Abstract
Vascular smooth muscle cell migration is important during vascular development and contributes to lesion formation in the adult vasculature. The mechanisms regulating migration of this cell type are therefore of great interest. Recent work has shown that reactive oxygen species (ROS) derived from NADPH oxidases are important mediators of promigratory signaling pathways. ROS regulate the intracellular signals responsible for lamellipodia formation, actin cytoskeleton remodeling, focal adhesion turnover, and contraction of the cell body. In addition, they contribute to matrix remodeling, a critical step to initiate and support vascular smooth muscle cell motility. Despite these recent advances in our understanding of the redox mechanisms that contribute to migration, additional work is needed to evaluate fully the potential of ROS-sensitive molecular signals as therapeutic targets to prevent inappropriate smooth muscle cell migration. Antioxid. Redox Signal. 12, 625–640.
Introduction
Directed cell migration is an integrated, dynamic, and cyclic process that guides the morphogenesis of the embryo during development. In the adult, cell migration plays a key role in mounting immune responses and the repair of injured tissues. In vascular remodeling associated with diseases such as hypertension, atherosclerosis, hyperlipidemia, diabetes, and postangioplasty restenosis, one of the most relevant cellular events underlying this process is the dedifferentiation of vascular smooth muscle cells (VSMCs) into a synthetic phenotype. A major characteristic of this latter phenotype is that it recoups its capacity to migrate and proliferate in response to a variety of extracellular stimuli.
Reactive oxygen species (ROS) production has been implicated in nearly every cardiovascular pathology, from hypertension to atherosclerosis and restenosis after angioplasty (53). Indeed, ROS mediate neointimal hyperplasia during restenosis (95, 171), angiotensin II–induced hypertension (39, 146), impaired endothelium-dependent vasorelaxation (96), and heart failure (16). Moreover, the expression of NADPH oxidase subunits is upregulated in aortas of hypertensive animals (46) and during restenosis in experimental models (175), suggesting an association between these oxidases and ROS-mediated events.
The strong relation of oxidant stress with vascular remodeling establishes a connection between ROS production and VSMC proliferation, hypertrophy, and migration. Although the role of ROS in vascular growth has been investigated in detail, surprisingly, only limited information is available regarding the role of ROS in VSMC migration.
This review summarizes the current knowledge of the impact of ROS-mediated signaling on a variety of molecular targets that participate in VSMC migration. The repercussions for pathology and the potential directions for future research are discussed.
Reactive Oxygen Species
ROS is the common name given to a heterogeneous group of highly reactive small molecules. An important fraction of these compounds have unpaired valence shell electrons in the oxygen atom, explaining, in part, their increased reactivity. Another important ROS is hydrogen peroxide (H2O2), which is not a free radical. Although it is one of the most stable ROS, it has a strong oxidizing capacity based on its high reduction potential. The reaction of H2O2 with thiol-containing proteins is a key redox-signaling event (202). Very often, the term ROS also is used to refer to reactive nitrogen species (RNS) such as nitric oxide (NO) and peroxynitrite (ONOO−), which are also highly reactive and have high oxidant capacity, but instead depend on the presence of a reactive nitrogen atom within the molecule.
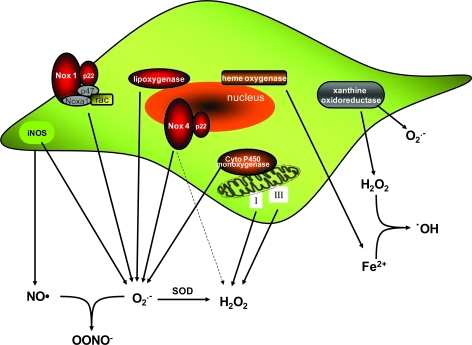
ROS are produced from a sequential one- or two-electron reduction of molecular oxygen. Among the major sources of intracellular ROS are the mitochondria, where they are produced as a by-product of the electron-transport chain (principally in complexes I and III) during cell metabolism (124). In addition to the mitochondrial respiratory chain, VSMCs contain abundant sources of ROS, including xanthine oxidase (205), lipoxygenases (126), nitric oxide synthases (155), and NADPH oxidases (51). VSMCs also contain hemoxygenases (122), which produce the Fe2+ that reacts with H2O2 to create the highly reactive hydroxyl radical through the Fenton reaction (103). Sources of ROS and the possible interactions among them are summarized in Fig. 1.
FIG. 1.
VSMCs contain multiple sources of ROS. ROS-producing enzymes include NADPH oxidases, lipoxygenases, xanthine oxidase, iNOS, mitochondrial electron-transport chain, and cytochrome p450 monoxygenase. VSMCs also contain hemoxygenase, which produces Fe that can react with H2O2 and generate hydroxyl radical (•OH) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Although they were originally known for their detrimental role in oxidation of biomolecules, such as proteins, lipids, and DNA, it is now widely accepted that ROS function as important intracellular and intercellular second messengers to modulate many downstream signaling molecules. ROS influence signaling molecules by altering the intracellular redox state and by oxidative modification of proteins, such as protein tyrosine phosphatases (30, 31), protein tyrosine kinases (52), transcription factors (155), mitogen-activated protein kinases (189), and ion channels (104, 151, 176). It is now well established that ROS such as superoxide (O2•−) and H2O2 play important roles regulating physiologic and pathophysiologic processes in vascular biology (52). Of importance for our review, they have been shown to have profound effects on VSMC growth and migration (65, 99, 119, 157, 173, 199, 208).
We and others have established that the ROS responsible for PDGF-induced migratory signaling is H2O2 (22, 173, 199). This makes sense because of its longer half-life and lower reactivity than other ROS. As noted earlier, despite its higher stability, H2O2 can induce protein oxidation, such as thiol modifications, that alter the activation state of proteins (207). Certain proteins contain cysteine thiols with a low pKa that are easily oxidized by H2O2 to form sulfenic (SOH), sulfinic (SO2H), and sulfonic (SO3H) acids or protein disulfides (PrSSPr).
Among the nonmitochondrial oxidases, NADPH oxidases are a major source of O2•− and H2O2 within the vessel wall (117, 145, 185, 208). NADPH oxidases produce ROS both extracellularly and intracellularly within endosomal compartments (113, 133). As a result, NADPH oxidase–derived ROS have been shown to modulate intracellular signaling pathways in a paracrine, autocrine, or even intracrine manner (145, 187, 193, 194). NADPH oxidases are multisubunit enzymes of which the catalytic subunit consists of one of the Nox proteins. The best-studied NADPH oxidase mediates the respiratory burst of neutrophils. The catalytic moiety of this enzyme is gp91phox (Nox2), which contains one FAD and two hemes, and catalyzes NADPH-dependent reduction of O2 to form O2•−. It is dormant in resting neutrophils and becomes activated on assembly with the cytosolic regulatory proteins p47phox, p67phox, and the small GTPase Rac (9).
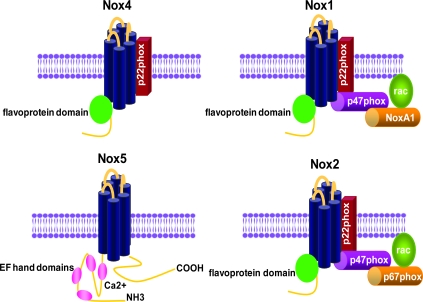
In VSMCs, NADPH oxidase activity is centered around novel gp91phox homologues as the catalytic subunits (93). VSMCs from large arteries express Nox1 and Nox4, whereas resistance and coronary arteries express Nox2 (56, 185), and human VSMCs also express Nox5 (14). In all vascular cells, these oxidases are low-output enzymes whose capacity is about one third that of the neutrophil (54), making them good candidates for participation in signaling. The kinetics of activation with cellular stimulation are also unique: O2•− is produced in minutes to hours, rather than in seconds to minutes as in the neutrophil (51, 134, 137). Like Nox2, Nox1 and Nox4 also interact with p22phox (4, 62, 190), and agonist-stimulated Nox1 activity requires Rac1 activation (92, 163, 197). Although the exact identity of the vascular Nox1 complex has not been proven in a single study, Lavigne et al. (97) showed that genetic deletion of p47phox attenuates angiotensin II– and PDGF-induced radical production in aortic VSMCs (which has been shown to be Nox1 dependent), whereas Ambasta et al. (4) found that p67phox mRNA is barely detectable in VSMCs and is instead functionally replaced by the p67phox homologue Nox-activator 1 (Noxa1). Taken together, these studies suggest that the VSMC Nox1 complex consists of Nox1, p22phox, p47phox, Noxa1, and Rac1. In contrast, Nox4 does not require any of the known cytosolic subunits for activity (17) but instead uses Polip2 as an activator (105). Structures of NADPH oxidases expressed in VSMCs are shown in Fig. 2.
FIG. 2.
Structures of NADPH oxidases found in VSMCs. NADPH oxidases are a family of multisubunit enzymes whose catalytic subunit consists of one of the Nox proteins. VSMCs from large arteries express Nox1 and Nox4, as well as Nox5 in humans. VSMCs from resistance arteries express Nox2 and Nox4. The Nox1 NADPH oxidase associates with two cytosolic factors, p47phox and Nox activator 1 (Noxa1), as well as the small-molecular-weight G protein Rac. Nox2 is regulated by p47phox and p67phox, whereas Nox4 is not. In the case of Nox1 and 2, activity requires assembly with the cytosolic subunits (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The need for more than one NADPH oxidase complex within the same cell is somewhat paradoxic. One likely explanation may be that the location of O2•− production is important, as expected when dealing with a signaling molecule with an extremely short half-life and diffusion distance. We have found that VSMC Noxes have different subcellular localizations (68) and differential regulation by agonists (92), suggesting different functions in VSMC biology as well. Similar observations have been made for Nox2 and Nox4 in endothelial cells (5). This is potentially extremely important for the regulation of migration, because, as noted later, subcellular location is exquisitely important in migratory signaling.
