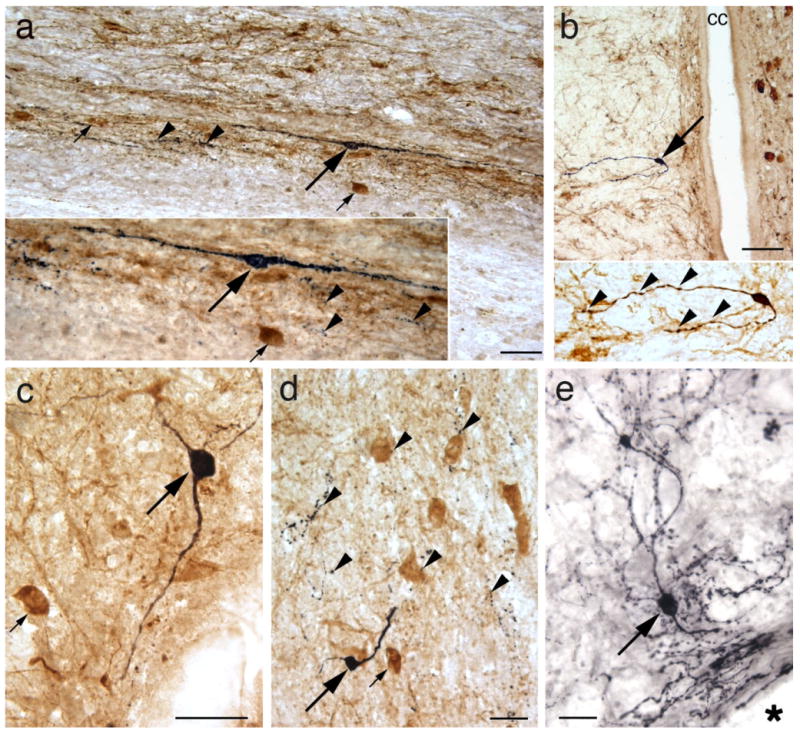
Serotonergic (5-HT) interneurons are found in thoracic, lumbar, and sacral sagittal sections of media- (a,c,d) and OEG-injected (b,e) adult spinal rats. 5-HT immunoreactivity is illustrated in black and choline acetyltransferase (ChAT) in amber-brown. a: A 5-HT-positive interneuron (large arrow) projects its processes rostrocaudally. Enlargement (inset) illustrates 5-HT-positive varicosities (arrowheads) detected near the 5-HT interneuron (large arrow) and a ChAT-positive central canal cluster cell in lateral lamina X (small arrow). b: A 5-HT-positive neuron (arrow) with two processes is detected just ventral to the central canal (cc). At higher magnification (inset) both neuronal processes display varicosities (arrowheads). c,d: 5-HT-labeled interneurons detected just lateral to the central canal are round and have two processes. Small arrows (c,d) designate ChAT-labeled central canal cluster cells and arrowheads (d) mark 5-HT-positive axon terminal-like structures. e: A 5-HT-labeled neuron (arrow) located just rostral to the transection site (asterisk) is surrounded by 5-HT positive axons, some of which likely arise from the raphespinal tract. Scale bars = 50 μm for a,b,d,e; 25 μm for c.
5-HT axons appose motor-associated cholinergic neurons in the caudal stump of adult spinal rats
In intact rats, 5-HT-positive axons often are associated with populations of motor-associated cholinergic spinal cord neurons, i.e., central canal cluster cells, SMNs, and partition cells. Since both media- and OEG-injected rats also have 5-HT-positive axons that reside near these three populations of cholinergic neurons, we asked if these appositions were more common in OEG- than in media-injected rats. Serotonergic fibers are highly concentrated in the dorsal half of lamina X and are located near ChAT-positive cholinergic somata (Fig. 3a–c). In the lower thoracic and upper lumber segments both central canal cluster cells and sympathetic preganglionic central autonomic (CA) neurons reside in dorsal lamina X (Barber et al., 1991). Those located ventral and lateral to the central canal are easily identified as central canal cluster cells, while the two populations are difficult to distinguish in the dorsal central gray matter, except possibly by their size. The smaller cholinergic neurons (<20 μm by 20 μm) are central canal cluster cells while the larger neurons are likely CA neurons (Barber et al., 1991). We quantified the number of 5-HT-positive varicosities immediately adjacent to the cholinergic neurons in lamina X in lower thoracic, middle to lower lumbar, and sacral segments of the media- and OEG-injected groups. In thoracic segments, where the two cholinergic populations intermingle in lamina X, the number of 5-HT appositions was not statistically different between media- and OEG-injected rats. In middle to lower lumbar and sacral segments, where no CA neurons are found, there were significantly more 5-HT-positive axons apposed to central canal cluster cells in OEG-than media-injected rats (Fig. 3d, thoracic: P = 0.08, middle to lower lumbar: P = 0.01, sacral: P = 0.04). Together, spinal rats averaged 3.9 appositions/mm3 to central cholinergic neurons in thoracic and 3.2 appositions/mm3 to the central canal cluster cells in middle to lower lumbar and sacral segments. To examine the relationship between long-term treadmill step training and the organization of serotonergic appositions along central cholinergic neurons, we compared the number of appositions with respect to training status, i.e., OEG- and media-trained vs. OEG- and media-untrained. We found no training effect on the number of 5-HT appositions to the central canal cluster cells at any of the spinal cord segments analyzed or when results from the three levels analyzed are combined (thoracic: P = 0.18, lumbar: P = 0.28, sacral: P = 0.49, combined: P = 0.42). Because we observed a training effect in OEG-transplanted rats (Kubasak et al., 2008), we then asked if there were any differences between the OEG-injected trained and untrained groups. There were no significant differences at any of the spinal cord levels examined, or when we combined data from all caudal levels (thoracic: P = 0.31, lumbar: P = 0.21, sacral: P = 0.41, combined: P = 0.49).
