Abstract
Background
The mushroom bodies (MBs) of Drosophila are required for complex behaviors and consist of three types of neurons, γ, α'/β' and α/β. Previously, roles for transcription factors in MB neuronal differentiation have only been described for a subset of MB neurons. We are investigating the roles of unfulfilled (unf; HR51, CG16801) in MB development. unf encodes a nuclear receptor that is orthologous to the nuclear receptors fasciculation of axons defective 1 (FAX-1) of the nematode and photoreceptor specific nuclear receptor (PNR) of mammals. Based on our previous observations that unf transcripts accumulate in MB neurons at all developmental stages and the presence of axon pathfinding defects in fax-1 mutants, we hypothesized that unf regulates MB axon growth and pathfinding.
Results
We show that unf mutants exhibit a range of highly penetrant axon stalling phenotypes affecting all neurons of the larval and adult MBs. Phenotypic analysis of unfX1 mutants revealed that α'/β' and α/β neurons initially project axons but stall prior to the formation of medial or dorsal MB lobes. unfZ0001 mutants form medial lobes, although these axons fail to branch, which results in a failure to form the α or α' dorsal lobes. In either mutant background, γ neurons fail to develop larval-specific dorsal projections. These mutant γ neurons undergo normal pruning, but fail to re-extend axons medially during pupal development. unfRNAi animals displayed phenotypes similar to those seen in unfZ0001 mutants. Unique asymmetrical phenotypes were observed in unfX1/unfZ0001 compound heterozygotes. Expression of UAS-unf transgenes in MB neurons rescues the larval and adult unf mutant phenotypes.
Conclusions
These data support the hypothesis that unf plays a common role in the development of all types of MB neurons. Our data indicate that unf is necessary for MB axon extension and branching and that the formation of dorsal collaterals is more sensitive to the loss of unf function than medial projections. The asymmetrical phenotypes observed in compound heterozygotes support the hypothesis that the earliest MB axons may serve as pioneers for the later-born MB neurons, providing evidence for pioneer MB axon guidance in post-embryonic development.
Background
The mushroom bodies (MBs) of Drosophila melanogaster, which are required for olfactory learning and other complex behaviors [1,2], are ideal for studying the transcriptional regulation of interneuronal development because they form discrete axonal projections that are well-characterized [3-5] and easily visualized [4,6-9]. Four neuroblasts in each brain hemisphere sequentially generate three types of Kenyon cells, the γ, α'/β', and α/β MB neurons that begin dividing during embryogenesis and continue to divide through development [10,11]. Each neuron projects dendrites that contribute to a large dendritic field in the calyx, and an axon that travels anteroventrally, forming a tightly bundled peduncle before branching medially to form the γ, β', and β lobes, and dorsally to form the α' and α lobes (Figure 1A). The earliest born γ neurons initially extend axons both medially and dorsally during late embryonic and early larval stages. These larval-specific γ axons are then pruned back to the peduncle by 18 hours after puparium formation (APF) and re-extend medially during pupal remodeling; the late larval-born α'/β' and pupal-born α/β neurons do not remodel their axonal projection patterns during metamorphosis [3-5].
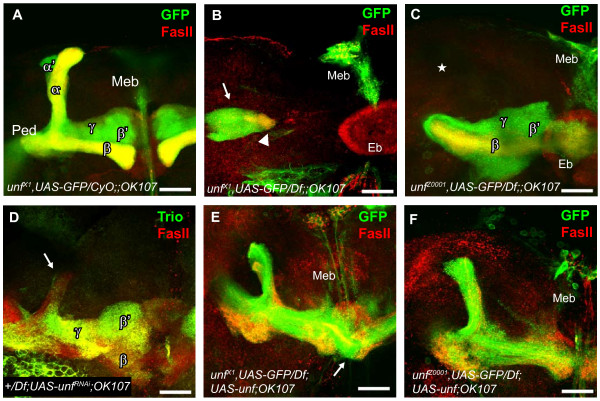
Figure 1.
unf is required for mushroom body lobe formation. In the adult brain, the mushroom body (MB) is a paired neuropil structure that comprises five axonal lobes, γ, α'/β', and α/β. Each neuron projects dendrites that contribute to a large dendritic field (calyx), and an axon that travels anteroventrally. MB axons fasciculate with other MB axons forming a peduncle (Ped) before projecting axons medially and dorsally. α' and α axons project dorsally, whereas γ, β', and β axons project medially, forming five distinctive lobes. To visualize the MB lobes, OK107-GAL4 was used to drive expression of the UAS-mCD8GFP transgene in all MB neurons (Kenyon cells) and their axons. Lobes were distinguished by using anti-Fasciclin II (anti-Fas II) to label α and β lobes and anti-Trio to label α', β', and γ lobes. (A) In adult UAS-mCD8GFP;;OK107-GAL4 control animals labeled with anti-Fas II (red), all MB lobes have formed. (B) In unfX1 mutants, MB axons have formed a peduncle (arrowhead), but have spread out and stalled prior to lobe formation (arrow). (C) In unfZ0001 mutants, γ, β', and β axons projected medially, but were disorganized. No dorsal lobes were formed (star). (D) This +/Df2426;UAS-unfRNAi;OK107-GAL4 adult displayed dramatically reduced dorsal lobes in one brain hemisphere (arrow). (E, F) In unfX1 and unfZ0001 rescue animals, in which a wild-type unf trangene was expressed in all MB neurons in an otherwise mutant background, all MB lobes were present. It is interesting to note that in rescued flies, MB lobes may have fewer axons, and that some medially projecting axons have extended past the midline (E, arrow). Eb, ellipsoid body; Meb, median bundle; Ped, peduncle. Scale bars = 10 μm.
Since the three different classes of MB neurons are born sequentially, generate a single dendritic field, project axons that fasciculate prior to branching medially and/or dorsally to form type-specific lobes, it is interesting to consider whether any differentiative events of the γ, α'/β', and α/β neurons are regulated by a common set of genes or whether they utilize independent transcriptional networks. Existing data on the role of transcription factors in MB differentiation provide little insight into this question. The genes eyeless [12-14], tramtrak [15], mushroom body miniature [16,17], chinmo [18], polyhomeotic [19], and tailless [20] regulate proliferation, specification, and viability of MB neurons, events that precede differentiation. dachshund (dac), ecdysone receptor B1 (EcR-B1), ultraspiracle (usp), and dSmad2 act in subtype-specific pathways [12,13,21-23], consistent with the hypothesis that the differentiation of the γ, α'/β', and α/β neurons utilize independent transcriptional pathways. dac mutants display axonal branching and pathfinding defects in subsets of α'/β' and α/β MB neurons [12,21]. EcR-B1 and its heterodimeric partner, Ultraspiracle (USP), both members of the nuclear receptor superfamily, form an ecdysone-regulated transcription factor that is required for the pruning of MB γ neurons at the outset of metamorphosis [22]. dSmad2 regulates the transcription of EcR-B1 in MB γ neurons during neuronal remodeling [24]. Thus, whether any differentiative events of the γ, α'/β', and α/β neurons are regulated by a common set of genes has not been previously reported.
In this study we show that the gene unfulfilled is required for the development of all three types of MB neurons, supporting the hypothesis that some differentiative events of the three types of MB neurons are regulated by a common set of genes. The unfulfilled gene (unf; HR51, CG16801) encodes the Drosophila NR2E3 member of the nuclear receptor superfamily [25]. UNF, like all classical nuclear receptors, contains an amino-terminal transactivational domain, a DNA-binding domain, a hinge region, and a carboxy-terminal ligand-binding domain [26]. unf is an ortholog of the Caenorhabditis elegans gene fasciculation of axons defective (fax-1) and the human gene photoreceptor specific nuclear receptor (PNR) [27]. Both fax-1 and PNR mutations disrupt developmental events in a limited number of neurons and result in behavioral or sensory deficits. fax-1 mutants are uncoordinated and display axon pathfinding and neurotransmitter defects [28-30]. The observed axon pathfinding defects are inferred to be due to the misregulation of fax-1 target genes. PNR impacts neuronal identity of vertebrate photoreceptors, functions as a dimer, and acts as a dual function transcriptional regulator, able to act as a transcriptional activator and a transcriptional repressor [31-37].