ROS and VSMC Migration: An Overview
The first clue that ROS might be important in VSMC migration came from a seminal study of Sundaresan et al. (173), who showed that H2O2 was required for PDGF-induced migration in VSMCs (173). We and others have expanded these observations, showing that PDGF-induced ROS production is dependent on Nox1 activation (22, 173, 181, 199). Subsequently, it was shown that migration in response to other agonists, such as phenylephrine and VEGF, is also ROS sensitive, as it is prevented by catalase treatment and antioxidants [N-acetylcysteine (NAC) and pyrrolidine dithiocarbamate] (127, 195). Moreover, thrombin-stimulated migration is blocked by the flavin-containing oxidase inhibitor diphenylene iodonium (DPI) and the NADPH oxidase inhibitor apocynin, implicating NADPH oxidase–derived ROS in this response (196). Recently, it was proven that the Nox1-based NADPH oxidase is required for VSMC migration induced by basic fibroblast growth factor (161) and PDGF (99, 157). Although Nox1 seems to be unequivocally involved in agonist-induced migration in VSMCs, Nox4 may play a role as well. It was recently published that Nox4 mediates insulin-like growth factor-I–induced migration (111) and angiotensin II–induced myofibroblast migration (65).
Peroxiredoxins are a family of multifunctional antioxidant thioredoxin-dependent peroxidases that eliminate H2O2. One of the members of this family, peroxiredoxin II (Prx II), has been shown to be a negative regulator of PDGF signaling. Prx II overexpression in VSMCs inhibits migration in vitro (32).
In parallel with the in vitro studies, data obtained in animal models support the role of ROS in VSMC migration. Superoxide and lipid peroxidation are elevated immediately after vascular injury, during the migratory phase of neointimal formation (8, 132, 171). In addition, an increase in nitrotyrosine, which is formed by reaction of nitric oxide and O2•−, has been detected with immunohistochemistry after vascular injury (184). Functionally, a number of investigators have shown that administration of antioxidants (75, 76, 89, 99, 132, 180), treatment with the NADPH oxidase inhibitor gp91ds-tat (74), genetic deletion of NADPH oxidase homologues (99), or treatment with the xanthine oxidase inhibitor allopurinol (205) significantly reduces neointimal hyperplasia formation during repair of vascular injury, a response that is heavily dependent on VSMC migration and proliferation. Likewise, it was recently reported that gene transfer of redox factor 1 inhibits neointimal formation in vivo because it blocks ROS-mediated protein tyrosine kinase activity in VSMCs (98). Similar results were obtained by adenovirus-mediated overexpression of peroxisome proliferator–activated receptor-γ coactivator (PGC)-1α, a protein that regulates mitochondrial antioxidant capacity and biogenesis. PGC-1α overexpression greatly reduced neointima formation in balloon-injured rat carotid artery (143). Conversely, wire-injured carotid arteries from Prx II−/− animals develop a thicker layer of neointima when compared with wild-type animals (32).
The Cycle of Migration
Our knowledge of the molecular mechanisms regulating VSMC migration is still somewhat limited, but much can be inferred from studies in fibroblasts. Fibroblast migration is a dynamic process that requires specialized signaling domains at the front and rear of the cell (11, 25, 144). First, a cell must sense a gradient and establish polarity (94). Plasma membrane is then extended in the direction of eventual movement in the form of lamellipodia (168). New focal complexes are established in the front of the cell under the protrusion by restructuring of the actin cytoskeleton. Then, a mechanical contraction force is induced by phosphorylation of myosin II, and the body of the cell contracts, moving it forward. Subsequently, focal adhesions in the rear of the cell are detached, and the trailing edge retracts. Finally, adhesion receptors are recycled by endocytosis and vesicular transport (164). These individual events are directed by activation of specific signals in the relevant subcellular compartment. Therefore, specialized signaling domains exist that serve to distinguish the front and rear of the cell (11, 25, 144). Successful migration is thus dependent on many molecules, the activation and actions of which are carefully timed in the pertinent subcellular compartments. In the remainder of this review, we consider how ROS regulate each of these steps in migration, starting with their effects on the actin cytoskeleton and microtubules, which are involved in all aspects of migration.
Cytoskeleton Dynamics and ROS
Actin-filament dynamics and reorganization are essential for cell-shape change, polarity formation, and all phases of cell migration (59). Organized and directed movement of the cell is based on an exquisite local and temporal regulation of the actin cytoskeleton. The Rho family of low-molecular-weight G proteins (especially, cdc42, Rho, and Rac) are intimately involved in most aspects of actin-filament turnover and assembly. Depending on the identity of the GTPase, different changes in the actin cytoskeleton will be induced. Cdc42 activation induces the formation of actin-rich surface protrusions called filopodia (90, 131). Rho activation leads to the assembly of contractile actin–myosin filaments (stress fibers) and of associated focal adhesion complexes (148). Finally, Rac induces the assembly of a meshwork of actin filaments at the cell periphery to produce lamellipodia and membrane ruffles (129–131, 148). As discussed in more detail later, ROS can influence actin dynamics both directly and indirectly through the alteration of intracellular signaling pathways during specific phases of migration.
ROS as Direct Regulators of Actin Polymerization
As noted earlier, H2O2 can oxidize reactive thiols in proteins. Oxidized thiols can also react with glutathione (GSH) to form glutathiolated disulfides (PrSSG). S-glutathiolation is reversible by enzymatic reduction through glutaredoxins, thioredoxin, or peroxiredoxins (207). Obviously, a number of proteins involved in cytoskeletal reorganization are potential targets for oxidation or glutathiolation, but oxidation of only a few has been verified, including Src (49), C-terminal Src kinase (Csk) (114), actin (35), and a number of phosphatases (PTP-PEST, LMW-PTP, and SHP-2) (30, 177). Of importance, β-actin itself can be directly oxidized, and this posttranslational modification has been shown to affect polymerization. In vitro treatment of β-actin with high (millimolar) concentrations of H2O2 or tert-butyl hydroperoxide decreases the maximal rate of polymerization, increases both the delay time and the time required for half-maximal assembly, decreases the elongation rate, increases the critical monomer concentration for polymerization, and inhibits binding of the actin capping protein filamin (35, 36, 115). Extensive mutational and mass-spectrometry analysis showed that the C-terminal cysteine (Cys374) of α- or β-actin can be oxidized in either G-actin monomers or after polymerization of F-actin (35, 36). Of importance, this C-terminal region of the molecule is the binding site for several actin-binding proteins (152). Cys374 has also been shown to be glutathiolated (81), which also leads to a reduced rate of polymerization, a relative instability of F-actin filaments, and a corresponding enhancement of steady-state ATPase activity in vitro (40, 172).
This effect of oxidants to cause cytoskeletal disorganization or impairment of actin–myosin functionality has also been demonstrated in cells and tissues treated with strong oxidants. Incubation of cardiomyocytes with 2,2-dithiodipyridine reduces contractile-force generation in parallel with oxidation of actin (67), whereas treatment of permeabilized rabbit psoas muscle fibers with 50 mM H2O2 decreases fiber contractility and impairs actomyosin enzyme activity (142). However, disruption of the actin cytoskeleton by oxidants is not a universal finding. Treatment of macrophage-like P388D1 cells with 1–5 mM H2O2 increases stress-fiber formation while decreasing actin nucleation activity (136). Slow oxidation of G-actin produces intermolecular disulfide-bonded actin dimers that can be incorporated into F-actin during polymerization, generating cross-links between actin filaments and thus enhancing the elasticity of the F-actin network (178). Moreover, additional work has shown that when oxidizing conditions favor sulfhydryl oxidation, a greater rate and extent of actin polymerization is observed (69).
It should be noted that all of these studies were performed with very high concentrations of oxidants, which do not necessarily mimic the physiologic state. When intact cells are exposed to millimolar concentrations of H2O2, cells undergo apoptosis or cell cycle arrest but not migration (38, 101). Conversely, generation of lower, physiologically relevant concentrations of H2O2 seems to promote actin polymerization and formation of stress fibers. For example, endothelial cells actively migrating into a wound produce elevated levels of ROS, and reduction of these molecules with DPI or the SOD-mimetic MnTMPyP abolishes actin monomer incorporation at the barbed end of growing actin filaments (118). Because these studies were performed in intact cells, it was not possible to determine whether ROS exert their effects by directly oxidizing actin or by affecting the oxidation state, phosphorylation, or binding of actin-binding proteins. However, the effects of oxidants on the actin cytoskeleton may be cell-type specific. Huot et al. (71) showed that the same concentration of H2O2 that induces fragmentation of F-actin in fibroblasts induces a reorganization of F-actin in endothelial cells, leading to the accumulation of stress fibers, the recruitment of vinculin to focal adhesions, and the loss of membrane ruffles. Fiaschi et al. (42) found that administration of an inhibitor of ROS generation during cell adhesion and spreading on fibronectin prevents the necessary remodeling of the actin cytoskeleton. They found that engagement of integrin receptors results in a transient glutathiolation of actin that is required for cytoskeletal reorganization. Similarly, our work showed that depletion of Nox4 by using siRNA results in dissolution of smooth muscle α-actin–based stress fibers (33), but the mechanism remains unclear. Clearly, more work is needed to determine the potential role of actin oxidation in VSMCs.
