Summary
Objective
Investigate the role of the superficial zone in regulating the frictional response of articular cartilage. This zone contains the superficial protein (SZP), a proteoglycan synthesized exclusively by superficial zone chondrocytes and implicated in reducing the friction coefficient of cartilage.
Design
Unconfined compression creep tests with sliding of cartilage against glass in saline were carried out on fresh bovine cylindrical plugs (Ø6mm, n=35) obtained from sixteen bovine shoulder joints (ages 1-3 months). In the first two experiments, friction tests were carried out before and after removal of the superficial zone (∼100 microns), in a control and treatment group, using two different applied load magnitudes (4.4N and 22.2N). In the third experiment, friction tests were conducted on intact surfaces and the corresponding microtomed deep zone of the same specimen.
Results
In all tests the friction coefficient exhibited a transient response, increasing from a minimum value (μmin) to a near-equilibrium final value (μeq). No statistical change (p>0.5) was found in μmin before and after removal of the superficial zone in both experiments 1 and 2. However, μeq was observed to decrease significantly (p<0.001) after removal of the surface zone. Results from the third experiment confirm that μeq is even lower at the deep zone. Surface roughness measurements with atomic-force microscopy revealed an increase in surface roughness after microtoming. Immunohistochemical staining confirmed the presence of SZP in intact specimens and its removal in microtomed specimens.
Conclusions
The topmost (∼100 micron) superficial zone of articular cartilage does not have special properties which enhance its frictional response.
Keywords: Cartilage, friction, interstitial fluid pressurization, superficial zone protein, atomic force microscopy
Introduction
The primary function of articular cartilage is to provide low friction and wear at contacting joint surfaces. Several mechanisms have been proposed to explain the remarkable frictional characteristics of cartilage (1-10). One of these proposed mechanisms is premised on the special tribological properties of a mucinous glycoprotein present in synovial fluid, lubricin, described by Swann, Radin and co-workers (4, 5). Recent studies have established a homology of lubricin with superficial zone protein (SZP) (11, 12), a proteoglycan synthesized exclusively by superficial zone chondrocytes (13-15). It has been inferred, but not directly verified, that the presence of SZP in the superficial zone of cartilage should impart beneficial tribological properties to contacting articular layers.
This study investigated the potential special role of the superficial zone in regulating the frictional response of articular cartilage, by measuring the friction coefficient of bovine articular cartilage against glass, before and after removal of the topmost superficial tangential zone, where SZP is localized. The hypothesis was that the friction coefficient of articular cartilage would increase after microtoming of the superficial zone. Additional control groups were tested to help minimize confounding effects in the interpretation of results. Atomic force microscopy (AFM) measurements were also performed to characterize the surface topography before and after microtoming.
Materials and Methods
Three friction experiments were conducted in this study. In the first two experiments, cartilage samples were divided into a control group and a treatment group. The friction coefficient was measured twice in each sample: In the treatment group, the first friction test on the sample was performed with the superficial tangential zone intact and the second test was performed after microtoming to remove the superficial zone. In the control group, both tests were performed with the surface intact. The second experiment was similar to the first, but employed a higher applied load in the friction test to investigate whether results might be dependent on load magnitude. In the third experiment, one group of samples was used; two friction tests were performed on each sample, one on the intact articular surface and the other on the microtomed deep zone.
Specimen Preparation
Fresh bovine shoulder joints were obtained from a local abattoir (19 joints, ages 1-4 months). Joints were stored at 4°C, with an intact capsule, for no more than four days prior to dissection. Following joint dissection, full thickness osteochondral plugs (Ø6mm) were harvested from the humeral head. Underlying bone and vascularized tissue (∼400 microns thick) were removed from the deep zone of the cartilage plugs using a sledge microtome (model 1400; Leitz, Rockleigh, NJ), leaving the articular surface intact. A total of 58 cylindrical cartilage samples were obtained. Twelve samples were used in each of the first two experiments and 13 in the third experiment. Nine samples were used for immunohistochemical staining for SZP and 12 were used for AFM measurements of surface topography.