Based on our previous observations that robust levels of unf transcripts accumulate in MB neurons at all developmental stages [25] and the axon pathfinding defects of fax-1 mutants [28-30], we hypothesized that unf regulates MB axon growth and pathfinding. Phenotypic analysis of unf mutants revealed that MB axons stall prior to the formation of the lobes with the exception of the larval-specific γ neurons, which project axons medially, but fail to project dorsally. These axons are pruned appropriately but fail to re-extend during pupal stages. Expression of an unf transgene in the MBs in a mutant background rescued the unf mutant phenotypes, demonstrating that MB defects of unf mutants are due to loss of unf function in the MB neurons. These data demonstrate that unf is required for the proper formation of γ, α'/β', and α/β lobes, consistent with the hypothesis that at least some differentiative events of the γ, α'/β', and α/β neurons are regulated by a common set of genes.
Results
unf mutants show a reduction or complete loss of mushroom body lobes
To test the hypothesis that unf regulates MB neuron development, flies of various mutant genotypes (unfX1/Df2426, unfX1/unfX1, unfZ0001/Df2426, unfZ0001/unfZ0001, unfX1/unfZ0001, unfMB05909/Df2426, +/Df2426;UAS-unfRNAi;OK107-GAL4) were analyzed for aberrant MB phenotypes. All MB axons were visualized by expressing the UAS-mCD8GFP (UAS-GFP) reporter [9] using the OK107-GAL4 transgene, which expresses GAL4-driven green fluorescent protein (GFP) in all MB neurons [38]. Specific lobes were unambiguously identified immunohistochemically using anti-Fasciclin II (anti-Fas II) to label the α/β lobes [6] or anti-Trio to label the α'/β' lobes; both antibodies weakly label the γ lobes [39]. The unfX1 and unfZ0001alleles have been characterized previously [25], while the unfMB05909 allele has been recently identified (FlyBase; Figure 2). All five MB lobes were present and morphologically normal in adult control animals: α and α' lobes projected dorsally, whereas γ, β, and β' axons projected medially stopping at the median bundle (Figure 1A). MB axons of adult unfX1/Df2426 hemizygous (unfX1, UAS-GFP/Df2426;;OK107-GAL4; Figure 1B) and unfX1/unfX1 homozygous (unfX1/unfX1, UAS-GFP;;OK107-GAL4) mutants labeled with anti-Fas II projected anteroventrally, forming a peduncle, but stalled prior to the formation of discrete lobes. Axons that reached the heel region of the MB tended to spread out, but did not extend axons medially or dorsally (Figure 1B; compare Additional file 1 to Additional files 2 and 3). unfX1/Df2426 hemizygotes labeled with anti-Trio rather than anti-Fas II confirmed that γ and α'/β' unf mutant MB neurons initially projected axons forming a peduncle, but that these axons stalled prior to the formation of lobes (data not shown). MB lobes were never observed in unfX1/Df2426 hemizygotes or unfX1/unfX1 homozygotes (Table 1, rows 8 and 9). In contrast, all MB lobes were observed in all control genotypes (w1118, unfX1/+, Df2426/CyOGFP, UAS-GFP;;OK107-GAL4 controls, and unfX1, UAS-GFP/CyOGFP;;OK107-GAL4 control siblings; Table 1, rows 1, 2, 4, 5, and 6). We did not observe a reduced number of MB neurons in unf mutants (compare Additional file 1 to Additional files 2 to 5).
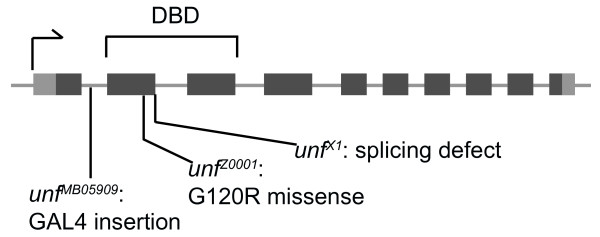
Figure 2.
Summary of unf alleles. The unfX1 allele disrupts the 5' donor splice site of intron 2, whereas the unfZ0001 allele has a missense mutation due to a guanine to adenine transition at base 312 of exon 2, resulting in a glycine to arginine substitution (G120R) [25]. The unfMB05909 line contains a GAL4 insertion in intron 1 of the unf gene (FlyBase). DBD, DNA-binding domain.
Table 1.
Mushroom body phenotypes in unf mutants and rescue animals.
| Row | Genotype | All lobes present adult/larvae (%) | All lobes missing adult/larvae (%) | Dorsal lobes missing adult/larvae (%) | Missing a dorsal or medial lobe adult/larvae (%) | n adult/larvae |
|---|---|---|---|---|---|---|
| Controls | ||||||
| 1 | w1118 | 100 | 0 | 0 | 0 | 10 |
| 2 | unfX1/+ | 100/100 | 0/0 | 0/0 | 0/0 | 12/8 |
| 3 | unfZ0001/+ | 100/100 | 0/0 | 0/0 | 0/0 | 7/15 |
| 4 | Df/CyOGFP | 100/100 | 0/0 | 0/0 | 0/0 | 8/8 |
| 5 | UAS-GFP;;OK107 | 100 | 0 | 0 | 0 | 10 |
| 6 | unfX1, UAS-GFP/CyOGFP;;OK107 | 100 | 0 | 0 | 0 | 15 |
| 7 | unfZ0001, UAS-GFP/CyOGFP;;OK107 | 90 | 0 | 10 | 0 | 10 |
| unf mutants and gene knock down | ||||||
| 8 | unfX1,UAS-GFP/Df;;OK107 | 0/0 | 100*/0 | 0/90* | 0/10 | 15/11 |
| 9 | unfX1,UAS-GFP/unfX1;;OK107 | 0 | 100* | 0 | 0 | 7 |
| 10 | unfZ0001/Df, UAS-GFP;;OK107 | 0/0 | 0/0 | 100*/100* | 0/0 | 13/6 |
| 11 | unfZ0001/unfZ0001,UAS-GFP;;OK107 | 37.5 | 0 | 37.5 | 25 | 8 |
| 12 | unfMB05909/Df, UAS-GFP;;OK107 | 100 | 0 | 0 | 0 | 14 |
| 13 | +/Df;UAS-unfRNAi;OK107 | 50 | 0 | 40 | 10 | 10 |
| 14 | unfX1,UAS-GFP/unfZ0001;;OK107 | 0 | 13 | 47 | 40 | 15 |
| unf transgenic rescues | ||||||
| 15a | unfX1,UAS-GFP/Df;UAS-unfrsqF/+;OK107 | 100*/83* | 0/0 | 0/0 | 0/17 | 11/6 |
| 16b | unfZ0001/Df, UAS-GFP/UAS-unfrsqF/+;OK107 | 100* | 0 | 0 | 0 | 8 |
| 17c | unfX1,UAS-GFP/Df;UAS-unfrsqC/+;OK107 | 86* | 0 | 0 | 14 | 7 |
| unf rescue controls | ||||||
| 18a | unfX1,UAS-GFP/Df;TM3Sb/+;OK107 | 0 | 100 | 0 | 0 | 6 |
| 19a | unfX1,UAS-GFP/CyO;UAS-unfrsqF/+;OK107 | 100 | 0 | 0 | 0 | 9 |
| 20a | unfX1,UAS-GFP/CyO;TM3Sb/+;OK107 | 100 | 0 | 0 | 0 | 4 |
| 21a | Df/CyOGFP;UAS-unfrsqF/+;OK107 | 100 | 0 | 0 | 0 | 5 |
| 22a | Df/CyOGFP;TM3Sb/+;OK107 | 100 | 0 | 0 | 0 | 7 |
| 23b | unfZ0001/Df, UAS-GFP;TM3Sb/+;OK107 | 0 | 0 | 100 | 0 | 7 |
| 24c | unfX1,UAS-GFP/Df;TM3Sb/+;OK107 | 0 | 100 | 0 | 0 | 9 |
| 25 | unfX1,UAS-GFP/Df;UAS-lacZ/+;OK107 | 0 | 100 | 0 | 0 | 9 |
| 26 | UAS-GFP;UAS-unfrsqF/+;OK107 | 100 | 0 | 0 | 0 | 5 |
Data are presented as percentages of whole brains that exhibit the phenotype. Control and mutant animals are 0- to 2-day adults, whereas rescue and rescue control animals are 72- to 96-hour pupae. All larvae are third instars. Single entries are adult or late pupae. Rescues and the corresponding control siblings are noted by matching subscript letters in rows. Asterisks indicate P-values of < 0.01 from the Fisher exact test. Abbreviations: Df, Df(2R)ED2426; UAS-GFP, UAS-mCD8GFP; OK107, OK107-GAL4.