The relation between the actin cytoskeleton and ROS seems to work both ways. Thus, cortactin, an actin-binding protein that has traditionally been found to regulate polymerization of the actin cortex, has also been shown to mediate p47phox translocation to the membrane during angiotensin II– and hyperoxia-induced of NADPH oxidase activation (186, 188). Moreover, actin activates Nox2 in neutrophils in a cell-free system, implying a direct effect on NADPH oxidase enzyme activity, and destabilization of the actin cytoskeleton robustly enhances the neutrophil respiratory burst activity (19, 121). A more complete understanding of this bidirectional relation between NADPH oxidases and the actin cytoskeleton may shed further light on how ROS mediate migration.
Microtubules, ROS, and Migration
Active remodeling of microtubules also is required during multiple phases of migration. Microtubules are the strongest of the cytoskeletal polymers and are made up of α/β-tubulin heterodimers. Microtubules are essential, not only because they reorganize the microtubule cytoskeleton during cell-cycle progression and cell motility, but also because they participate in the modulation of signal transduction within the cell and regulate remodeling of the actin cytoskeleton.
To migrate directionally, cells must be polarized. The microtubule-organizing center (MTOC) and other microtubule-containing apparatuses orient toward the direction of migration. Treatment with the microtubule-stabilizing agent taxol has been shown to inhibit VSMC migration in vivo and in vitro (7, 169). The pathways involved in microtubule dynamics in VSMCs have not been well studied, and no direct evidence suggests that ROS participate in those dynamics. Most of the available mechanistic information has been inferred from the mechanism of action of microtubule inhibitors. As in the relation between actin and NADPH oxidases, microtubules seem both to influence ROS production and to be regulated by it. It has been reported that in melanoma cells, taxol induces downregulation of uncoupling protein 2, thus increasing mitochondrial ROS production, in a mechanism that involves activation of the JNK and p38 pathways, and is blocked by N-acetylcysteine (NAC) (162). In addition, it has been shown that taxol promotes ROS generation by enhancing the activity of NADPH oxidase in neurons (77) and in cancer cells through translocation of Rac1 (3). Moreover, the flavoenzyme inhibitor diphenylene iodonium (DPI), which blocks NADPH oxidases and mitochondrial ROS production, has been shown to inhibit mitotic cell division by impairment of centrosome maturation (159). Finally, depolymerization of microtubules activates NF-κB (150), a transcription factor widely implicated in the regulation of oxidative stress–related proteins. Because of the critical role of microtubules in migration in other cell types, clearly the relation of ROS with microtubules during VSMC migration deserves further study.
ROS and the Initiation of Migration
VSMC migration is influenced by many factors, but in vivo, PDGF is the major promigratory stimulus (55, 73, 78), largely as a consequence of PDGF-β receptor activation (24). PDGF-α and PDGF-β are expressed at very low or undetectable levels in normal vessels (66). Likewise, PDGF-α and PDGF-β receptor mRNAs are present in SMCs in the vessel wall (108), but their proteins are barely detectable (43, 153). In atherosclerosis, during the initial response to injury or even during the phenotypic transformation of VSMCs in culture, PDGF-β receptor synthesis is induced (15, 108). This may in part be mediated by ROS as a result of a positive feedback of the increased ROS production in these conditions (63, 138). Agents that inhibit the PDGF-induced ROS formation in VSMCs (139) are also capable of blocking autocrine PDGF-β synthesis in mesangial cells (154). Furthermore, preincubation of VSMCs with NADPH oxidase inhibitors DPI and apocynin partially blocks PDGF-induced PDGF-β–receptor phosphorylation (98), suggesting that ROS may be involved as early as the initial activation of the receptor.
Several migratory stimuli can induce a positive redox feedback in the expression of other migratory signals. For instance, after the engagement of PDGF with its receptor, Nox1-mediated ROS are produced over minutes to hours (92, 99). Not only do these ROS mediate cytoskeletal-associated signal transduction (see later), but they also participate in the induction of other promigratory growth factors (109), such as FGF-2 (21, 140). Likewise, angiotensin II, which is a weak migratory factor that also activates Nox1, can enhance EGF-receptor expression levels through an ROS-mediated mechanism in a nontumorigenic human keratinocyte cell line (125). Thus, in the course of their action, and most likely through ROS-dependent pathways, growth factors and cytokines can stimulate the synthesis of other promigratory stimuli, amplifying their cellular responses.
The production of ROS extracellularly can also increase migratory signals. For instance, nitrotyrosine can stimulate VSMC migration through a mechanism blocked by antioxidants or the SOD mimetic MnTBAP (123). Similarly, oxidant stress can increase levels of homocysteine (Hcys), an amino acid associated with a high risk for atherosclerosis and restenosis after angioplasty (110). Hcys activates MAP kinases and induces migration in VSMCs by a mechanism blocked by pretreatment with the flavoenzyme inhibitor DPI and the free-radical scavenger NAC (102). Moreover, in cultured VSMCs, Hcys can upregulate monocyte chemoattractant protein-1 (MCP-1), a potent chemokine that stimulates VSMC migration (192).
Finally, biomechanical forces such as hemodynamic changes also can affect VSMC migration (58). Although the mechanisms remain to be elucidated, focal adhesion sites, integrins, and cellular junctions can act as sensors of these mechanical changes, which have been reported to activate ROS-sensitive signal-transduction pathways (88).
Lamellipodium Formation and ROS
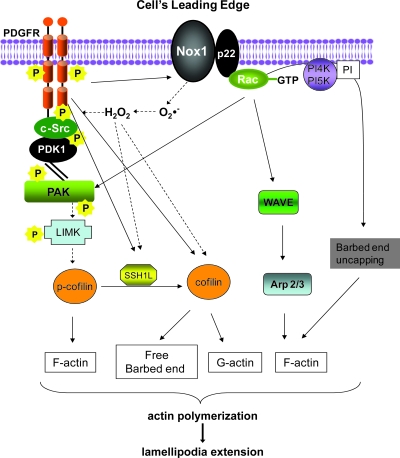
After the cell senses a signal, lamellipodia formation, or localized protrusion of the cell membrane in the direction of the chemotactic stimulus, is driven by the extension of F-actin–rich fibers (1, 28, 191, 204). Protrusion of such actin-rich lamellipodia in moving cells requires cycles of actin polymerization and depolymerization (actin polymerization transients). ROS-dependent pathways leading to lamellipodium formation are summarized in Fig. 3.
FIG. 3.
ROS-dependent pathways leading to lamellipodium formation in VSMCs. After agonist stimulation, VSMCs initiate a cascade of events leading to lamellipodium formation. PDGF-induced ROS activate the Src/PAK/LIMK pathways to induce cofilin inactivation and therefore F-actin stabilization. PDGF-induced migration requires the activation of SSH1L, which activates cofilin and thus supplies G-actin and free barbed ends continuously to the leading edge. Branching and elongation of preexisting filaments are induced through Rac and WAVE/ARP2/3. Interrupted arrows, ROS-mediated pathways. SSH1L = slingshot phosphatase 1L; LIMK = LIM domain kinase 1; WAVE =WASP family verprolin-homologous protein; ARP2/3 = actin-related protein 2/3; PAK =p21-activated kinase. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In lamellipodia, chemoattractants bind to receptors to activate a specific guanine nucleotide exchange factor (GEF), leading to an increase in the GTP-bound active form of Rac (10, 94, 116). Rac stimulates actin polymerization by several mechanisms, including NADPH oxidase–mediated ROS production (118), nucleation of new actin filaments by activation of WAVE/Arp2/3 (106, 112), or barbed-end uncapping and extension of existing filaments (64).
Lamellipodium formation in vivo has been shown to be essentially dependent on the formation of free barbed ends on existing actin filaments (198). Cofilin, a key player in agonist-induced lamellipodium protrusion, is a protein capable of severing actin filaments at or near the pointed ends, and binds to both G- and F-actin to increase the rate of depolymerization of actin filaments (12, 13). This leads to the formation of free barbed ends, a continuous supply of actin monomers for polymerization, and rapid turnover of new actin filaments (27). Activation of cofilin has been shown to play an essential role in maintaining and protruding lamellipodia at the leading edge of migrating cells.
Cofilin activity is negatively regulated through phosphorylation at Ser-3 by the LIM kinase (LIMK) family of serine/threonine kinases (6, 182, 206). Suppression of cofilin activity by LIMK overexpression abolishes lamellipodium formation and polarized cell migration (37, 210). LIMKs are activated by phosphorylation in response to various extracellular stimuli, including lysophosphatidic acid (107), stromal cell-derived factor-1 (128), insulin (206), and PDGF (157). LIMK phosphorylation is mediated by Rho, Rac, Cdc42, and their downstream protein kinases, such as Rho kinase (ROCK) and p21-activated kinase (PAK) (128, 135, 206). It has been reported that ROCK is activated by ROS in VSMCs (80), and our work showed that PAK activation in PDGF-induced migration occurs through a ROS-sensitive Src activation (199).
