In the absence of p110β function, spermatogenesis is dramatically disturbed because of a progressive reduction of differentiating spermatogones. Genetically modified mice and pharmacological inhibition of p110β confirmed this enzyme as the main PI3K isoform activated downstream of c-Kit.
Abstract
Phosphoinositide 3-kinases (PI3K) are key molecular players in male fertility. However, the specific roles of different p110 PI3K catalytic subunits within the spermatogenic lineage have not been characterized so far. Herein, we report that male mice expressing a catalytically inactive p110β develop testicular hypotrophy and impaired spermatogenesis, leading to a phenotype of oligo-azoospermia and defective fertility. The examination of testes from p110β-defective tubules demonstrates a widespread loss in spermatogenic cells, due to defective proliferation and survival of pre- and postmeiotic cells. In particular, p110β is crucially needed in c-Kit–mediated spermatogonial expansion, as c-Kit–positive cells are lost in the adult testis and activation of Akt by SCF is blocked by a p110β inhibitor. These data establish that activation of the p110β PI3K isoform by c-Kit is required during spermatogenesis, thus opening the way to new treatments for c-Kit positive testicular cancers.
INTRODUCTION
Phosphoinositide 3-kinases (PI3Ks) are a conserved family of enzymes (Engelman et al., 2006; Hirsch et al., 2008). Although all PI3Ks share the capacity to phosphorylate the D3 hydroxyl group of phosphatidylinositols (PtdIns), their substrate specificity and molecular structure allow to distinguish different classes of PI3Ks. Class IA PI3Ks (PI3Kα, β, and δ) are heterodimers composed of a catalytic subunit (p110α, β, or δ) and an adaptor protein comprising a Src homology 2 domain (p85α, p50α, p55α, p85β, or p55γ). This class of enzymes is engaged by receptor or Ras activation to produce the lipid second messenger PtdIns-3,4,5-triphosphate and to evoke a complex series of signal transduction pathways, including that of the protein kinase B/Akt (Wymann and Marone, 2005).
Although the catalytic activity of class I PI3Ks has been widely involved in several biological processes, including cell viability and metabolism, the specific contribution of individual PI3Ks is only beginning to emerge. In particular, limited data are currently available with respect to p110β in physiology and disease. Although the genetic deletion of p110β is embryonic lethal (Bi et al., 2002), we have recently reported the successful generation of a viable genetically engineered mouse model expressing a catalytically inactive form of p110β (Pik3cbK805R mutant; Ciraolo et al., 2008). We and others have further demonstrated that p110β is specifically required in cell signaling downstream of G protein–coupled receptors (GPCRs) and tyrosine kinase receptors (RTKs; Ciraolo et al., 2008; Guillermet-Guibert et al., 2008; Jia et al., 2008). With respect to RTK pathways, p110β has been shown to sustain long-term insulin signaling. Accordingly, the loss of the catalytic activity of p110β results in a phenotype of growth retardation and insulin resistance in vivo. Furthermore, the kinase activity of p110β is specifically required for PTEN-loss or ERBB2-driven carcinogenesis, suggesting that the pharmacological targeting of p110β may be a promising therapeutic approach for cancer treatment (Ciraolo et al., 2008; Jia et al., 2008; Wee et al., 2008).
In the testis, the PI3K-Akt signaling pathway is activated at different steps of the spermatogenetic process by key mediators such as the stem cell factor (SCF) and the glial cell line-derived neurotrophic factor (GDNF; Serve et al., 1994; Lee et al., 2007). GDNF is secreted by Sertoli cells and has been shown to stimulate the self-renewal of spermatogonial stem cells (Kanatsu-Shinohara et al., 2003), whereas SCF exists in a soluble form and as a membrane-bound growth factor expressed by Sertoli cells within seminiferous tubules. The biological effects of SCF are mediated by its RTK receptor c-Kit. In the postnatal testis, c-Kit essentially marks Leydig cells, differentiated spermatogonia, and to a lesser extent, spermatides and spermatozoa (Besmer et al., 1993; Rothschild et al., 2003). Through binding to c-Kit, SCF stimulates the proliferation and meiotic progression of spermatogonia (Besmer et al., 1993; Lyman and Jacobsen, 1998; Sette et al., 2000). Although without indicating the precise p110 isoform involved, two studies have provided conclusive evidence that PI3Ks are required for the c-Kit/SCF signaling pathway in the spermatogenic process (Blume-Jensen et al., 2000; Kissel et al., 2000). In both reports, the genetic disruption of the c-Kit receptor domain mediating the molecular interaction with PI3K was associated with male sterility due to defective proliferation and meiosis, as well as with increased apoptosis of spermatogonia. Moreover, c-Kit–induced PI3K signaling has also been detected in Leydig cells, where it controls baseline and stimulated testosterone secretion (Rothschild et al., 2003).