unfZ0001/Df2426 hemizygous (unfZ0001,UAS-GFP/Df2426;;OK107-GAL4; Figure 1C) adults formed only medial lobes and displayed γ axons that splayed as they approached the midline compared to the compact bulb-like organization of γ lobes in wild-type animals (Figure 1C; Table 1, row 10; Additional files 4 and 5). α'/β' and α/β neurons of unfZ0001/Df2426 hemizygotes projected axons medially, but rarely projected dorsal collateral axons (data not shown). Dorsal lobes were observed more frequently in unfZ0001/unfZ0001 homozygotes (unfZ0001/unfZ0001,UAS-GFP/Df2426;;OK107-GAL4; Table 1, row 11). The observation that dorsal lobes were observed more frequently in unfZ0001/unfZ0001 homozygotes than in unfZ0001/Df2426 hemizygotes supports the hypothesis that the unfZ0001 allele is a hypomorph [25]. All MB lobes were observed in control genotypes (w1118, unfZ0001/+, Df2426/CyOGFP, UAS-GFP;;OK107-GAL4 controls); however, missing dorsal lobes were observed in 1 of 10 unfZ0001,UAS-GFP/CyOGFP;;OK107-GAL4 control siblings (Table 1, rows 1, 3, 4, 5, and 7). unfMB05909/Df2426 hemizygotes (unfMB05909/Df2426,UAS-GFP;;OK107-GAL4) displayed all MB lobes and did not display any abnormal MB phenotypes (Table 1, row 12), suggesting that the GAL4 insertion in the unfMB05909 line does not disrupt unf gene function.
To independently test whether unf plays a role in MB neuron development, a UAS-unfRNAi line was crossed to the OK107-GAL4 line to generate animals in which unf levels were reduced in the MBs via RNA interference (RNAi). Adult brains were double-labeled with anti-Fas II and anti-Trio to visualize the five MB lobes. When OK107-GAL4 was used to drive UAS-unfRNAi in a wild-type background, normal MBs were observed (data not shown). However, when OK107-GAL4 was used to drive UAS-unfRNAi in unf+/Df2426 hemizygotes (unf+/Df2426;UAS-unfRNAi;OK107-GAL4) 50% of brains displayed dramatically reduced dorsal lobes or were missing dorsal lobes bilaterally or unilaterally (Figure 1D; Table 1, row 13). These RNAi data are consistent with the analyses of unfX1 and unfZ0001 mutants, supporting the hypothesis that unf is necessary for axon extension and branching in all MB neurons and that dorsal collaterals are more sensitive to loss of unf function than medial projections.
unf expression in the mushroom bodies rescues lobe formation
We tested whether expression of unf in the MBs was sufficient to rescue the phenotypes observed in unf mutants by driving expression of a UAS-unf transgene with the OK107-GAL4 transgene. Interestingly, all UAS-unfrsqF;OK107-GAL4 flies developed to late pupal stages, but failed to eclose. The failure to eclose is probably due to OK107-GAL4-driven expression of unf in regions other than the MBs causing a disruption that prevents further development. We therefore assessed the MBs of rescued animals at late pupal stages, 72 to 96 hours APF. At this late developmental time in wild-type pupae, the MBs are indistinguishable from those of adult MBs [4]. Medial and dorsal MB lobes were observed in all unfX1/Df2426 rescued (unfX1,UAS-GFP/Df2426;UAS-unfrsqF/+;OK107-GAL4; Figure 1E) and unfZ0001/Df2426 (unfZ0001,UAS-GFP/Df2426;UAS-unfrsqF/+;OK107-GAL4; Figure 1F) rescued pupae. In contrast, MB lobes were not observed in unfX1/Df2426 control siblings (unfX1,UAS-GFP/Df2426;TM3/+;OK107-GAL4) that lacked the UAS-unfrsqF transgene (Table 1, compare rows 15 and 18). Similarly, only medial lobes were observed in unfZ0001/Df2426 control siblings (unfZ0001/Df2426,UAS-GFP;TM3/+;OK107-GAL4) (Table 1, compare rows 16 and 23) that lacked the UAS-unfrsqF transgene. All MB lobes were observed in all other control pupae (Table 1, rows 19 to 22). MB lobes appeared thin and less robust in some unfX1 and unfZ0001 rescued animals, suggesting that the rescue was imperfect. Nonetheless, MB axons contributing to each of the five MB lobes could be identified in all rescued animals. An independent rescue line, UAS-unfrsqC, was tested with OK107-GAL4 to express unf in the MBs of unfX1/Df2426 hemizygotes. All MB lobes were observed in six of seven rescued unfX1/Df2426 pupae with the UAS-unfrsqC transgene (unfX1,UAS-GFP/Df2426;UAS-unfrsqC/+;OK107-GAL4). In the seventh pupa, medial lobes were observed bilaterally, while dorsal lobes were observed only in one hemisphere (Table 1, row 17). MB lobes were not detected in any unfX1/Df2426 control siblings that lacked the UAS-unfrsqC transgene (unfX1,UAS-GFP/Df2426;TM3/+;OK107-GAL4; Table 1, row 24). To confirm that the rescue depended upon the expression of an unf open reading frame, we tested the ability of a UAS-lacZ transgene to rescue unfX1/Df2426 hemizygotes. As expected, MB lobes were not observed in unfX1/Df2426 pupae containing the UAS-lacZ transgene (unfX1,UAS-GFP/Df2426;UAS-lacZ/+;OK107-GAL4; Table 1, row 25). These data demonstrate that the axonal defects observed in unfX1 mutants are due to the lack of UNF function.
It is interesting to note that the medially projecting axons of any genotype carrying the UAS-unfrsqF and OK107-GAL4 transgenes failed to stop appropriately, extending axons past the midline. Midline crossing was observed in mutants carrying UAS-unfrsqF and OK107-GAL4 (Figure 1E) as well as controls carrying these two transgenes. Midline crossing was observed approximately 50% of the time: unfX1,UAS-GFP/Df2426;UAS-unfrsqF/+;OK107-GAL4 rescue animals (55%, n = 11), unfX1,UAS-GFP/CyO;UAS-unfrsqF/+;OK107-GAL4 control siblings (44%, n = 9), and UAS-GFP/+;UAS-unfrsqF/+;OK107-GAL4 controls (60%, n = 5). This observation suggests that MB axons may be sensitive to levels of UNF expression.