Friction Measurements
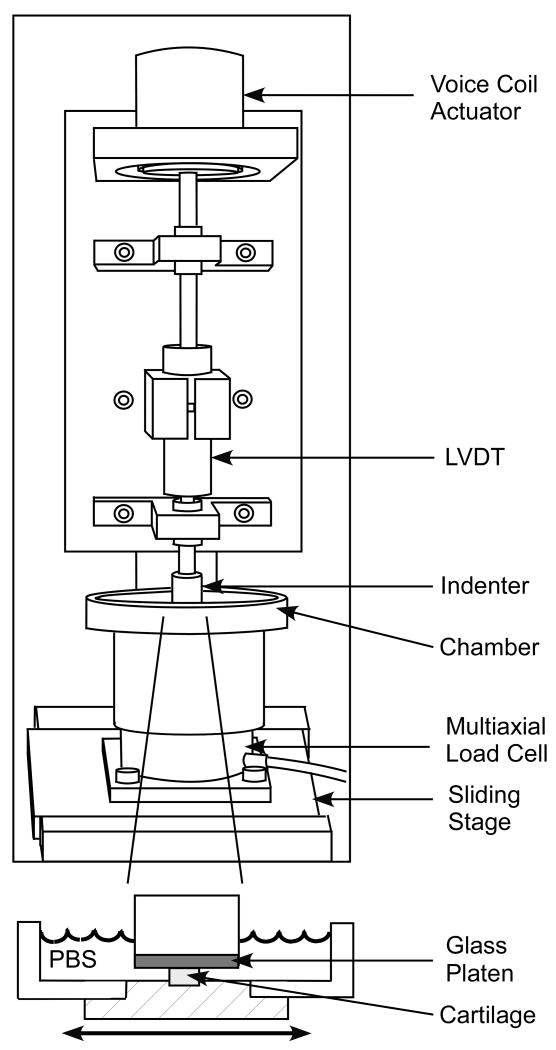
The friction coefficient between cartilage and glass was measured in phosphate buffered saline (PBS), under the configuration of unconfined compression creep (Figure 1), with intermittent sliding over logarithmic time increments (sliding velocity = 1 mm/s). Reciprocal sliding motion was provided by a computer controlled translation stage (Model PM500-1L, Newport Corporation, CA). The range of translation for the sliding stage was ± 1.5 mm in the first experiment (where the normal applied load was 4.4 N) and ± 4.5 mm in the second and third experiments (normal load of 22.2 N). The greater range of translation in the second and third experiments was prescribed to better overcome the shear deformation of the sample and ensure relative sliding between the surfaces. The normal load was applied using a voicecoil load actuator (model LA17-28-000A, BEI Kimco Magnetics Division, San Marcos, CA), ramped up from zero to its prescribed value over a period of 10 seconds, then maintained constant over the remaining duration of the test. Normal and frictional loads were measured with a multi axial load cell mounted on the translation stage (Model 20E12A-M25B,JR3 Inc., Woodland, CA). The friction force and normal force were averaged over the back and forth portions of each cycle of reciprocal motion and the friction coefficient was obtained from the ratio of these values. When plotting the friction coefficient, the values at consecutive logarithmic increments were connected with a straight line, producing continuous curves as a function of time. Cartilage creep displacement was monitored with a linear variable differential transformer (model PR 812, Macro Sensors, Pennsauken, NJ). All tests were terminated after 2,500 seconds. The normal force, frictional force and creep displacement were monitored throughout the test with data acquisition hardware and software (model PCI-6030E & Labview v6.1; National Instruments, Austin, TX). The time-dependent friction coefficient, μeff, was calculated from the ratio of the friction force to the normal force. The minimum value of μeff was denoted by μmin and the final near-equilibrium value, achieved at 2,500 s, was denoted by μeq. Between tests, the glass platen was thoroughly cleaned with distilled water, alcohol and scrubbing.
Figure 1.
Schematic of friction device.
Experiments
In the first two experiments, samples were equally divided into the control and treatment groups; averages and standard deviations of specimen thickness (h) in each group are reported in Table 1. Two friction tests were performed on each sample. In the first test, the articular surface was left intact in both control and treatment groups. At the completion of the test, the sample was equilibrated in PBS for two hours to recover from creep deformation. In the treatment group, the sample was then frozen in an embedding matrix on the freezing stage of a sledge microtome (∼10 minutes), and ∼80-180 microns of the superficial tangential zone was removed (Table 1). Control group specimens were similarly embedded on the freezing stage but were not microtomed.
Table 1.