All these data support the involvement of PI3K in different steps of spermatogenesis, such as spermatogonial stem cell (SSC) as well as spermatogonial proliferation and survival. However, the specific p110 isoform involved is still unknown. To develop a greater understanding of the PI3K isoform implicated in these processes, we studied, using knock-in mice, the effects of p110β inactivation on testicular development. We found that in the absence of p110β function spermatogenesis is dramatically disturbed because of a progressive reduction in differentiating SSCs as well as a loss of c-Kit–positive cells. Further in vitro analysis by pharmacological inhibition of p110β confirmed this enzyme as the main PI3K isoform activated downstream of c-Kit. Overall, this demonstrates a crucial and nonredundant role of p110β in spermatogenesis.
MATERIALS AND METHODS
Mice
The targeting strategy used to generate the PIK3CB805R allele was previously described (Ciraolo et al., 2008). Results were obtained by using wild-type and mutant littermates derived from heterozygous crosses of 129/Sv-C57Bl6 genetic background.
Reagents
Antibodies against p110β (sc-602, Western blotting), Erk (sc-93), c-Kit-receptor (sc-168), and GATA-4 (sc-9053; LaVoie, 2003) were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-p110β antibody used for immunostaining was developed by this laboratory. Antibodies against p85 (4257), p-akt (Ser473 9271), Akt (9272), and p-Erk (p42–44 MAPK 4695) were from Cell Signaling Technology (Beverly, MA). The anti-TRA98 antibody was kindly provided by Dr. Tanaka (Department of Science for Laboratory Animal Experimentation, Osaka University, Osaka, Japan; Tanaka et al., 1997). The anti-PLZF antibody was as described (Kovalovsky et al., 2008). Serum testosterone and follicle-stimulating hormone (FSH) were measured using immunoradiometric assays from IBL International GmbH (Hamburg, Germany).
Protein Analysis
Testes were removed, frozen in liquid nitrogen, and homogenized in lysis buffer (50 mM Tris-HCl, pH 8, and 150 mM NaCl) supplemented with 2 mg/ml aprotinin, 1 mM pepstatin, 1 ng/ml leupeptin, 50 mM NaF, 2 mM sodium orthovanadate, 1 mM sodium pyrophosphate, and 1% Triton X-100. The same buffer was used to solubilize protein extracts from cultured cells. Homogenates were clarified by centrifugation in a microcentrifuge at 4°C. Supernatants were analyzed by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Blots were probed with the indicated antibodies and developed by enhanced chemiluminescence (ECL; Millipore, Bedford, MA).
Histological Analysis, Apoptosis, and Proliferation Assays
For histological analysis, testes were fixed in 4% paraformaldehyde (PFA) or Carnoy (3:1 methanol and acetic acid), embedded in paraffin, and cut into 5-μm-thick sections. Sections were stained with hematoxylin and eosin following standard protocols and with specific antibodies.
For the apoptotic assay, 4% paraformaldehyde–fixed and embedded testes were subjected to TUNEL assay (Roche Applied Science, Indianapolis, IN; 11684817910). For quantization, TUNEL-positive cells were counted for each tubule in different sections.
For the proliferation assay, sections of fixed testes were stained with an antibody specific for the proliferation cell nuclear antigen (PCNA; Santa Cruz Biotechnology; sc-7907) and cyclin D1 (rabbit monoclonal, Novus Biologicals, Baltimore, MD). Positively stained nuclei were then counted in each tubule by microscopic examination.
Cell Proliferation and Apoptosis
SSCs were obtained from C57Bl6 mice as previously described by Guan et al. (2006). SSCs were cultivated in αMEM supplemented with 9% FBS and 10 ng/ml GDNF. SSCs were treated with a PI3K (PIK75, 25 nM) and a p110β specific inhibitor (TGX221, 200 nM) or vehicle (DMSO) for 72 h. After 72 h cells were trypsinized and counted. For the proliferation assay, SSCs were fixed with 4% paraformaldehyde and stained with the proliferation marker Ki67 (Novocastra, Newcastle upon Tyne, United Kingdom), numbers were counted, and the percentage of proliferating cells was calculated in comparison to not-stained cells. Similarly, the analysis of apoptosis of SSC was performed after treatment with TGX221, PIK75, or DMSO for 72 h. After fixation cells were subjected to TUNEL assay, and apoptotic cells were counted in different microscopic fields. The percentage of apoptotic cells was calculated in comparison to not stained cells.
Phosphoprotein Analysis
SSCs were starved with α-MEM containing 9% of FBS for 24 h, treated with TGX221 and PIK75 or DMSO for 2 h, and stimulated for 5 min with 10 ng/ml GDNF.
Spermatogones were obtained as following: testes of prepuberal mice were removed, decapsulated, and digested with a mixture of enzyme that included collagenase, trypsin, and hyaluronidase. Isolated cells were suspended in DMEM with addition of 20% FBS and then incubated in a cell culture plate for 2 h. Spermatogones were then collected from the supernatant and starved in F12 medium for 4 h. Subsequently, cells were stimulated with 100 ng/ml SCF in the presence p110β inhibitor TGX221 or vehicle.
Statistical Analysis
Statistical significance was calculated with Student's t test and one-way analysis of variance tests followed by Bonferroni post hoc analysis. Values are reported as the mean ± SEM.