Figure 3.
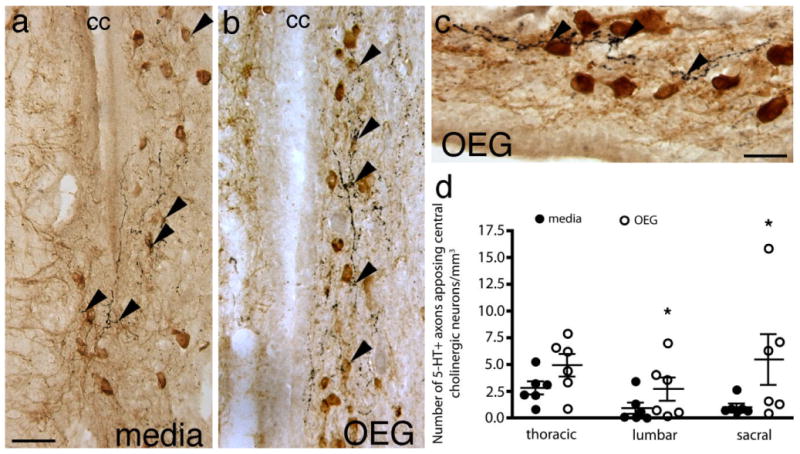
Serotonergic (5-HT) axons appose cholinergic neurons in lamina X of sagittal sections caudal to the complete transection site in spinal rats. a,b: Often 5-HT-positive fibers (arrowheads) course near centrally located (cc, central canal) cholinergic neurons in both media- (a) and OEG-injected (b) rats. c: 5-HT-labeled varicosities (arrowheads) course near central cholinergic neurons in an OEG-injected rat. d: OEG-injected rats contain significantly more appositions of 5-HT-positive fibers on central canal cluster cells than media-injected rats at the middle to lower lumbar (P = 0.01) and sacral (P = 0.04) levels, but not at the lower thoracic level (P = 0.08). Bars in (d), mean ± SEM for six media- and six OEG-injected rats. *Significant difference between media- and OEG-injected rats. Scale bars = 50 μm in a (applies to b); 25 μm in c.
In intact spinal cord 5-HT-positive axons directly appose SMNs (Fig. 4a). Compared to the central cholinergic neurons, fewer 5-HT-positive axons are found coursing near SMNs in adult spinal rats (mean of 1.2 appositions/mm3; Fig. 4b,c). More SMNs received 5-HT appositions in OEG- than media-injected rats at the lower thoracic segment, but not at the other segmental levels analyzed (Fig. 4g, thoracic: P = 0.018, lumbar: P = 0.21, sacral: P = 0.21). When the total number of 5-HT appositions near SMNs is combined for all three segments, however, significantly more appositions are detected in OEG- than media-injected rats (Fig. 4g, combined: P = 0.007). In contrast, step training did not influence the number of 5-HT appositions along SMNs at any spinal cord segment analyzed or when data from all three spinal segments are combined (thoracic: P = 0.26; lumbar: P = 0.33; sacral: P = 0.32; combined: P = 0.38). SMNs in the spinal cord of intact rats are outlined by extensive cholinergic synaptic terminals or C-boutons (Houser et al., 1983; Nagy et al., 1993), presumably derived from the cholinergic interneuron populations. These C-boutons are still prominent after a complete spinal cord transection in both the media- and OEG-injected spinal cord (Fig. 4e,f) and in a similar distribution to those detected in the intact spinal cord (Fig. 4d).
Figure 4.