The Ser-3–phosphorylated cofilin (p-cofilin) is dephosphorylated and thus activated by Slingshots (SSH), a relatively new family of protein phosphatases (83), or the novel HAD-type serine protein phosphatase chronophin (50). We recently showed that Slingshot1L (SSH1L) phosphatase activation is required for PDGF-induced cofilin activation (dephosphorylation) and VSMC migration (157). Intriguingly, we observed that cofilin activation by PDGF is dependent on Nox1 expression (99, 157) through ROS-mediated activation of SSH1L, in a mechanism that involves oxidation of its inhibitory partner protein 14-3-3 (85, 156). α6β4 Integrin signaling through Rac-1 activation also participates in the regulation of SSH-mediated cofilin activation (87).
Focal Adhesion Assembly/Disassembly and ROS
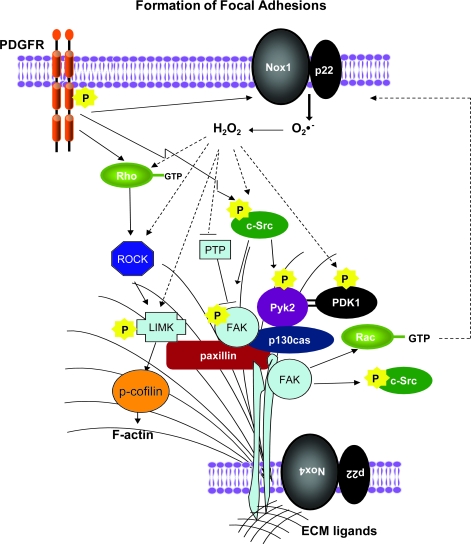
After lamellipodia have formed, the next step in migration is the attachment of the leading edge of the cell to the substrate, which occurs through integrin engagement. Integrins are cell-surface receptors that serve to bridge the extracellular matrix (ECM) with the cell cytoskeleton (72). The specialized sites of cell attachment through integrins to the ECM are known as focal adhesions (FAs). It is important to note that FA turnover that is required for cell motility, so that FAs must form and dissolve properly for normal migration. FA-turnover pathways that use ROS as signaling molecules are summarized in Fig. 4.
FIG. 4.
ROS-dependent pathways leading to focal adhesion formation in VSMCs. Integrin stimulation through inside-out or outside-in stimuli induces clustering of FA proteins and strengthening of stress fibers. Simultaneous stimulation with PDGF activates a series of tyrosine kinases and inhibits phosphatases that contribute to FA formation. Both PDGF-mediated signaling and local production of ROS by Nox4 in FAs coordinate FA formation. Interrupted arrows, ROS-mediated pathways. FAs = focal adhesions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Whether integrin activation occurs primarily through the engagement of ECM-bound promigratory stimuli (outside in), or as a consequence of an intracellular cascade initiated by a soluble factor (inside out), activated integrins cluster and consequently recruit actin filaments. This recruitment is achieved as the cytoplasmic domains of integrins associate with a group of effectors, which include talin, vinculin, α-actinin, filamin, and paxillin (34, 91, 209). One of the two homologues of LIMK, LIMK1, is also localized mainly to focal adhesions (2). Interestingly, in Drosophila, paxillin can negatively modulate LIMK function within focal adhesions by regulating the Rho pathway (26), which is activated by ROS in VSMCs (79, 80). These findings suggest a role for LIMK in FAs at the time that it participates in the formation of lamellipodia.
The FA protein complex organizes the actomyosin contractile apparatus and attracts signaling molecules such as focal adhesion kinase (FAK), Src family kinases, and integrin-linked kinase (91, 209). These kinases link integrins to the actin cytoskeleton and coordinate the formation and strengthening of FAs in the lamellipodium, as well as their recycling from the rear of the cell. FAK is of particular importance. Integrin-mediated activation of FAK leads to phosphorylation of paxillin and p130Cas, thereby regulating their translocation to FAs (203) and enhancing FA formation. At the same time, FAK autophosphorylates on tyrosine 397, which is essential for FAK-induced FA disassembly (60). Depletion of FAK in fibroblasts results in enhanced FAs and impaired migration (167).
It is well established that integrin signaling involves ROS, and, at the same time, that ROS can mediate integrin activation (29, 174). In different cell types, integrins have been shown to activate small Rho-GTPases (20, 61, 141). During fibronectin (FN)/integrin-mediated cell adhesion, ROS are dramatically increased by a Rac1-dependent activation of NADPH oxidase (31). Other sources of integrin-induced ROS include mitochondria (201) and lipoxygenase (177). As a result of this oxidative burst, the activity of low-molecular-weight protein tyrosine phosphatase (LMW-PTP) is transiently inhibited by thiol oxidation within the active site of the enzyme (30, 31). LMW-PTP also has been shown to associate with, dephosphorylate, and thus inactivate FAK (149). Therefore, LMW-PTP activity must be tightly regulated, probably through ROS-dependent mechanisms, to ensure proper focal complex/adhesion dynamics. Indeed, overexpression of LMW-PTP has been shown to inhibit VSMC migration (165). Similarly, the participation of ROS modulating the activity of PTPs has been studied in an in vivo model in which the participation of PDGF-induced ROS inhibition of PTP activity was reversed with the use of antioxidants (84).
FAs provide a support against which cell contraction can occur. They begin life as focal complexes, and the mechanisms regulating the conversion of focal complexes to FAs are unclear. Our work has shown that in angiotensin II–treated cells, ROS regulate the Src-dependent activation of PDK1, which is essential for this process (179). In addition, stress fiber formation and contraction, which are involved in FA strengthening, require activation of Rho (130, 200). Shinohara et al. (166) found that in Ras-transformed cells, Nox1-generated ROS mediate downregulation of Rho activity through oxidative inactivation of the LMW-PTP.
We recently found that Nox4 is also a key player in the regulation of stress fiber formation and focal adhesion turnover in VSMCs (33). Our group just reported the identification of Poldip2, a new regulator of Nox4 (105). Poldip2 is an activator of Nox4-mediated ROS production in VSMCs, and either upregulation or downregulation of Poldip2/Nox4 negatively affects FA turnover and inhibits VSMCs migration. These findings suggest a potentially novel mechanism, local ROS production, by which FA turnover is coordinated.
Contraction and ROS
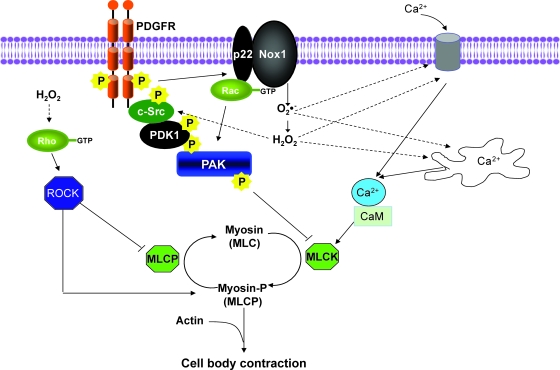
The next phase in SMC motility is contraction of the cell body to create forward movement. As with cell contraction to regulate vascular tone, ATPase activity associated with myosin II is required for contraction to occur. After phosphorylation of the myosin regulatory light chain by a calcium-calmodulin (Ca2+/CaM)-dependent myosin light-chain kinase (MLCK), actin activates myosin II ATPase activity, and contraction proceeds (47, 48, 170). In Fig. 5, ROS-mediated signaling pathways leading to VSMCs contraction are summarized.
FIG. 5.
ROS-dependent pathways leading to cell-body contraction in VSMCs. The final step in motility is the production of forward movement through regulation of myosin II phosphorylation and actin–myosin interaction. Several major regulators of myosin phosphorylation are ROS sensitive, including mobilization of calcium from intracellular or extracellular compartments, Src activation, and the Rho-ROCK pathway. Interrupted arrows, ROS-mediated pathways. Src = Rous sarcoma virus kinase homologue; ROCK = Rho-associated protein kinase. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In VSMCs, ROS appear to be both upstream and downstream of intracellular Ca2+ release and calcium influx. After growth-factor stimulation, VSMCs exhibit waves of cytosolic calcium release that are required for migration (160); blockers of calcium channels reduce both migration and ROS production. However, it also was shown that H2O2 and superoxide can increase the intracellular Ca2+ concentration in VSMCs and endothelial cells (104, 151, 176), apparently by regulating Ca2+ release from 1,4,5-trisphosphate–sensitive Ca2+ stores.
Although Ca2+-dependent activation of MLCK is the major mechanism initiating cell contraction, additional pathways have been shown to regulate actin–myosin function. One such pathway in VSMCs is PAK. After activation by either Rac or Cdc42, PAK1 phosphorylates MLCK, resulting in decreased MLCK activity, thereby inhibiting myosin light chain (MLC) phosphorylation and cell contractility (158). This mechanism may be ROS mediated, because PAK1 activation in VSMCs is dependent on Nox1-derived ROS (99). Another important mechanism that regulates the contractile apparatus is the Rho/ROCK pathway, which has shown to be activated by ROS in both aorta and VSMCs (79, 80). Importantly, this pathway promotes MLC phosphorylation by phosphorylating and thus inhibiting the regulatory subunit of myosin light chain phosphatase (79, 80, 86), and by direct ROCK-mediated myosin II phosphorylation in fibroblasts (183).
Actual movement of the cell occurs through engagement of actin–myosin interactions. As the body of the cell moves forward, the newly formed FAs become stronger and arrive at the rear of the cell, where they are dissociated, allowing their components to recycle to the leading edge of the cell for the next wave of migration (11). Virtually nothing is known about the role of ROS in FA dissociation.
