Abstract
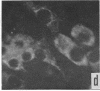
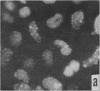
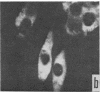
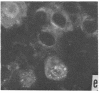
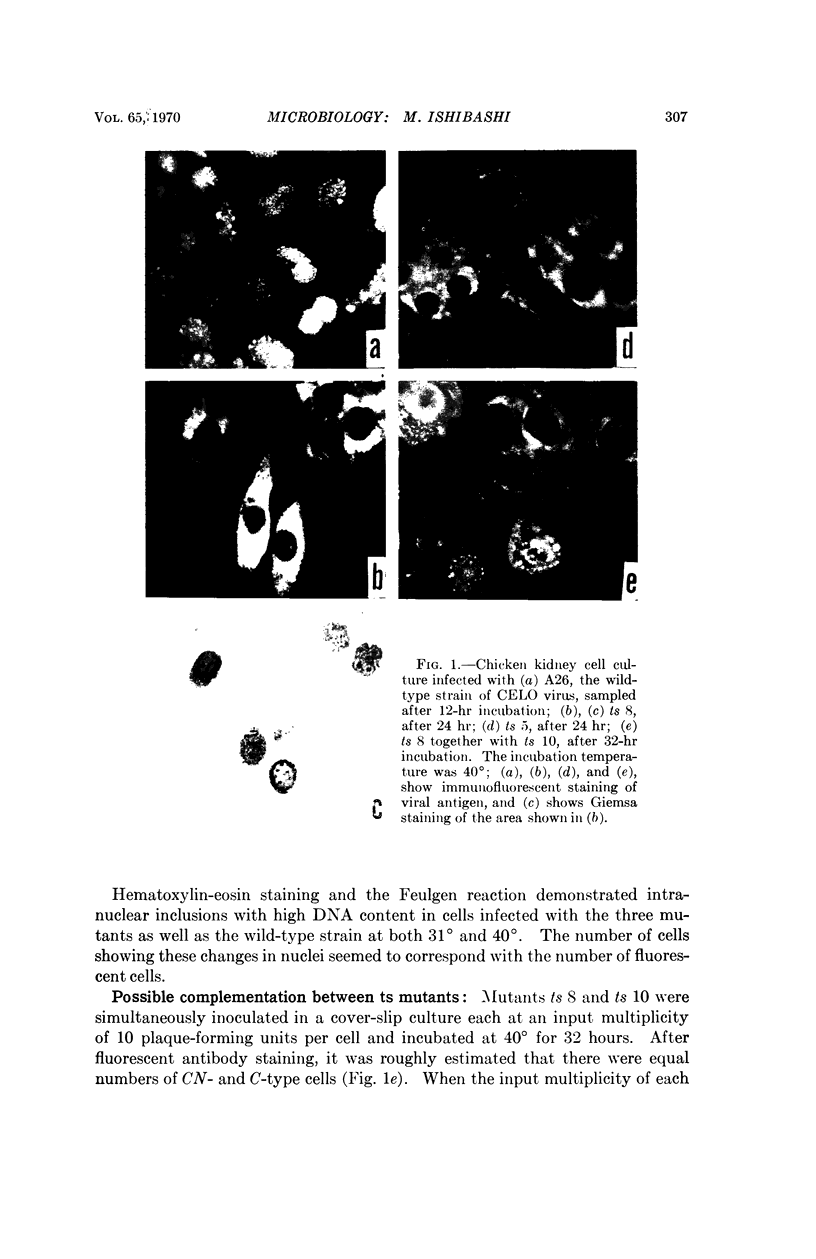
Immunofluorescent studies were made on chicken kidney cell cultures infected with various temperature-sensitive (ts) mutants of an avian adenovirus, chicken embryo lethal orphan (CELO), which grow well at the nonrestrictive temperature of 31°, but fail to grow at the restrictive temperature of 40°. At 40° some mutants (ts 5, 8, and 10) accumulated viral antigen in the cytoplasm, but scarcely at all in the nucleus. However, at 31° they accumulated the antigen in the nucleus like the wild-type strain at either 31° or 40°. These mutants seemed to complement each other in nuclear accumulation of antigen at 40°. In cells infected with ts 8, the antigen remaining in the cytoplasm during incubation at 40° was partially transferred to the nucleus even in the presence of an inhibitor of protein synthesis when the temperature was lowered to 31°. The results suggest that viral antigen produced in the cytoplasm is transported to the nucleus and that these ts mutants are defective in this process at 40°.