RESULTS
Loss of p110β Activity Impairs Male Fertility and Fecundity
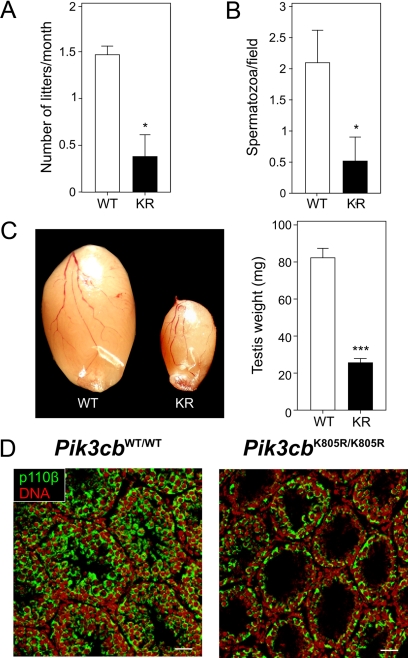
Homozygous mice expressing a catalytically inactive p110β (Pik3cbK805R/K805R) were born and reached adulthood (Ciraolo et al., 2008). Pik3cbK805R/K805R males exhibited a normal libido and were able to mate with receptive females. However, the number of litters obtained from mutant males was significantly reduced (Figure 1A), indicating a defect in male fertility. On the contrary, homozygous mutant females were fully fertile. To assess the fecundity of Pik3cbK805R/K805R males, sperm counts were next performed after isolation and flushing of the deferens ducts. Indeed, the sperm of Pik3cbK805R/K805R mice contained significantly fewer spermatozoa than wild-type controls (Figure 1B), ranging from oligozoospermia to azoospermia (67% of Pik3cbK805R/K805R).
Figure 1.
The absence of p110β activity causes severe testicular hypotrophy, defective fertility, and fecundity. (A) Number of litters from mutant mice. Mutant and control mice (n = 6) were mated with receptive female for 6 mo, and the number of litters produced was counted. (B) Number of spermatozoa obtained from the flushing of the deferens ducts of wild-type (WT) and Pik3cbK805R/K805R (KR) mice (n = 5). Spermatozoa were counted as the number of cells per microscopic field. Data (number of cells/microscopic field) are reported as average ± SEM; *p < 0.05, KR versus WT. (C) Average weight of testes from Pik3cbWT/WT (WT) and Pik3cbK805R/K805R (KR) mice (n = 10). A representative image of testes dissected from a WT and a KR mouse is presented. The data (mg/testis) is reported as average ± SEM; ***p < 0.001, KR versus WT. (D) Representative fluorescent immunostaining of testicular sections obtained from Pik3cbWT/WT and Pik3cbK805R/K805R mice. p110β was detected with a specific mAb (green), whereas nuclei were stained in red with DAPI. Scale bar, 50 μm.
At 24 wk of age, when wild-type and mutant mice show equal body weights (Ciraolo et al., 2008), the testes of Pik3cbK805R/K805R males were severely reduced in size and mass compared with those of Pik3cbWT/WT animals (Figure 1C). Immunostaining of Pik3cbK805R/K805R testes confirmed the widespread expression of p110β in the cells of the seminiferous tubules and interstitium (Figure 1D). It was previously reported that Pik3cbK805R/K805R mutants show reduction of the kinase-dead p110β protein levels compared with WT p110β (Ciraolo et al., 2008). Through Western blotting (Supplemental Figure 1), we confirmed that the average expression of p110β also was reduced by 50% in the testes of Pik3cbK805R/K805R, whereas as previously reported (Ciraolo et al., 2008), the abundance of other class I PI3Ks was unchanged (data not shown).
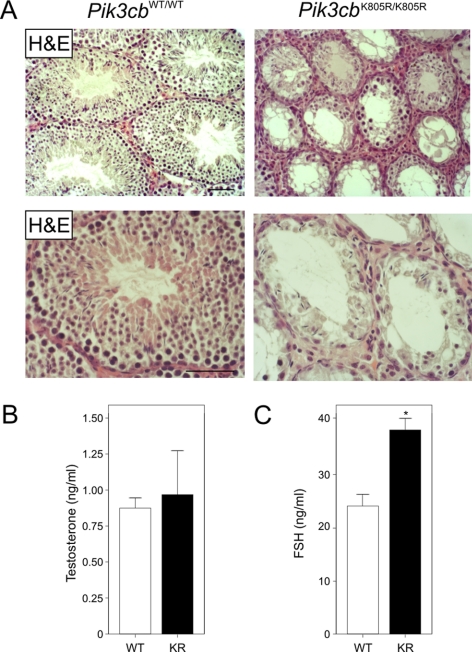
The histological architecture of Pik3cbK805R/K805R and Pik3cbWT/WT testes was next analyzed. As suggested by the gross gonadic hypotrophy of Pik3cbK805R/K805R testes, the histological organization was severely impaired in the absence of p110β catalytic activity (Figure 2A). Most strikingly, the seminiferous tubules of Pik3cbK805R/K805R mice appeared severely hypocellular compared with those of Pik3cbWT/WT testes. In addition, focal hyperplasia of the interstitial components was observed in mutant testes. Taken together, the paucity of cells within seminiferous tubules and the oligo-azoospermia of Pik3cbK805R/K805R males indicate that the loss of p110β kinase activity is associated with a major spermatogenic defect.