unfX1/unfZ0001 compound heterozygotes exhibit a range of mushroom body phenotypes
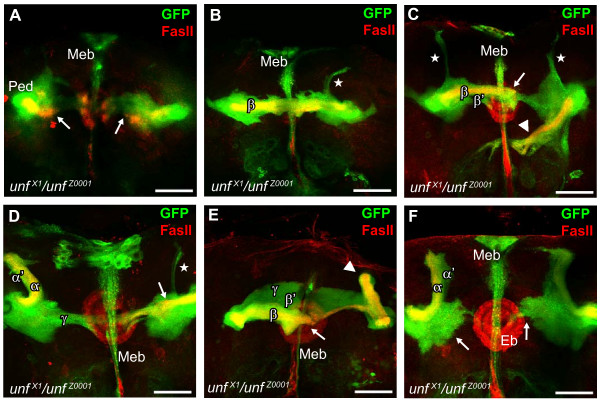
Phenotypic analysis of unfX1/unfZ0001 compound heterozygotes (unfX1,UAS-GFP/unfZ0001;;OK107-GAL4) revealed a range of aberrant MB phenotypes. Thirteen percent of unfX1/unfZ0001 compound heterozygotes lacked all MB lobes (Figure 3A), similar to the unfX1 mutant phenotype. Forty-seven percent of the unfX1/unfZ0001 compound heterozygotes developed only medial lobes and were missing dorsal lobes (Figure 3B, C), similar to the unfZ0001 mutant phenotype. These unfXl/unfZ0001 compound heterozygotes occasionally displayed a thin fascicle of dorsally projecting α' axons (Figure 3B, C). Interestingly, 40% of unfX1/unfZ0001 compound heterozygotes exhibited asymmetrical phenotypes in which a dorsal and/or medial lobe were present in one hemisphere but missing in the other (Table 1, row 14). In some cases, medial axons misprojected or extended past the midline (Figure 3B, C, E). γ neurons were also variably affected in unfX1/unfZ0001 compound heterozygotes and often appeared defasciculated, and stalled at various points along their medial trajectory (Figure 3C, D, F; Additional files 6, 7 and 8). The novel phenotypes of unfX1/unfZ0001 compound heterozygotes that are different from either unfX1/Df2426 or unfZ0001/Df2426 hemizygotes demonstrate that the unfX1 and unfZ0001 alleles interact.
Figure 3.
unfX1/unfZ0001 compound heterozygotes display a range of aberrant mushroom body phenotypes, suggesting that unfX1 and unfZ0001 alleles interact. OK107-GAL4 was used to drive expression of the UAS-mCD8GFP transgene in all MB neurons and their axons (green), and anti-Fas II (red) to label the α and β axons. (A) In this unfX1/unfZ0001 compound heterozygote all MB axons stall (arrows), similar to the unfX1 mutant phenotype. (B) In this unfX1/unfZ0001 heterozygote only medial lobes are present, similar to the unfZ0001 mutant phenotype; a thin fascicle of α' axons is present in the right hemisphere (star). (C) In this unfX1/unfZ0001 heterozygote, left hemisphere β' and β axons extend medially beyond the midline (arrow), whereas γ axons appear to stall; right hemisphere β and β' axons misproject ventrally (arrowhead) and γ axons are highly disorganized; only a few α' dorsal axon projections are present in either hemisphere (stars). (D) In this unfX1/unfZ0001 heterozygote α and α' dorsal axon projections are present in the left hemisphere, whereas only a thin fascicle of α' axons is present in the right hemisphere (star); β axons are present in the right hemisphere and appear to stall (arrow), whereas they are completely absent in the left hemisphere. (E) In this unfX1/unfZ0001 heterozygote β', β, and γ axons project medially and cross the midline (arrow), but dorsal axons are missing in the left hemisphere; in the right hemisphere γ axons project medially and α and α' axons project dorsally but appear to stall (arrowhead). (F) In this unfX1/unfZ0001 heterozygote, α and α' dorsal axon projections are present in the left hemisphere, whereas only α' dorsal axons are present in the right hemisphere; medial axon projections are disorganized and stall before reaching the midline in both right and left hemispheres (arrows). Eb, ellipsoid body; Meb, median bundle; Ped, peduncle. Scale bars = 25 μm.
unf is required for larval-specific γ dorsal collaterals and the re-extension of γ axons during metamorphosis
The axon stalling phenotypes of unfX1/Df2426 hemizygotes suggested that unf is required in all MB neurons for axons to extend in any direction beyond the heel region of the MB. This region is the branching point for dorsal collateral projections from medially projecting axons. The MB axons of adult unfX1/Df2426 hemizygotes may have all stalled during the initial phase of their outgrowth, either during larval or pupal development. Alternatively, it is possible that MB neurons may initially project axons medially and dorsally, but these axons may not be maintained into the adult. To determine whether unf is required for the initial projection patterns of all MB neurons, the MBs of experimental and control animals were analyzed at various larval and pupal stages.
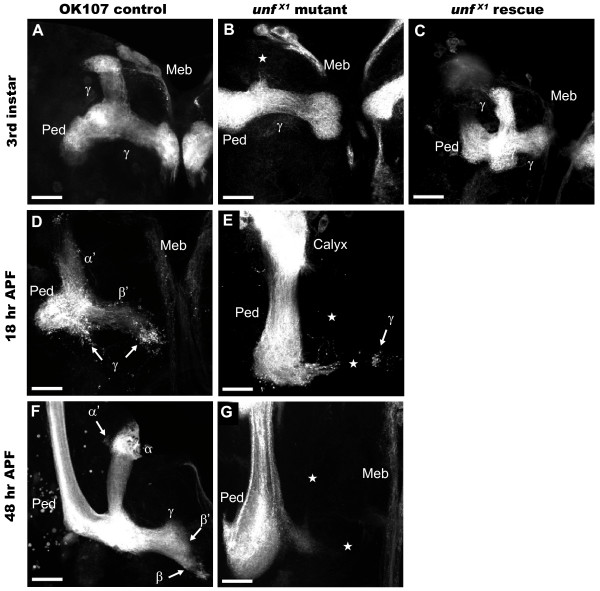
During late embryonic development, γ neurons normally begin to extend axons both medially and dorsally. These medial and dorsal projections persist throughout larval development. By 18 hours APF, γ axons have been pruned to the branching point and they subsequently re-extend medially only [3-5]. unfX1/Df2426 (unfX1,UAS-GFP/Df2426;;OK107-GAL4) early-, mid-, and late-third instar larvae displayed only medial axons (n = 17), whereas control (UAS-GFP;;OK107-GAL4) third instar larvae displayed normal bifurcated larval-specific γ projection patterns (n = 5) (compare Figure 4A and Figure 4B). Examination of unfX1/Df2426 hemizygotes (n = 4) and control (n = 6) pupae at 18 hours APF revealed that both medial and dorsal γ axons had been pruned (compare Figure 4D and Figure 4E). At 48 hours APF, MB lobes were not observed in unfX1/Df2426 hemizygotes (n = 3), whereas control pupae exhibited γ axons that had re-extended medially, forming the adult γ lobe (n = 5) (compare Figure 4F and Figure 4G). These data suggest that γ neurons extend axons medially but not dorsally in unfX1/Df2426 larvae and that they undergo pruning like wild-type axons at approximately 16 hours APF but that they fail to re-extend during pupal development.
Figure 4.
unf impacts all mushroom body neurons early during development. OK107-GAL4 was used to drive expression of the UAS-mCD8GFP transgene in all MB neurons and their axons (white). (A) In UAS-mCD8GFP;;OK107-GAL4 late third instar larvae controls, γ neurons project larval-specific axons medially and dorsally. (B) In unfX1 mutant late third instar larvae, γ neurons project medially only; dorsal axons are missing (star). (C) Medial and dorsal γ axons are present in unfX1 rescue third instar larvae. (D) In UAS-mCD8GFP;;OK107-GAL4 control pupae at 18 hours APF, γ axons have been pruned back to the peduncle and only axon fragments are visible; some β' and α' axons project medially and dorsally, respectively. (E) In unfX1 mutant pupae at 18 hours APF, γ axons have been pruned with some fragments visible (arrow); β' and α' axons are completely missing (stars). (F) All MB lobes are visible in UAS-mCD8GFP;;OK107-GAL4 control pupae at 48 hours APF. (G) All MB lobes are absent in unfX1 mutant pupae at 48 hours APF (stars). Scale bars = 10 μm.
