Abstract
Objective:
Idiopathic REM sleep behavior disorder (RBD) is a potential preclinical marker for the development of neurodegenerative diseases, particularly Parkinson disease (PD) and Lewy body dementia. However, the long-term risk of developing neurodegeneration in patients with idiopathic RBD has not been established. Obtaining an accurate picture of this risk is essential for counseling patients and for development of potential neuroprotective therapies.
Methods:
We conducted a follow-up study of all patients seen at the sleep disorders laboratory at the Hôpital du Sacré Coeur with a diagnosis of idiopathic RBD. Diagnoses of parkinsonism and dementia were defined according to standard criteria. Survival curves were constructed to estimate the 5-, 10-, and 12-year risk of developing neurodegenerative disease.
Results:
Of 113 patients, 93 (82%) met inclusion criteria. The mean age of participants was 65.4 years and 75 patients (80.4%) were men. Over the follow-up period, 26/93 patients developed a neurodegenerative disorder. A total of 14 patients developed PD, 7 developed Lewy body dementia, 4 developed dementia that met clinical criteria for AD, and 1 developed multiple system atrophy. The estimated 5-year risk of neurodegenerative disease was 17.7%, the 10-year risk was 40.6%, and the 12-year risk was 52.4%.
Conclusions:
Although we have found a slightly lower risk than other reports, the risk of developing neurodegenerative disease in idiopathic REM sleep behavior disorder is substantial, with the majority of patients developing Parkinson disease and Lewy body dementia.
GLOSSARY
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- LBD
= Lewy body dementia;
- MMSE
= Mini-Mental State Examination;
- MSA
= multiple system atrophy;
- PD
= Parkinson disease;
- PSG
= polysomnogram;
- RBD
= REM sleep behavior disorder;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
REM sleep behavior disorder (RBD) is characterized by a loss of the normal muscle atonia that accompanies REM sleep.1,2 Affected patients have excessive motor activity such as punching, kicking, or crying out in association with dream content. RBD is commonly associated with neurodegenerative disorders characterized by α-synuclein deposition, including PD, multiple system atrophy (MSA), and Lewy body dementia (LBD).2–5 Recent studies have suggested that it may be related to α-synuclein-mediated degeneration of sleep-regulating nuclei in the brainstem, especially in the pontine tegmentum.6,7
In a substantial proportion of cases, RBD can occur before the development of dementia or parkinsonism.5,8,9 This has major implications in the understanding of the pathophysiology of PD and LBD, and suggests that an opportunity exists for neuroprotective measures in the preclinical stage of disease. However, the risk of developing neurodegenerative disease in patients with idiopathic RBD has not been fully defined. To assess this risk, we conducted a clinical follow-up study of 93 patients seen at the Hôpital du Sacré-Coeur sleep disorders center, using life-table analysis to define disease risk over 5, 10, and 12 years.
METHODS
Subjects.
All procedures were carried out at the sleep disorders laboratory at the Hôpital du Sacré Coeur, Montreal, Quebec, Canada, and ethics approval was obtained from the REB of the hospital. All patients with idiopathic RBD diagnosed between 1989 and 2006 were potential candidates for this study—these patients were identified from a computerized database that tracks all diagnoses of patients seen at the sleep disorders center since 1989. Patients were generally referred from general practitioners or neurologists to evaluate abnormal sleep behaviors, although some patients were referred from other sleep specialists and neurologists in the province of Quebec specifically for inclusion into ongoing prospective research protocols (which have been continuing in our clinic since 1999). To mitigate important selection bias created by selective participation in research protocols, the inclusion criteria were set as broad as possible. Therefore the minimal criteria for inclusion were as follows:
Polysomnogram (PSG)-confirmed RBD, as defined by standard criteria as REM sleep without atonia,10 and at least one of a) history of harmful or potentially harmful motor manifestations or b) complex motor behaviors during REM sleep on PSG-synchronized videotape recording.11 Note that patients were withdrawn from any medication known to affect sleep or motor behavior for at least 2 weeks prior to PSG. RBD symptom onset was defined by patient self-report.