Summary of cartilage sample thicknesses in Experiments 1 and 2.
| h [mm] |
Experiment 1 4.4 N |
Experiment 2 22.2 N |
||
|---|---|---|---|---|
| Control | Treated | Control | Treated | |
| Test 1 | 1.85 ± 0.17 | 1.80 ± 0.22 | 2.15 ± 0.40 | 2.04 ± 0.45 |
| Test 2 | 1.85 ± 0.17 | 1.62 ± 0.23 | 2.15 ± 0.40 | 1.96 ± 0.44 |
In the third experiment, frictional tests were performed on the articular surface and the corresponding microtomed deep zone of each sample. A two hour recovery period was similarly used between tests. The order of testing of surfaces was randomized.
Immunohistochemical Staining for SZP
Samples were divided into three groups: (I) Osteochondral plugs with intact superficial tangential zone (n=3); (II) chondral plugs with intact superficial tangential zone and microtomed deep zone (n=3); and (III) chondral plugs microtomed both at the superficial tangential zone and at the deep zone (n=3). Samples were fixed overnight at 4°C in acid formalin ethanol (70% ethanol, 3.7% formaldehyde, 5% acetic acid). Fixed samples were dehydrated with a graded series of ethanol, embedded in paraffin blocks (Tissue Prep, Fisher Scientific, Fair Lawn, NJ), sectioned on a rotary microtome (model 2030, Leica, Bannockburn, IL) into 10 μm thick sections, and affixed onto glass slides. Prior to staining, specimens were deparaffinized with Citrosolv (Fisher Scientific, Fair Lawn, NJ) and rehydrated. Immunohistochemical analysis was carried out with a polyclonal antibody directed against SZP (06A10, kindly provided by Dr. Carl Flannery, Wyeth Research Division, Cambridge, MA). Sections were washed in PBS and blocked with 10% normal goat serum (NGS, in PBS) for 10 minutes at room temperature, followed by incubation with primary antibody (16 μg/ml in 10% NGS) for 12 hours at 4°C. Sections were then washed with PBS, and incubated with Alexa Fluor 488 conjugated goat anti-rabbit secondary antibody (Molecular Probes, Eugene, OR) at 20 μg/ml in 10% NGS for 1 hour at room temperature. After extensive washing with dH2O, samples were then treated with Propidium Iodide Nucleic Acid Stain (Molecular Probes, Eugene, OR) at 10 μg/ml for 5 minutes to visualize nuclei, washed three times with dH2O, and cover-slipped with Gel/Mount (Biomedia, Foster City, CA). On each slide, one section was maintained as a non-immune control, following the procedure described above with 10% NGS substituted for primary antibody. Sections were viewed using an inverted microscope (model IX-70, Olympus, Melvilles, NY) equipped with a confocal imaging system with dual wavelength excitation at 488 and 568 nm and imaged at 20× and 50× magnification using a 20× objective with digital magnification. All images were acquired using the Fluoview control software (Olympus, Japan).
Imaging with Atomic Force Microscopy
Samples, which were stored at 4°C for no more than 24 hours before imaging, were divided into three groups: (1) Intact articular surface (n=4); (2) microtomed superficial zone (n=4); (3) microtomed deep zone (n=4). Each sample was affixed to a polystyrene disk with cyanoacrylate adhesive, and imaged in PBS, at 2-4 locations on the surface of interest (Bioscope AFM, Digital Instruments, Santa Barbara, CA), following our recently described protocol (16). A scan size of 100×100×12 μm was obtained in contact mode, with an image size of 256×256 pixels using an unsharpened silicon-nitride microlever probe (Veeco Instruments, Santa Barbara, CA). Average surface roughness was determined using a centerline average (Ra) measured using Nanoscope software (Digital Instruments, Santa Barbara, CA). Ra was determined for the entire 100×100 μm image.
Statistical Analyses
In Experiments 1 and 2, three way analysis of variance (ANOVA) was used to analyze differences in μmin and μeq between control and treatment groups, between the first and second friction test (repeated measure), and between the different loading magnitudes (experiment 1 and experiment 2). When significant differences were detected, Bonferroni-corrected post-hoc testing of the means was performed. In Experiment 3, one-way ANOVA was performed to detect differences in μmin and μeq between the articular surface and the deep zone. For surface roughness measurements, Ra at the intact or microtomed articular surface and (when applicable) at the microtomed deep zone was compared among the three tested groups using one-way ANOVA (n=12 for group 1, n=11 for group 2 and n=11 for group 3). For all analyses, statistical significance was accepted for p≤0.05, with α = 0.05.