Expression of the UAS-unfrsqF transgene in the MBs in unfX1/Df2426 hemizygotes (unfX1,UAS-GFP/Df2426;UAS-unfrsqF/+;OK107-GAL4) rescued the dorsal collaterals of the larval γ neurons in five of six third instar larvae (Figure 4C; Table 1, row 15) and supported the re-extension of γ medial axon projections in pupae (Figure 1E, 1F). These data confirm that the unf function in MBs is necessary for the formation of dorsal collaterals in γ neurons during larval development.
unf is required during the early development of α'/β' and α/β mushroom body neurons
The α'/β' neurons develop medial and dorsal projections between mid-third instar and puparium formation. The α/β axons develop during early pupal stages [3-5]. α'/β' medial or dorsal projections were never observed in unfX1/Df2426 (unfX1,UAS-GFP/Df2426;;OK107-GAL4) late-third instar larvae (n = 5) or pupae at 18 hours APF (n = 6; Figure 4E). Similarly, α'/β' and α/β projections were never observed in unfX1/Df2426 pupae at 48 hours APF (n = 3; Figure 4G). MB axon projections were normal in control (UAS-GFP;;OK107-GAL4) late-third instar larvae (n = 5), pupae at 18 hours APF (n = 6; Figure 4D), and pupae at 48 hours APF (n = 5; Figure 4F). These data indicate that unf is necessary for the differentiation of all α'/β' and α/β MB neurons early during their development.
201Y-GAL4 and c739-GAL4 driven GFP expression in the mushroom bodies is unf-dependent
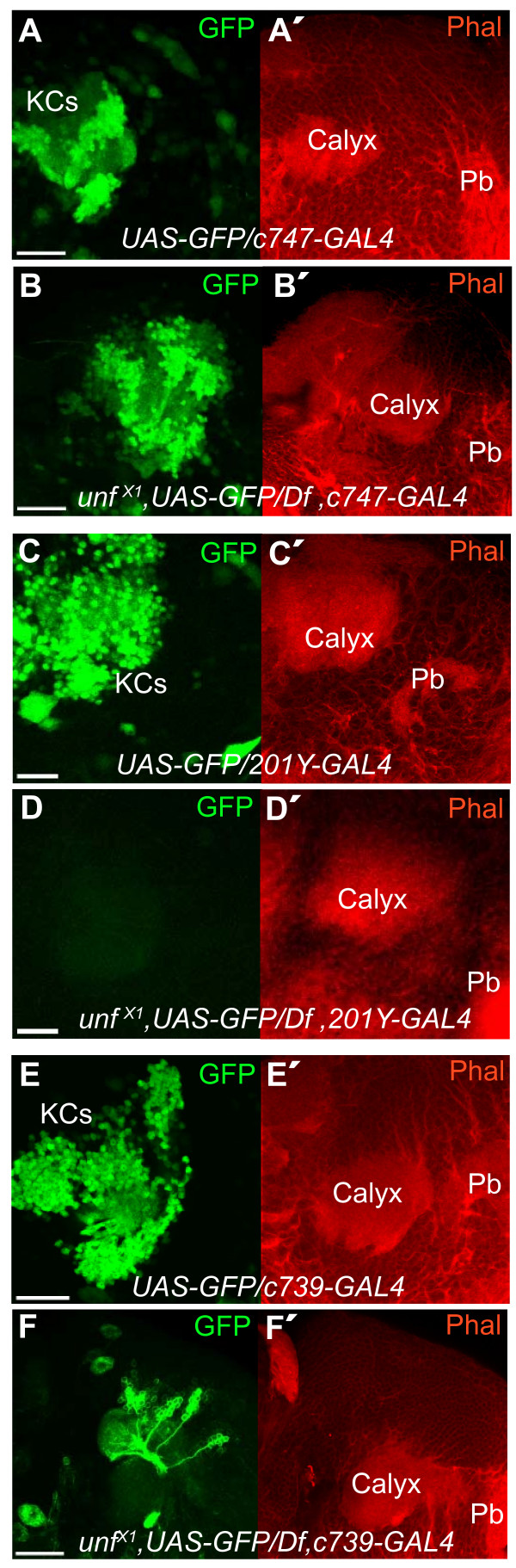
To independently confirm that the axon stalling phenotypes of unfX1/Df2426 hemizygotes were not an artifact associated with the OK107-GAL4 transgene (Figure 1), we examined UAS-GFP expression in the MBs of unfX1/Df2426 hemizygotes using three other GAL4 drivers known to express in all or subsets of MB neurons, 201Y-GAL4, c739-GAL4, and c747-GAL4 [7,40]. In each case, adult brains were counterstained with fluorescently labeled phalloidin to visualize actin-rich structures, including the extensive dendritic arbors of MB neurons that fill the calyces. The c747-GAL4 line showed GFP expression in MB neurons in control and unfX1/Df2426 MB neurons (Figure 5A, 5B). The unfX1/Df2426 hemizygotes (unfX1,UAS-GFP/Df2426,c747-GAL4) displayed stalled axons and failed to develop any MB lobes, confirming the unfX1 mutant phenotypes described using the OK107-GAL4 transgene to drive the reporter transgene in MB neurons. Surprisingly, unfX1/Df2426 hemizygotes carrying the 201Y-GAL4 transgene failed to express GFP in MB neurons (compare Figure 5C and Figure 5D), and GFP expression in unfX1/Df2426 hemizygotes carrying the c739-GAL4 transgene was greatly diminished (compare Figure 5E and Figure 5F). These observations indicate that 201Y-GAL4 and c739-GAL4 expression is unf-dependent. The 201Y-GAL4 transgene is an insertion in the TAK1-associated binding protein 2 (Tab2) gene (FlyBase). Inverse PCR revealed that the c739-GAL4 transgene is inserted in the second intron of hormone receptor-like in 39 (HR39; data not shown).
Figure 5.
201Y-GAL4 and c739-GAL4 driven GFP expression is unf-dependent. The UAS-mCD8GFP reporter was used with three enhancer trap constructs to visualize MB neurons (Kenyon cells (KCs), green). Fluorescently labeled phalloidin (red) was used to visualize actin-rich structures, including the dendritic fields (calyces) of the MBs and the protocerebral bridge (Pb) that lies in the same plane. (A, B) The c747-GAL4 transgene drives the expression of GFP in MB neurons in UAS-mCD8GFP/c747-GAL4 controls and in unfX1,UAS-mCD8GFP/Df2426,c747-GAL4 mutants. (C, D) The 201Y-GAL4 transgene drives the expression of GFP in a subset of the MB neurons in UAS-mCD8GFP/201Y-GAL4 controls (C), but unfX1,UAS-mCD8GFP/Df2426,201Y-GAL4 mutants do not express 201Y-GAL4-driven GFP in MB neurons (D). (E, F) UAS-mCD8GFP/c739-GAL4 controls express GFP in MB neurons (E). GFP expression is greatly reduced in unfX1,UAS-mCD8GFP/Df2426,c739-GAL4 mutants (F). The calyx and Pb are positively labeled with phalloidin in all mutants and controls. Scale bars = 10 μm.
Discussion
The unfX1 and unfZ0001 alleles interact showing that both alleles are at least partially functional
unf mutants exhibit a range of highly penetrant axon stalling phenotypes affecting all neurons (γ, α'/β' and α/β) of the larval and adult MBs. Similar phenotypes have been observed in unf microRNA knockdown animals [41]. unfX1/Df2426 hemizygotes and unfX1/unfX1 homozygotes fail to project larval-specific γ dorsal collaterals, fail to re-extend γ axons medially during metamorphosis, and fail to project any medial and dorsal axons of α'/β' and α/β neurons. The γ, α'/β' and α/β axons of unfZ0001/Df2426 hemizygotes only project medially, whereas MBs were normal in some unfZ0001/unfZ0001 homozygotes. These data together with previous observations [25] would seem to support the hypothesis that the unfX1 allele is an amorph, a null allele, and that the unfZ0001 allele is a hypomorph, a partial loss of function allele. However, while the unfZ0001 allele behaves as a hypomorph with respect to sterility, it displays dominant properties with respect to wing expansion [25]. Interestingly, the G56R allele of PNR, which displays dominant properties [42], is structurally equivalent to the unfZ0001 allele (G120R) [25]. The observation that the unfX1/unfZ0001 compound heterozygotes display unique phenotypes was unexpected and demonstrates that these alleles interact, compelling us to conclude that the unfX1 allele is not a null allele. These data strongly suggest that the unfX1 allele encodes a unique isoform of the UNF protein, UNFX1, which is predicted to contain the 110 residue amino-terminal domain and the complete first zinc finger of the DNA-binding domain [25]. These data do not allow us to infer the functional nature of the unfX1 allele or the mechanism of this genetic interaction.