Extracellular Matrix and ROS
As mentioned earlier, cell adhesion and migration are dependent on integrin binding to the extracellular matrix (ECM). Cell migration is, in its essence, an invasive process that requires degradation of the ECM. This is achieved by activation of matrix metalloproteinases (MMPs) and simultaneous inhibition of tissue inhibitors of metalloproteinases (TIMPs). Accordingly, MMP inhibitors have been shown to attenuate migration and delay neointimal formation (18). Moreover, genetic deletion of either MMP-2 or MMP-9 reduces VSMC migration (82). MMP activity is regulated by transcriptional and posttranscriptional mechanisms, both of which are mediated by ROS. Although ROS has been reported to downregulate MMP2 and 14 activities (41), most of the compelling data indicate that ROS can directly or indirectly activate MMPs. In VSMCs, ROS activate MMP-9 (120) and MMP-2 (70). The stimulation of MMP-9 activity by direct incubation with H2O2 (147) proves unequivocally that the redox state of MMPs is at least part of their mechanism of regulation. MMP-2 activity also is increased by H2O2, as well as by ONOO− (147). Conversely, MnSOD and NO inhibit IL-1β–stimulated MMP-9 activity (57). It should be noted that MMP-7, an MMP with high degradative ability, is activated (45) or inactivated (44) by hypochloric acid (HOCl−) depending on the system.
Like activity, expression of MMPs has shown to be sensitive to ROS. MMP-1, which is important in collagen degradation, is increased by angiotensin II stimulation through the redox-sensitive transcription factors NF-κB and activating protein-1 (AP-1) (23). TNF-α stimulation has similar effects (23). Similarly, 4-hydroxynonenal (HNE), a by-product of oxidative damage that frequently accumulates in atherosclerotic lesions, increases mitochondrial ROS production, and consequently enhances MMP-2 activity in VSMCs by a mechanism that involves the Akt/NF-κB signaling pathway (100). Thus, ROS not only regulate the mechanics of cell migration, but also regulate the expression and activity of the enzymes necessary to create a path for the migrating cell.
Conclusions and Future Directions
The findings discussed herein undoubtedly support a key role of ROS as signaling molecules that regulate VSMC migration. Because migration requires carefully coordinated, tightly regulated signaling within particular subcellular locations, ROS are potentially excellent candidates for such regulation. They have short half-lives and are degraded shortly after being produced, most likely only a few atomic ratios away from the site of production.
As is evident throughout this review, much work remains to be done to understand better the participation of ROS at different levels of cell migration. Particularly interesting will be to understand how the redox state of actin and actin-associated proteins affects their protein function/polymeri-zation properties. It will also be important to understand the spatial and temporal relations of ROS production from specific sources of ROS and their specific subcellular targets. At this point, one of the major challenges is to be able to visualize these localized events. New live-cell imaging techniques and new probes that allow us to study this process in real time are essential.
We also need to understand the contribution of variations in cell type as well as particular extracellular environments that can differentially affect cellular movement. Our present paradigm of VSMC migration is based on a model developed in other cell types, principally, but not exclusively, fibroblasts. It is very likely that VSMCs, and even potentially VSMCs from different vascular beds, have unique regulatory mechanisms for their migratory behavior. Ultimately, knowledge gained in the in vitro systems will have to be translated to animal models to allow us to understand how ROS-mediated signaling contributes to phenotypic modulation and wound healing.
Abbreviations Used
- AP-1
activating protein-1
- Arp 2/3
actin-related protein 2/3
- Cdc42
cell-division cycle 42
- Csk
C-terminal Src kinase
- DPI
diphenylene iodonium
- ECM
extracellular matrix
- FA
focal adhesion
- FAD
flavin adenine dinucleotide
- FAK
focal adhesion kinase
- FN
fibronectin
- GEF
guanine nucleotide exchange factor
- GSH
glutathione
- Hcys
homocysteine
- H2O2
hydrogen peroxide
- HOCl−
hypochloric acid
- JNK
JUN NH2-terminal kinase
- LIMK
LIM-domain kinase
- LMW-PTP
low-molecular-weight protein tyrosine phosphatase
- MCP-1
monocyte chemoattractant protein-1
- MLC
myosin light chain
- MLCK
myosin light-chain kinase
- MMP
metalloproteinase
- MnTBAP
Mn(III)tetrakis(4-benzoic acid) porphyrin chloride
- MTOC
microtubule organizing center
- NAC
N-acetyl cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor κB
- NO
nitric oxide
- Noxa1
NADPH oxidase-activator 1
- O2•−
superoxide radical
- ONOO−
peroxynitrite
- p38
p38 mitogen-activated protein kinase
- PAK
p21-activated kinase
- PDGF
platelet-derived growth factor
- PDK1
3'-phosphoinositide-dependent kinase-1
- PGC-1α
peroxisome proliferator–activated receptor-γ coactivator
- Poldip2
polymerase (DNA-directed), delta-interacting protein 2
- PrSSPr
protein disulfide
- Prx II
peroxiredoxin II
- PTP-PEST
PEST sequence containing protein tyrosine phosphatase
- Rho
Ras homologue
- RNS
reactive nitrogen species
- ROCK
Rho-associated kinase
- ROS
reactive oxygen species
- SHP-2
Src homology 2–containing protein tyrosine phosphatase
- SOD
superoxide dismutase
- SOH
sulfenic acid
- SO2H
sulfinic acid
- SO3H
sulfonic acid
- SSH1L
slingshot 1L
- TIMP
tissue inhibitor of metalloproteinase
- TNF-α
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cell
- Wave
Wiskott-Aldrich syndrome protein
Acknowledgments
This work was supported by National Institutes of Health grants HL38206, HL093115, and HL058863.
References
- 1.Abercrombie M. Heaysman JE. Pegrum SM. The locomotion of fibroblasts in culture, 3: Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970;62:389–398. doi: 10.1016/0014-4827(70)90570-7. [DOI] [PubMed] [Google Scholar]
- 2.Acevedo K. Moussi N. Li R. Soo P. Bernard O. LIM kinase 2 is widely expressed in all tissues. J Histochem Cytochem. 2006;54:487–501. doi: 10.1369/jhc.5C6813.2006. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre J. Hu Y. Lu W. Pelicano H. Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 4.Ambasta RK. Schreiber JG. Janiszewski M. Busse R. Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Anilkumar N. Weber R. Zhang M. Brewer A. Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 6.Arber S. Barbayannis FA. Hanser H. Schneider C. Stanyon CA. Bernard O. Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 7.Axel DI. Kunert W. Goggelmann C. Oberhoff M. Herdeg C. Kuttner A. Wild DH. Brehm BR. Riessen R. Koveker G. Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo LC. Pedro MA. Souza LC. de Souza HP. Janiszewski M. da Luz PL. Laurindo FR. Oxidative stress as a signaling mechanism of the vascular response to injury: the redox hypothesis of restenosis. Cardiovasc Res. 2000;47:436–445. doi: 10.1016/s0008-6363(00)00091-2. [DOI] [PubMed] [Google Scholar]
- 9.Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995;2:55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Bailly M. Condeelis JS. Segall JE. Chemoattractant-induced lamellipod extension. Microsc Res Tech. 1998;43:433–443. doi: 10.1002/(SICI)1097-0029(19981201)43:5<433::AID-JEMT9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Ballestrem C. Hinz B. Imhof BA. Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 13.Bamburg JR. McGough A. Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- 14.Banfi B. Molnar G. Maturana A. Steger K. Hegedus B. Demaurex N. Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 15.Barrett TB. Benditt EP. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988;85:2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauersachs J. Bouloumie A. Fraccarollo D. Hu K. Busse R. Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100:292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bendeck MP. Irvin C. Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78:38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson T. Orselius K. Wettero J. Role of the actin cytoskeleton during respiratory burst in chemoattractant-stimulated neutrophils. Cell Biol Int. 2006;30:154–163. doi: 10.1016/j.cellbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Bialkowska K. Kulkarni S. Du X. Goll DE. Saido TC. Fox JE. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J Cell Biol. 2000;151:685–696. doi: 10.1083/jcb.151.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black SM. DeVol JM. Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am J Physiol Cell Physiol. 2008;294:C345–C354. doi: 10.1152/ajpcell.00216.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes RP. Viedt C. Nguyen K. Beer S. Kreuzer J. Busse R. Gorlach A. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost. 2001;85:1104–1110. [PubMed] [Google Scholar]
- 23.Browatzki M. Larsen D. Pfeiffer CA. Gehrke SG. Schmidt J. Kranzhofer A. Katus HA. Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 24.Buetow BS. Tappan KA. Crosby JR. Seifert RA. Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163:979–984. doi: 10.1016/s0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter CL. Actin cytoskeleton and cell signaling. Crit Care Med. 2000;28:N94–N99. doi: 10.1097/00003246-200004001-00011. [DOI] [PubMed] [Google Scholar]
