We look inside neurons in vivo and identify major cytoskeletal rearrangements that allow a dendrite to become a regenerating axon.
Abstract
Axon regeneration is crucial for recovery after trauma to the nervous system. For neurons to recover from complete axon removal they must respecify a dendrite as an axon: a complete reversal of polarity. We show that Drosophila neurons in vivo can convert a dendrite to a regenerating axon and that this process involves rebuilding the entire neuronal microtubule cytoskeleton. Two major microtubule rearrangements are specifically induced by axon and not dendrite removal: 1) 10-fold up-regulation of the number of growing microtubules and 2) microtubule polarity reversal. After one dendrite reverses its microtubules, it initiates tip growth and takes on morphological and molecular characteristics of an axon. Only neurons with a single dendrite that reverses polarity are able to initiate tip growth, and normal microtubule plus-end dynamics are required to initiate this growth. In addition, we find that JNK signaling is required for both the up-regulation of microtubule dynamics and microtubule polarity reversal initiated by axon injury. We conclude that regulation of microtubule dynamics and polarity in response to JNK signaling is key to initiating regeneration of an axon from a dendrite.
INTRODUCTION
Neurons are highly polarized cells; many neurons have several dendrites that receive information and a single axon to send information. Axons and dendrites have distinct proteins targeted to them, as well as different cytoskeletal arrangements (Craig and Banker, 1994). Unlike many other cell types, most neurons are not replaced during an animal's lifetime, yet they can be damaged by physical traumas or immune attack. Complete axon removal is the most severe type of axon injury a neuron can sustain, and it renders the cell nonfunctional as it can no longer send signals. Studies performed in several systems suggest that neurons have a tremendous capacity for axon regeneration, even in response to total axon removal.
In sea lampreys, complete removal of axons from hindbrain neurons triggers new growth from dendrites that extends beyond the normal dendritic field (Hall and Cohen, 1983). This is in contrast to axon injuries further from the cell body that induce regrowth from the axon as well as increased dendritic growth. Subsequent analysis showed that at the ultrastructural level the new processes emerging from dendrites after axon removal had axonal features, for example, abundant neurofilaments (Hall et al., 1989). Similar observations of growth of axon-like processes from dendrites in vivo have been made in cat motoneurons and interneurons after proximal axotomy (Rose et al., 2001; MacDermid et al., 2002; Fenrich et al., 2007), as well as several types of rodent neurons (Cho and So, 1992; Hoang et al., 2005). Axon removal experiments have also been performed in cultured neurons. Axons of mouse hippocampal neurons in dissociated and slice culture were severed at different distances from the cell body (Gomis-Ruth et al., 2008). As in earlier in vivo studies, close axotomy induced new growth from dendrites. These new processes contain tau immunoreactivity, like axons, and are presynaptic (Gomis-Ruth et al., 2008). Thus in several systems, neurons initiate regeneration after axon removal by converting a dendrite to a new axon, or at least growing a new axon from the tip of a dendrite.
It is not known how neuronal polarity is completely rearranged after axon removal to allow a dendrite to become or grow a new axon. Microtubules in axons and dendrites have different polarity, and several different approaches have suggested that this arrangement is crucial for overall neuronal polarity. In cultured mammalian neurons, axons have uniform polarity microtubules with plus ends distal to the cell body (plus-end-out), whereas dendrites have mixed polarity with about half plus-end-out and half minus-end-out microtubules (Baas et al., 1988; Stepanova et al., 2003). The minus-end-out microtubules in dendrites have been linked to dendritic identity. In dissociated hippocampal neurons, dendrite specification and growth occurs at the same time that minus-end-out microtubules enter dendrites (Baas et al., 1989), and loss of the minus-end-out population of microtubules from dendrites results in overall loss of dendritic character (Yu et al., 2000). Thus if a dendrite becomes or grows an axon one might expect that microtubule polarity would have to be rearranged.
In fact, the process of regenerating an axon from a dendrite does seem to be accompanied by changing the microtubule cytoskeleton from the dendritic polarity to the axonal one. When cortical neurons from newborn rat are dissociated, the axon is lost, but a region of the apical dendrite that has mixed microtubule polarity can remain attached to the cell body. Thus dissociation of the cells causes total axon removal. Within 24 h of plating in culture, growth initiates from the tip of the dendritic process, and the process has switched to the axonal plus-end-out microtubule polarity (Takahashi et al., 2007). It was suggested that this shift in microtubule polarity is accomplished by loss of the minus-end-out microtubules from the growing process (Takahashi et al., 2007). It has also been suggested that an increase in microtubule stability in the growing process is key to the conversion of a dendrite to a regenerating axon (Gomis-Ruth et al., 2008). So the best current model for conversion of a dendrite to an axon after complete axotomy is loss of the minus-end-out microtubules from the mixed population and a concomitant increase in stability of the remaining plus-end-out ones. As this model derives from examining the arrangement of microtubules in fixed preparations at single time points, we wanted to determine how microtubules are rearranged over time as neurons respond to axon injury. We also wanted to examine neurons in vivo in their normal environment to eliminate any confounding changes induced by cell culture.
Like vertebrate neurons, Drosophila neurons are very polarized (Sanchez-Soriano et al., 2005; Rolls et al., 2007); moreover their cytoskeleton can be studied in vivo. We and others have previously used live imaging to study the layout of Drosophila microtubules in neurons in living larvae (Rolls et al., 2007; Satoh et al., 2008; Stone et al., 2008; Zheng et al., 2008) and have found that microtubules in all major classes of Drosophila neurons have opposite polarity in axons and dendrites (Stone et al., 2008). The axonal arrangement of microtubules is plus-end-out, as in other animals, but the dendrites have a more polarized microtubule cytoskeleton than cultured mammalian neurons and have greater than 90% minus-end-out microtubules. This arrangement of dendritic microtubules suggests either that Drosophila neurons will not be able to regenerate an axon from a dendrite because they do not have a significant population of plus-end-out microtubules in dendrites, or that the current model for conversion of a dendrite to a regenerating axon is incomplete. Using this tractable model system, we tested whether highly polarized neurons in vivo can really reverse polarity and identified major microtubule rearrangements that are required for initiation of regeneration of an axon from a dendrite.