Figure 2.
Histological architecture and endocrine profile of Pik3cbK805R/K805R mice. (A) Histological architecture of Pik3cbWT/WT (WT) and Pik3cbK805R/K805R (KR) mice (hematoxylin-eosin staining). Scale bar, 50 μm. (B) Plasma testosterone levels (ng/ml) of adult WT and KR mice (n = 9 WT, 5 KR), measured by ELISA. The difference is not statistically significant. (C) Plasma FSH levels (ng/ml) of adult WT and KR mice (WT, n = 9; KR, n = 5), measured by IRMA. *p < 0.05, KR versus WT.
Pik3cbK805R/K805R and Pik3cbWT/WT presented comparable levels of plasma testosterone (Figure 2B), ruling out a state of endocrine hypogonadism in mutant mice. Thus, the catalytic activity of p110β does not appear to be strictly required for male steroidogenesis. Instead, FSH was significantly higher in Pik3cbK805R/K805R males (Figure 2C), confirming a condition of primary gonadic failure in mutant mice. These findings imply that the testicular phenotype of Pik3cbK805R/K805R males cannot be attributed to an endocrine defect.
Loss of p110β Activity Impairs Spermatogenesis
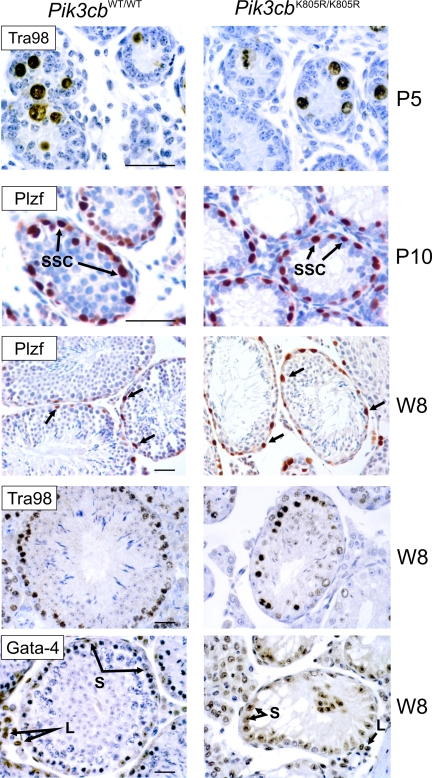
The histological architecture of Pik3cbK805R/K805R mutants prompted to a specific loss in one or more cell types within the seminiferous compartment. To rule out a primitive defect in the migration and development of germ cells in the testes, postnatal day 5 (P5) testes (i.e., earlier to the beginning of meiotic divisions) were stained for TRA98 (which marks all types of spermatogonia; Tanaka et al., 1997; Figure 3). At P5, the histological organization of the seminiferous tubules of Pik3cbK805R/K805R mice was normal and the number of TRA98-positive cells was similar in mutant and wild-type tubules, indicating normal PGC migration into gonads during embryogenesis.
Figure 3.
Loss of spermatogones in adult Pik3cbK805R/K805R testes. Representative immunostaining for Tra98 (marking undifferentiated spermatogones), PLZF (marking SSCs), and Gata-4 (L, Leydig cells; S, Sertoli cells) of testicular sections obtained from postnatal days 5 and 10 (P5 and P10) and week 16 (W16) Pik3cbWT/WT (left) and Pik3cbK805R/K805R mice (right). Testes were fixed in Carnoy and PFA, and immunostaining was obtained with a secondary antibody linked to HRP (appearing in brown and red), whereas counterstaining was performed with hematoxylin. Black arrows, SSCs. Scale bar, 50 μm.
Immunostaining for PLZF (a selective SSC marker) was then used to examine the stem cell compartment at later stages. At P10, the number of PLZF-positive cells was comparable in Pik3cbK805R/K805R and Pik3cbWT/WT mice, indicating that the absence of p110β kinase activity allows normal development of germ cells including SSCs. Instead, in adult Pik3cbK805R/K805R testes, whereas the number of PLZF-positive cells was unchanged, the number of TRA98-positive cells was significantly reduced. Thus, adult mice lacking p110β kinase activity presented a significant loss of spermatogenic cells, without detectable changes in the SSC subpopulation. These findings rule out a primary role for p110β in the maintenance of the SSC population in vivo, whereas p110β kinase activity appears necessary for the normal development of SSC-derived cells, including spermatogonia.