There are apparently two other types of ts mutant: one type (ts 3, 7, and 12), like the wild-type strain, showing nuclear accumulation of antigen at 40° and the other (ts 6, 11, and 17) showing no, or only slight, formation of the antigen at 40°.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER G. S., DENNY F. W., Jr, GINSBERG H. S. Sequential cellular changes produced by types 5 and 7 adenoviruses in HeLa cells and in human amniotic cells; cytological studies aided by fluorescein-labelled antibody. J Exp Med. 1959 Nov 1;110:827–844. doi: 10.1084/jem.110.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968 Feb;40(2):319–327. [PubMed] [Google Scholar]
- Burke C. N., Luginbuhl R. E., Williams L. F. Avian adeno-like viruses--characterization and comparison of seven isolates. Avian Dis. 1968 Aug;12(3):483–505. [PubMed] [Google Scholar]
- DUTTA S. K., POMEROY B. S. ELECTRON MICROSCOPIC STRUCTURE OF CHICKEN EMBRYO LETHAL ORPHAN VIRUS. Proc Soc Exp Biol Med. 1963 Nov;114:539–541. doi: 10.3181/00379727-114-28726. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Characterization of a Tumorlike Antigen in Type 12 and Type 18 Adenovirus-Infected Cells. J Bacteriol. 1965 Jul;90(1):120–125. doi: 10.1128/jb.90.1.120-125.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Russell W. C. A study of the development of adenovirus antigens by the immunofluorescent technique. Virology. 1968 Mar;34(3):470–480. doi: 10.1016/0042-6822(68)90067-6. [DOI] [PubMed] [Google Scholar]
- KAWAMURA H., SHIMIZU F., TSUBAHARA H. AVIAN ADENOVIRUS: ITS PROPERTIES AND SEROLOGICAL CLASSIFICATION. Natl Inst Anim Health Q (Tokyo) 1964;4:183–193. [PubMed] [Google Scholar]
- Levinthal J. D., Ahmad-Zadeh C., Van Hoosier G., Jr, Trentin J. J. Immunofluorescence of human adenovirus type 12 in various cell types. Proc Soc Exp Biol Med. 1966 Feb;121(2):405–414. doi: 10.3181/00379727-121-30791. [DOI] [PubMed] [Google Scholar]
- Levinthal J. D., Cerottini J. C., Ahmad-Zadeh C., Wicker R. The detection of intracellular adenovirus type 12 antigens by indirect immunoferritin technique. Int J Cancer. 1967 Mar 15;2(2):85–102. doi: 10.1002/ijc.2910020204. [DOI] [PubMed] [Google Scholar]
- Maeda M., Okaniwa A., Kawamura H. Morphological studies on intranuclear inclusion bodies in chicken kidney cell culture infected with avian adenovirus. Natl Inst Anim Health Q (Tokyo) 1967 Fall;7(3):164–177. [PubMed] [Google Scholar]
- Monreal G. Untersuchungen über die Vermehrung eines adeno-älnlichen Hühnervirus (CELO) Arch Gesamte Virusforsch. 1967;20(2):143–163. [PubMed] [Google Scholar]
- PEREIRA H. G., ALLISON A. C., BALFOUR B. Multiplication of adenovirus type 5 studied by infectivity titrations and by the fluorescent antibody technique. Virology. 1959 Mar;7(3):300–314. doi: 10.1016/0042-6822(59)90200-4. [DOI] [PubMed] [Google Scholar]
- PETEK M., FELLUGA B., ZOLETTO R., BERSANI G. FURTHER STUDIES ON CELO VIRUS: ITS RELATIONSHIP TO THE ADENOVIRUS GROUP. Arch Gesamte Virusforsch. 1964 Jun 17;14:637–649. doi: 10.1007/BF01555121. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petek M., Felluga B., Zoletto R. Biological properties of CELO virus: stability to various agents, and electron-microscopic study. Avian Dis. 1963 Feb;7(1):38–49. [PubMed] [Google Scholar]
- Shimojo H., Yamamoto H., Abe C. Differentiation of adenovirus 12 antigens in cultured cells with immunofluorescent analysis. Virology. 1967 Apr;31(4):748–752. doi: 10.1016/0042-6822(67)90213-9. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Kalnins V. I., MacKinnon E., Yohn D. S. Electron microscopic localization of adenovirus type 12 antigens. J Ultrastruct Res. 1967 Aug 30;19(5):556–562. doi: 10.1016/s0022-5320(67)80081-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication, xi. Evidence of a cytoplasmic site for the synthesis of viral-coded proteins. Proc Natl Acad Sci U S A. 1966 Jul;56(1):243–246. doi: 10.1073/pnas.56.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES V. J., FRY D. E. Observations on a chicken embryo lethal orphan (CELO) virus. Am J Vet Res. 1957 Jul;18(68):657–660. [PubMed] [Google Scholar]