Asymmetrical phenotypes suggest a role for unfulfilled in pioneer axon guidance
The phenotypic variation and asymmetry observed in the MBs of unfX1/unfZ0001 compound heterozygotes supports the hypothesis that pioneer axons are established early during MB development and that the pathfinding of these pioneers is unf-dependent. The observation that the β lobe axons in one hemisphere project medially while the β lobe axons in the other hemisphere project ventromedially (Figure 3C, 45° angle down, arrowhead) demonstrates that the projection of the β lobes in these unfX1/unfZ0001 compound heterozygotes is independent of their genotype. In an independent, yet genetically identical animal, unfX1/unfZ0001 α/β MB neurons assume a different fate and fail to project any medially projecting β axons (Figure 3F). Similar observations can be made for all unfX1/unfZ0001 MB lobe axons when these and other samples are examined (Figure 3). The fact that the axons of the later-born α/β neurons consistently stall or misproject whenever the axons of the earlier-born α'/β' neurons stall or misproject suggests that the α'/β' axons may be acting as pioneers for the α/β axons. These data support a model of MB lobe formation in which unf is required for MB pioneer axons to navigate to their targets, and that later-born MB neurons project axons that fasciculate along these established axons. We propose that the variable and asymmetric phenotypes observed in unfX1/unfZ0001 compound heterozygotes are due to inappropriate targeting of pioneer axons of the MB or the stalling of pioneer axons prematurely as a result of insufficient unf function in α'/β' pioneer axons. Thus, the asymmetric β lobe projection in Figure 3C may be due to asymmetric projections of pioneer axons, while the lack of β lobes in Figure 3F may be due to the stalling of these pioneer axons in the peduncle. These data are supported by an analysis of non-autonomous effects of Dscam mutant clones, which suggests that the α'/β' axons may be acting as pioneers for the α/β axons at least some of the time [43]. Our observations that larval γ dorsal axons, and the α' and α dorsal lobes all fail to develop in unfZ0001/Df2426 mutants not only supports the hypothesis that the α'/β' axons act as pioneers for the α/β axons, but suggests that the larval γ axons act as pioneers for the α'/β' axons. Discerning the roles and mechanisms of unf in MB pioneers during post-embryonic MB development requires further investigation.
unf plays a common role in the early development of all mushroom body neurons
The data presented here demonstrate that unf plays a common role in the early development of all three subtypes of MB neurons by regulating axon extension and branching. While we cannot rule out the possibility that single axons, which normally project dorsally, may be misguided and project medially, our analysis is consistent with the hypothesis that unf mutant γ, α'β', and α/β neurons fail to project dorsal axon branches. Our observations that unf mutant MB neurons express subtype-specific epitopes such as Fas II and Trio suggest that unf does not impact MB neuronal subtype identity. Interestingly, Lin et al. [41] disagree and conclude that unf does regulate MB neuronal subtype identity based on a series of unf RNAi knockdown experiments. We argue that until the transcriptional codes that distinguish MB neuronal subtypes are defined, one cannot conclusively determine whether the identity of these neurons has been impacted [44-47]. While we cannot yet place unf at any specific position in a transcriptional hierarchy that regulates MB development, our data suggest that unf acts earlier than dac, EcR-B1, and usp, since these genes regulate differentiation in specific subsets of MB neurons while unf regulates the differentiation of all three MB subtypes.
Based on the homology of UNF with PNR, we anticipate that UNF is likely to act as a dual function transcription factor with the ability to activate the transcription of some target genes, while repressing others [32,33,35,36]. Palanker et al. [48] have previously proposed that UNF may function as a transcriptional repressor. We propose that the axon stalling phenotype observed in unf mutants is due to the misregulation of target genes. Two potential target genes are Tab2 and HR39 based on the observation that the expression of the 201Y-GAL4, an enhancer trap insertion in Tab2, and c747-GAL4, an enhancer trap insertion in HR39, is unf-dependent. If the transcriptional regulation of these two enhancer trap transgenes reflects the transcriptional regulation of the genes in which they are inserted, then it follows that Tab2 and HR39 are expressed in the MBs and that this expression is unf-dependent, a hypothesis that remains to be tested.
Axon stalling and branching phenotypes in unf mutants suggest that genes encoding axon guidance cues are likely to be regulated by unf. Genetic screens have already identified a number of guidance genes that are expressed in the MBs. For example, genes encoding cell adhesion molecules like volado, an α-integrin, Notch, a transmembrane receptor and transcription factor [1], and semaphorinla [49] and plexinA [15], which encode a ligand-receptor pair that is largely involved in axonal repulsion, have been identified. Ephrin receptor tyrosine kinase [50] and Dscam [43,51], other well-known guidance cues, are necessary for the proper guidance of MB axons. Misregulation of these or other guidance genes could disrupt the normal balance of attractive and repulsive cues resulting in inappropriate axon pathfinding and stalling.
Conclusions
These data support the hypothesis that unf plays a common role in the early development of all three subtypes of MB neurons, γ, α'/β', and α/β, by regulating axon extension and branching during the initial phases of larval and pupal outgrowth. Expression of a UAS-unf transgene in MB neurons of unf mutants rescues the unf mutant MB phenotypes, demonstrating that the MB defects are due to the lack of unf. The phenotypic variation and asymmetry observed in the MBs of unfX1/unfZ0001 compound heterozygotes suggests a role for unf in the targeting of pioneer axons.
Materials and methods
Drosophila stocks
All stocks were raised on standard cornmeal and sugar medium. The unfX1 and unfZ0001 stocks have been characterized previously: the unfX1 allele disrupts the 5' donor splice site of intron 2, whereas the unfZ0001 allele has a missense mutation due to a guanine to adenine transition at base 312 of exon 2 resulting in a glycine to arginine substitution (G120R) [25]. The Df(2R)ED2426 (Df2426) chromosome carries a deletion of 482,016 bp on the second chromosome that removes 57 genes or annotated genes in their entirety, including unf [52]. The Hr51MB05909 (unfMB05909) line contains a GAL4 insertion in intron 1 of the unf gene (FlyBase). The Df(2R)ED2426, Hr51MB05909 (unfMB05909), UAS-CD8::GFP;;OK107-GAL4, 201Y-GAL4, c747-GAL4, and c739-GAL4 lines were obtained from Bloomington Stock Center. The UAS-unfRNAi line was obtained from Vienna Drosophila RNAi Center (VDRC). All mutant or transgenic stocks were maintained over GFP-marked chromosomes to facilitate genotyping. Animals were reared at 25°C with the exception of UAS-unfRNAi crosses, which were reared at 29°C.