Finally, to uncover any concomitant defect in Sertoli and Leydig cells, testicular sections were stained for GATA-4, a transcription factor expressed in the testis which marks Sertoli and Leydig cells, but not the spermatogenic lineage (LaVoie, 2003). As shown in Figure 3, Pik3cbK805R/K805R mice presented a normal number of Sertoli cells in the seminiferous tubules, and no apparent defect was evident in their position, cell volume, and morphology. In addition, the GATA-4 staining of the interstitium of Pik3cbK805R/K805R testes did not reveal a loss of Leydig cells (Figure 3). Thus, the disruption of the seminiferous tubules in Pik3cbK805R/K805R mice is attributable to an intrinsic defect of the spermatogenic lineage and does not involve Sertoli and Leydig cells.
p110β Is Not Required for SSC Proliferation
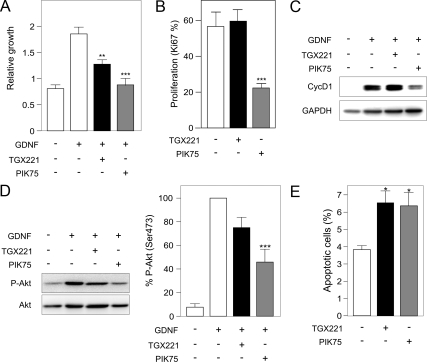
SSC proliferation is known to critically depend on the activation by GDNF of the PI3K pathway leading to Akt phosphorylation (Lee et al., 2007). To gain insight into the specific function of different PI3K isoforms in this process, primary cultures of SSCs were established as previously described and cultured in the presence of GDNF (Guan et al., 2009). Because p110α is a known key player in the proliferative response to growth factors, we tested the effects of a selective p110β inhibitor (TGX221) as well as of a less selective PI3K inhibitor mainly blocking p110α (PIK75) on the GDNF-driven cell growth of SSC. After 3 d of culture, the growth response to GDNF was slightly reduced by treatment with TGX221 but completely blunted by PIK75 (Figure 4A). In agreement, PIK75 but not TGX221 administration reduced expression of the proliferation marker Ki67 (Figure 4B and Supplemental Figure 2). Furthermore, PIK75-treated SSC showed reduced levels of cyclin D1 compared with vehicle-treated cells, whereas cyclin D1 expression was unaffected by TGX221 (Figure 4C). Taken together, these findings indicate that p110α and not p110β is required for GDNF-induced SSC proliferation in culture. Accordingly, PIK75 and not TGX221 blunted GDNF-induced Akt phosphorylation, suggesting that p110α is the main PI3K isoform activated by GDNF (Figure 4D).
Figure 4.
The absence of p110β activity reduces the survival but does not affect the proliferation of SSCs. (A) Inhibition of cell proliferation of SSCs by a specific p110β or p110α inhibitor: TGX221 (200 nM) or PIK75 (25 nM), respectively. Cells were treated with TGX221 or PIK75 for 72 h in the presence or absence of GDNF, and the relative growth was calculated in comparison to day 0. (B) Proliferation analysis. SSCs were treated with TGX221 and PIK75 for 72 h in the presence of GDNF, fixed with 5% PFA, and stained for Ki67, a positive marker for cell proliferation. The percentage of Ki67-positive cells was measured in more than 10 microscope fields. (C) Analysis of cell cycle markers. Cells were treated with TGX221 or PIK75 for 72 h in the presence or absence of GDNF, and the expression of cyclin D1 was analyzed by Western blot. (D) Analysis of Akt phosphorylation in response to GDNF after treatment with TGX221 or PIK75. SSCs were treated for 1 h with inhibitors and stimulated with 10 ng/ml GDNF. Left, a representative Western blot is presented. Right, bars represent the quantification of phosphorylated Akt on Ser473 (n = 6; **p < 0.001). (E) Analysis of apoptosis after treatment with TGX221 or PIK75. After 72 h of treatment, the percentage of apoptotic cells was quantified by TUNEL assay.
Finally, because the PI3K pathway controls both cell proliferation and death, the apoptosis of SSC was next assessed in the presence of both inhibitors. As shown in Figure 4E and Supplemental Figure 2, incubation with either TGX221 or PIK75 slightly but significantly increased the apoptotic rate of cultured SSC.
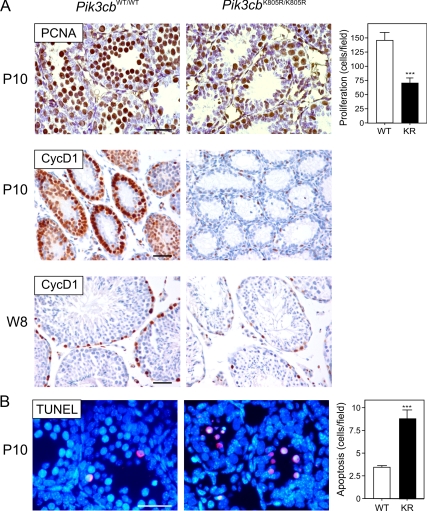
p110β Activity Is Required for the Proliferation and Survival of Spermatogones
Our findings suggested that the role of p110β in the spermatogenic process involves SSC-derived cells and not the stem cell compartment. To assess whether p110β is required for the growth and maintenance of spermatogones, we then analyzed markers of proliferation and survival at P10, a time characterized by high grade proliferation and differentiation of type A spermatogones. In P10 Pik3cbK805R/K805R pups, actively proliferating PCNA-positive cells within the seminiferous tubules were indeed reduced by nearly 50% compared with controls (Figure 5A). Moreover, the proportion of cyclin D1–positive cells was strikingly reduced in mutant tubules compared with the widespread expression of cyclin D1 in wild-type controls. A similar finding was also observed in adult testes, thus confirming that p110β is strictly required in vivo for the proliferation of spermatogones. Furthermore, TUNEL staining of P10 testes uncovered a 2.5-fold increase in apoptosis within the seminiferous tubules of Pik3cbK805R/K805R mice, whereas only a few cells were TUNEL-positive in Pik3cbWT/WT testes (Figure 5B). These findings suggested that the loss p110β kinase activity results in a dual disruption of spermatogonial proliferation and survival in the mouse testis.