MATERIALS AND METHODS
System to Study Neuronal Responses to Injury
Drosophila dendritic arborization (da) neurons tile the larval body wall in a stereotyped manner (Grueber et al., 2002). Class I da neurons have a simple dendritic tree and exhibit little structural plasticity over the course of larval life (Sugimura et al., 2003). Two class I da neurons are present in the dorsal region of each hemisegment. We used ddaE as our model neuron as it is easy to identify and visualize. EB1-green fluorescent protein (GFP) can be used to monitor microtubule polarity as it only binds to growing microtubule plus ends. The direction of EB1-GFP comet movement over time therefore points toward the plus end. We scored direction of comets that were visible for three or more consecutive frames. Comets that moved toward the cell body were at tips of minus-end-out microtubules; comets that moved away from the cell body were indicative of plus-end-out microtubules.
Axons were severed in larvae with a UV laser. This method has previously been used to sever da neuron dendrites (Sugimura et al., 2003) and axons of Caenorhabditis elegans neurons in vivo (Wu et al., 2007). After axon severing, we acquired movies of EB1-GFP and then put the animals back in food to recover. They could be mounted for imaging multiple times after this treatment and followed over 4 d, at which point they initiated pupariation.
Genetic Background, Imaging, and Culturing Conditions
Whole, live larvae heterozygous for the class I da neuron specific driver 221-Gal4 and UAS-EB1-GFP were collected 48–72 h after egg laying, mounted on a microscope slide with a dry agarose pad, and covered with a glass coverslip. Animals containing 221-Gal4, UAS-EB1-red fluorescent protein (RFP), and UAS-Apc2-GFP transgenes were collected similarly. Dendrites and axons of class I dendritic arborization neurons were severed in these larvae using a Micropoint UV laser (Photonic Instruments, St. Charles, IL). Live imaging was performed using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY). Single frames were collected every 2 s to image EB1 dynamics in the cell bodies, dendrites, and axons of injured and uninjured neurons. Immediately after imaging, animals were recovered from microscope slides by adding Schneider's insect media to release them from the agarose pad and placed into standard Drosophila media for recovery until further imaging. Images were analyzed using ImageJ software (http://rsb.info.nih.gov/ij/; NIH).
Quantitation of Microtubule Number and Polarity
For all experiments one neuron per animal was analyzed, and the number of animals for each condition is indicated in the figures as n's. To analyze microtubule orientation, EB1-GFP comets were tracked manually in the region between the cell body and the first branch point of the dendrite for wild type and throughout the whole dendrite for bsk RNAi or bskDN (dominant negative) and only comets that were tracked over a minimum of three consecutive frames were counted.
Quantitation of number of EB1-GFP comets in the cell body and dendrites of injured and uninjured neurons was done by taking the first in-focus frame in a movie and subsequent fifth in-focus frames. This was done until a total of three frames were analyzed. EB1-GFP comets were counted in the still images of these frames.
Neuronal RNAi and Dominant Negative Experiments
For the RNAi experiments we crossed a tester line, UAS-dicer2; 221-Gal4, UAS-EB1-GFP, to lines that express hairpin RNAs under UAS control and analyzed microtubule dynamics as above in the ddaE neuron in larval progeny. The 221-Gal4 drives expression of the GFP, dicer2, and RNA hairpin only in class I dendritic arborization neurons and a few other cells, meaning that most of the animal is normal. The RNA hairpin lines were from the VDRC collection (Dietzl et al., 2007). The Vienna Drosophila RNAi Center (VDRC, Vienna, Austria) stock numbers used to target proteins of interest were as follows: 34138 (bsk) and 21982 (msps). As a control the tester line was crossed to a line containing a different transgene, UAS-mCD8-RFP.
For bskDN expression we crossed the line w1118, P{UAS-bsk.DN}2 (Bloomington Drosophila Stock Center, Bloomington, IN) to 221-Gal4, UAS-EB1-GFP to generate larvae heterozygous for all transgenes. As a control for this experiment, we crossed the 221-Gal4, UAS-EB1-GFP line to a line containing a different transgene: UAS-mCD8-RFP, so that the 221-Gal4 would be driving expression of the same number of transgenes in both cases.
RESULTS
The Number of Growing Microtubules Is Dramatically Up-regulated by Axon, But Not Dendrite, Severing
To test how neurons in vivo respond to axon removal, we generated Drosophila larvae that express a microtubule plus end–binding protein tagged with a GFP, EB1-GFP, in a small subset of dendritic arborization sensory neurons. These neurons have branched dendrites reminiscent of mammalian dendrites (Grueber et al., 2002) and are ideal for live imaging because they lie immediately under the larval cuticle. Moreover, although these cells are sensory, the arrangement of microtubules in their dendrites is similar to that in Drosophila central neurons (Stone et al., 2008). To further simplify the analysis, we focused on the ddaE cell on the dorsal side of the larva. This cell is one of the class I dendritic arborization neurons and has a relatively simple dendrite branching pattern (Grueber et al., 2002).
To sever axons in these neurons in vivo, we mounted larvae on a slide and focused a pulsed UV laser on the region of the cell we wanted to cut. This method has previously been used to sever Drosophila dendrites (Sugimura et al., 2003) and Caenorhabditis elegans axons (Wu et al., 2007). Using this method, we successfully generated small cuts in axons or dendrites (Figure 1A) that resulted in degeneration of the region distal to the cut site by 24 h (Figure 1A). After injury larvae were recovered to media and allowed to develop as normal. They were remounted for imaging at 24-h intervals.
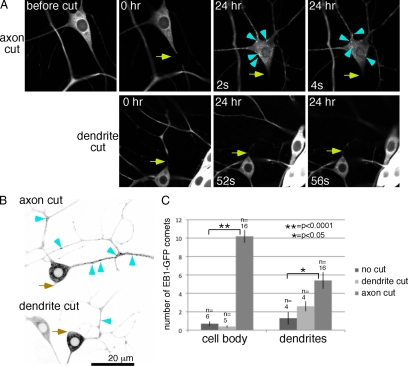
Figure 1.