Transgenic flies and rescue experiment
The transgenic rescue constructs UAS-unfrsqF and UAS-unfrsqC are two independent isolates of the same transgene, which was generated by cloning the unf cDNA [25] into the vector pUAST [40]. Transgenic flies were generated by P-element-mediated transformation [53]. A homozygous w1118 stock was used for all P-element-mediated transformations. Df2426/CyO;UAS-unfrsq/TM3Sb flies were crossed to unfX1,UAS-GFP/CyOGFP;;OK107-GAL4 or unfZ0001,UAS-GFP/CyOGFP;;OK107-GAL4 flies to generate rescues and control siblings. Df2426/CyO;UAS-lacZ/TM3Sb flies were crossed to unfX1,UAS-GFP/CyOGFP;;OK107-GAL4 flies for additional rescue controls. All rescue and control larvae and pupae were genotyped using PCR. The following primers were used to detect the presence of unfX1, unfZ0001, Df2426, and UAS-unfrsq. unfX1, 5' CAGCGGCATTGCTACACTC 3' (fx1b1) and 5' GGAAAATTCCCACGTCAAAA 3' (R947) followed by XbaI digest; unfZ001, 5' CTGAGCTGGAATCACAGTGC 3' (L150Z1) and 5' GGATTCCGTAGTGCTTTCT 3' (R330Z1); Df2426, 5' TCATTTAATTTTAGTGCCGGA 3' (2426A) and 5' CAATCATATCGCTGTCTCACTCA 3' (PRY4); UAS-unfrsq, 5' CAGCGGCATTGCTACACTC 3' (fx1b1) and 5' GATTCCGATGAGCTTTGTCCACCACAC 3' (XR941).
Immunohistochemistry and microscopy
Third instar larvae and pupae were staged as described [54,55]. The central nervous system of third instar larvae, pupae, and 0- to 2-day adults were collected, fixed in 4% paraformaldehyde, and processed using standard protocols [9]. mAb1D4 [56] (anti-Fas II; 1:10) and mAb9.4A [39] (anti-Trio; 1:4) were obtained from the Developmental Studies Hybridoma Bank (DSHB). The rabbit anti-Fas II (1:2,000) was a gift from Dr Vivian Budnik (University of Massachusetts). Biotinylated anti-mouse and anti-rabbit IgG (1:200) were obtained from Vector Labs (Burlingame, CA USA). Streptavidin Alexa Fluor 488, 546 (1:200), and Alexa Fluor 546 phalloidin (1:40) were obtained from Invitrogen Molecular Probes (Carlsbad, CA USA). Preparations were examined and imaged using an Olympus Fluoview FV-1000 laser scanning confocal system mounted on an Olympus IX-81 inverted microscope. Images were processed using Image J and Adobe Photoshop. Movies of z stacks were processed using QuickTime. During z stack collections for Additional files 1 to 3, the photomultiplier tube was manually adjusted for optimal brightness at different focal planes.
Statistics
The Fisher exact test was used to determine whether the frequency of defects in experimental animals was significantly different from the frequency of defects in control animals. Relevant genotypes were tested in pair-wise combinations. P-values less than 0.01 were considered significant.
Abbreviations
APF: after puparium formation; dac: dachshund; EcR-B1: ecdysone receptor B1; Fas II: Fasciclin II; fax-1: fasciculation of axons defective; GFP: green fluorescent protein; HR39: hormone receptor 39; MB: mushroom body; PNR: photoreceptor specific nuclear receptor; RNAi: RNA interference; Tab2: Tak1-associated binding protein; usp: ultraspiracle; unf: unfulfilled.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KEB and CSS were responsible for all technical work. CSS was solely responsible for generating the rescue animals. KEB was largely responsible for phenotypic analyses and writing the manuscript. SR conceived of, directed, and obtained funding for the study, and participated in writing the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Figure S1. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/CyO;;OK107-GAL4 control animal labeled with anti-Fas II (red), in which all MB lobes are properly formed. OK107-GAL4 driven GFP expression is visible in the Kenyon cells (green) and all MB lobes. Images depict the right hemisphere at 90× magnification.
Figure S2. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). The Kenyon cells (green) are visible in posterior planes. A rarely observed medial projection is visible in the region of the calyx. Axons travel anteroventrally then spread out, and stall prior to lobe formation. Images depict the left hemisphere at 90× magnification.
Figure S3. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons project anteroventrally in a poorly organized peduncle, spread out, and stall at the end of the peduncle. The median bundle (green) is to the left. Images depict the right hemisphere at 90× magnification.
Figure S4. Movie of a z stack of an adult unfZ0001,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons travel anteroventrally. γ, β', and β axons projected medially but were disorganized. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) axons. Images depict the right hemisphere at 90× magnification.
Figure S5. Movie of a z stack of an adult unfZ0001,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons travel anteroventrally in a tightly organized peduncle. γ, β', and β axons projected medially. A thin fascicle of axons projects dorsally but stalls. Images depict the left hemisphere at 90× magnification.
Figure S6. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S6 corresponds to Figure 3C in the text. Images were obtained at a magnification of 60×.
Figure S7. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S7 corresponds to Figure 3D. Images were obtained at a magnification of 60×.
Figure S8. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S8 corresponds to Figure 3F. Images were obtained at a magnification of 60×.
Contributor Information
Karen E Bates, Email: kbates2@hawaii.edu.
Carl S Sung, Email: carlsung@hawaii.edu.
Steven Robinow, Email: robinow@hawaii.edu.
Acknowledgements
We thank Suewei Lin and Tzumin Lee for sharing unpublished results. We thank Oren Schuldiner, Shiri Yaniv and Paul Whitington for thoughtful discussions and critical reading of the manuscript. We thank Vivian Budnik for the rabbit anti-Fas II, Tina Carvalho at Pacific Biosciences Research Center for technical support, and Andrew Taylor for statistical advice. This work was funded by grants to SR from the George F Straub Trust and the Victoria S and Bradley L Geist Foundation administered through the Hawaii Community Foundation, and the National Science Foundation (IOB05-17765).
References
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–795. doi: 10.1016/S0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Armstrong JD, de Belle JS, Wang Z, Kaiser K. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn Mem. 1998;5:102–114. [PMC free article] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Technau G, Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature. 1982;295:405–407. doi: 10.1038/295405a0. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/S0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-X. [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-Q. [DOI] [PubMed] [Google Scholar]
- Martini SR, Roman G, Meuser S, Mardon G, Davis RL. The retinal determination gene, dachshund, is required for mushroom body cell differentiation. Development. 2000;127:2663–2672. doi: 10.1242/dev.127.12.2663. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Nagao T, Walldorf U, Flister S, Gehring WJ, Furukubo-Tokunaga K. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and Dachshund genes. Proc Natl Acad Sci USA. 2000;97:2140–2144. doi: 10.1073/pnas.040564497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveen A, Daniel A, Hartenstein V. Early development of the Drosophila mushroom body: the roles of eyeless and dachshund. Development. 2000;127:3475–3488. doi: 10.1242/dev.127.16.3475. [DOI] [PubMed] [Google Scholar]
- Nicolai M, Lasbleiz C, Dura JM. Gain-of-function screen identifies a role of the Src64 oncogene in Drosophila mushroom body development. J Neurobiol. 2003;57:291–302. doi: 10.1002/neu.10277. [DOI] [PubMed] [Google Scholar]
- Raabe T, Clemens-Richter S, Twardzik T, Ebert A, Gramlich G, Heisenberg M. Identification of mushroom body miniature, a zinc-finger protein implicated in brain development of Drosophila. Proc Natl Acad Sci USA. 2004;101:14276–14281. doi: 10.1073/pnas.0405887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm) Proc Natl Acad Sci USA. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Wang J, Lee CH, Lin S, Lee T. Steroid hormone-dependent transformation of polyhomeotic mutant neurons in the Drosophila brain. Development. 2006;133:1231–1240. doi: 10.1242/dev.02299. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Maruyama Y, Adachi Y, Okabe M, Suzuki E, Furukubo-Tokunaga K. A conserved nuclear receptor, Tailless, is required for efficient proliferation and prolonged maintenance of mushroom body progenitors in the Drosophila brain. Dev Biol. 2009;326:224–236. doi: 10.1016/j.ydbio.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Martini SR, Davis RL. The dachshund gene is required for the proper guidance and branching of mushroom body axons in Drosophila melanogaster. J Neurobiol. 2005;64:133–144. doi: 10.1002/neu.20130. [DOI] [PubMed] [Google Scholar]
- Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. doi: 10.1016/S0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang J, Haerry TE, Wu AY, Martin J, O'Connor MB, Lee CH, Lee T. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/S0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Zheng J, Edelman SW, Tharmarajah G, Walker DW, Pletcher SD, Seroude L. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci USA. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C, Wong LE, Chang Sen LQ, Nguyen E, Lazaga N, Ganzer G, McNabb SL, Robinow S. The unfulfilled/DHR51 gene of Drosophila melanogaster modulates wing expansion and fertility. Dev Dyn. 2009;238:171–182. doi: 10.1002/dvdy.21817. [DOI] [PubMed] [Google Scholar]
- Laudet VaGH. The Nuclear Receptor Factsbook. San Diego: Academic Press; 2002. [Google Scholar]
- DeMeo SD, Lombel RM, Cronin M, Smith EL, Snowflack DR, Reinert K, Clever S, Wightman B. Specificity of DNA-binding by the FAX-1 and NHR-67 nuclear receptors of Caenorhabditis elegans is partially mediated via a subclass-specific P-box residue. BMC Mol Biol. 2008;9:2. doi: 10.1186/1471-2199-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Baran R, Garriga G. Genes that guide growth cones along the C. elegans ventral nerve cord. Development. 1997;124:2571–2580. doi: 10.1242/dev.124.13.2571. [DOI] [PubMed] [Google Scholar]
- Much JW, Slade DJ, Klampert K, Garriga G, Wightman B. The fax-1 nuclear hormone receptor regulates axon pathfinding and neurotransmitter expression. Development. 2000;127:703–712. doi: 10.1242/dev.127.4.703. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ebert B, Carmean N, Weber K, Clever S. The C. elegans nuclear receptor gene fax-1 and homeobox gene unc-42 coordinate interneuron identity by regulating the expression of glutamate receptor subunits and other neuron-specific genes. Dev Biol. 2005;287:74–85. doi: 10.1016/j.ydbio.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- Haider NB, Mollema N, Gaule M, Yuan Y, Sachs AJ, Nystuen AM, Naggert JK, Nishina PM. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res. 2009;89:365–372. doi: 10.1016/j.exer.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–764. doi: 10.1093/hmg/ddi070. [DOI] [PubMed] [Google Scholar]
- Chen F, Figueroa DJ, Marmorstein AD, Zhang Q, Petrukhin K, Caskey CT, Austin CP. Retina-specific nuclear receptor: A potential regulator of cellular retinaldehyde-binding protein expressed in retinal pigment epithelium and Muller glial cells. Proc Natl Acad Sci USA. 1999;96:15149–15154. doi: 10.1073/pnas.96.26.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takezawa S, Hara K, Yu RT, Umesono Y, Agata K, Taniwaki M, Yasuda K, Umesono K. Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci USA. 1999;96:4814–4819. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O'Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, Hama C. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron. 2000;26:119–131. doi: 10.1016/S0896-6273(00)81143-5. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Lin S, Huang Y, Lee T. Nuclear receptor unfulfilled regulates axonal guidance and cell identity of Drosophila mushroom body neurons. PLoS One. 2009;4:e8392. doi: 10.1371/journal.pone.0008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters F, Leroy BP, Beysen D, Hellemans J, De Bosscher K, Haegeman G, Robberecht K, Wuyts W, Coucke PJ, De Baere E. Recurrent mutation in the first zinc finger of the orphan nuclear receptor NR2E3 causes autosomal dominant retinitis pigmentosa. Am J Hum Genet. 2007;81:147–157. doi: 10.1086/518426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33:559–571. doi: 10.1016/S0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- Brody T, Odenwald WF. Cellular diversity in the developing nervous system: a temporal view from Drosophila. Development. 2002;129:3763–3770. doi: 10.1242/dev.129.16.3763. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- Kao CF, Lee T. Birth time/order-dependent neuron type specification. Curr Opin Neurobiol. 2009. in press . [DOI] [PMC free article] [PubMed]
- Yu HH, Lee T. Neuronal temporal identity in post-embryonic Drosophila brain. Trends Neurosci. 2007;30:520–526. doi: 10.1016/j.tins.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Palanker L, Necakov AS, Sampson HM, Ni R, Hu C, Thummel CS, Krause HM. Dynamic regulation of Drosophila nuclear receptor activity in vivo. Development. 2006;133:3549–3562. doi: 10.1242/dev.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Michaut L, Ino A, Honjo K, Nakajima T, Maruyama Y, Mochizuki H, Ando M, Ghangrekar I, Takahashi K, Saigo K, Ueda R, Gehring WJ, Furukubo-Tokunaga K. Differential microarray analysis of Drosophila mushroom body transcripts using chemical ablation. Proc Natl Acad Sci USA. 2006;103:14417–14422. doi: 10.1073/pnas.0606571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Nighorn A, Thomas JB. Drosophila Eph receptor guides specific axon branches of mushroom body neurons. Development. 2006;133:1845–1854. doi: 10.1242/dev.02353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CH, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Ryder E, Blows F, Ashburner M, Bautista-Llacer R, Coulson D, Drummond J, Webster J, Gubb D, Gunton N, Johnson G, O'Kane CJ, Huen D, Sharma P, Asztalos Z, Baisch H, Schulze J, Kube M, Kittlaus K, Reuter G, Maroy P, Szidonya J, Rasmuson-Lestander A, Ekström K, Dickson B, Hugentobler C, Stocker H, Hafen E, Lepesant JA, Pflugfelder G, Heisenberg M. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 2004;167:797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Andres AJ, Thummel CS. Methods for quantitative analysis of transcription in larvae and prepupae. Methods Cell Biol. 1994;44:565–573. doi: 10.1016/s0091-679x(08)60932-2. full_text. [DOI] [PubMed] [Google Scholar]
- Van Vactor D, Sink H, Fambrough D, Tsoo R, Goodman CS. Genes that control neuromuscular specificity in Drosophila. Cell. 1993;73:1137–1153. doi: 10.1016/0092-8674(93)90643-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/CyO;;OK107-GAL4 control animal labeled with anti-Fas II (red), in which all MB lobes are properly formed. OK107-GAL4 driven GFP expression is visible in the Kenyon cells (green) and all MB lobes. Images depict the right hemisphere at 90× magnification.
Figure S2. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). The Kenyon cells (green) are visible in posterior planes. A rarely observed medial projection is visible in the region of the calyx. Axons travel anteroventrally then spread out, and stall prior to lobe formation. Images depict the left hemisphere at 90× magnification.
Figure S3. Movie of a z stack of an adult unfX1,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons project anteroventrally in a poorly organized peduncle, spread out, and stall at the end of the peduncle. The median bundle (green) is to the left. Images depict the right hemisphere at 90× magnification.
Figure S4. Movie of a z stack of an adult unfZ0001,UAS-mCD8GFP/Df2426;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons travel anteroventrally. γ, β', and β axons projected medially but were disorganized. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) axons. Images depict the right hemisphere at 90× magnification.
Figure S5. Movie of a z stack of an adult unfZ0001,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 mutant labeled with anti-Fas II (red). Axons travel anteroventrally in a tightly organized peduncle. γ, β', and β axons projected medially. A thin fascicle of axons projects dorsally but stalls. Images depict the left hemisphere at 90× magnification.
Figure S6. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S6 corresponds to Figure 3C in the text. Images were obtained at a magnification of 60×.
Figure S7. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S7 corresponds to Figure 3D. Images were obtained at a magnification of 60×.
Figure S8. Movie of z stacks of adult unfX1,UAS-mCD8GFP/unfZ0001;;OK107-GAL4 compound heterozygotes. The γ axons occupy the most superficial plane and can be distinguished from the more posterior β' (green) and Fas II-positive β (yellow) medial axons; the Fas II-positive α' dorsal axons (yellow) can be distinguished from the α dorsal axons (green), which do not express Fas II. Figure S8 corresponds to Figure 3F. Images were obtained at a magnification of 60×.