- 26.Chen GC. Turano B. Ruest PJ. Hagel M. Settleman J. Thomas SM. Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol Cell Biol. 2005;25:979–987. doi: 10.1128/MCB.25.3.979-987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H. Bernstein BW. Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/s0968-0004(99)01511-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen P. Gupta K. Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiarugi P. Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Chiarugi P. Fiaschi T. Taddei ML. Talini D. Giannoni E. Raugei G. Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 31.Chiarugi P. Pani G. Giannoni E. Taddei L. Colavitti R. Raugei G. Symons M. Borrello S. Galeotti T. Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi MH. Lee IK. Kim GW. Kim BU. Han YH. Yu DY. Park HS. Kim KY. Lee JS. Choi C. Bae YS. Lee BI. Rhee SG. Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 33.Clempus RE. Sorescu D. Dikalova AE. Pounkova L. Jo P. Sorescu GP. Schmidt HH. Lassegue B. Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley DR. Focal adhesions: the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 35.DalleDonne I. Milzani A. Colombo R. H2O2-treated actin: assembly and polymer interactions with cross-linking proteins. Biophys J. 1995;69:2710–2719. doi: 10.1016/S0006-3495(95)80142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DalleDonne I. Milzani A. Colombo R. The tert-butyl hydroperoxide-induced oxidation of actin Cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry. 1999;38:12471–12480. doi: 10.1021/bi990367k. [DOI] [PubMed] [Google Scholar]
- 37.Dawe HR. Minamide LS. Bamburg JR. Cramer LP. ADF/cofilin controls cell polarity during fibroblast migration. Curr Biol. 2003;13:252–257. doi: 10.1016/s0960-9822(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 38.Deshpande NN. Sorescu D. Seshiah P. Ushio-Fukai M. Akers M. Yin Q. Griendling KK. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal. 2002;4:845–854. doi: 10.1089/152308602760599007. [DOI] [PubMed] [Google Scholar]
- 39.Dikalova A. Clempus R. Lassegue B. Cheng G. McCoy J. Dikalov S. San Martin A. Lyle A. Weber DS. Weiss D. Taylor WR. Schmidt HH. Owens GK. Lambeth JD. Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 40.Drewes G. Faulstich H. The enhanced ATPase activity of glutathione-substituted actin provides a quantitative approach to filament stabilization. J Biol Chem. 1990;265:3017–3021. [PubMed] [Google Scholar]
- 41.Elliot S. Catanuto P. Stetler-Stevenson W. Cousins SW. Retinal pigment epithelium protection from oxidant-mediated loss of MMP-2 activation requires both MMP-14 and TIMP-2. Invest Ophthalmol Vis Sci. 2006;47:1696–1702. doi: 10.1167/iovs.05-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiaschi T. Cozzi G. Raugei G. Formigli L. Ramponi G. Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 43.Floege J. Hudkins KL. Davis CL. Schwartz SM. Alpers CE. Expression of PDGF alpha-receptor in renal arteriosclerosis and rejecting renal transplants. J Am Soc Nephrol. 1998;9:211–223. doi: 10.1681/ASN.V92211. [DOI] [PubMed] [Google Scholar]
- 44.Fu X. Kassim SY. Parks WC. Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- 45.Fu X. Kassim SY. Parks WC. Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7): a mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 46.Fukui T. Ishizaka N. Rajagopalan S. Laursen JB. Capers QT. Taylor WR. Harrison DG. de Leon H. Wilcox JN. Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher PJ. Herring BP. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- 48.Gallagher PJ. Herring BP. Griffin SA. Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 49.Giannoni E. Buricchi F. Raugei G. Ramponi G. Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gohla A. Birkenfeld J. Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- 51.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 52.Griendling KK. Sorescu D. Lassegue B. Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 53.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 54.Griendling KK. Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 55.Grotendorst GR. Seppa HE. Kleinman HK. Martin GR. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981;78:3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupte SA. Kaminski PM. George S. Kouznestova L. Olson SC. Matthew R. Hintze TH. Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol. 2009;296:H1048–H1057. doi: 10.1152/ajpheart.00491.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurjar MV. Deleon J. Sharma RV. Bhalla RC. Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am J Physiol Heart Circ Physiol. 2001;281:H2568–H2574. doi: 10.1152/ajpheart.2001.281.6.H2568. [DOI] [PubMed] [Google Scholar]
- 58.Halka AT. Turner NJ. Carter A. Ghosh J. Murphy MO. Kirton JP. Kielty CM. Walker MG. The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovasc Pathol. 2008;17:98–102. doi: 10.1016/j.carpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 60.Hamadi A. Bouali M. Dontenwill M. Stoeckel H. Takeda K. Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–4425. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- 61.Hamelers IH. Olivo C. Mertens AE. Pegtel DM. van der Kammen RA. Sonnenberg A. Collard JG. The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna IR. Hilenski LL. Dikalova A. Taniyama Y. Dikalov S. Lyle A. Quinn MT. Lassegue B. Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Harrison D. Griendling KK. Landmesser U. Hornig B. Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 64.Hartwig JH. Bokoch GM. Carpenter CL. Janmey PA. Taylor LA. Toker A. Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 65.Haurani MJ. Cifuentes ME. Shepard AD. Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heldin CH. Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 67.Hertelendi Z. Toth A. Borbely A. Galajda Z. van der Velden J. Stienen GJ. Edes I. Papp Z. Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid Redox Signal. 2008;10:1175–1184. doi: 10.1089/ars.2007.2014. [DOI] [PubMed] [Google Scholar]
- 68.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells [see comment] Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 69.Hinshaw DB. Burger JM. Beals TF. Armstrong BC. Hyslop PA. Actin polymerization in cellular oxidant injury. Arch Biochem Biophys. 1991;288:311–316. doi: 10.1016/0003-9861(91)90200-3. [DOI] [PubMed] [Google Scholar]
- 70.Hu T. Luan R. Zhang H. Lau WB. Wang Q. Zhang Y. Wang HC. Tao L. Hydrogen peroxide enhances the osteopontin expression and matrix metalloproteinase activity in aortic vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 2008;36:626–630. doi: 10.1111/j.1440-1681.2008.05124.x. [DOI] [PubMed] [Google Scholar]
- 71.Huot J. Houle F. Marceau F. Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 72.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 73.Jackson CL. Raines EW. Ross R. Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- 74.Jacobson GM. Dourron HM. Liu J. Carretero OA. Reddy DJ. Andrzejewski T. Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res. 2003;92:637–643. doi: 10.1161/01.RES.0000063423.94645.8A. [DOI] [PubMed] [Google Scholar]
- 75.Jagadeesha DK. Lindley TE. Deleon J. Sharma RV. Miller F. Bhalla RC. Tempol therapy attenuates medial smooth muscle cell apoptosis and neointima formation after balloon catheter injury in carotid artery of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1047–H1053. doi: 10.1152/ajpheart.01071.2004. [DOI] [PubMed] [Google Scholar]
- 76.Jagadeesha DK. Miller FJ Jr. Bhalla RC. Inhibition of apoptotic signaling and neointimal hyperplasia by tempol and nitric oxide synthase following vascular injury. J Vasc Res. 2008;46:109–118. doi: 10.1159/000151444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jang HJ. Hwang S. Cho KY. Kim do K. Chay KO. Kim JK. Taxol induces oxidative neuronal cell death by enhancing the activity of NADPH oxidase in mouse cortical cultures. Neurosci Lett. 2008;443:17–22. doi: 10.1016/j.neulet.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 78.Jawien A. Bowen-Pope DF. Lindner V. Schwartz SM. Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89:507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jernigan NL. Walker BR. Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin L. Ying Z. Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 81.Johansson M. Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson C. Galis ZS. Matrix metalloproteinase-2 and −9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 83.Kaji N. Ohashi K. Shuin M. Niwa R. Uemura T. Mizuno K. Cell cycle-associated changes in Slingshot phosphatase activity and roles in cytokinesis in animal cells. J Biol Chem. 2003;278:33450–33455. doi: 10.1074/jbc.M305802200. [DOI] [PubMed] [Google Scholar]
- 84.Kappert K. Sparwel J. Sandin A. Seiler A. Siebolts U. Leppanen O. Rosenkranz S. Ostman A. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 85.Kim JS. Huang TY. Bokoch GM. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol Biol Cell. 2009;20:2650–2660. doi: 10.1091/mbc.E09-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimura K. Ito M. Amano M. Chihara K. Fukata Y. Nakafuku M. Yamamori B. Feng J. Nakano T. Okawa K. Iwamatsu A. Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 87.Kligys K. Claiborne JN. Debiase PJ. Hopkinson SB. Wu Y. Mizuno K. Jones JC. The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization and motility behavior of keratinocytes. J Biol Chem. 2007;282:32520–32528. doi: 10.1074/jbc.M707041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- 89.Konneh MK. Rutherford C. Li SR. Anggard EE. Ferns GA. Vitamin E inhibits the intimal response to balloon catheter injury in the carotid artery of the cholesterol-fed rat. Atherosclerosis. 1995;113:29–39. doi: 10.1016/0021-9150(94)05423-g. [DOI] [PubMed] [Google Scholar]
- 90.Kozma R. Ahmed S. Best A. Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 92.Lassegue B. Sorescu D. Szocs K. Yin Q. Akers M. Zhang Y. Grant SL. Lambeth JD. Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 93.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 94.Lauffenburger DA. Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 95.Laurindo FR. da Luz PL. Uint L. Rocha TF. Jaeger RG. Lopes EA. Evidence for superoxide radical-dependent coronary vasospasm after angioplasty in intact dogs. Circulation. 1991;83:1705–1715. doi: 10.1161/01.cir.83.5.1705. [DOI] [PubMed] [Google Scholar]