Absence of signs of neurodegenerative disease confirmed on a baseline neurologic examination.
At least one follow-up examination ≥1 year after the baseline examination. If distance or inability to travel prevented an in-person examination, telephone follow-up could be offered.
Given concerns that telephone or clinic-based follow-up could inaccurately assess the presence of disease, a subcohort of patients was defined according to stricter inclusion criteria, namely that all patients must have had a systematic research-based in-person follow-up examination, as described below.
Follow-up protocol.
In-person research-based examination protocols have been conducted since 1999 in our clinic. Although specific aspects of the examination varied, each in-person examination protocol included, at minimum, a complete medical history and neurologic examination, assessment of the Unified Parkinson's Disease Rating Scale Part III (UPDRS), and cognitive testing (at minimum, the Folstein Mini-Mental State Examination [MMSE]). The clinical examination was conducted by a movement disorders specialist (R.P. or M.L.F.). Neuropsychological evaluation12 and extensive evaluation of nonmotor symptoms13 was offered to all patients, but was not required for inclusion.
To prevent bias due to selective attendance in clinic, a systematic telephone interview was conducted by a neurologist (R.P.) for the subset of patients who were unable to attend an in-person evaluation. This interview consisted of a clinical history of current RBD status and symptoms, medications used, family history, past medical history review, and review of diagnoses of parkinsonism or dementia. Undiagnosed parkinsonism was screened for using the Tanner questionnaire (a nine-item interview with a sensitivity and specificity of over 90%14). All components of the UPDRS Parts I and II were assessed. As a screen for dementia, the Telephone Interview for Cognitive Status was administered. This is a mental status test adapted for the telephone, with reported sensitivity of 83–100%.15–17 A score of ≤26/39 was considered suggestive of cognitive impairment.
Diagnosis of disease.
At the conclusion of the study, all data on each patient were collected in a centralized database. Parkinsonism was diagnosed according to UK brain bank criteria as bradykinesia in association with rest tremor, rigidity, or postural instability. The most likely cause for the parkinsonism was delineated based on standard criteria.18 Dementia was diagnosed as the presence of cognitive impairment (MMSE <24) in association with impairment of activities of daily living. Probable LBD was defined according to McKeith criteria19 as cognitive decline, the presence of RBD (present in all patients), in association with at least one of parkinsonism, visual hallucinations, or fluctuations. Possible LBD was not included as a diagnostic category, since all RBD patients with dementia would, by definition, carry this diagnosis. The diagnosis of Alzheimer dementia was defined according to DSM-IV criteria as the presence of progressive impairment of memory and at least one other cognitive domain of sufficient severity to impact social or occupation functioning, without alternate explanation.20 To ensure reliability of disease assessment, and given the very high prevalence of subtle signs in idiopathic RBD,13 parkinsonism and dementia were strictly defined; therefore, possible parkinsonism (i.e., one cardinal feature only) or mild cognitive impairment were not considered disease outcomes.
Statistical analysis.
Statistical analysis was performed by R.P. The primary outcome measure was defined as the risk of developing parkinsonism or dementia. This was calculated using a life table (Kaplan-Meier) survival analysis. For this analysis, Time = 0 was set at the year that PSG confirmed the diagnosis of idiopathic RBD (year of self-reported symptom onset was not chosen as Time = 0 because of concerns about reliability of symptom onset reports, and because patients who developed disease before PSG diagnosis would be systematically excluded from these calculations). For parkinsonism, time of disease onset was the first of 1) the first self-reported symptom of parkinsonism,21 or 2) if asymptomatic, the demonstration of parkinsonism on examination. For dementia, time of disease onset was set as the time of onset of cognitive impairment severe enough to affect activities of daily living (by report of either the patient or caregiver). Linear regression of disease risk over time was performed to ensure no violation of the proportional hazards assumption. Analysis of categorical variables was conducted with the Fisher exact test, and continuous variables were analyzed with the two-sided t test.