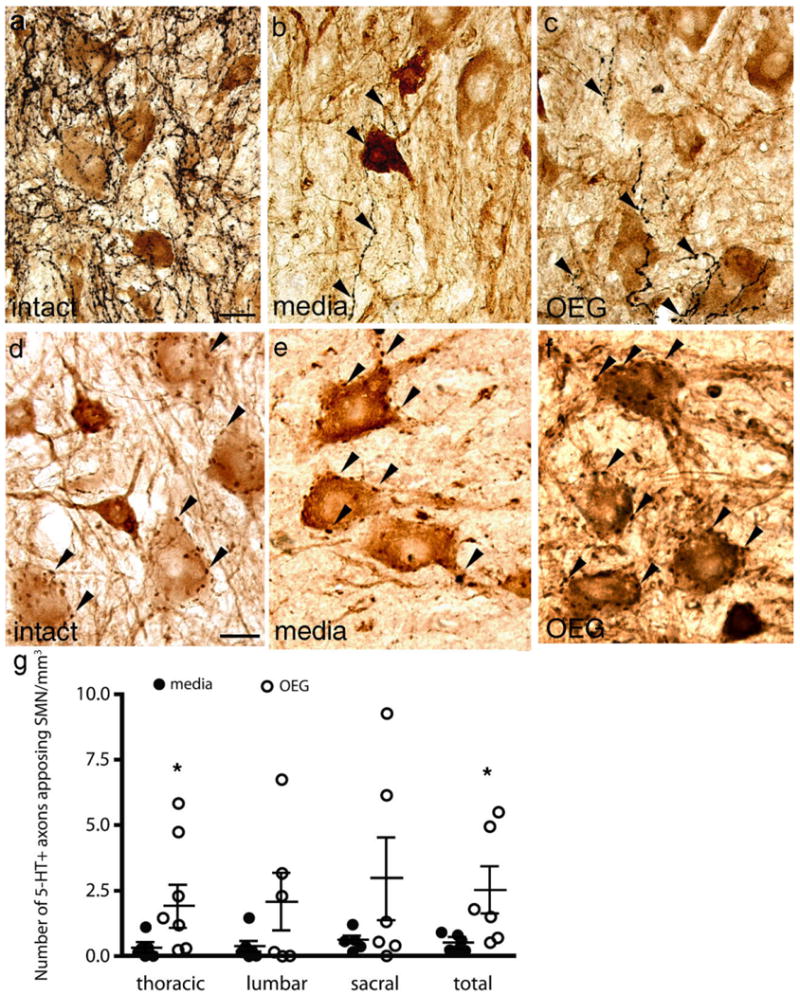
Serotonergic (5-HT) axons and cholinergic boutons appose somatic motor neurons (SMNs) in sagittal sections of intact and spinal rats caudal to the complete transection site. a–c: Varicose 5-HT-immunolabeled axons normally appose SMNs in the ventral horn at lower thoracic levels in intact rats a: Only a few 5-HT-positive axons (arrowheads) appose SMNs in media- (b) and OEG-injected (c) spinal rats. d–f: Cholinergic boutons (arrowheads) appose large SMNs in intact (d) as well as media- (e) and OEG-injected (f) spinal rats. g: The total number (across lower thoracic, lumbar, and sacral levels) of 5-HT-labeled fibers near or apposed to SMN in the caudal stump of transected rats is significantly higher in OEG- than media-injected rats (P = 0.007). At individual segmental levels this difference is significant only for the lower thoracic spinal cord (P = 0.018). Bars in (g), mean ± SEM for six media- and six OEG-injected rats. *Significant difference between media- and OEG-injected rats. Scale bars = 50 μm in a (applies to b,c); 25 μm in d (applies to e,f).
To clearly distinguish partition cells from the cholinergic sympathetic and parasympathetic preganglionic neurons located in thoracic and sacral segments, respectively, we chose to analyze only the lumbar enlargement where partition cells do not intermingle with the preganglionic populations. In the intact spinal cord, 5-HT-positive axons directly appose partition cells (Fig. 5a). We also found occasional 5-HT-labeled axons that coursed near the somata and large dendrites of partition cells in both media- and OEG-injected rats (Fig. 5b,c). However, the density of appositions between 5-HT-positive fibers and partition cells was low (0.2 appositions/mm3; Fig. 5d) and did not differ between media- and OEG-injected rats (P = 0.08). Step training did not affect the number of 5-HT axons apposing the partition cells (P = 0.27).
Figure 5.
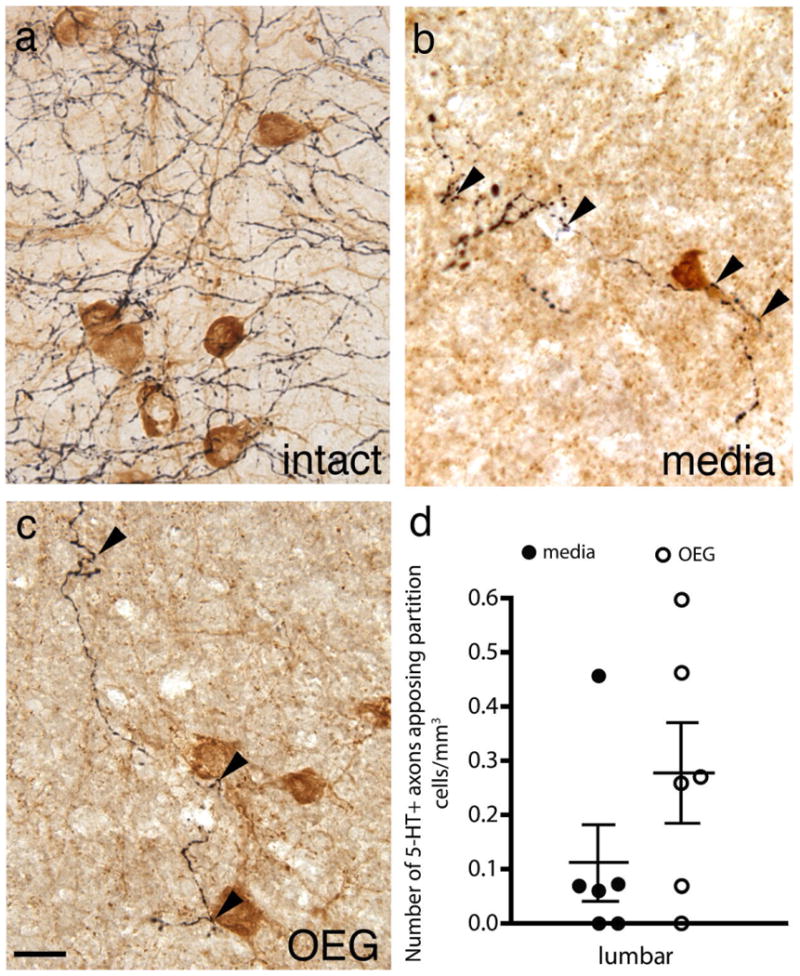
Appositions between serotonergic (5-HT) axons (black) and cholinergic partition cells (amber-brown) in the lumbosacral region of intact and spinal rats. a: Many 5-HT-labeled axons course near partition cells in the lower lumbar cord of intact rats. b,c: 5-HT-positive axons (arrowheads) course near partition cells in the caudal stumps of media- (b) and OEG-injected (c) spinal rats. d: The density of 5-HT-labeled axons that appose partition cells did not differ between the media- and OEG-injected groups. Bars in (d), mean ± SEM for six media- and six OEG-injected rats. Scale bar = 50 μm in c (applies to a,b).
5-HT axons frequently appose sympathetic preganglionic neurons (SPNs) in adult spinal rats