Results
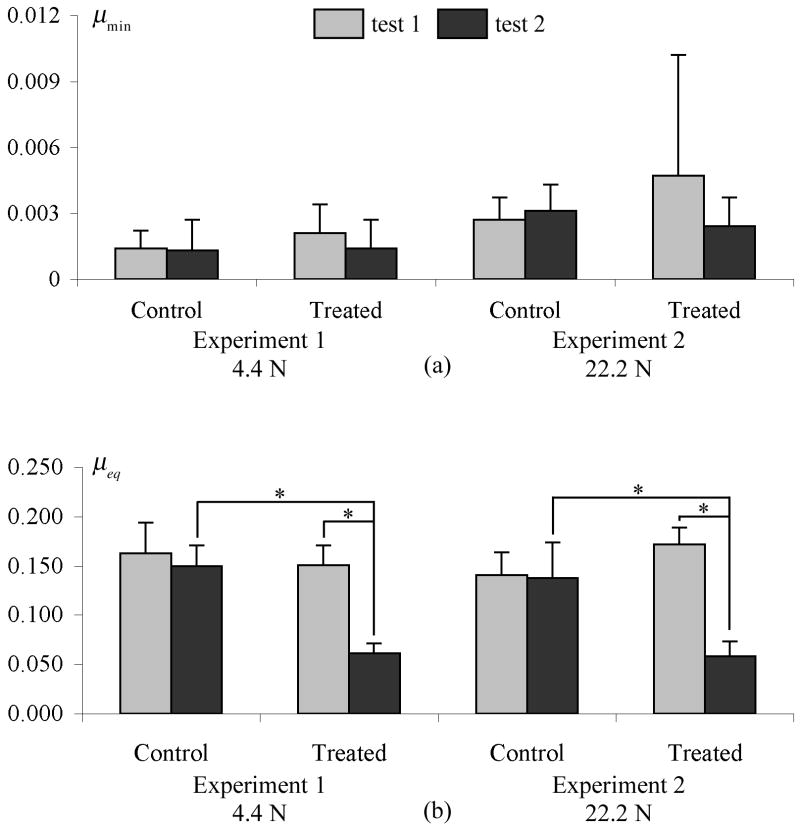
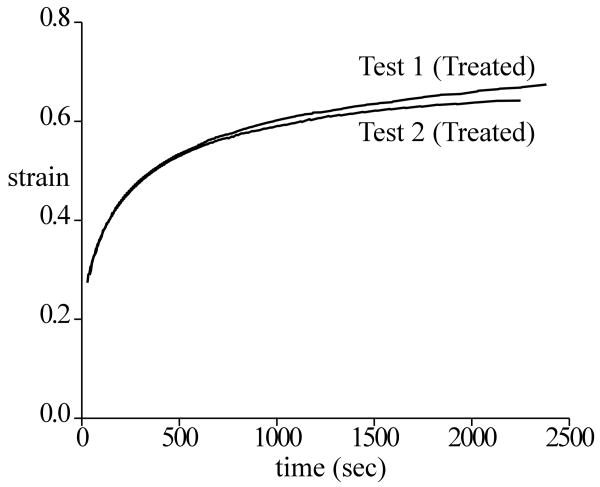
In all tests, the friction coefficient μeff increased with time (Figure 2). The minimum and near-equilibrium values of μeff are reported in Figure 3 for Experiments 1 and 2, and Table 2 for Experiment 3, along with statistical differences. In both surface removal experiments (Figure 3), no significant change was observed in μmin between the first and second test, either in the control group or in the treated group. For μeq, no statistical difference was observed between the first and second test in the control group (where the surface remained intact), or between the control and treated group in the first test (intact surfaces). However, a significant decrease was noted between the first and second test in the treated group (before and after surface removal, p<0.001), and between the control and treated group in the second test (intact versus removed surface, p<0.001). A statistical increase was found in μmin between experiment 1 and experiment 2 (p<0.05), indicating increasing friction coefficient with normal force. However, no statistical difference was found in μeq between the two experiments. No statistical difference was observed in the creep response before and after surface removal, as shown for example in the specimens of Experiment 2 (Figure 4).
Figure 2.