The number of growing microtubules is up-regulated by axon, but not dendrite, severing. (A) Images of EB1-GFP in the ddaE neuron were acquired before, immediately after (0 h), and 24 h after axon (top row) or dendrite (bottom row) severing. Two frames are shown from each movie from the 24-h time point. Arrows indicate the site of UV laser-mediated severing. Arrowheads point out examples of EB1-GFP comets in the cell body. In all figures dorsal is up. (B) Panels from movies acquired 24 h after axon or dendrite severing are shown. Images were inverted for ease of identifying EB1-GFP comets in dendrites; examples are marked with arrowheads. (C) The number of EB1-GFP comets in the cell body or a region of dendrite 2 was counted in single frames from movies of uninjured neurons and from neurons 24 h after axon or dendrite severing. Three frames were averaged for each animal, error bars, SD of the average from all animals. n = number of animals scored (one neuron per animal). Unpaired t tests were used to determine whether the number of comets was significantly increased after dendrite or axon cutting. No significant difference between number of dots in the cell body before and after dendrite cutting was found. Significant differences were found for cell bodies and dendrites before and after axon cutting.
The first alteration in EB1-GFP dynamics we observed after axon severing was unexpected. Rather than an increase in microtubule stability, which would be detected as fewer growing microtubule ends labeled with EB1-GFP, we saw a large increase in the number of EB1-GFP comets. This increase was initiated in some cells immediately after axon severing (Movie 1) and was always seen at 24 h (Figure 1 and Movies 1–3). Before axotomy, EB1-GFP comets at tips of growing microtubules were seen occasionally in the cell body. Twenty-four hours after axotomy, many EB1-GFP comets were seen swirling like a whirlpool in the neuronal cell body (Figure 1A and Movies 1 and 2). The number of EB1-GFP comets has previously been used as a readout of total number of dynamic microtubules (Piehl et al., 2004), so we interpret this increase in comet number as an increase in number of growing microtubules, and it is likely that all neuronal microtubules exhibit growth at their plus ends.
Comparison of the number of EB1-GFP comets in uninjured neurons with neurons after axotomy revealed a more than 10-fold increase in number of comets in the cell body 24 h after axon severing (Figure 1C). The number of comets also increased throughout the dendritic tree (Figure 1, B and C). Although the number of EB1-GFP comets was increased by axon removal, the rate of microtubule growth was not: before severing the average velocity of EB1-GFP comets was 0.225 μm/s (SD, 0.043, data are from 40 microtubules in six animals), and after severing it was 0.229 μm/s (SD, 0.068, data are from 37 microtubules in eight animals).
Because this increase in number of growing microtubules was unexpected, we wanted to determine whether it was a general response to neuronal injury or a specific response to axon severing. We therefore severed dendrites close to the cell body and monitored EB1-GFP dynamics after this injury. Dendrite severing did not increase number of EB1-GFP comets in the cell body or remaining dendrites (Figure 1 and Movie 2), indicating that this is a specific response to axon injury. Thus although injury to both axons and dendrites is predicted to cause a breach in the plasma membrane and ion influx, only axon injury triggers a global increase in dynamic microtubule number.
Microtubule Polarity Switching in Dendrites after Axon Removal
As well as monitoring the number of EB1-GFP comets after axon removal, we monitored their direction of movement in dendrites to determine whether a change in microtubule polarity might be triggered by loss of the axon (Figure 2A and Movie 3). EB1-GFP binds only to growing microtubule plus ends, so the direction of movement gives a readout of microtubule polarity (Stepanova et al., 2003; Stone et al., 2008). To simplify comparisons between neurons, we numbered each dendrite of the ddaE dendritic arborization neuron (Figure 2). The dendrite with a comb-like-branching pattern was numbered 1; the dendrite closest to the axon site was numbered 2. The presence of a third (or fourth) dendrite was variable. If these were present, they were numbered 3 and 4.
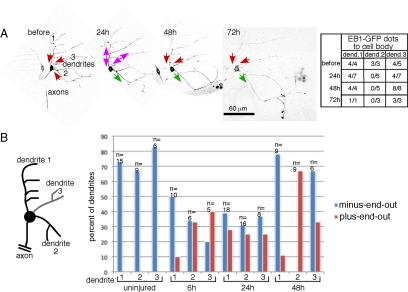
Figure 2.
Axon injury induces orientation switching of dendritic microtubules. (A) Images of EB1-GFP in a single ddaE neuron at different times before or after axon injury. Several confocal images were projected to give a complete overview of the dendrite arbor. In zoomed in movies, the direction of EB1-GFP comet movement was scored. Comets moving toward the cell body represent minus-end-out microtubules and comets moving away from the cell body represent plus-end-out microtubules. The raw data are shown in the table and represented by arrows on the overview pictures. A green arrow indicates plus-end-out microtubule orientation, a red arrow indicates minus-end-out orientation and double arrow indicates mixed orientation. Movie 3 shows microtubule dynamics in this cell. (B) Microtubule orientation was quantitated in dendrites of uninjured neurons and neurons at different times after axon severing. Comets were scored in each dendrite as in A: EB1-GFP dots in the region of the dendrite between the cell body and first dendrite branch point were counted; dendrites with four or more comets were classified as plus-end-out if 75% or more comets moved away from the cell body and minus-end-out if 75% or more moved toward the cell body. The class in between was classified as mixed and is not shown explicitly in the table. n = number of dendrites classified for each time point.
In uninjured neurons, comets in most dendrites moved toward the cell body consistent with minus-end-out microtubule orientation (Figure 2). Minus-end-out microtubule orientation is found in all types of Drosophila dendrites and distinguishes them from axons that have plus-end-out microtubules (Stone et al., 2008). Dendrites with plus-end-out microtubule orientation were never observed before axon injury (Figure 2B). By 6 h after proximal axon severing, dendrites were frequently observed to have the axonal plus-end-out microtubule orientation (Figure 2B). The time period from 6 to 24 h seemed to be a transition period; by 48 h many of the neurons had one dendrite with plus-end-out microtubules, and the remaining dendrites had reverted to minus-end-out orientation (Figure 2B). In most cases dendrite 2 was the one that acquired the axonal microtubule arrangement. Thus microtubule polarity could be completely reversed in a dendrite after axon removal.
