Abstract
Vascular endothelial growth factor (VEGF, VEGF-A) is a major regulator of physiological and pathological angiogenesis. One feature of VEGF is the existence of multiple isoforms arising from alternative exon splicing. Our initial biochemical and biological studies indicated that such isoforms are uniquely suited to generate angiogenic gradients by virtue of their differential ability to interact with the extracellular matrix (ECM). Although ECM-bound VEGF was bioactive, processing by physiologically relevant proteases such as plasmin was identified as a key mechanism to convert ECM-bound VEGF into freely diffusible forms. This retrospective article examines the early studies and also emphasizes the subsequent progress in our understanding of these processes in health and disease.
INTRODUCTION
The development of a vascular supply is essential for a wide a variety of physiological processes. It is also well established that uncontrolled growth of blood vessels plays a pathogenic role in several disorders including cancer and intraocular neovascular diseases (Ferrara, 2002). Over the past decade, much work has been devoted to the biology of vascular endothelial growth factor (VEGF)-A (VEGF hereafter). Although new blood vessel growth is a highly complex process requiring the action of multiple regulators, there is agreement that VEGF signaling through its tyrosine kinase receptors (VEGFR-1 and VEGFR-2) often represents a rate-limiting step (Coultas et al., 2005). The crucial role of VEGF in developmental angiogenesis is underscored by early embryonic lethality after inactivation of a single VEGF allele in mice (Ferrara et al., 1996). VEGF inhibition also results in tumor growth suppression (Kim et al., 1993). Several VEGF pathway inhibitors have been approved by the FDA as therapy for advanced cancer and age-related macular degeneration (AMD; reviewed in Ferrara et al., 2007).
MULTIPLE ISOFORMS FOR MULTIPLE ROLES?
VEGF was independently cloned 20 years ago as an angiogenic factor (Leung et al., 1989) and as a vascular permeability factor (VPF; Keck et al., 1989). For a historical review, see (Ferrara, 2009). From screening a cDNA library from HL-60 human promyelocytic leukemia cells, we identified VEGF clones encoding three different isoforms: VEGF121, VEGF165, and VEGF189 (Leung et al., 1989). Keck et al. (1989) described a VPF clone, from a cDNA library–derived form U937 cells, that encodes a protein identical to VEGF189. The existence of multiple VEGF isoforms raised the possibility that these may play different roles.
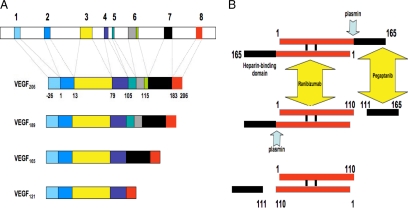
VEGF165, the most abundant isoform, has a cluster of basic residues within its COOH- terminus, which are encoded by exon 7. The shortest isoform, VEGF121, lacks the 44 amino acids encoded by exon 7. Compared with VEGF165, VEGF189 (and a subsequently identified even longer isoform, VEGF206) has a 24-amino acid insertion highly enriched in Arg and Lys, which is encoded by exon 6 (Keck et al., 1989; Leung et al., 1989; Houck et al., 1991; Tischer et al., 1991). Figure 1A illustrates alternative splicing in the human VEGF gene.
Figure 1.
(A) Organization of the human VEGF-A gene. The gene comprises of eight exons. Alternative splicing results in the generation of multiple isoforms (Houck et al., 1991; Tischer et al., 1991). The monomeric precursor of each isoform is represented here. The most frequently detected isoforms are VEGF121, VEGF165, and VEGF189. Less abundant splice variants have been described, including VEGF145, VEGF162, VEGF183, and VEGF165b. VEGF165 interacts with HSPG through exon 7–encoded sequences. VEGF189 and VEGF206 have one additional heparin-binding domain, encoded by exon 6, explaining the particularly strong binding of these isoforms to HSPG in the ECM. (B) Plasmin-mediated cleavage of VEGF165. The mature VEGF homodimer is represented here. Note the sequential cleavage, generating first the heterodimer VEGF165/110 (Keyt et al., 1996). The final product is VEGF110, lacking the sequences encoded by exons 7 and 8 and part of exon 5. This protein is biologically and biochemically similar to alternatively spliced VEGF121. The diagram also illustrates the site-specific binding of two inhibitors, ranibizumab and pegaptanib. VEGF110 activity is blocked by ranibizumab but not by pegaptanib.
A sequence highly homologous to the exon 6-encoded basic domain in VEGF is present in the COOH-terminal region of PDGF-B and in the long, alternatively spliced, variant of PDGF-A (Ostman et al., 1991). Initially, on the basis of the analysis of deletion mutants of PDGF-B lacking the signal peptide, such a motif, termed “nuclear targeting site,” was implicated in directing nuclear localization of the protein (Lee et al., 1987). However, such a localization was difficult to reconcile with a secreted polypeptide that operates in the extracellular milieu, raising the possibility that, physiologically, such a sequence confers targeting into alternative compartments.