Average curves showing variation of the effective friction coefficient (μeff) as a function of time for (a) test 1 and test 2 in control and treated groups from experiment 1; (b) test 1 and test 2 in control and treated groups from experiment 2; (c) friction tests on articular surface and corresponding side opposite the surface (microtomed deep zone) from experiment 3.
Figure 3.
Summary of frictional results from surface removal tests. Microtomed specimens are in Test 2 of the treated group. (a) μmin; (b) μeq; *p≤0.001.
Table 2.
Summary of frictional results from articular surface vs deep zone. Last column shows p value of Surface vs Deep zone. p-value for surface vs deep appears in the first row for μmin and second row for μeq
| Surface | Deep | ||||||
|---|---|---|---|---|---|---|---|
| h[mm] | μmin | μeq | h[mm] | μmin | μeq | p value | |
| Experiment 3 22.2 N | 1.96 ±0.24 | .003 ±.0012 | 0.152 ±0.013 | 1.96 ±0.24 | .023 ±.021 | 0.060 ±0.023 | p<0.01 p<0.001 |
Figure 4.
Average compressive strain response (creep displacement/original thickness) over all specimens in Experiment 2, for test 1 and test 2 of the treated group (before and after surface removal). The creep strain was 0.645±0.082 in test 1 and 0.653±0.157 in test 2, with no statistical difference observed (p=0.91).
In Experiment 3, μmin was significantly smaller (p<0.01) and μeq was significantly higher (p<0.001) at the articular surface than on the microtomed deep zone.
Immunohistochemical staining showed SZP present in groups I and II, at the intact articular surface and within the top 20 μm from the surface (Figure 5). In group III, SZP was absent at the microtomed surface. No staining was found in the deep zone in any of the three groups.
Figure 5.
Immunohistochemistry: Fluorescent images of representative samples from (a),(b) group I and (c),(d) group II, showed SZP present at the articular surface and within the superficial zone; (e),(f) group III (microtomed surface) and (g),(h) group III (microtomed deep zone) showed negligible SZP at the microtomed surface or the deep zone. (a),(c),(e),(g) 20× magnification, scale bar=100μm; (b),(d),(f),(h) 50× magnification, scale bar = 50 μm. Arrow indicates surface of interest in each image.
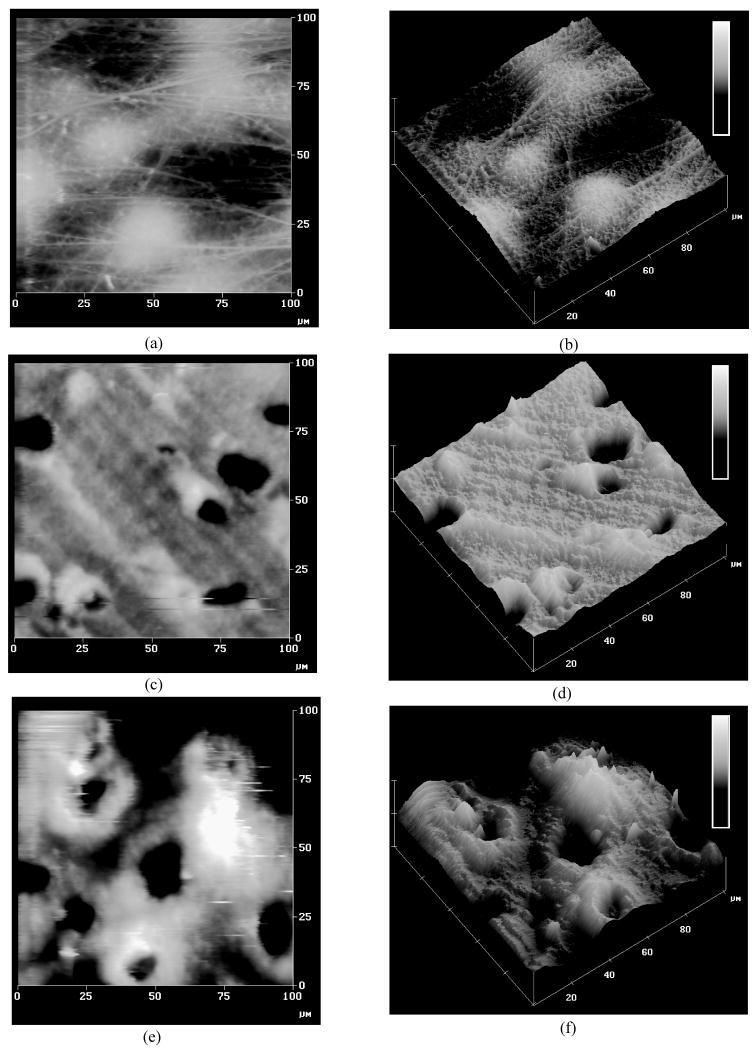
The average surface roughness was Ra=379±83 nm for samples with intact articular surface (group 1), Ra=615±143 nm for samples with a microtomed superficial zone (group 2), and Ra=795±208 nm at the microtomed deep zone (group 3). Significant differences were found in the surface roughness between groups 1 and 2 (p<0.001) and between groups 1 and 3 (p<0.001). The surface topography of microtomed surfaces was also found to be quite different from intact cartilage (Figure 6). The articular surface of intact specimens was largely fibrillar, showing scattered protuberances likely representing subsurface chondrocytes. Microtomed surfaces revealed no distinct collagen network, though distinct pits were apparent which might have resulted from chondrocytes sheared away during surface removal. Parallel grooves were also observed, likely produced by the microtome blade.