A Dendrite That Switches to Plus-End-Out Microtubules Can Become a Growing Axon
To determine whether the dendrite that acquired the axonal plus-end-out microtubule orientation had other axonal properties and could initiate growth, we performed experiments over a longer time course. At 72 and 96 h after axon severing, many processes with plus-end-out microtubules had a bulbous structure at the end. The plus-end-out process also initiated extension in many cases (Figures 2A and 3, C and D, and Figure S1B). Six of nine neurons in one experiment initiated growth from a dendrite tip. The neurons that initiated tip growth were distinguished by having a single process that switched to plus-end-out microtubule orientation (Figure S2). In these neurons, the remaining minus-end-out processes did not extend from their tips; they continued the normal behavior of ddaE dendrites, which is to expand all over as the animal grows (Sugimura et al., 2003). In this normal, all-over expansion, the distance between the last branch point, and dendrite tip does not increase at a greater rate than the distance between internal branch point. Normal dendrite expansion rather than tip growth was also observed in time-course experiments after dendrite removal (Figure 3, A and B; n = 5; note the same V-shape is seen at the remaining dendrite tips at all time points). Thus tip growth is a specific response to axon removal rather than a general response to injury.
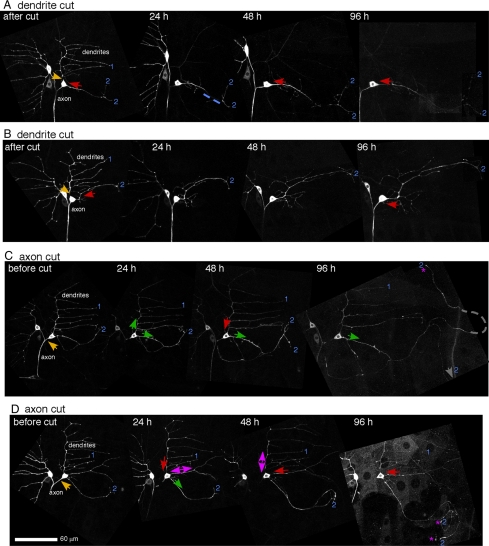
Figure 3.
Axon, but not dendrite, injury induces extensive tip growth from a dendrite. The axon or dendrite of the ddaE neuron was severed with a UV laser, and EB1-GFP was imaged at different time points. Overview images were compiled from movies of EB1-GFP, and microtubule orientation was scored as in Figure 2. Yellow arrows, site of laser severing; red arrows, minus-end-out microtubules; green arrows, plus-end-out microtubules; double arrows, mixed orientation. Stars label tips of processes that have extended by 96 h. Six of nine cells in which the axon was removed initiated tip growth and 0 of 5 in which a dendrite was removed initiated tip growth. The dendrites are numbered as in Figure 2. Movie frames were Z projected, maximum method, to show the entire dendritic tree. In some cases several frames of a movie had to be assembled next to one another to cover the complete area of the dendrites. The images were also rotated and placed on a black background so that the neuron would be seen in the same orientation at all time points. Scale is the same for all images.
As well as initiating tip growth, the plus-end-out process generated after axon removal lost key dendritic features. Class I da neuron dendrites normally exhibit tiling behavior with one another and do not cross (Grueber et al., 2003). Growing processes with plus-end-out microtubules could cross neighboring processes from the same cell (Figure S1B). Dendrites from these cells are also normally restricted to a subregion of the larval body, but growing processes often extended beyond normal dendritic territory (Figure 3C and Figure S1A).
To test more rigorously whether dendrites that initiated tip growth had the molecular characteristics of axons, we tracked the behavior of Apc2-GFP in ddaE after axon severing. There are very few markers that localize robustly to axons or dendrites in Drosophila when tagged and overexpressed. One of the most robust dendrite markers is Apc2-GFP (Rolls et al., 2007). In Drosophila there are two APC (adenomatous polyposis coli) proteins. A tagged version of one of these, Apc2-GFP, expressed in Drosophila central neurons localizes to spots in dendrites, to the cell body, and to the first part of the axon, but is cleanly excluded from distal axons (Rolls et al., 2007). When we expressed Apc2-GFP in da neurons we saw a similar pattern of fluorescence, with distinct spots of Apc2-GFP in dendrites, Apc2-GFP in some, but not all, proximal axons (Figure 4, A–C), but not in distal axons (Figure 4D). At 96 h after axon removal, when one process with plus-end-out microtubules exhibited significant growth, Apc2-GFP was seen in puncta throughout most of the dendritic tree, but was not seen in the new region (Figure 4, B and C; of 12 animals in which tip growth was initiated, Apc2-GFP was never seen in the new region). Apc2-GFP was seen at the base of the process with plus-end-out microtubule orientation, consistent with localization of Apc2-GFP in proximal axons or failure to clear Apc2-GFP from the region that was previously a dendrite. This data strongly argues that the process with plus-end-out microtubules has been respecified as a regenerating axon. Thus even a mature dendrite in vivo in a cell that normally exhibits no structural plasticity (Sugimura et al., 2003) can be respecified as an axon and part of this conversion is total reversal of microtubule polarity throughout the entire process, including the region that was previously a dendrite.
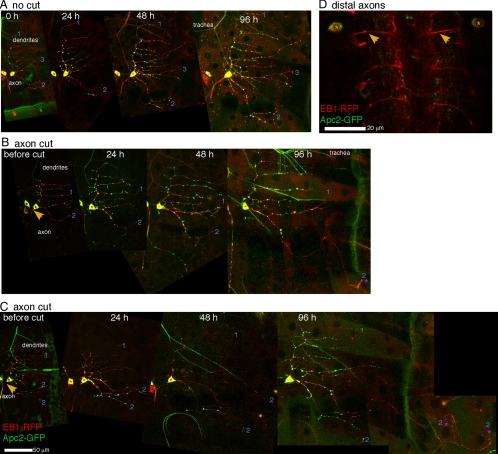
Figure 4.