In addition to CA neurons, distinct populations of cholinergic SPNs are found in the intermediolateral region of lamina VII in the lower thoracic segments. In the intact spinal cord, 5-HT-positive axons densely innervate these cholinergic SPNs (Fig. 6a; Bacon et al., 1990). Although 5-HT appositions along SPNs were less dense in the spinal cord of media- and OEG-injected rats than in intact rats, we observed frequent 5-HT appositions (8.1 appositions/mm3; Fig. 6b,c). 5-HT-positive axons often coursed within and apposed a cluster of SPN somata. Neither transplantation nor step training status affected the number of 5-HT appositions along these SPNs (media vs. OEG: P = 0.38, trained vs. untrained: P = 0.31). We detected a small number of 5-HT appositions to cholinergic parasympathetic preganglionic neurons in sacral segments (data not shown).
Figure 6.
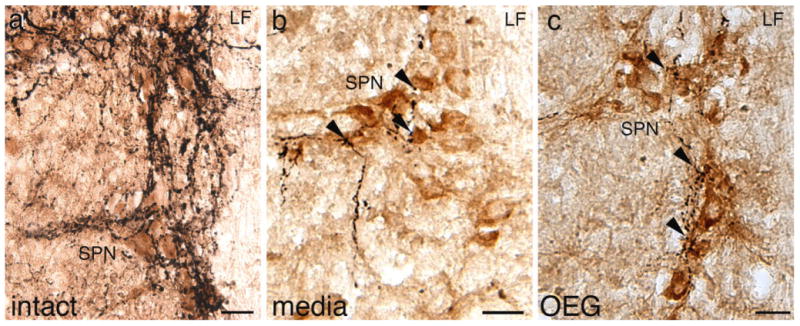
Serotonergic (5-HT) axons course near sympathetic preganglionic neurons (SPNs) in horizontal sections of the thoracic spinal cord in intact and spinal rats. a: 5-HT-labeled axons, presumably from the raphespinal pathways, densely innervate SPNs in intact rats. b,c: 5-HT-positive axons (arrowheads) course near clusters of ChAT-positive somata and dendritic processes in the caudal stump of media-injected (b) and OEG-injected (c) spinal rats. LF, lateral funiculus. Scale bars = 50 μm.
Discussion
The objectives of this study were to determine if the 5-HT-labeled axons observed in the caudal stump of spinal rats: 1) are derived from a source other than regeneration of the raphespinal pathway; 2) vary in density depending on the OEG or training status of the spinal rats; and 3) could interact with motor-associated cholinergic neurons. One source of the caudal 5-HT-positive processes is the serotonergic interneurons, originally described by Newton et al. (1986) in adult spinal rats. The presence of 5-HT-labeled somata, dendrites, and axons found in the caudal stump of all spinal rats analyzed in this study is consistent with the biochemical evidence reporting low amounts of 5-HT present in the caudal stump after a complete transection (Hadjiconstantinou et al., 1984). Based on our data, combined with previous studies (Newton et al., 1986; Newton and Hamill, 1988), we conclude that the presence of 5-HT-positive axons in the caudal stump is not a reliable indicator of raphespinal regeneration. Despite the significantly higher level of locomotor recovery in the OEG-injected rats, the overall density of 5-HT-positive axons present in the entire caudal stump does not vary between media- and OEG-injected spinal rats. However, we found more 5-HT-positive varicosities adjacent to motor-associated central canal cluster cells and SMNs in OEG- than media-injected spinal rats, interactions that may contribute to the improvement in treadmill stepping reported previously in adult OEG-injected spinal rats (Kubasak et al., 2008).