VEGF BOUND AND UNBOUND
In 1991 we reported that, unlike VEGF121 and VEGF165, but similar to PDGF-B and the long form of PDGF-A (Ostman et al., 1991), VEGF189 and VEGF206 are undetectable in the conditioned medium of transfected cells and remain “cell-associated” (Houck et al., 1991). However, the precise localization and fate of these proteins remained to be elucidated.
In a subsequent study (Houck et al., 1992), we determined that VEGF189 (or VEGF206) is not stored intracellularly but instead is bound in the cell surface or in the extracellular matrix (ECM), as assessed by the ability of suramin to displace the protein in a soluble form in the medium. The finding that addition of heparin or heparinase to the medium also resulted in release of VEGF189 indicated that the binding sites consisted of heparan sulfate proteoglycans (HSPG; Houck et al., 1992). A somewhat surprising finding was that as much as 50–70% of VEGF165 could be released by heparin, suggesting that although this isoform is secreted, a substantial fraction remains bound to HSPG. In contrast, VEGF121 appeared freely diffusible and the amount released in the medium was not affected by heparin (Houck et al., 1992). These studies indicated that alternative splicing of VEGF is responsible for turning a weakly acidic polypeptide, VEGF121, into increasingly more basic heparin-binding ones.
Another important finding was that the serine protease plasmin is able to induce the release of proteolytically cleaved VEGF species of both VEGF165 and VEGF189 that are freely soluble in the medium and are biologically active (Houck et al., 1992), suggesting that the proteolytic cascade of plasminogen activation, a key step during angiogenesis, can cleave the bound forms of VEGF. Thus, these early studies suggested that the VEGF proteins may become available to endothelial cells by at least two different mechanisms: alternative splicing and proteolytic cleavage, generating non-heparin-binding, diffusible fragments.
In our 1993 Molecular Biology of the Cell article (Park et al., 1993), we followed up on these observations and investigated the localization of the VEGF isoforms in various cellular and extracellular compartments by a variety of techniques, including immunoelectron microscopy. Our model was CEN-4 cells stably expressing each of the aforementioned four VEGF isoforms or control vector. We also prepared cell-free subepithelial ECM from various transfectants for biochemical and biological analysis.
We found that VEGF189 and VEGF206 are localized to the ECM. Accordingly, endothelial cells cultured on ECM derived from cells expressing these isoforms were markedly stimulated to proliferate compared with ECM derived from vector-transfected or VEGF121-expressing cells, demonstrating that not only soluble but also ECM-bound VEGF is bioactive. Furthermore, we characterized the effects of plasmin on intact monolayers or ECM derived from VEGF189- or VEGF206-expressing cells. In both cases, the protease induced in a dose-dependent manner the release of a diffusible VEGF fragment, which is devoid of binding to heparin (Park et al., 1993).
Interestingly, ECM derived from cells expressing VEGF165 was also able to stimulate endothelial cell growth (although less effectively than that derived from cells expressing the two longer isoforms), in spite of the fact that the VEGF165 protein was undetectable by the methods we used. A possibility was that VEGF189 or VEGF206, in order to exert mitogenic activity, must be released locally from its bound state. In contrast, VEGF165, being less strongly bound to the ECM, exists in equilibrium with a soluble form and therefore lower amounts may be effective at promoting endothelial cell proliferation.
These and other findings suggested that VEGF165, by virtue of its intermediate ECM-binding properties, has optimal characteristics of bioavailability and biological potency. We hypothesized that the longer forms of VEGF might represent primarily a storage form of the growth factor, releasable for example after degradation of the ECM, whereas the diffusible forms play a more dynamic role, being readily available to endothelial cells.
In summary, the interplay among the VEGF isoforms appeared uniquely suited to create biochemical gradients that radiate from angiogenic areas such as ischemic tissues (Park et al., 1993; Keyt et al., 1996). Compared with the longer forms of VEGF, the diffusible forms would migrate a greater distance, bind VEGF receptors, and trigger endothelial cell proliferation and migration. The intensity of the angiogenic signal would be weakest at the most distant sites, given the lesser mitogenic potency of VEGF121 and the plasmin-generated fragment, which subsequent studies showed to be comprised of the first 110 NH2-terminal amino acids of VEGF (VEGF110), and therefore lacking all the exon 7–encoded sequences (Keyt et al., 1996; see Figure 1B).
SUBSEQUENT STUDIES
What have we learned since these initial studies? In 1998, the discovery that Neuropilin-1, a molecule implicated in axon guidance, functions also as an isoform-specific VEGF receptor that interacts with heparin-binding VEGF and modulates activation of VEGFR-2 (Soker et al., 1998) added a new level of complexity to our understanding of the significance of the VEGF isoforms. It also provided an explanation for the greater biological potency of VEGF165 as compared with VEGF121 or VEGF110 (Keyt et al., 1996). Also, elegant genetic studies, employing isoform-specific knockouts in mice, provided a compelling verification of the hypothesis that VEGF gradients, driven by the balance between diffusible and HSPG-bound VEGF, have an impact on development and patterning of the vascular system.