Apc2-GFP is excluded from growing processes. Apc2-GFP and EB1-RFP were expressed in ddaE neurons. (A) Uninjured neurons were imaged over the same time course used in other experiments. At all times Apc2-GFP is seen in spots throughout the dendritic arbor. (B and C) Axon-severing experiments were performed as in Figure 3. In both cells shown tip growth is initiated from dendrite 2. Apc2-GFP is only found in the proximal region of this process at 96 h after axon removal, and this pattern was seen in a total of 12 of 12 neurons which initiated tip growth. (D) The ddaE neuron extends its axon from the body wall to the ventral ganglion. In live animals expressing Apc2-GFP and EB1-RFP in class I DA neurons, EB1-RFP can be seen in the distal axons that enter the ventral ganglion. Apc2-GFP is not seen in these axons. See Figure S4 for greyscale images of Apc2-GFP alone.
Respecification of a Dendrite to an Axon Requires msps-stimulated Microtubule Dynamics
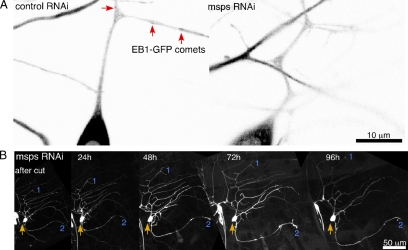
As the microtubule cytoskeleton was dramatically rearranged after axon removal, we hypothesized that regulation of microtubule dynamics would be essential to respecifying a dendrite as an axon and initiating growth. To test this hypothesis we searched for genetic methods to reduce microtubule dynamics. We found that targeting the msps transcript by RNAi almost completely eliminated EB1-GFP comets from neurons (Figure 5A and Movie 4). In control (rtnl2) RNAi neurons EB1-GFP comets are seen frequently throughout the dendritic tree (Figure 5A and Movie 5), similar to uninjured neurons that do not express hairpin RNAs or neurons after dendrite severing (Figure 1 and Movies 1 and 3). This result is consistent with models of microtubule growth in which msps (or XMAP215 in vertebrates) acts as a microtubule polymerase (Brouhard et al., 2008; Howard and Hyman, 2009), and loss of msps in vivo results in increased microtubule pausing (Brittle and Ohkura, 2005). We confirmed that although microtubule plus ends do not behave normally in neurons with reduced msps, stable microtubules are present (Figure S3A), and neuronal structure is quite normal throughout larval life, with the exception that in some larvae increased dendritic branching was observed close to the cell body (Figure S3B).
Figure 5.
RNAi targeting msps blocks regeneration from a dendrite after axon removal. (A) The cell body and proximal dendrite of ddaE neurons expressing EB1-GFP and hairpin RNAs that target rtnl2 (control) or msps are shown. rtnl2 RNAi was used as a control as its loss has no known consequences in flies. EB1-GFP comets (red arrows) can be seen in control, but not msps RNAi neurons. (B) An axon-severing experiment as in Figure 3 was performed on a ddaE neuron expressing EB1-GFP and msps hairpin RNA. Cell shape (but not microtubule polarity, as no EB1-GFP comets were present) were tracked over time. No tip growth was observed (n = 5).
When ddaE neurons expressing msps RNAi hairpins were subjected to axon removal, in all cases they failed to initiate growth from a dendrite tip (Figure 5B, n = 5). As in uninjured larvae, the shape of the dendritic tree remained constant through larval life. We therefore conclude that msps-stimulated microtubule dynamics are not required for maintenance of normal dendrite structure during larval life, but are required to convert a dendrite into a regenerating axon, supporting the role of microtubule plus-end dynamics in this process.
c-Jun N-terminal Kinase Activation Is Required for All Identified Responses to Axon Removal
We have identified two major types of microtubule reorganization in response to axon removal: increased number of growing microtubules and polarity reversal. To identify the upstream signals that trigger these rearrangements, we took a candidate approach. c-Jun N-terminal kinase (JNK), or related mitogen-activated protein (MAP) kinases, have been implicated in axon regeneration in multiple systems, including C. elegans, Drosophila, and mammals (Ayaz et al., 2008; Hammarlund et al., 2009; Itoh et al., 2009). In each of these systems these kinases were shown to be important for initiating growth from an axon stump after distal axon severing, and it has been hypothesized that unidentified rearrangements of microtubules might be important for this growth (Hammarlund et al., 2009). We therefore hypothesized that JNK signaling could play a role in the response to proximal axon severing, although this initiates growth from a dendrite rather than extension from the axon stump.
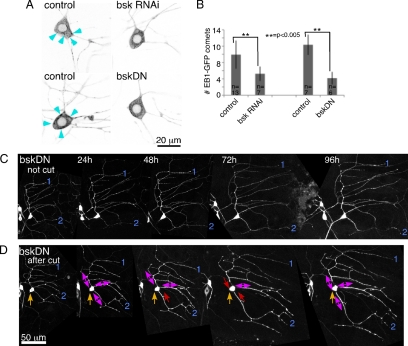
We used two methods to block JNK signaling, expression of RNA hairpins to target bsk, the Drosophila JNK, and expression of a nonphosphorylatable dominant negative JNK (bskDN; Adachi-Yamada et al., 1999). Control neurons expressing a different hairpin RNA (targeting rtnl2) had normal shape, and the dendrite arbor remained stable through larval life (not shown). After axon severing, the increase in number of growing microtubules was significantly blocked in bsk RNAi and bskDN neurons compared with neurons expressing a different transgene, mCD8-RFP (Figure 6, A and B). We conclude that one of the earliest changes in the microtubule cytoskeleton induced by axon injury, up-regulation of dynamic microtubule number, requires JNK activity.
Figure 6.