Serotonergic interneurons in the adult rat spinal cord
The presence, frequency, and location of serotonergic intraspinal neurons are dependent on age, species, and possibly the strain within a species. Adult lampreys, stingrays, long-nose garfish, newts, chicks, monkeys, and young mice commonly have 5-HT-positive spinal cord interneurons (Lamotte et al., 1982; Ritchie et al., 1983; Van Dongen et al., 1985; Sako et al., 1986; Branchereau et al., 2000). Interestingly, Branchereau et al. (2002) reported that supraspinal 5-HT innervation inhibits the expression of 5-HT interneurons in the developing mouse spinal cord. Conversely, the expression of 5-HT interneurons is upregulated when the spinal cord is isolated in vitro, potentially to compensate for the lack of supraspinal 5-HT innervation (Branchereau et al., 2002). Innervation by the raphespinal pathways does not preclude the presence of 5-HT interneurons since we found several immunopositive somata rostral to the transection site in spinal cord tissue still innervated by the raphespinal axons (e.g., Fig. 2e; fig. 5B in Kubasak et al., 2008).
Both strain and technical variations may contribute to the differences in the number and location of 5-HT interneurons between previous studies and the current investigation, just as there are substrain differences in descending pathways from different vendors (Clark and Proudfit, 1992). In both intact and spinal adult Sprague–Dawley rats, Newton et al. (1986) detected 3–9 serotonergic interneurons per rat between the upper thoracic and coccygeal spinal cord levels. In addition, they found 5-HT-labeled neurons primarily in lamina VII associated with SPNs, in dorsolateral lamina X, and less frequently in Clarke's column (Newton et al., 1986; Newton and Hamill, 1988). In contrast, we detected 5-HT-positive somata exclusively in lamina X in Wistar Hannover rats. It is possible that the variability in location is due to the different rat strains and/or to the injection of monoamine oxidase inhibitors used by Newton et al. (1986, 1988) to enhance the detection of 5-HT-immunoreactivity. We detected these rare 5-HT interneurons using a highly sensitive and permanent DAB method (Shu et al., 1988) rather than the standard DAB or immunofluorescence techniques. Several other studies reported the presence of 5-HT-positive axons caudal to a complete spinal cord transection in control and treated adult rats of the Fisher and Sprague–Dawley strains (Fouad et al., 2005; Steward et al., 2006), but no evidence of serotonergic spinal interneurons. While some of the 5-HT-positive fibers may be derived from serotonergic interneurons, there may be additional yet unidentified sources. Our current results suggest at least two interpretations of the 5-HT-immunolabeled axons in the caudal stump of spinal rats. First, previous studies reported that OEGs promote regeneration of 5-HT-labeled fibers across the injury site into the caudal stump (Ramón-Cueto et al., 2000; Kubasak et al., 2008). Second, we now show that 5-HT interneurons in the spinal cord contribute to immunopositive axons in the caudal stump regardless of transplantation status. In order to unambiguously distinguish between a raphespinal or intrinsic origin of the 5-HT-labeled axons in the caudal stump of spinal rats, tracing experiments will be required.
5-HT in the caudal stump of adult spinal rats may contribute to locomotion
In addition to its role at the neuromuscular junction, acetylcholine also interacts with the locomotor central pattern generators. Studies reported that the bath application of acetylcholine induces alternating rhythmic bursting in Xenopus embryos and in rodent in vitro models, and that muscarinic antagonists reverse this effect (Smith and Feldman, 1987; Panchin et al., 1991; Cowley and Schmidt, 1994). Furthermore, stimulation of the mesencephalic locomotor region activates cholinergic central canal cluster and partition cells and induces fictive locomotion (Carr et al., 1995; Huang et al., 2000).
5-HT is strongly implicated in locomotion as serotonergic grafts (Ribotta et al., 2000) and pharmacological interventions (Barbeau and Rossignol, 1991; Fong et al., 2005; Gerasimenko et al., 2007) improve the locomotor ability of adult spinal animals. Nonsynaptic release, i.e., volume transmission, of 5-HT readily diffuses between a few microns and a millimeter in the extracellular space and can act on multiple targets until its removal by extrasynaptic transporters (Bunin and Wightman, 1998, 1999; Tao-Cheng and Zhou, 1999). Although Lopez-Vales et al. (2006) reported serotonergic axons apposing SMNs in OEG- but not media-injected adult spinal rats, we detected such appositions regardless of the transplantation status. We did detect, however, significantly more 5-HT-positive varicosities associated with central canal cluster cells and SMNs in the caudal stump of OEG- than media-injected rats and, importantly, these two groups of cholinergic neurons do express 5-HT2A receptors after a complete spinal cord transection (unpubl. data). Furthermore, when Musienko et al. (2008) administered the 5-HT2A receptor antagonist ketanserin to spinal rats during epidural stimulation-induced hindlimb locomotion, their stepping ability was severely impaired, a finding that reveals the fundamental contribution of intrinsic 5-HT in the caudal stump of spinal rats. Therefore, appositions of 5-HT-labeled varicosities along central canal cluster cells and SMNs could contribute to the generation of locomotor activity by either synaptic or nonsynaptic release of 5-HT. However, further morphological studies with electron microscopy as well as pharmacological studies are necessary to demonstrate that the relationships between 5-HT varicosities and cholinergic neurons result in functional synaptic or volume transmission. Possibly, serotonergic axons also interact with other populations of locomotor pattern generating neurons that reside in lamina VII or further ventrally (MacLean et al., 1995; Kjaerulff and Kiehn, 1996). In addition, noradrenergic axons, which also utilize volume transmission, are in close proximity to motor-associated cholinergic neurons in the caudal stump of OEG-injected adult spinal rats (Takeoka et al., 2007). Therefore, at least noradrenaline and possibly other neurotransmitters as well may contribute to the locomotor recovery observed in OEG-injected spinal rats.