- 96.Laursen JB. Rajagopalan S. Galis Z. Tarpey M. Freeman BA. Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 97.Lavigne MC. Malech HL. Holland SM. Leto TL. Genetic requirement of p47phox for superoxide production by murine microglia. FASEB J. 2001;15:285–287. doi: 10.1096/fj.00-0608fje. [DOI] [PubMed] [Google Scholar]
- 98.Lee HM. Jeon BH. Won KJ. Lee CK. Park TK. Choi WS. Bae YM. Kim HS. Lee SK. Park SH. Irani K. Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-{beta} receptor signaling via the inhibition of reactive oxygen species-mediated syk pathway. Circ Res. 2008;104:219–227. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 99.Lee MY. San Martin A. Mehta PK. Dikalova AE. Garrido AM. Lyons E. Krause KH. Banfi B. Lambeth JD. Lassegue B. Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SJ. Seo KW. Yun MR. Bae SS. Lee WS. Hong KW. Kim CD. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic Biol Med. 2008;45:1487–1492. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 101.Li J. Li W. Su J. Liu W. Altura BT. Altura BM. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: possible relation to neurodegenerative diseases and strokes. Brain Res Bull. 2003;62:101–106. doi: 10.1016/j.brainresbull.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Li L. Gao PJ. Xi R. Wu CF. Zhu DL. Yan J. Lu GP. Pioglitazone inhibits homocysteine-induced migration of vascular smooth muscle cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Clin Exp Pharmacol Physiol. 2008;35:1471–1476. doi: 10.1111/j.1440-1681.2008.05025.x. [DOI] [PubMed] [Google Scholar]
- 103.Liochev SL. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic Res. 1996;25:369–384. doi: 10.3109/10715769609149059. [DOI] [PubMed] [Google Scholar]
- 104.Lounsbury KM. Hu Q. Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med. 2000;28:1362–1369. doi: 10.1016/s0891-5849(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 105.Lyle AN. Deshpande NN. Taniyama Y. Seidel-Rogol B. Pounkova L. Du P. Papaharalambus C. Lassegue B. Griendling KK. Poldip2, a novel regulator of nex1 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Machesky LM. Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 107.Maekawa M. Ishizaki T. Boku S. Watanabe N. Fujita A. Iwamatsu A. Obinata T. Ohashi K. Mizuno K. Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 108.Majesky MW. Reidy MA. Bowen-Pope DF. Hart CE. Wilcox JN. Schwartz SM. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990;111:2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marmur JD. Poon M. Rossikhina M. Taubman MB. Induction of PDGF-responsive genes in vascular smooth muscle: implications for the early response to vessel injury. Circulation. 1992;86:III53–III60. [PubMed] [Google Scholar]
- 110.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 111.Meng D. Lv DD. Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 112.Miki H. Suetsugu S. Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller FJ., Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 114.Mills JE. Whitford PC. Shaffer J. Onuchic JN. Adams JA. Jennings PA. A novel disulfide bond in the SH2 domain of the C-terminal Src kinase controls catalytic activity. J Mol Biol. 2007;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milzani A. DalleDonne I. Colombo R. Prolonged oxidative stress on actin. Arch Biochem Biophys. 1997;339:267–274. doi: 10.1006/abbi.1996.9847. [DOI] [PubMed] [Google Scholar]
- 116.Mitchison TJ. Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 117.Mohazzab KM. Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol. 1994;267:L815–L822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- 118.Moldovan L. Moldovan NI. Sohn RH. Parikh SA. Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 119.Montezano AC. Callera GE. Yogi A. He Y. Tostes RC. He G. Schiffrin EL. Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 120.Moon SK. Kang SK. Kim CH. Reactive oxygen species mediates disialoganglioside GD3-induced inhibition of ERK1/2 and matrix metalloproteinase-9 expression in vascular smooth muscle cells. FASEB J. 2006;20:1387–1395. doi: 10.1096/fj.05-4618com. [DOI] [PubMed] [Google Scholar]
- 121.Morimatsu T. Kawagoshi A. Yoshida K. Tamura M. Actin enhances the activation of human neutrophil NADPH oxidase in a cell-free system. Biochem Biophys Res Commun. 1997;230:206–210. doi: 10.1006/bbrc.1996.5881. [DOI] [PubMed] [Google Scholar]
- 122.Morita T. Perrella MA. Lee ME. Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mu H. Wang X. Lin P. Yao Q. Chen C. Nitrotyrosine promotes human aortic smooth muscle cell migration through oxidative stress and ERK1/2 activation. Biochim Biophys Acta. 2008;1783:1576–1584. doi: 10.1016/j.bbamcr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakai K. Yoneda K. Igarashi J. Moriue T. Kosaka H. Kubota Y. Angiotensin II enhances EGF receptor expression levels via ROS formation in HaCaT cells. J Dermatol Sci. 2008;51:181–189. doi: 10.1016/j.jdermsci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Natarajan R. Nadler JL. Lipoxygenases and lipid signaling in vascular cells in diabetes. Front Biosci. 2003;8:s783–s795. doi: 10.2741/1144. [DOI] [PubMed] [Google Scholar]
- 127.Nishio E. Watanabe Y. The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol. 1997;121:665–670. doi: 10.1038/sj.bjp.0701171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishita M. Aizawa H. Mizuno K. Stromal cell-derived factor 1alpha activates LIM kinase 1 and induces cofilin phosphorylation for T-cell chemotaxis. Mol Cell Biol. 2002;22:774–783. doi: 10.1128/MCB.22.3.774-783.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nobes CD. Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nobes CD. Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- 131.Nobes CD. Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 132.Nunes GL. Sgoutas DS. Redden RA. Sigman SR. Gravanis MB. King SB., 3rd Berk BC. Combination of vitamins C and E alters the response to coronary balloon injury in the pig. Arterioscler Thromb Vasc Biol. 1995;15:156–165. doi: 10.1161/01.atv.15.1.156. [DOI] [PubMed] [Google Scholar]
- 133.Oakley FD. Abbott D. Li Q. Engelhardt J. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2008;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ohara Y. Peterson TE. Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ohashi K. Nagata K. Maekawa M. Ishizaki T. Narumiya S. Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 136.Omann GM. Harter JM. Burger JM. Hinshaw DB. H2O2-induced increases in cellular F-actin occur without increases in actin nucleation activity. Arch Biochem Biophys. 1994;308:407–412. doi: 10.1006/abbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- 137.Pagano PJ. Chanock SJ. Siwik DA. Colucci WS. Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 138.Papaharalambus CA. Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park J. Ha H. Seo J. Kim MS. Kim HJ. Huh KH. Park K. Kim YS. Mycophenolic acid inhibits platelet-derived growth factor-induced reactive oxygen species and mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Am J Transplant. 2004;4:1982–1990. doi: 10.1111/j.1600-6143.2004.00610.x. [DOI] [PubMed] [Google Scholar]
- 140.Pintucci G. Yu PJ. Saponara F. Kadian-Dodov DL. Galloway AC. Mignatti P. PDGF-BB induces vascular smooth muscle cell expression of high molecular weight FGF-2, which accumulates in the nucleus. J Cell Biochem. 2005;95:1292–1300. doi: 10.1002/jcb.20505. [DOI] [PubMed] [Google Scholar]
- 141.Price LS. Leng J. Schwartz MA. Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prochniewicz E. Lowe DA. Spakowicz DJ. Higgins L. O'Conor K. Thompson LV. Ferrington DA. Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–C626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qu A. Jiang C. Xu M. Zhang Y. Zhu Y. Xu Q. Zhang C. Wang X. PGC-1α attenuates neointimal formation via inhibition of vascular smooth muscle cell migration in the injured rat carotid artery. Am J Physiol Cell Physiol. 2009;297:C645–C653. doi: 10.1152/ajpcell.00469.2008. [DOI] [PubMed] [Google Scholar]
- 144.Raftopoulou M. Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Rajagopalan S. Kurz S. Munzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rajagopalan S. Kurz S. Munzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rajagopalan S. Meng XP. Ramasamy S. Harrison DG. Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ridley AJ. Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 149.Rigacci S. Rovida E. Dello Sbarba P. Berti A. Low Mr phosphotyrosine protein phosphatase associates and dephosphorylates p125 focal adhesion kinase, interfering with cell motility and spreading. J Biol Chem. 2002;277:41631–41636. doi: 10.1074/jbc.M201709200. [DOI] [PubMed] [Google Scholar]
- 150.Rosette C. Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roveri A. Coassin M. Maiorino M. Zamburlini A. van Amsterdam FT. Ratti E. Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch Biochem Biophys. 1992;297:265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- 152.Rozycki MD. Myslik JC. Schutt CE. Lindberg U. Structural aspects of actin-binding proteins. Curr Opin Cell Biol. 1994;6:87–95. doi: 10.1016/0955-0674(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 153.Rubin K. Tingstrom A. Hansson GK. Larsson E. Ronnstrand L. Klareskog L. Claesson-Welsh L. Heldin CH. Fellstrom B. Terracio L. Induction of B-type receptors for platelet-derived growth factor in vascular inflammation: possible implications for development of vascular proliferative lesions. Lancet. 1988;1:1353–1356. doi: 10.1016/s0140-6736(88)92177-0. [DOI] [PubMed] [Google Scholar]