Figure 5.
The absence of p110β activity reduces the survival of the spermatogenic lineage. (A) Number of actively proliferating PCNA and cyclin D1–positive cells in testicular sections of Pik3cbWT/WT (WT) and Pik3cbK805R/K805R (KR) testes obtained from postnatal day 10 (P10) and adult (W8) mice. Representative immunostaining for PCNA and cyclin D1 of a WT and a KR section are presented. PCNA and cyclin D1–positive cells appear in brown and red, respectively, whereas counterstaining was performed with hematoxylin. PCNA-positive cells were counted for each tubule in different sections. Data are presented as average ± SEM (n = 5 animals per genotype); ***p < 0.001 KR versus WT. (B) Number of TUNEL-positive cells in tubules from WT and KR mice. Representative micrographs are shown. TUNEL staining appears as a red fluorescence, whereas nuclei were stained with DAPI (blue). The data (number of TUNEL-positive cells/microscopic field) are reported as average ± SEM (n = 5 animals per genotype); *p < 0.05 KR versus WT. Scale bar, 50 μm.
SCF Requires p110β to Activate Akt Downstream of the c-Kit Receptor
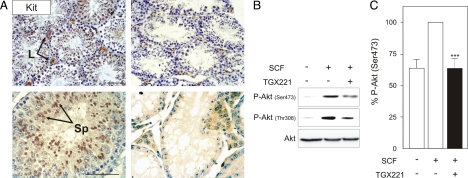
Similar to p110β inactivation, the genetic disruption of PI3K signaling downstream of the c-Kit receptor (c-KitY719F mutant) causes an early spermatogonial differentiation block, associated with reduced proliferation and extensive apoptosis of spermatogones (Blume-Jensen et al., 2000; Kissel et al., 2000). To test the involvement of p110β in c-Kit dependent spermatogenesis, we first analyzed the expression of c-Kit in Pik3cbK805R/K805R adult testes. As shown in Figure 6A, immunostaining for the c-Kit receptor revealed that mutant seminiferous tubules lost c-Kit–positive cells, whereas extensive c-Kit immunoreactivity was evident within the seminiferous tubules of Pik3cbWT/WT testes. Nonetheless, mutant testes regularly showed c-Kit–positive interstitial Leydig cells. Because the c-Kit receptor also marks type A and type B spermatogones, and to a lesser extent spermatides and spermatozoa, these findings confirmed that the blockade of p110β function selectively affects the spermatogenic lineage and leads to the loss of c-Kit–positive cells.
Figure 6.
p110β activates Akt downstream of the c-Kit receptor in spermatogones (A) Analysis of c-Kit–positive cells in Pik3cbWT/WT and Pik3cbK805R/K805R adult testis. Testes were fixed with 4% PFA (top) or Carnoy (bottom), and sections were stained with an antibody specific for the c-Kit receptor (marking Leydig cells and spermatogones). Scale bar, 50 μm. (B) Analysis of Akt and Erk phosphorylation after treatment with TGX221. Isolated spermatogones were treated with 200 nM TGX221 and stimulated with 100 ng/ml SCF. A representative Western blot is presented. (C) Bars represent the quantification of Akt phosphorylation on Ser473 (n = 6; **p < 0.01).
The present finding suggested a key role for the p110β signaling downstream of the c-Kit receptor in spermatogones. To directly test this hypothesis and to better define the involvement of p110β in the SCF/c-Kit signaling pathway, we next isolated nonadherent primary spermatogonial cells from prepubertal Pik3cbWT/WT males. As previously reported (Besmer et al., 1993), these cells expressed the c-Kit receptor (not shown). Cells were thus treated with SCF (100 ng/ml, 5 min) in the presence of a selective p110β inhibitor (TGX221, 200 nM) or vehicle (DMSO), and triggering of Akt phosphorylation was next assayed. As shown in Figure 6, B and C, treatment of primary spermatogonia with SCF induced a rapid phosphorylation of Akt, which indicated signaling through PI3K downstream of the c-Kit receptor. Of note, the SCF-dependent Akt phosphorylation was blunted by cotreatment of primary spermatogonia with TGX221. Taken together, these findings provide in vitro evidence that p110β is critically required for c-Kit signaling in spermatogones.