Another interpretation of our results is that OEG facilitate plasticity within the caudal spinal cord that may lead to the functional recovery in hindlimb treadmill stepping. For example, OEG transplantation could promote intraspinal reorganization by acting on interneurons near the transection site that ultimately influence components of the lumbosacral central pattern generators. While most in vivo studies transplanting OEG focus on how these glia promote axonal regeneration (Cheng and Olson, 1995; Chen et al., 1996; Ramon-Cueto et al., 1998, 2000; Lu et al., 2001, 2002; Fouad et al., 2005; Lopez-Vales et al., 2006, 2007; Kubasak et al., 2008), they also may promote the reorganization of 5-HT-positive axons that then interact with the existing spinal locomotor networks in the caudal stump.
A number of studies report that task-specific training and exercise promote spinal cord reorganization (de Leon et al., 1999; Tillakaratne et al., 2000, 2002; Edgerton et al., 2004). Although we observed a training effect in bipedal hindlimb stepping ability in adult spinal rats that received OEG transplantation (Kubasak et al., 2008), we did not detect a relationship between the training effect and the density of serotonergic axons in the caudal stump. Possibly, treadmill step training for 20 min/day, 5 days/week for 6 months is not sufficient to induce activity-dependent axonal sprouting of the limited numbers of 5-HT-positive axons present. Alternatively, the effect of task-specific training may be mediated by other mechanisms such as: 1) reorganization and regeneration of other neurotransmitter pathways; 2) changes in SMN excitability involving a decrease in inhibitory input (de Leon et al., 1999; Tillakaratne et al., 2002) or an increase in C-bouton innervation (Feng-Chen and Wolpaw, 1996); 3) a change in the synaptic strength of the central pattern generation circuits; and/or 4) altered 5-HT receptor expression levels that contribute to differential locomotor recovery (Antri et al., 2003; Otoshi et al., 2009).
Additional roles of 5-HT axons in the caudal stump of adult spinal rats
Intact raphespinal pathways usually provide a major innervation to the SPNs (Holets and Elde, 1982; Appel et al., 1986; Hosoya et al., 1991). While the evidence shows that activity of SMNs can be coupled with the sympathetic motor outflow in vitro (Chizh et al., 1998), the activity of SPNs is not directly associated with locomotion. Yet SPNs are active in adult spinal rats despite their loss of supraspinal innervation, suggesting that these neurons have an intrinsic rhythmic generation (Taylor and Schramm, 1987; Osborn et al., 1989). Normally the raphespinal pathway provides excitatory stimulation to the sympathetic and parasympathetic preganglionic neurons (Bacon et al., 1990; Lewis and Coote, 1990; Lewis et al., 1993; Pickering et al., 1994; Ranson et al., 2006). Additionally, in an isolated in vitro spinal cord preparation, 5-HT application induces rhythmic sympathetic discharges (Marina et al., 2006). Thus, the serotonergic axons found near SPNs in our study may contribute to the intrinsic sympathetic rhythmic activity reported after a complete spinal cord transection in the presence or absence of axonal regeneration. The intraspinal 5-HT-positive axons may facilitate autonomic recovery of bladder function, for example, as 5-HT is implicated in alleviating autonomic dysreflexia after a spinal cord injury (Marsh et al., 2006).
Conclusions
The present results, in combination with those from Newton et al. (1986, 1988), demonstrate that the presence of 5-HT-positive axons in the caudal stump of rats with a complete spinal cord transection is not a reliable indicator of raphespinal axon regeneration. Importantly, the residual 5-HT in the spinal cord after a complete spinal cord transection, whether from intrinsic or supraspinal sources, could contribute to the improvements detected in hindlimb stepping ability following OEG transplantation. The excitability of the intrinsic locomotor networks that reside in the lumbosacral spinal cord may be mediated by the direct excitation of SMNs by the serotonergic axons, or indirectly through the excitation of cholinergic or other interneurons that then terminate on SMNs. The regeneration and reorganization of other neurotransmitter systems within the caudal stump also could contribute to the functional recovery.
Acknowledgments
We thank Kacie MacFarland, Jenny Kaplan, Justin Hopkins, and Lauren Mamer for assistance with many aspects of the study.