RESULTS
A total of 113 patients were diagnosed with idiopathic RBD at the Hôpital du Sacré Coeur from 1989 to 2006. Of these patients, 93 (82.3%) met inclusion criteria. Of the 20 not participating, 13 patients could not be contacted, 6 had died without clinical follow-up (2 within the first year), and 1 refused further follow-up. Of the 93 patients included, 78 (83.9%) met the strictest inclusion criteria of a full research evaluation follow-up examination. Of the remaining 15 patients, 6 had an in-person follow-up supplemented by a more recent telephone follow-up, 3 had in-person clinical follow-up only (with J.M.), and 6 had telephone follow-up only.
The mean age of participants was 65.4 years and 75 patients (80.4%) were men (table). The mean duration of disease from PSG diagnosis to last evaluation was 5.2 years. The mean duration between RBD symptom onset and PSG diagnosis was 7.2 years.
Table Patient demographics
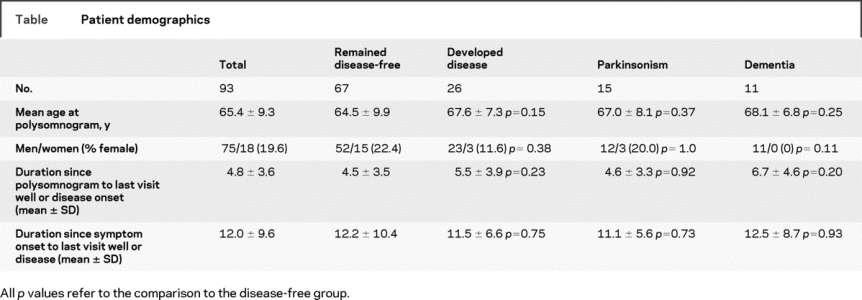
Of the 93 patients included, 26 developed neurodegenerative disease, 15 developed parkinsonism, and 11 developed dementia. Of the patients with parkinsonism, 14 had a diagnosis of idiopathic PD, and 1 was diagnosed with MSA. Of the patients with dementia, 7 met clinical criteria for LBD, and 4 had no clinical hallmarks of LBD and met clinical criteria for AD. There were no significant differences in age or sex between those who did or did not develop disease, although there may have been a tendency toward decreased risk of dementia in women (0/11 dementia patients, p = 0.11). There was no difference in RBD duration between those who did or did not develop disease.
The life table survival curve is presented in figure 1. The estimated 5-year risk of developing neurodegenerative disease (parkinsonism or dementia) was 17.7%. The estimated 10-year risk of disease was 40.6%, and the 12-year risk was 52.4%. Linear regression of the disease risk vs time suggested that the risk of developing disease remained constant over the follow-up period (R2 = 0.038, p = 0.54). In the strict-inclusion cohort (n = 78), the estimated risk was similar; 5-year risk was 19.5%, 10-year risk was 38.0%, and 12-year risk was 55.0%.
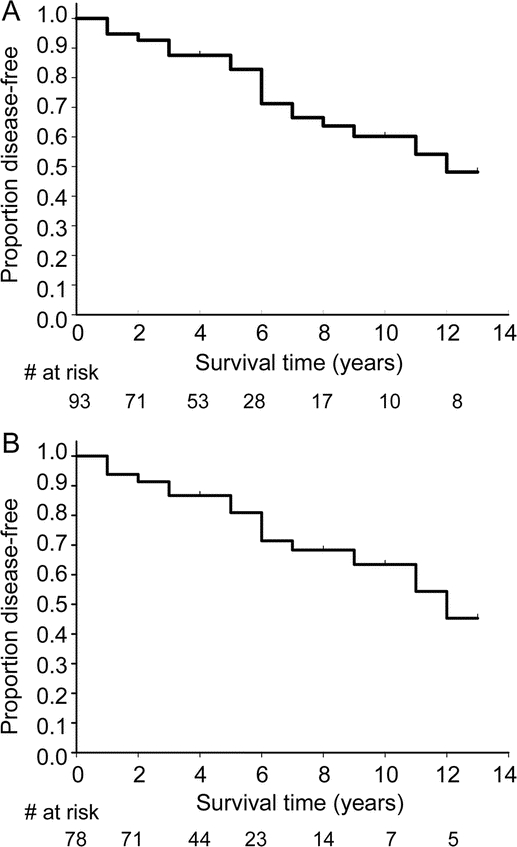
Figure Survival curve of all patients with idiopathic REM sleep behavior disorder (RBD) (A), and the survival curve of patients with idiopathic RBD for whom a systematic research-based examination was performed by a movement disorders specialist (B)
Disease outcome is defined as the development of parkinsonism or dementia.