JNK is required for up-regulation of microtubule number and initiation of growth in response to axon removal. (A) ddaE neurons expressing EB1-GFP and mCD8-RFP (control), RNAi hairpins to target bsk, or bskDN, were imaged 24 h after axon removal. Numerous EB1-GFP comets (arrowheads) were seen in control neurons, and many fewer were seen is bskRNAi or bskDN neurons. (B) EB1-GFP comets in the cell body were quantitated 24 h after axon injury. Number of comets in individual frames of movies was counted as in Figure 1C. Genotypes of the larvae in order shown in the table were as follows: 1) UAS-Dicer2/UAS-mCD8-RFP; 221-Gal4, UAS-EB1-GFP/+; 2) UAS-Dicer2/UAS-bskRNAi; 221-Gal4, UAS-EB1-GFP/+; 3) UAS-mCD8-RFP/+; 221-Gal4, UAS-EB1-GFP/+; and 4) UAS-bskDN/+;; 221-Gal4; UAS-EB1-GFP. (C and D) A ddaE neuron expressing EB1-GFP and bskDN was tracked over time. The dendrite arbor of these cells retained the same shape over time as in controls (n = 6). In D, microtubule orientation was determine as in Figure 3, except that comets were quantitated throughout major dendrites because fewer comets were present. Red arrows, minus-end-out polarity (>75% of comets to the cell body); double arrows, mixed polarity (25–75% of comets to the cell body).
To determine whether the other intracellular events we mapped during conversion of a dendrite to a regenerating axon were also initiated by JNK signaling, we assayed neuron shape and microtubule polarity for 4 d after axon removal. Expression of either bsk RNAi or bskDN blocked growth from the tip of a dendrite (n = 10 for RNAi, n = 6 for DN, Figure 6, C and D); the only change in shape after injury was an increase in proximal branching in some animals. Both treatments also blocked most polarity changes in dendrites after axon removal. Out of five ddaE neurons assayed for microtubule polarity, only one had any dendrites that acquired the axonal plus-end-out microtubule orientation, and this one actually had two processes that became plus-end-out. In the rest of the neurons, dendrite microtubule polarity was mixed or minus-end-out at all time points as shown in Figure 6D.
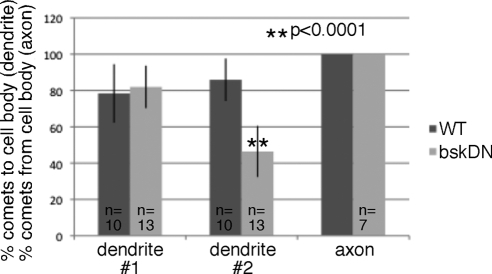
As so many dendrites had mixed polarity, we wondered whether there was any change in polarity in uninjured neurons expressing bskDN. Interestingly, the dendrite 2, which has a simpler branching pattern than dendrite 1 (or comb) dendrite, was significantly more mixed in cells expressing bskDN (Figure 7). This suggests that JNK signaling may play a role in dendritic microtubule polarity even in uninjured neurons. We hypothesize that the difference was only seen in the dendrite 2 and not the dendrite 1 because another mechanism is present that relies on dendrite branching to maintain microtubule polarity (F. Mattie and M. Rolls, unpublished results). Axon polarity was unchanged by expression of bskDN (not shown). We conclude that activation of JNK initiates microtubule rearrangements that precede conversion of a dendrite to a growing axon and that JNK may also play a role in controlling microtubule polarity in uninjured dendrites.
Figure 7.
Reduction of JNK signaling affects microtubule polarity in uninjured neurons. Microtubule polarity was assayed by tracking direction of EB1-GFP comet movement in uninjured neurons. Comets were counted in main trunk of the comb dendrite (1), and in dendrite 2 (see Figure 2B). The percent of dots moving toward the cell body is shown for dendrites. The percent was calculated for each cell; error bars, SD. An unpaired t test was used to calculate the significance of the difference between wild type and bskDN. For axons, the percent of comets moving away from the cell body is shown.
DISCUSSION
Model for Polarity Reversal of a Dendrite to a Regenerating Axon
Using this simple system to study neuronal responses to injury, we have found that even mature dendrites that normally exhibit little plasticity can undergo polarity reversal to be respecified as axons in vivo. Vertebrate neurons can also initiate axon formation from a dendrite (Hall and Cohen, 1983; Hall et al., 1989; Cho and So, 1992; Rose et al., 2001; MacDermid et al., 2002; Hoang et al., 2005; Fenrich et al., 2007; Takahashi et al., 2007; Gomis-Ruth et al., 2008), and thus this process is likely to be evolutionarily ancient and widely conserved.
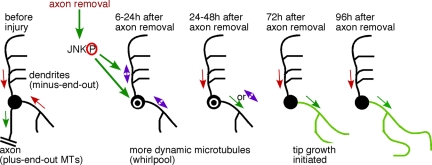
On the basis of our data, we propose a model for respecifying a dendrite as a regenerating axon (Figure 8). First, axon injury elicits a specific signal that is not generated by similar injury to dendrites. This signal turns on a kinase cascade that leads to JNK activation. Phosphorylated JNK up-regulates the number of growing microtubules, perhaps by increasing microtubule nucleation or severing. We hypothesize that this results in overall shorter microtubules that may be more likely to undergo complete catastrophe, facilitating polarity changes. After a transition phase during which multiple microtubule orientation switches can occur, a single dendrite takes on the axonal microtubule arrangement. JNK signaling also regulates the acquisition of plus-end-out microtubule polarity in this dendrite. Growth from the tip of the new axon is initiated, presumably as a result of transport of new components along the reoriented microtubules. We think that these changes in microtubule dynamics and polarity are required to initiate regeneration of an axon from a dendrite because only those neurons that switch one dendrite to the axonal microtubule polarity initiate tip growth, and genetically disrupting normal plus-end microtubule dynamics blocks tip growth.
Figure 8.
Model for conversion of a dendrite to a regenerating axon after axon removal. Before axon injury, microtubules in dendrites have minus-end-out polarity (red arrows) and axonal microtubules are plus-end-out. Axon removal up-regulates JNK signaling, which switches microtubule polarity in dendrites, frequently resulting in mixed polarity (purple double arrows) and up-regulates the number of microtubules in the cell body (white circle) and throughout the dendrites. Over several days polarity resolves such that one dendrite takes on the axonal microtubule polarity and the rest return to minus-end-out polarity. After this point the process with axonal microtubule polarity initiates tip growth.