Grant sponsor: Christopher Reeve Paralysis Foundation; Grant numbers: PA-1-0102-2, PAC1-0102-2; Grant sponsor: National Institute of Neurological Disorders and Stroke (NINDS); Grant numbers: R21NS42000-01, RO1NS54159.
Literature Cited
- Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988;274:32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- Anderson CR, McLachlan EM, Srb-Christie O. Distribution of sympathetic preganglionic neurons and monoaminergic nerve terminals in the spinal cord of the rat. J Comp Neurol. 1989;283:269–284. doi: 10.1002/cne.902830208. [DOI] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Appel NM, Wessendorf MW, Elde RP. Coexistence of serotonin- and substance P-like immunoreactivity in nerve fibers apposing identified sympathoadrenal preganglionic neurons in rat intermediolateral cell column. Neurosci Lett. 1986;65:241–246. doi: 10.1016/0304-3940(86)90268-5. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Zagon A, Smith AD. Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res. 1990;79:589–602. doi: 10.1007/BF00229327. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Julien C, Rossignol S. The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res. 1987;437:83–96. doi: 10.1016/0006-8993(87)91529-0. [DOI] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Vaughn JE. Generation patterns of immunocytochemically identified cholinergic neurons at autonomic levels of the rat spinal cord. J Comp Neurol. 1991;311:509–519. doi: 10.1002/cne.903110406. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Rodriguez JJ, Delvolve I, Abrous DN, Le Moal M, Cabelguen JM. Serotonergic systems in the spinal cord of the amphibian urodele Pleurodeles waltl. J Comp Neurol. 2000;419:49–60. doi: 10.1002/(sici)1096-9861(20000327)419:1<49::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Carr PA, Huang A, Noga BR, Jordan LM. Cytochemical characteristics of cat spinal neurons activated during fictive locomotion. Brain Res Bull. 1995;37:213–218. doi: 10.1016/0361-9230(94)00271-2. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Chen A, Xu XM, Kleitman N, Bunge MB. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Cheng H, Olson L. A new surgical technique that allows proximodistal regeneration of 5-HT fibers after complete transection of the rat spinal cord. Exp Neurol. 1995;136:149–161. doi: 10.1006/exnr.1995.1092. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Paton JFR. Coupling of sympathetic and somatic motor outflows from the spinal cord in a perfused preparation of adult mouse in vitro. J Physiol. 1998;508:907–918. doi: 10.1111/j.1469-7793.1998.907bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Anatomical evidence for genetic differences in the innervation of the rat spinal cord by noradrenergic locus coeruleus neurons. Brain Res. 1992;591:44–53. doi: 10.1016/0006-8993(92)90976-g. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Crawford GD, Correa L, Salvaterra PM. Interaction of monoclonal antibodies with mammalian choline acetyltransferase. Proc Natl Acad Sci U S A. 1982;79:7031–7035. doi: 10.1073/pnas.79.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, Verge D, Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol. 2004;472:496–511. doi: 10.1002/cne.20082. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Verge D, Conrath M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol. 2005;490:256–269. doi: 10.1002/cne.20667. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Statistical data analysis in the computer age. Science. 1991;253:390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- Feng-Chen KC, Wolpaw JR. Operant conditioning of H-reflex changes synaptic terminals on primate motoneurons. Proc Natl Acad Sci U S A. 1996;93:9206–9211. doi: 10.1073/pnas.93.17.9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res. 1999;55:87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–2536. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Panula P, Lackovic Z, Neff NH. Spinal cord serotonin: a biochemical and immunohistochemical study following transection. Brain Res. 1984;322:245–254. doi: 10.1016/0006-8993(84)90114-8. [DOI] [PubMed] [Google Scholar]
- Hentall ID, Pinzon A, Noga BR. Spatial and temporal patterns of serotonin release in the rat's lumbar spinal cord following electrical stimulation of the nucleus raphe magnus. Neuroscience. 2006;142:893–903. doi: 10.1016/j.neuroscience.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holets V, Elde R. The differential distribution and relationship of serotoninergic and peptidergic fibers to sympathoadrenal neurons in the intermediolateral cell column of the rat: a combined retrograde axonal transport and immunofluorescence study. Neuroscience. 1982;7:1155–1174. doi: 10.1016/0306-4522(82)91123-x. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Okado N, Sugiura Y, Kohno K. Coincidence of “ladderlike patterns” in distributions of monoaminergic terminals and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res. 1991;86:224–228. doi: 10.1007/BF00231058. [DOI] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266:97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Joynes RL, de Leon RD, Tillakaratne NJ, Roy RR, Tobin AJ, Edgerton VR. Recovery of locomotion in rats after neonatal spinal transection is not attributable to growth across the lesion. Soc Neurosci Abstr 467.18 1999 [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasak MD, Hedlund E, Roy RR, Carpenter EM, Edgerton VR, Phelps PE. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte CC, Johns DR, de Lanerolle NC. Immunohistochemical evidence of indolamine neurons in monkey spinal cord. J Comp Neurol. 1982;206:359–370. doi: 10.1002/cne.902060404. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurones in the neonate rat spinal cord in vitro. Brain Res. 1993;610:267–275. doi: 10.1016/0006-8993(93)91410-t. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Olfactory ensheathing glia graft in combination with FK506 administration promote repair after spinal cord injury. Neurobiol Dis. 2006;24:443–454. doi: 10.1016/j.nbd.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303–311. doi: 10.1002/glia.20457. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 2001;889:344–357. doi: 10.1016/s0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Hochman S, Magnuson DS. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neurosci Lett. 1995;197:9–12. doi: 10.1016/0304-3940(95)11882-w. [DOI] [PubMed] [Google Scholar]
- Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol. 2006;571:441–450. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DR, Marsh AD, Walker GA, Mukhida K. Lack of serotonergic axons in the intermediolateral cell column is associated with autonomic dysreflexia after spinal cord injury. Soc Neurosci Abstr 474.5. 2006 doi: 10.1089/neu.2010.1441. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Salvatierra AT. Apposition of enkephalin- and neurotensin-immunoreactive neurons by serotonin-immunoreactive varicosities in the rat spinal cord. Neuroscience. 1998;85:837–846. doi: 10.1016/s0306-4522(97)00522-8. [DOI] [PubMed] [Google Scholar]
- Mullner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008;27:326–333. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Musienko P, Gerasimenko Y, Brand RVD, Roy RR, Zhong H, Edgerton VR, Courtine G. Monoaminergic modulation of locomotion facilitated by epidural stimulation (ES) in spinal rats. Soc Neurosci Abstr 573.2 2008 [Google Scholar]
- Nagy JI, Yamamoto T, Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Newton BW, Maley BE, Hamill RW. Immunohistochemical demonstration of serotonin neurons in autonomic regions of the rat spinal cord. Brain Res. 1986;376:155–163. doi: 10.1016/0006-8993(86)90910-8. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Taylor RF, Schramm LP. Determinants of arterial pressure after chronic spinal transection in rats. Am J Physiol. 1989;256:R666–673. doi: 10.1152/ajpregu.1989.256.3.R666. [DOI] [PubMed] [Google Scholar]
- Otoshi CK, Walwyn WM, Tillakaratne NJ, Zhong H, Roy RR, Edgerton VR. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. J Neurotrauma. 2009;26:575–584. doi: 10.1089/neu.2008.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchin YY, Perrins RJ, Roberts A. The action of acetylcholine on the locomotor central pattern generator for swimming in Xenopus embryos. J Exp Biol. 1991;161:527–531. doi: 10.1242/jeb.161.1.527. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Barber RP, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:347–361. doi: 10.1002/cne.902290306. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Barber RP, Brennan LA, Maines VM, Salvaterra PM, Vaughn JE. Embryonic development of four different subsets of cholinergic neurons in rat cervical spinal cord. J Comp Neurol. 1990;291:9–26. doi: 10.1002/cne.902910103. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480:109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Santer RM, Watson AH. The relationship between serotonin, dopamine beta hydroxylase and GABA immunoreactive inputs and spinal preganglionic neurones projecting to the major pelvic ganglion of Wistar rats. Neuroscience. 2006;141:1935–1949. doi: 10.1016/j.neuroscience.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Ritchie TC, Livingston CA, Hughes MG, McAdoo DJ, Leonard RB. The distribution of serotonin in the CNS of an elasmobranch fish: immunocytochemical and biochemical studies in the Atlantic stingray, Dasyatis sabina. J Comp Neurol. 1983;221:429–443. doi: 10.1002/cne.902210406. [DOI] [PubMed] [Google Scholar]
- Sako H, Kojima T, Okado N. Immunohistochemical study on the development of serotoninergic neurons in the chick: II. Distribution of cell bodies and fibers in the spinal cord. J Comp Neurol. 1986;253:79–91. doi: 10.1002/cne.902530107. [DOI] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Selvan G, Hadden A, Hofstadter M, Au E, Roskams J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp Neurol. 2006;198:483–499. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Kubasak MD, McFarland KC, Kaplan J, Zhong H, Roy RR, Phelps PE. Noradrenergic axons in the caudal stump of completely transected adult rat spinal cords: regeneration and peripheral innervation. Soc Neurosci Abstr 801.5 2007 [Google Scholar]
- Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94:821–630. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- Taylor RF, Schramm LP. Differential effects of spinal transection on sympathetic nerve activities in rats. Am J Physiol. 1987;253:R611–618. doi: 10.1152/ajpregu.1987.253.4.R611. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen PA, Hokfelt T, Grillner S, Verhofstad AA, Steinbusch HW, Cuello AC, Terenius L. Immunohistochemical demonstration of some putative neurotransmitters in the lamprey spinal cord and spinal ganglia: 5-hydroxytryptamine-, tachykinin-, and neuropeptide-Y-immunoreactive neurons and fibers. J Comp Neurol. 1985;234:501–522. doi: 10.1002/cne.902340408. [DOI] [PubMed] [Google Scholar]
- Wu W, Elde R, Wessendorf MW. Organization of the serotonergic innervation of spinal neurons in rats. III. Differential serotonergic innervation of somatic and parasympathetic preganglionic motoneurons as determined by patterns of co-existing peptides. Neuroscience. 1993;55:223–233. doi: 10.1016/0306-4522(93)90468-u. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]