Figure 6.
Atomic Force Microscopy: (a),(b) Intact articular surface from group 1; (c),(d) Microtomed surface zone from group 2; (e),(f) Microtomed deep zone from group 3. Z-direction scale bar = 5μm.
Discussion
The objective of the current study was to test the hypothesis that the topmost superficial zone of articular cartilage, where SZP is localized (13), has special frictional properties. This was investigated by performing in-vitro frictional tests between articular cartilage and glass, before and after removal of the superficial tangential zone (∼100 microns thick) in cylindrical cartilage plugs. Results confirm previous findings that the friction coefficient increases with time under a constant applied load (Figure 2) (10, 17, 18). This time-dependent response of the friction coefficient has been shown to be inversely correlated with the time-dependent pressurization of the interstitial water of cartilage (9, 19) according to the formula
| (1) |
where WP/W is the time-varying interstitial fluid load support and 1 − φ is related to the porosity of the articular surfaces. When load is first applied onto cartilage, the maximum value of WP/W is normally very high (>90%), producing a low friction coefficient μeff = μmin. Over time, if the interstitial fluid pressure subsides (WP/W =0), the friction coefficient achieves its highest value, μeff=μeq. Under physiological loading conditions it is believed that the interstitial fluid pressurization of cartilage always remains elevated (9, 19-21), so that μmin is more representative of the values of the friction coefficient in vivo. This is supported by findings that prolonged frictional loading of cartilage under near-equilibrium conditions produces measurable wear (22).
The current study shows that removal of the superficial tangential zone produces no significant change in the minimum friction coefficient, μmin, which is achieved immediately after loading (Figure 3). In a counter-intuitive finding, results also indicate that the long-term (near-equilibrium) friction coefficient, μeq, decreases after removal of the superficial tangential zone. Based on the inclusion of control groups in the experimental design, it can be concluded that observed differences arise exclusively from the removal of the superficial zone, since neither repeated testing of intact samples nor short-term freezing of samples on the microtome stage alter the frictional response. Coupled with the histological confirmation that SZP was present in intact specimens and absent in microtomed specimens, and the consistency between the results of Experiment 1 (4.4 N normal load) and Experiment 2 (22.2 N normal load), these results do not support the hypothesis that the topmost superficial zone of cartilage, where SZP is localized, has special properties which reduce the friction coefficient at the articular surface. It should be noted however that this study does not address whether SZP can help reduce wear at the articular surfaces. Similarly, it does not address the potential interaction between SZP and synovial fluid, since friction measurements were performed in saline.
To further investigate the counter-intuitive decrease in μeq after removal of the superficial tangential zone, surface roughness measurements were used to assess whether microtoming might have produced a smoother surface than in the intact specimen. However, AFM measurements demonstrated that surface roughness increased after microtoming, thus discounting this possibility. For intact specimens, AFM roughness measurements were comparable to measurements made on newborn calf cartilage using more traditional methods (23).
