Abstract
Immunoglobulin M (IgM, 19S macroglobulin) is a high molecular weight antibody molecule currently thought to be composed of five identical 7S subunits linked by disulfide bonds, perhaps in a cyclic conformation. Reported molecular weights of IgM vary from 8 × 105 to 106. Proteolytic cleavage of this molecule under standard conditions with several enzymes results in the formation of ten antigen-binding (Fab) fragments per mole, each Fab having a molecular weight of 40,000. The remainder of the molecule, designated Fc by analogy with the structure of immunoglobulin G (IgG), is highly susceptible to further enzyme cleavage and breaks down to dialyzable peptides. However, under carefully controlled conditions, an intact Fc-like fragment in low yield can be isolated from short-term papain digests of IgM at 37°C. Availability of the Fc portion of the molecule is important not only for structural studies but because it may determine certain important biological properties of the molecule such as complement fixation and placental permeability.
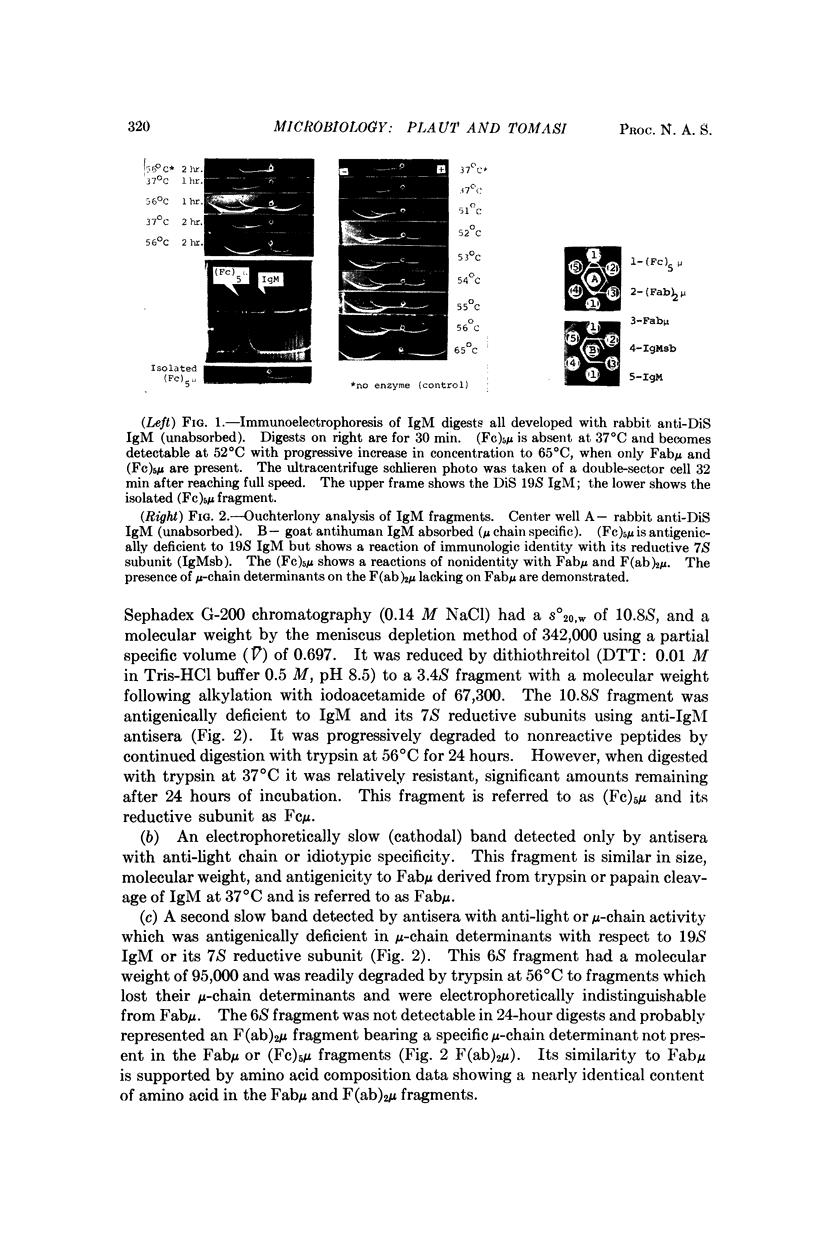
We here report that trypsin cleavage of IgM at temperatures exceeding 50°C results in the production of excellent yields of an intact Fc fragment, whereas no detectable Fc fragment is produced by trypsin cleavage at 37°C. Fab fragments are obtained under both temperature conditions, and an Fab dimer has been identified in high temperature digests. This unusual difference in the products of enzyme digestion with temperature is unexplained but may be related to steric changes induced in the IgM molecule by heat. Fc fragment isolated from high temperature digests contains two-thirds of the carbohydrate of the intact molecule, and has a molecular weight of 342,000. The molecular weight falls to 67,300 after treatment with disulfide reducing agents, thus supporting the concept of a pentameric structure for IgM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chen J. P., Reichlin M., Tomasi T. B., Jr Studies on the chymotrypsin C and papain fragments of human immunoglobulin M. Biochemistry. 1969 Jun;8(6):2246–2254. doi: 10.1021/bi00834a003. [DOI] [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- Mihaesco C., Seligmann M. Papain digestion fragments of human IgM globulins. J Exp Med. 1968 Mar 1;127(3):431–453. doi: 10.1084/jem.127.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. 3. The products of tryptic digestion. J Biol Chem. 1966 Apr 25;241(8):1732–1740. [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Onoue K., Kishimoto T., Yamamura Y. Papain fragmentation of the subunits of human macroglobulin. J Immunol. 1967 Feb;98(2):303–313. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]