- 154.Sabuda-Widemann D. Grabensee B. Schwandt C. Blume C. Mycophenolic acid inhibits the autocrine PDGF-B synthesis and PDGF-BB-induced mRNA expression of Egr-1 in rat mesangial cells. Nephrol Dial Transplant. 2009;24:52–61. doi: 10.1093/ndt/gfn462. [DOI] [PubMed] [Google Scholar]
- 155.San Martin A. Foncea R. Laurindo FR. Ebensperger R. Griendling KK. Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 156.San Martin A. Lee MY. Griendling KK. Novel nox1-mediated mechanism of SShil activation in VSMC: role in cell migration. Atheroscler Thromb Vasc Biol. 2008;28:e109. [Google Scholar]
- 157.San Martin A. Lee MY. Williams HC. Mizuno K. Lassegue B. Griendling KK. Dual regulation of cofilin activity by LIM kinase and slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res. 2008;102:432–438. doi: 10.1161/CIRCRESAHA.107.158923. [DOI] [PubMed] [Google Scholar]
- 158.Sanders LC. Matsumura F. Bokoch GM. de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 159.Scaife RM. G2 cell cycle arrest, down-regulation of cyclin B, and induction of mitotic catastrophe by the flavoprotein inhibitor diphenyleneiodonium. Mol Cancer Ther. 2004;3:1229–1237. [PubMed] [Google Scholar]
- 160.Scherberich A. Campos-Toimil M. Ronde P. Takeda K. Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci. 2000;113:653–662. doi: 10.1242/jcs.113.4.653. [DOI] [PubMed] [Google Scholar]
- 161.Schroder K. Helmcke I. Palfi K. Krause KH. Busse R. Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 162.Selimovic D. Hassan M. Haikel Y. Hengge UR. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008;20:311–322. doi: 10.1016/j.cellsig.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 163.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 164.Sheetz MP. Felsenfeld DP. Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 165.Shimizu H. Shiota M. Yamada N. Miyazaki K. Ishida N. Kim S. Miyazaki H. Low M(r) protein tyrosine phosphatase inhibits growth and migration of vascular smooth muscle cells induced by platelet-derived growth factor. Biochem Biophys Res Commun. 2001;289:602–607. doi: 10.1006/bbrc.2001.6007. [DOI] [PubMed] [Google Scholar]
- 166.Shinohara M. Shang WH. Kubodera M. Harada S. Mitsushita J. Kato M. Miyazaki H. Sumimoto H. Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J Biol Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- 167.Sieg DJ. Hauck CR. Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 168.Small JV. Stradal T. Vignal E. Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 169.Sollott SJ. Cheng L. Pauly RR. Jenkins GM. Monticone RE. Kuzuya M. Froehlich JP. Crow MT. Lakatta EG. Rowinsky EK. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95:1869–1876. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Somlyo AP. Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 171.Souza HP. Souza LC. Anastacio VM. Pereira AC. Junqueira ML. Krieger JE. da Luz PL. Augusto O. Laurindo FR. Vascular oxidant stress early after balloon injury: evidence for increased NAD(P)H oxidoreductase activity. Free Radic Biol Med. 2000;28:1232–1242. doi: 10.1016/s0891-5849(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 172.Stournaras C. Drewes G. Blackholm H. Merkler I. Faulstich H. Glutathionyl(cysteine-374) actin forms filaments of low mechanical stability. Biochim Biophys Acta. 1990;1037:86–91. doi: 10.1016/0167-4838(90)90105-o. [DOI] [PubMed] [Google Scholar]
- 173.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 174.Svineng G. Ravuri C. Rikardsen O. Huseby NE. Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49:197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- 175.Szocs K. Lassegue B. Sorescu D. Hilenski LL. Valppu L. Couse TL. Wilcox JN. Quinn MT. Lambeth JD. Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 176.Tabet F. Savoia C. Schiffrin EL. Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 177.Taddei ML. Parri M. Mello T. Catalano A. Levine AD. Raugei G. Ramponi G. Chiarugi P. Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxid Redox Signal. 2007;9:469–481. doi: 10.1089/ars.2006.1392. [DOI] [PubMed] [Google Scholar]
- 178.Tang JX. Janmey PA. Stossel TP. Ito T. Thiol oxidation of actin produces dimers that enhance the elasticity of the F-actin network. Biophys J. 1999;76:2208–2215. doi: 10.1016/S0006-3495(99)77376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taniyama Y. Weber DS. Rocic P. Hilenski L. Akers ML. Park J. Hemmings BA. Alexander RW. Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23:8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tardif JC. Cote G. Lesperance J. Bourassa M. Lambert J. Doucet S. Bilodeau L. Nattel S. de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty: Multivitamins and Probucol Study Group. N Engl J Med. 1997;337:365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- 181.ten Freyhaus H. Huntgeburth M. Wingler K. Schnitker J. Baumer AT. Vantler M. Bekhite MM. Wartenberg M. Sauer H. Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 182.Toshima J. Toshima JY. Amano T. Yang N. Narumiya S. Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol Biol Cell. 2001;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Totsukawa G. Wu Y. Sasaki Y. Hartshorne DJ. Yamakita Y. Yamashiro S. Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Toumpoulis IK. Malamou-Mitsi VD. Michalis LK. Katsouras C. Gloustianou G. Galaris D. Bai M. Vardakas D. Agnantis NJ. Sideris DA. Apoptosis bcl-2 and nitrotyrosine expression in an angioplasty-restenosis rabbit: an experimental model. Int J Surg. 2007;5:260–266. doi: 10.1016/j.ijsu.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 185.Touyz RM. Chen X. Tabet F. Yao G. He G. Quinn MT. Pagano PJ. Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 186.Touyz RM. Yao G. Quinn MT. Pagano PJ. Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 187.Tummala PE. Chen XL. Sundell CL. Laursen JB. Hammes CP. Alexander RW. Harrison DG. Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 188.Usatyuk PV. Romer LH. He D. Parinandi NL. Kleinberg ME. Zhan S. Jacobson JR. Dudek SM. Pendyala S. Garcia JG. Natarajan V. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem. 2007;282:23284–23295. doi: 10.1074/jbc.M700535200. [DOI] [PubMed] [Google Scholar]
- 189.Ushio-Fukai M. Alexander RW. Akers M. Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II: role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 190.Ushio-Fukai M. Zafari AM. Fukui T. Ishizaka N. Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 191.Verschueren H. Dewit J. De Braekeleer J. Schirrmacher V. De Baetselier P. Motility and invasive potency of murine T-lymphoma cells: effect of microtubule inhibitors. Cell Biol Int. 1994;18:11–19. doi: 10.1006/cbir.1994.1002. [DOI] [PubMed] [Google Scholar]
- 192.Wang G. Siow YL O K. Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem J. 2000;352:817–826. [PMC free article] [PubMed] [Google Scholar]
- 193.Wang HD. Pagano PJ. Du Y. Cayatte AJ. Quinn MT. Brecher P. Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 194.Wang HD. Xu S. Johns DG. Du Y. Quinn MT. Cayatte AJ. Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice [see comment] Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 195.Wang Z. Castresana MR. Newman WH. Reactive oxygen and NF-kappaB in VEGF-induced migration of human vascular smooth muscle cells. Biochem Biophys Res Commun. 2001;285:669–674. doi: 10.1006/bbrc.2001.5232. [DOI] [PubMed] [Google Scholar]
- 196.Wang Z. Castresana MR. Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 197.Wassmann S. Laufs U. Muller K. Konkol C. Ahlbory K. Baumer AT. Linz W. Bohm M. Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 198.Wear MA. Schafer DA. Cooper JA. Actin dynamics: assembly and disassembly of actin networks. Curr Biol. 2000;10:R891–R895. doi: 10.1016/s0960-9822(00)00845-9. [DOI] [PubMed] [Google Scholar]
- 199.Weber DS. Taniyama Y. Rocic P. Seshiah PN. Dechert MA. Gerthoffer WT. Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 200.Weiss S. Frischknecht K. Greutert H. Payeli S. Steffel J. Luscher TF. Carrel TP. Tanner FC. Different migration of vascular smooth muscle cells from human coronary artery bypass vessels: role of Rho/ROCK pathway. J Vasc Res. 2007;44:149–156. doi: 10.1159/000099141. [DOI] [PubMed] [Google Scholar]
- 201.Werner E. Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 203.Wozniak MA. Modzelewska K. Kwong L. Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 204.Xie H. Turner T. Wang MH. Singh RK. Siegal GP. Wells A. In vitro invasiveness of DU-145 human prostate carcinoma cells is modulated by EGF receptor-mediated signals. Clin Exp Metastasis. 1995;13:407–419. doi: 10.1007/BF00118180. [DOI] [PubMed] [Google Scholar]
- 205.Yamamoto Y. Ogino K. Igawa G. Matsuura T. Kaetsu Y. Sugihara S. Matsubara K. Miake J. Hamada T. Yoshida A. Igawa O. Yamamoto T. Shigemasa C. Hisatome I. Allopurinol reduces neointimal hyperplasia in the carotid artery ligation model in spontaneously hypertensive rats. Hypertens Res. 2006;29:915–921. doi: 10.1291/hypres.29.915. [DOI] [PubMed] [Google Scholar]
- 206.Yang N. Higuchi O. Ohashi K. Nagata K. Wada A. Kangawa K. Nishida E. Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 207.Ying J. Clavreul N. Sethuraman M. Adachi T. Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zafari AM. Ushio-Fukai M. Akers M. Yin Q. Shah A. Harrison DG. Taylor WR. Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 209.Zaidel-Bar R. Ballestrem C. Kam Z. Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 210.Zebda N. Bernard O. Bailly M. Welti S. Lawrence DS. Condeelis JS. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol. 2000;151:1119–1128. doi: 10.1083/jcb.151.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]