DISCUSSION
It has been commonly noted that RBD often precedes the development of parkinsonism and dementia.2,8 Obtaining an accurate picture of the risk of developing a neurodegenerative disorder is essential for accurate counseling of patients and for planning of any potential neuroprotective trials. However, definition of the risk of developing a neurodegenerative condition has been published only in two relatively small-scale studies.5,9 The initial report of RBD as a predictor of neurodegenerative disease found that 38% of 29 male patients had developed a parkinsonian disorder 5 years after the diagnosis of idiopathic RBD. More recently, a study of 44 patients with an initial diagnosis of idiopathic REM sleep behavior disorder found that after a median of 5 years of follow-up, 20 of 44 patients (45%) went on to develop a neurodegenerative condition; the commonest diagnoses were PD,9 LBD,6 and mild cognitive impairment.4 Two case series, reported in abstract form only, have suggested that at a mean follow-up of 10 years, 65% of patients had developed neurodegenerative disease.22,23 No published studies have described 10- and 12-year follow-up of idiopathic RBD.
We have demonstrated a risk of disease that is somewhat lower than found in the two previous case series. Some of this variation may be due to differences in disease definition—mild cognitive impairment was not considered a disease outcome in our study, and we used a systematic strict definition of parkinsonism which excluded those with mild parkinsonian signs or a single cardinal manifestation of parkinsonism. It is also possible that disease risk can be changing with time, perhaps related to earlier diagnosis and recognition of milder cases. In support of this notion, the interval between RBD symptom onset and diagnosis in those patients diagnosed between 1989 and 1996 was 10.3 ± 9.2 years (n = 18) compared to an interval of 5.7 ± 5.0 years in those diagnosed from 2004 to 2006 (n = 22, p = 0.052). Therefore symptom to diagnosis latency seems to be changing in time, which no doubt reflects increased awareness and recognition of the disorder. Finally, differences in analytic method between studies can result in important differences in estimation of disease risk. Whereas our method of analysis was a life table analysis, the two previous case series estimating risk of disease in RBD described the proportion developing disease, with mean follow-up duration. If we analyze our results in the same way, we find a similar, although slightly lower proportion developing neurodegenerative disease (i.e., 27.9% over a mean of 5.2 years follow-up [to last clinical visit]). An important advantage of the life table method is that it utilizes censored data—that is, patients, who are censored because of death, loss to follow-up, or recent diagnoses, can contribute in a systematic manner to estimation of risk at later time points. Because of this method and the size of the cohort, we were able to estimate disease risk at 10- and 12-year time points.
Some limitations of this study should be noted. Because persons who agree to participate in prospective annual protocols may have important differences in disease risk between those who refuse or who are unable to participate, we felt that it was essential to include all patients for whom follow-up information was available. This warranted the inclusion of 15 (of 93) patients for whom at least some information was collected from clinical records or telephone follow-up. This information is probably less reliable than that gleaned from a systematic research-based in-person examination—in particular, subtle parkinsonism is often missed by patients, and can be picked up only on examination. However, the large majority of patients had a thorough standardized examination by a movement disorders specialist, and a second analysis that included only these individuals did not find differences in disease risk. All diagnoses were clinical, without autopsy confirmation; inevitably, some patients may have been misdiagnosed. In particular, the diagnosis of LBD requires the presence of clinical hallmarks which may not be apparent early in disease. Given that pathologic studies have found Lewy bodies in all cases of RBD with dementia,24 it will be of exceptional interest to see if the four patients diagnosed with clinical AD will eventually develop hallmarks of LBD. Preliminary analysis of our patients with dementia (presently in progress) has suggested that our patients with clinical diagnosis of AD are indistinguishable from our LBD patients, suggesting that our clinical AD patients in fact have LBD (data not shown). Finally, although this study is the largest study following patients with idiopathic RBD, sample size restrictions prevented analysis of subgroups—in particular, it would be of interest to assess whether risk of developing dementia may be lower in women with idiopathic RBD.