Since μeq was found to be lower just below the articular surface, the next logical step was to explore whether it might decrease further toward the deep zone. Results from Experiment 3 (Table 2) confirmed that this is indeed the case, showing a sixty percent reduction from 0.15 at the articular surface to 0.06 at the deep zone. Whether this decrease is monotonic across the entire depth of the articular layer would need to be confirmed from additional experiments. Interestingly however, the minimum friction coefficient, which represents the more functional measure of friction under physiological loading conditions, increased from 0.003 at the intact articular surface to 0.023 at the microtomed deep zone. This finding is consistent with our expectation that the inhomogeneity of the cartilage ultrastructural organization, biochemical composition, and biomechanical properties through the depth is related to its function as a bearing material (24), and our earlier observation that the peak interstitial fluid load support is significantly lower in the deep zone (WP/W =71±8%) than in the surface zone (WP/W =94±4%) (25).
No specific mechanism is proposed at this time to explain the reduction in μeq with depth from the articular surface; any proposed mechanism would need to be consistent with our previous studies where it was observed that the equilibrium friction coefficient increases with decreasing ionic strength of the saline bath, and decreases with increasing compressive strain (26).
One potential weakness of this study is that it measures the frictional response of cartilage against glass, whereas it might be argued that SZP is an effective boundary lubricant only for cartilage-against-cartilage. While this might indeed be the case, it should be noted that the minimum friction coefficients observed for intact cartilage in the current study (μmin∼0.0013-0.0047) remain in the very low range of coefficients reported in the literature for a variety of testing configurations, including cartilage-against-cartilage in synovial fluid (2, 27, 28). Thus testing against glass does not appear to have an adverse effect on the frictional response of cartilage.
Another potential weakness is that microtoming of the surface zone does not simply remove SZP but also alters the structure of the articular surface. This can produce confounding effects that make it difficult to reach a definitive conclusion on the role of SZP in cartilage lubrication. However, we believe that alternative approaches, such as enzymatic degradation of SZP, may not be sufficiently selective and could produce other confounding effects, such as alterations in material properties and interstitial fluid load support, that may similarly complicate the interpretation of results. For example, in our recent study (29), it has been shown that proteoglycan degradation with chondroitinase ABC produces a statistically significant increase in μmin and μeq, and a corresponding decrease in interstitial fluid load support. While enzymatic degradation studies may provide valuable insight, the current mechanical approach is equally necessary and useful in assessing the role of the superficial zone where SZP is localized. Importantly, neither the porosity (30) nor the mechanical properties of bovine cartilage vary significantly in the topmost ∼100-200 μm region (31, 32), as supported by our finding that the creep response remained virtually the same before and after surface removal (Figure 4).
Despite the homology of SZP with lubricin (11, 12), the current study does not discount the tribological properties of the lubricin present in synovial fluid (4, 5, 33). All measurements in this study were performed in PBS to reduce the potentially confounding effect of lubricin in synovial fluid. Other investigators have shown that testing the frictional response of articular cartilage in synovial fluid produces a moderately smaller friction coefficient than in Ringer's solution (10, 22), which is likely due to the presence in synovial fluid of boundary lubricants such as lubricin, or phospholipids (34).
Acknowledgments
This study was supported by a grant from the National Institutes of Health (AR43628). The authors would also like to thank Dr. Carl Flannery of Wyeth Research Division, Cambridge, MA for providing polyclonal antibody against SZP and Ms. Terri-Ann Kelly of Columbia University for her assistance.