The importance of the heparin-binding VEGF isoforms was emphasized by the finding that 50% of the mice expressing exclusively VEGF120 (mouse VEGF is one amino acid shorter that human) die perinatally and show impaired myocardial angiogenesis and ischemic cardiomyopathy (Carmeliet et al., 1999). Interestingly, in such mice endothelial cells are recruited into existing vessels, resulting in increased caliber and reduced complexity of vascular branching. These findings indicated that the heparin-binding VEGF isoforms provide essential stimulatory cues to initiate vascular branching as well as endothelial tip cell filopodia emission (Ruhrberg et al., 2002; Gerhardt et al., 2003).
Conversely, mice expressing only VEGF188 also display abnormalities in vessel branching, but in the opposite direction from those mice expressing only VEGF120, with an excess of thin and disorganized branches (Ruhrberg et al., 2002); they also have a number of defects in the skeletal system, resulting in stunted growth and reduced survival (Maes et al., 2004). Remarkably, mice expressing only VEGF164 are apparently normal (Maes et al., 2004), perhaps reflecting the “perfect balance” between diffusibility and HSPG binding achieved by this isoform.
Significant progress has been made also in our understanding of the pathophysiological implications of the proteolytic processing of VEGF. In 2005 Lee et al. (2005) reported that various matrix metalloproteinases (MMPs), especially MMP-3, can cleave VEGF165 to generate diffusible, non-heparin-binding fragments. Interestingly, the final product of VEGF165 processing by MMP-3 is VEGF113, which is biologically and biochemically very similar to the plasmin-generated fragment, VEGF110 (Keyt et al., 1996).
Considering the widespread activation of proteases such as plasminogen and MMPs during angiogenesis (Coussens and Werb, 2002), there is reason to believe that such fragments play important pathophysiological roles. Indeed, there is already some evidence from clinical trials. Two VEGF inhibitors have been approved by the FDA as therapy for neovascular AMD: pegaptanib, an aptamer that interacts selectively with the exon 7-encoded heparin-binding domain and ranibizumab, a Fab that neutralizes all VEGF isoforms and proteolytic fragments (reviewed in Ferrara et al., 2007). Treatment with ranibizumab resulted in greater clinical benefit, including improved visual acuity. It seems likely that such difference was due, at least in part, to the ability of ranibizumab to neutralize not only intact VEGF165 but also fragments resulting from plasmin or MMP processing.
CONCLUSION
There is now clear evidence for the importance of angiogenic gradients dependent on VEGF isoforms in a variety of pathophysiological circumstances. However, several questions remain to be addressed. For example, the role of the long isoforms (VEGF189 or VEGF206) in pathological angiogenesis is still unclear. Can they induce angiogenesis while bound to the ECM, as our early in vitro studies suggested (Park et al., 1993), or do they need to be first processed by proteases? Confounding factors are the dominance of VEGF165 and the fact that proteolytic processing of multiple isoforms may result in similar bioactive fragments. The availability of antibodies or other reagents that can target specifically exon 6–encoded sequences may allow answering some of these questions. Finally, elucidating the significance of other, less frequent, VEGF isoforms (e.g., VEGF145, VEGF162, and VEGF165b; reviewed in Ferrara et al., 2007) is an active and potentially fruitful area of research.
REFERENCES
- Carmeliet P., et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat. Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- Coultas L., Chawengsaksophak K., Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Coussens L. M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell Braxton L., Hillan K. J., Moore M. W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Mass R. D., Campa C., Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu. Rev. Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- Gerhardt H., et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Keyt B. A., Berleau L. T., Nguyen H. V., Chen H., Heinsohn H., Vandlen R., Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J. Biol. Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Lee B. A., Maher D. W., Hannink M., Donoghue D. J. Identification of a signal for nuclear targeting in platelet-derived-growth-factor related molecules. Mol. Cell. Biol. 1987;7:3527–3537. doi: 10.1128/mcb.7.10.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Jilani S. M., Nikolova G. V., Carpizo D., Iruela-Arispe M. L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Maes C., Stockmans I., Moermans K., Van Looveren R., Smets N., Carmeliet P., Bouillon R., Carmeliet G. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J. Clin. Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman A., Andersson M., Betsholtz C., Westermark B., Heldin C. H. Identification of a cell retention signal in the B-chain of platelet-derived growth factor and in the long splice version of the A-chain. Cell Regul. 1991;2:503–512. doi: 10.1091/mbc.2.7.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Keller G.-A., Ferrara N. The vascular endothelial growth factor isoforms (VEGF): differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C., Gerhardt H., Golding M., W. R, Ioannidou S., Fujisawa H., Betsholtz C., Shima D. T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]