Microtubule Polarity Reversal
Although our results suggest that reversing microtubule polarity is crucial for respecifying a dendrite as an axon, we do not yet know how this is accomplished at the molecular level. In the dendrite that switches from minus-end-out to plus-end-out polarity, it is unlikely existing microtubules could be turned around in the narrow dendrite, and so all the minus-end-out microtubules must be completely depolymerized over the 48-h time period in which respecification occurs. Depolymerization could be accomplished by severing minus-end-out microtubules or increasing catastrophe rates. Alternately, the normal microtubule turnover rate could be maintained, but addition of new minus-end-out microtubules could be blocked. During this switchover time, new plus-end-out microtubules must be added. One way this could be done is by nucleation of new microtubules in dendrites, although, for this to result in addition of only plus-end-out microtubules, orientation of nucleation sites would need to be controlled. Very little is known about mechanisms that generate uniform microtubule polarity in neurons, and so it will be extremely exciting to test whether oriented nucleation or selective depolymerization could play a role in determining polarity.
Microtubule Dynamics and Stability in Regenerating Neurons
Massive up-regulation of the number of growing microtubules, and most likely overall microtubule number, in neurons after axon injury is extremely surprising. The number of growing microtubules is known to be up-regulated at centrosomes upon entry into mitosis (Piehl et al., 2004), but this type of regulation has not been described in terminally differentiated cells like neurons. Our results suggest that neurons maintain a pathway to up-regulate microtubule number and activate it when injured.
A previous study suggested that increased microtubule stability, rather than an increase in microtubule dynamics, might be key for axon regeneration from a dendrite (Gomis-Ruth et al., 2008) consistent with a role for microtubule stability in initial axon specification (Witte and Bradke, 2008; Conde and Caceres, 2009). How can we reconcile this with our finding that increased microtubule dynamics is a robust early step in the response to axon removal?
The increase in microtubule number that we observe happens before tip growth is initiated, and number of growing microtubules generally decreases by 72 h after axon injury (Movie 3); tip growth is typically first obvious at 72 h, and is extensive by 96 h. Quantitation of the number of growing microtubules in dendrites confirms this impression: at 24 h after axon removal an average of 4.9 (SD 1.8) and 5.0 (SD 2.9) comets per 20 μm were observed in the comb and no. 2 dendrites, respectively. At 96 h after axon removal these numbers were reduced to 2.7 (SD 1.9) and 2.1 (SD 1.5) comets per 20 μm, respectively (the difference between the number of comets in the comb or no. 2 dendrites at both time points in unpaired t test; p < 0.05). Microtubule stability thus increases in the days after the initial up-regulation of dynamics. Our results support a model in which reduced microtubule stability plays a role in respecification of a dendrite to an axon. After respecification, microtubule stability is increased, and this increase could be important for growth, although we have not tested this idea in the current study.
Control of Axon Number
A previous study on responses to axon severing in slice and dissociated neuronal cultures found that more than one axon could be generated by respecification (Gomis-Ruth et al., 2008). In contrast, we find that in vivo the control on axon number is maintained: in cells that initiated tip growth, only one process arising from the cell body acquired axonal microtubule orientation. Importantly, the control on axon number was in the proximal region of the process: only one plus-end-out process arose from the cell body, but more than one tip of this process could have a growth cone and extend (Figure 3, C and D). In fact, this was often the case, and the growing tips tended to extend in different directions across the body wall. This could maximize their chance of finding the nerve and route to the target in the brain. Because of the onset of pupariation, we were only able to follow neurons for 4 d after axon severing, and therefore we could not determine whether extending axons were ever successful in finding the nerve or targets.
Most of the time the single dendrite that was respecified as an axon was the one closest to the site of the previous axon, dendrite 2. There are several possible models to explain this. One is that the side of the cell nearest the previous axon experiences some type of inductive signal so that the new axon is closest to the original one and thus has the best chance of finding the target. We prefer an alternative model as there is no evidence that the respecified process receives any directional information once it initiates growth. In this model, the key factor is the branching complexity of the dendrite. The dendrite opposite the axon has a comb shape with many more branches off the main trunk than dendrite 2. It may be more straightforward to generate uniform plus-end-out polarity in a process with a simpler branching pattern.
Involvement of JNK Signaling in Respecification of a Dendrite to a Regenerating Axon
The finding that JNK regulates number of growing microtubules, as well as microtubule polarity and tip growth are significant for several reasons. JNK, or other similar MAP kinases, are established initiators of axon regeneration from an axon stump in other systems (Ayaz et al., 2008; Hammarlund et al., 2009; Itoh et al., 2009), and the fact that it is also required to respond to axon severing near the cell body suggests these responses may be closely related. In regeneration of an axon from its stump the cytoskeletal rearrangements initiated by JNK signaling have not been identified. Control of microtubule number and polarity are also possible JNK targets in this response. In fact, in Aplysia californica, local polarity reversal of microtubules near axon cut sites are seen (Erez et al., 2007). Although the classic role for JNK is modulation of transcription by phosphorylation of transcription factors, it can also phosphorylate cytoskeletal proteins (Bogoyevitch and Kobe, 2006). The fact that we observe extremely rapid (<5 min) changes in microtubule dynamics in some cells (Movie 1) suggests that JNK may regulate microtubules in injured neurons by direct regulation of cytoskeletal proteins rather than through transcription changes.
Regulation of microtubule number by JNK is also significant because very little is known about what controls the number of growing microtubules in interphase cells. New work suggests that reducing levels of the stathmin protein in interphase cultured cells can increase microtubule nucleation and thus microtubule number (Ringhoff and Cassimeris, 2009), but no circumstances have been identified in which regulation of microtubule number might be important in nondividing cells. Our results show that studying control of microtubule number will be key for understanding neuronal responses to injury and neuronal polarity.
Supplementary Material
ACKNOWLEDGMENTS
We thank W. Grueber for the 221-Gal4 line and W. Hanna-Rose for sharing her UV laser and for stimulating discussions. We also acknowledge the Bloomington Drosophila Stock Center and the VDRC; they are amazing resources. Funding for this work was provided by an American Heart Association Scientist Development Grant, a March of Dimes Basil O'Connor Starter Scholar Award, and National Institutes of Health Grant R21NS066216. M.M.R. is a Pew Scholar in the Biomedical Sciences.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-11-0967) on January 6, 2010.