Supplementary Material
Address correspondence and reprint requests to Dr. Ronald B. Postuma, Department of Neurology, L7-305 Montreal General Hospital, 1650 Cedar Ave., Montreal, Quebec, Canada H3G 1A4 ronald.postuma@mcgill.ca
Editorial, page 1294
e-Pub ahead of print on December 24, 2008, at www.neurology.org.
Supported by a Canadian Institutes of Health Research grant to J.M. and J.F.G., and by a grant from the Fonds de la recherche en santé du Québec to R.P.
Disclosure: J.Y. Montplaisir received personal compensation as consultant (Boehringer Ingelheim, Servier, Shire Biochem), speaker (Boehringer, Shire), and received financial support for research activities from Sanofi Synthelabo, GlaxoSmithKline. R.B. Postuma, J.F. Gagnon, M. Vendette, M.L. Fantini, and J. Massicotte-Marquez have nothing to disclose.
Received May 21, 2008. Accepted in final form September 18, 2008.
REFERENCES
- 1.Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA 1987;257:1786–1789. [PubMed] [Google Scholar]
- 2.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain 2000;123:331–339. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 2001;16:622–630. [DOI] [PubMed] [Google Scholar]
- 4.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 2005;128:126–137. [DOI] [PubMed] [Google Scholar]
- 5.of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996;46:388–393. [DOI] [PubMed] [Google Scholar]
- 6.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–138. [DOI] [PubMed] [Google Scholar]
- 7.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130:2770–2788. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol 2006;5:424–432. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 10.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology 1992;42:1371–1374. [DOI] [PubMed] [Google Scholar]
- 11.International Classification of Sleep Disorders 2. Westchester, IL: American Academy of Sleep Disorders, 2005. [Google Scholar]
- 12.Massicotte-Marquez J, Decary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology 2008;70:1250–1257. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006;66:845–851. [DOI] [PubMed] [Google Scholar]
- 14.Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: an etiologic study. JAMA 1999;281:341–346. [DOI] [PubMed] [Google Scholar]
- 15.Konagaya Y, Washimi Y, Hattori H, Takeda A, Watanabe T, Ohta T. Validation of the Telephone Interview for Cognitive Status (TICS) in Japanese. Int J Geriatr Psychiatry 2007;22:695–700. [DOI] [PubMed] [Google Scholar]
- 16.Crooks VC, Clark L, Petitti DB, Chui H, Chiu V. Validation of multi-stage telephone-based identification of cognitive impairment and dementia. BMC Neurol 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 2003;18:318–324. [DOI] [PubMed] [Google Scholar]
- 18.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
- 21.Reider CR, Halter CA, Castelluccio PF, Oakes D, Nichols WC, Foroud T. Reliability of reported age at onset for Parkinson's disease. Mov Disord 2003;18:275–279. [DOI] [PubMed] [Google Scholar]
- 22.of older men initially diagnosed with idiopathic RBD, and an analysis of the minimum & maximum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep 2003;26:A316Abs. [Google Scholar]
- 23.Tippmann-Peikert M, Olson EJ, Boeve B, Silber MH. Idiopathic REM sleep behavior disorder: a follow-up of 39 patients. Sleep 2006;29:A272Abs. [Google Scholar]
- 24.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003;61:40–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