References
- 1.MacConaill MA. The function of inter-articular fibrocartilages, with special references to the knee and inferior radio-ulnar joints. J Anat. 1932;66:210–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Charnley J. Symposium on Biomechanics. London: Inst of Mech Engrs; 1959. The lubrication of animal joints; pp. 12–22. [Google Scholar]
- 3.Hills BA. Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol. 1989;16(1):82–91. [PubMed] [Google Scholar]
- 4.Radin EL, Swann DA, Weisser PA. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970;228(269):377–8. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- 5.Swann DA, Bloch KJ, Swindell D, Shore E. The lubricating activity of human synovial fluids. Arthritis Rheum. 1984;27(5):552–6. doi: 10.1002/art.1780270511. [DOI] [PubMed] [Google Scholar]
- 6.McCutchen CW. Sponge-hydrostatic and weeping bearings. Nature. 1959;184:1284. doi: 10.1038/1841284a0. [DOI] [PubMed] [Google Scholar]
- 7.Mow VC, Lai WM. Recent developments in synovial joint biomechanics. SIAM Review. 1980;22:275–317. [Google Scholar]
- 8.Dowson D, Jin ZM. Micro-elastohydrodynamic lubrication of synovial joints. Eng Med. 1986;15(2):63–5. doi: 10.1243/emed_jour_1986_015_019_02. [DOI] [PubMed] [Google Scholar]
- 9.Ateshian GA, Wang H, Lai WM. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. J Tribology. 1998;120:241–51. [Google Scholar]
- 10.Forster H, Fisher J. The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng [H] 1996;210(2):109–19. doi: 10.1243/PIME_PROC_1996_210_399_02. [DOI] [PubMed] [Google Scholar]
- 11.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254(3):535–41. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 12.Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19(4):677–87. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17(1):110–20. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher BL, Su JL, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec. 2002;266(4):241–8. doi: 10.1002/ar.10063. [DOI] [PubMed] [Google Scholar]
- 15.Su JL, Schumacher BL, Lindley KM, Soloveychik V, Burkhart W, Triantafillou JA, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20(3):149–57. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 16.Moa-Anderson BJ, Costa KD, Hung CT, Ateshian GA. Bovine articular cartilage surface topography and roughness in fresh versus frozen tissue samples using atomic force microscopy. Summer Bioengineering Conference; 2003; Key Biscayne, FL. 2003. Poster 1207. [Google Scholar]
- 17.McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:1–17. [Google Scholar]
- 18.Malcom LL. An experimental investigation of the frictional and deformational response of articular cartilage interfaces to static and dynamic loading [PhD] San Diego: University of California; 1976. [Google Scholar]
- 19.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ateshian GA, Wang H. A theoretical solution for the frictionless rolling contact of cylindrical biphasic articular cartilage layers. J Biomech. 1995;28(11):1341–55. doi: 10.1016/0021-9290(95)00008-6. [DOI] [PubMed] [Google Scholar]
- 21.Kelkar R, Ateshian GA. Contact creep of biphasic cartilage layers. Journal of Applied Mechanics, Transactions ASME. 1999;66(1):137–145. [Google Scholar]
- 22.Forster H, Fisher J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc Inst Mech Eng [H] 1999;213(4):329–45. doi: 10.1243/0954411991535167. [DOI] [PubMed] [Google Scholar]
- 23.Sayles RS, Thomas TR, Anderson J, Haslock I, Unsworth A. Measurement of the surface microgeometry of articular cartilage. J Biomech. 1979;12(4):257–67. doi: 10.1016/0021-9290(79)90068-x. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan R, Park S, Eckstein F, Ateshian GA. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Eng. 2003;125(5):569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression. J Biomech. 2003;36(12):1785–96. doi: 10.1016/s0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transport in Porous Media. 2003;50:5–33. [Google Scholar]
- 27.Charnley J. The lubrication of animal joints in relation to surgical reconstruction by arthroplasty. Ann Rheum Dis. 1960;19:10–19. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linn FC, Radin EL. Lubrication of animal joints. 3. The effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum. 1968;11(5):674–82. doi: 10.1002/art.1780110510. [DOI] [PubMed] [Google Scholar]
- 29.Basalo IM, Raj D, Krishnan R, Chen FH, Hung CT, Ateshian GA. Effects of enzymatic degradation on the frictional response of articular cartilage in stress relaxation. J Biomech. 2004 doi: 10.1016/j.jbiomech.2004.05.045. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torzilli PA, Askari E, Jenkins JT. Water content and solute diffusion properties in articular cartilage. In: Mow VC, Ratcliffe A, Woo SLY, editors. Biomechanics of diarthrodial joints. New York: Springer-Verlag; 1990. pp. 363–90. [Google Scholar]
- 31.Wang CC, Deng JM, Ateshian GA, Hung CT. An automated approach for direct measurement of two-dimensional strain distributions within articular cartilage under unconfined compression. J Biomech Eng. 2002;124(5):557–67. doi: 10.1115/1.1503795. [DOI] [PubMed] [Google Scholar]
- 32.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 33.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J Biomed Mater Res. 1998;40(3):414–8. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Hills BA. Boundary lubrication in vivo. Proc Inst Mech Eng [H] 2000;214(1):83–94. doi: 10.1243/0954411001535264. [DOI] [PubMed] [Google Scholar]