REFERENCES
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz D., Leyssen M., Koch M., Yan J., Srahna M., Sheeba V., Fogle K. J., Holmes T. C., Hassan B. A. Axonal injury and regeneration in the adult brain of Drosophila. J. Neurosci. 2008;28:6010–6021. doi: 10.1523/JNEUROSCI.0101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Black M. M., Banker G. A. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J. Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Deitch J. S., Black M. M., Banker G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M. A., Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A. L., Ohkura H. Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in Drosophila. EMBO J. 2005;24:1387–1396. doi: 10.1038/sj.emboj.7600629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard G. J., Stear J. H., Noetzel T. L., Al-Bassam J., Kinoshita K., Harrison S. C., Howard J., Hyman A. A. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E. Y., So K. F. Characterization of the sprouting response of axon-like processes from retinal ganglion cells after axotomy in adult hamsters: a model using intravitreal implantation of a peripheral nerve. J. Neurocytol. 1992;21:589–603. doi: 10.1007/BF01187119. [DOI] [PubMed] [Google Scholar]
- Conde C., Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Banker G. Neuronal polarity. Annu. Rev. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Dietzl G., et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Erez H., Malkinson G., Prager-Khoutorsky M., De Zeeuw C. I., Hoogenraad C. C., Spira M. E. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J. Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich K. K., Skelton N., MacDermid V. E., Meehan C. F., Armstrong S., Neuber-Hess M. S., Rose P. K. Axonal regeneration and development of de novo axons from distal dendrites of adult feline commissural interneurons after a proximal axotomy. J. Comp. Neurol. 2007;502:1079–1097. doi: 10.1002/cne.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Ruth S., Wierenga C. J., Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Grueber W. B., Jan L. Y., Jan Y. N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber W. B., Ye B., Moore A. W., Jan L. Y., Jan Y. N. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr. Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Hall G. F., Cohen M. J. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science. 1983;222:518–521. doi: 10.1126/science.6623092. [DOI] [PubMed] [Google Scholar]
- Hall G. F., Poulos A., Cohen M. J. Sprouts emerging from the dendrites of axotomized lamprey central neurons have axonlike ultrastructure. J. Neurosci. 1989;9:588–599. doi: 10.1523/JNEUROSCI.09-02-00588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M., Nix P., Hauth L., Jorgensen E. M., Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. X., Nieto J. H., Havton L. A. Regenerating supernumerary axons are cholinergic and emerge from both autonomic and motor neurons in the rat spinal cord. Neuroscience. 2005;136:417–423. doi: 10.1016/j.neuroscience.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Howard J., Hyman A. A. Growth, fluctuation and switching at microtubule plus ends. Nat. Rev. Mol. Cell Biol. 2009;10:569–574. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- Hummel T., Krukkert K., Roos J., Davis G., Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- Itoh A., Horiuchi M., Bannerman P., Pleasure D., Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem. Biophys. Res. Commun. 2009;383:258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- MacDermid V., Neuber-Hess M., Short C., Rose P. K. Alterations to neuronal polarity following permanent axotomy: a quantitative analysis of changes to MAP2a/b and GAP-43 distributions in axotomized motoneurons in the adult cat. J. Comp. Neurol. 2002;450:318–333. doi: 10.1002/cne.10324. [DOI] [PubMed] [Google Scholar]
- Piehl M., Tulu U. S., Wadsworth P., Cassimeris L. Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc. Natl. Acad. Sci. USA. 2004;101:1584–1588. doi: 10.1073/pnas.0308205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringhoff D. N., Cassimeris L. Stathmin regulates centrosomal nucleation of microtubules and tubulin dimer/polymer partitioning. Mol. Biol. Cell. 2009;20:3451–3458. doi: 10.1091/mbc.E09-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M. M., Satoh D., Clyne P. J., Henner A. L., Uemura T., Doe C. Q. Polarity and compartmentalization of Drosophila neurons. Neural Dev. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P. K., MacDermid V., Joshi M., Neuber-Hess M. Emergence of axons from distal dendrites of adult mammalian neurons following a permanent axotomy. Eur. J. Neurosci. 2001;13:1166–1176. doi: 10.1046/j.0953-816x.2001.1490.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Soriano N., Bottenberg W., Fiala A., Haessler U., Kerassoviti A., Knust E., Lohr R., Prokop A. Are dendrites in Drosophila homologous to vertebrate dendrites? Dev. Biol. 2005;288:126–138. doi: 10.1016/j.ydbio.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Satoh D., Sato D., Tsuyama T., Saito M., Ohkura H., Rolls M. M., Ishikawa F., Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C. C., Lansbergen G., Dortland B., De Zeeuw C. I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J. Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. C., Roegiers F., Rolls M. M. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Yamamoto M., Niwa R., Satoh D., Goto S., Taniguchi M., Hayashi S., Uemura T. Distinct developmental modes and lesion-induced reactions of dendrites of two classes of Drosophila sensory neurons. J. Neurosci. 2003;23:3752–3760. doi: 10.1523/JNEUROSCI.23-09-03752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D., Yu W., Baas P. W., Kawai-Hirai R., Hayashi K. Rearrangement of microtubule polarity orientation during conversion of dendrites to axons in cultured pyramidal neurons. Cell Motil. Cytoskelet. 2007;64:347–359. doi: 10.1002/cm.20188. [DOI] [PubMed] [Google Scholar]
- Witte H., Bradke F. The role of the cytoskeleton during neuronal polarization. Curr. Opin. Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Wu Z., Ghosh-Roy A., Yanik M. F., Zhang J. Z., Jin Y., Chisholm A. D. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc. Natl. Acad. Sci. USA. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Cook C., Sauter C., Kuriyama R., Kaplan P. L., Baas P. W. Depletion of a microtubule-associated motor protein induces the loss of dendritic identity. J. Neurosci. 2000;20:5782–5791. doi: 10.1523/JNEUROSCI.20-15-05782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wildonger J., Ye B., Zhang Y., Kita A., Younger S. H., Zimmerman S., Jan L. Y., Jan Y. N. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.