DISCUSSION
The present findings establish p110β as a crucial PI3K isoform controlling spermatogenesis. In mice expressing a kinase-dead p110β (Pik3cbK805R/K805R), testicles were hypotrophic and spermatogenesis was severely impaired, whereas somatic cells (Sertoli and Leydig) and plasma testosterone levels were unaffected. Therefore, the oligo-azoospermia of Pik3cbK805R/K805R mice was associated with the absence of p110β activity specifically within the spermatogenic lineage. In agreement, the increased FSH levels in mutant mice might indicate feedback pituitary activation in response to primary gonadic failure. Furthermore, this is in line with previous reports, where increased levels of gonadotropins have been observed in mice devoid of PI3K signaling downstream of the c-Kit receptor (Rothschild et al., 2003).
Despite a major loss within the spermatogenic lineage, Pik3cbK805R/K805R mice did not exhibit a detectable decrease in PLZF-positive spermatogenic cells, which represent the SSC population. In line with this observation, the selective pharmacological inhibition of p110β on cultured SSC did not significantly affect their capacity to proliferate, although a minor increase in their apoptotic rate was observed. However, this subtle change appears to be fully compensated in vivo. The negligible role of p110β in SSC survival and proliferation could be supported by the marginal contribution of p110β to Akt phosphorylation upon GDNF stimulation, as shown by the minor effect of TGX221 in this context. During embryonic development, SSC originate from a pool of embryonic precursors, the primordial germ cells (PGCs; De Felici, 2000; Saga, 2008). At 10 d postcoitum (dpc), PGCs begin to move by active migration from the endoderm into the gonadal ridges, where they start to differentiate (12.5 dpc) in SSCs. During this stage, the SCF is the major growth factor sustaining PGC migration, survival, and proliferation through its binding and activation of the c-Kit receptor (Saga, 2008). Although we demonstrate that c-Kit is potentially able to signal through p110β in spermatogenic cells, we did not observe alterations in the migration and number of PGCs. This is in line with previous reports, which have shown that the c-Kit receptor does not require PI3K signaling during the earlier stages of gonadic development (Blume-Jensen et al., 2000; Kissel et al., 2000). Taken together, our results thus support the view that p110β is neither necessary for the migration and proliferation of PGCs in the embryonic gonads, nor for the maintenance of the SSC population in the adult testis, whereas this enzyme is critically involved in later developmental stages. This finding is relevant, because p110β has been implied in the regulation of the self-renewal and pluripotency of embryonic stem cells (Kingham and Welham, 2009). Thus, the participation of p110β in the biology of stem cells may be strictly dependent upon their specific machinery. Instead, we report that the pharmacological inhibition with the potent PI3K inhibitor PIK75 could limit SSC proliferation in vitro, by blunting GDNF-induced Akt activation. Although in vivo studies are necessary to fully address this issue, it is reasonable that p110α was the isoform blocked by PIK75 and involved in controlling the self-renewal of SSC. Accordingly, treatment with other pan-PI3K inhibitors is already known to prevent SSC self-renewal (Lee et al., 2007).
The absence of p110β activity caused a dramatic loss of spermatogones and their more differentiated progeny, thus implying the involvement of p110β in the signaling of a crucial agonist of spermatogonial expansion and differentiation such as SCF. This growth factor is known to trigger the class IA PI3K/Akt axis to promote spermatogenesis, as genetic deletion of the PI3K-binding site on c-Kit (c-KitY719F) causes azoospermia (Blume-Jensen et al., 2000; Kissel et al., 2000). Interestingly, our results indicate that the lack of p110β activity closely phenocopied this c-Kit mutation. Indeed, because SCF-dependent Akt activation was significantly blocked in spermatogones after treatment with TGX221, it is reasonable that Akt phosphorylation downstream of c-Kit mostly depended on p110β. Consistent with the role of p110β downstream c-Kit, adult Pik3cbK805R/K805R testes lost both c-Kit–positive type B spermatogones and spermatocytes. However, the presence of a residual spermatozoa production, albeit not adequate to support full fertility, might not completely rule out a minor role for p110α in spermatogenesis.
Recent studies have reported that p110β activity is mainly associated with GPCRs (Guillermet-Guibert et al., 2008). However, the engagement of PI3Kβ by RTKs has also emerged in multiple cell types (Ciraolo et al., 2008; Guillermet-Guibert et al., 2008; Jia et al., 2008; Canobbio et al., 2009). In mast cells, c-Kit selectively couples to PI3Kδ, modulating the proliferation, adhesion, and migration of these cells (Ali et al., 2004). However, p110δ expression is restricted to leukocytes and is absent in the spermatogenic lineage. Overall, these observations support the view that c-Kit drives different PI3K isoenzymes in distinct cell types and that in general, selected RTKs may preferentially signal through p110β or p110δ and not through p110α, depending on the cellular contexts. Because fully selective p110α inhibitors are not yet commercially available, further studies are needed to understand whether this isoform cooperates with p110β in c-Kit–mediated spermatogenesis. However, independently of p110α, p110β may be engaged by RTKs through a possible cross-talk between c-Kit and a GPCR yet to be determined. Similarly, it cannot be excluded that p110β activation by c-Kit receptor may be mediated by Ras activation (Marques et al., 2009).
The present findings may have critical implications for the development and therapeutic use of the PI3K inhibitors currently under development and/or assessment in clinical trials. Our data, indeed, imply potential side effects on male fertility of drugs targeting the p110β isoform but also open the way to the use of more selective PI3Kβ inhibitors in c-Kit–positive testicular cancers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alessia Perino and Laura Braccini for technical help; Peter Shepherd (Department of Molecular Medicine and Pathology, University of Auckland, Auckland, New Zealand) for inhibitors; and Guido Tarone for constructive discussions. This work was supported by a grant from University of Torino (ex 60%), PRIN, Telethon, Italian Association for Cancer Research (AIRC) to E.H. and G.F., the Sixth Framework Programme EUGeneHeart and Fondation Leducq to E.H., National Institutes of Health Grant GM55692 to J.M.B., German Federal Ministry of Education and Research (BMBF) to K.G., the EU FP6 Grant BBW 03.0441-3/LSHG-CT-2003-502935, Swiss Natl. Foundation grant 3100A0-109718 and Oncosuisse OCS-01924-08-2006 to M.P.W., and the Regione Piemonte Ricerca Sanitaria Finalizzata 2008 bis and 2009 to F.M.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-08-0744) on January 6, 2010.
REFERENCES
- Ali K., et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- Besmer P., Manova K., Duttlinger R., Huang E. J., Packer A., Gyssler C., Bachvarova R. F. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. 1993;(Suppl.):125–137. [PubMed] [Google Scholar]
- Bi L., Okabe I., Bernard D. J., Nussbaum R. L. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm. Genome. 2002;13:169–172. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Jiang G., Hyman R., Lee K. F., O'Gorman S., Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet. 2000;24:157–162. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- Canobbio I., Stefanini L., Cipolla L., Ciraolo E., Gruppi C., Balduini C., Hirsch E., Torti M. Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114:2193–2196. doi: 10.1182/blood-2009-03-208074. [DOI] [PubMed] [Google Scholar]
- Ciraolo E., et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci. Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M. Regulation of primordial germ cell development in the mouse. Int. J. Dev. Biol. 2000;44:575–580. [PubMed] [Google Scholar]
- Engelman J. A., Luo J., Cantley L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Guan K., Nayernia K., Maier L. S., Wagner S., Dressel R., Lee J. H., Nolte J., Wolf F., Li M., Engel W., Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Guan K., Wolf F., Becker A., Engel W., Nayernia K., Hasenfuss G. Isolation and cultivation of stem cells from adult mouse testes. Nat. Protoc. 2009;4:143–154. doi: 10.1038/nprot.2008.242. [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. USA. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E., Ciraolo E., Ghigo A., Costa C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacol. Ther. 2008;118:192–205. doi: 10.1016/j.pharmthera.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Jia S., et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kingham E., Welham M. Distinct roles for isoforms of the catalytic subunit of class-IA PI3K in the regulation of behaviour of murine embryonic stem cells. J. Cell Sci. 2009;122:2311–2321. doi: 10.1242/jcs.046557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel H., Timokhina I., Hardy M. P., Rothschild G., Tajima Y., Soares V., Angeles M., Whitlow S. R., Manova K., Besmer P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D., et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie H. A. The role of GATA in mammalian reproduction. Exp. Biol. Med. 2003;228:1282–1290. doi: 10.1177/153537020322801107. [DOI] [PubMed] [Google Scholar]
- Lee J., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Kimura T., Nakano T., Ogura A., Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lyman S. D., Jacobsen S. E. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91:1101–1134. [PubMed] [Google Scholar]
- Marques M., Kumar A., Poveda A. M., Zuluaga S., Hernandez C., Jackson S., Pasero P., Carrera A. C. Specific function of phosphoinositide 3-kinase beta in the control of DNA replication. Proc. Natl. Acad. Sci. USA. 2009;106:7525–7530. doi: 10.1073/pnas.0812000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G., Sottas C. M., Kissel H., Agosti V., Manova K., Hardy M. P., Besmer P. A role for kit receptor signaling in Leydig cell steroidogenesis. Biol. Reprod. 2003;69:925–932. doi: 10.1095/biolreprod.102.014548. [DOI] [PubMed] [Google Scholar]
- Saga Y. Mouse germ cell development during embryogenesis. Curr. Opin. Genet. Dev. 2008;18:337–341. doi: 10.1016/j.gde.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Serve H., Hsu Y. C., Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3-kinase activity in COS-1 cells. J. Biol. Chem. 1994;269:6026–6030. [PubMed] [Google Scholar]
- Sette C., Dolci S., Geremia R., Rossi P. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int. J. Dev. Biol. 2000;44:599–608. [PubMed] [Google Scholar]
- Tanaka H., Pereira L. A., Nozaki M., Tsuchida J., Sawada K., Mori H., Nishimune Y. A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int. J. Androl. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Wee S., Wiederschain D., Maira S. M., Loo A., Miller C., deBeaumont R., Stegmeier F., Yao Y. M., Lengauer C. PTEN-deficient cancers depend on PIK3CB. Proc. Natl. Acad. Sci. USA. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann M. P., Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr. Opin. Cell Biol. 2005;17:141–149. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.