Abstract
Thymidylate kinase (TMPK) is a nucleoside monophosphate kinase that catalyzes phosphorylation of thymidine monophosphate (TMP) to thymidine diphosphate (TDP). TMPK also mediates phosphorylation of monophosphates of thymidine nucleoside analog (NA) prodrugs on the pathway to their active triphosphate antiviral or anti-tumor moieties. Novel transgenic mice (TG) expressing human (h) TMPK were genetically engineered using the α-myosin heavy chain promoter to drive its cardiac-targeted over-expression. In “2 by 2” protocols, TMPK TGs and wild type (WT) littermates were treated with the NA zidovudine (a deoxythymidine analog, AZT) or vehicle for 35 days. Alternatively, TGs and WTs were treated with a deoxycytidine NA (racivir, RCV) or vehicle. Changes in mitochondrial DNA (mtDNA) abundance and mitochondrial ultrastructure were defined quantitatively by real-time PCR and transmission electron microscopy, respectively. Cardiac performance was determined echocardiographically. Results showed TMPK TGs treated with either AZT or RCV exhibited decreased cardiac mtDNA abundance. Cardiac ultrastructural changes were seen only with AZT. AZT-treated TGs exhibited increased left ventricle (LV) mass. In contrast, LV mass in RCV treated TGs and WTs remained unchanged. In all cohorts, LV end-diastolic dimension (LVEDD) remained unchanged. This novel cardiac-targeted overexpression of hTMPK helps define the role of TMPK in mitochondrial toxicity of antiretrovirals.
INTRODUCTION
Mitochondria are unique cellular organelles with their own DNA (mtDNA), replication machinery (polymerase gamma, pol γ) (1), and mitochondrial deoxynucleotide triphosphate (dNTP) pools essential for the synthesis of mtDNA (2). mtDNA synthesis occurs throughout the life of the cell, independent of nuclear DNA synthesis and is essential for mitochondrial energy production. Cardiomyocytes (terminally differentiated, non-replicating cells) require constant high energy phosphates to sustain muscular contractions. Disrupted mtDNA replication can lead to left ventricle hypertrophy, cardiomegaly and organ dysfunction.
The importance of maintaining the dNTP precursor pools is underscored by human genetic diseases in which mutations in enzymes for dNTP synthesis adversely affect the rate and/or fidelity of mtDNA replication (3-5). Mitochondrial deoxythymidine triphosphate (dTTP) may be generated by two separate pathways in mammalian cells. De novo synthesis is accomplished from ribonucleotides in the cytosol and is followed by import into mitochondria (6). Alternatively, deoxythymidine (dThd) is imported into the mitochondria and three sequential kinase-mediated phosphorylations generate dTTP. Cycling cells largely depend on the first pathway (de novo synthesis) whereas resting cells use the alternative pathway mediated by three mitochondrial kinases (7). Intramitochondrially, the first phosphorylation is mediated by the mitochondrial thymidine kinase (TK2) (8) which we have studied previously (9, 10). The second phosphorylation is mediated by the cytosolic thymidylate kinase (TMPK) (11). In this study, we targeted cytoplasmic overexpression of TMPK. We hypothesized that in these TMPK TGs, TDP precursors in the cytoplasm would accumulate as the myocytes are quiescent. These precursors ultimately would be delivered into the mitochondria via specific nucleotide transporters (e.g. SLC25 (12)) where they could potentially impact mtDNA replication.
Nucleoside analogs (NA) are well-recognized anticancer and antiviral agents (13). The thymidine NA zidovudine (3′-azido-3′deoxythymidine, AZT) is a bona fide treatment for HIV/AIDS. NAs are “prodrugs” whose therapeutic activity relates to conversion to the active triphosphated N (e.g., AZTTP) (11, 14, 15). Like their native counterparts, NAs are phosphorylated serially into TPs mediated by the same kinases (e.g., TK2 and TMPK). For AZT, the rate-limiting step is the conversion of AZTMP to AZTDP by TMPK.
Because treatment with AZT is associated with mitochondrial toxicity to tissues, a novel in vivo model using overexpression of TMPK was engineered to delineate the role of TMPK in phosphorylation of native nucleosides and NAs. Using “2 by 2” protocols, TGs and WTs were treated by daily gavage with each deoxythymidine analog solubolized in appropriate vehicles (AZT in carboxymethylcellulose [CMC], or RCV in saline), and compared to respective vehicle controls. Experiments here characterize a novel cardiac-targeted TMPK TG and highlight the role of TMPK in mtDNA replication, cardiac function, and how TMPK determines toxicity of NAs.
Materials and Methods
Generation of alpha-myosin heavy chain (α-MyHC) promoter-driven hTMPK transgenic mice (TG)
Established methods (16) were employed as described previously (17). They applied to the hTMPK cDNA construct (18). The TMPK gene included the R200A mutation. This mutation was initially done to facilitate structural studies on TMPK, and it does not affect the kinetic behavior of the enzyme (19). Three transgenic lines (A, B, C) were established for the targeted over-expression of hTMPK. FVB/n (JAX stock, Jackson Lab, Bar Harbor, ME) was the inbred background. Animals bred true for 5 generations (to ensure germline transgenesis) prior to beginning experimental studies. No gross phenotype was recognized in TGs or WT littermates. No changes in behavior, growth, maturation, breeding behavior, or Mendelian distribution of TG occurred.
TG gene copy analysis
To determine the relative copy number in each line, the level of hTMPK was analyzed semi-quantitatively from murine tail DNA extracts using real-time PCR and Light Cycler TaqMan Master kit. Target genes were amplified using specific primers for hTMPK (forward TMPK/LC: 5′-ATGAGAACGGGGCTTTCC-3′, reverse TMPK/LCR: 5′-TTTGGAAGCATCCACCATCT-3′, and Universal Probe Library probe #31; Roche Diagnostics Corp., Indianapolis, IN) and a “housekeeping” gene, GAPDH (forward: 5′-GATGCTACAAGCAGGCCTTT-3′, reverse: 5′-GCAGAAAGCAAGGGCAAA-3′, and Universal ProbeLibrary probe #4; Roche Diagnostics Corp.). DNA amplification was performed using LightCycler 480 (Roche Diagnostics Corp.) on individual tissues extracted from at least 5-7 mice within each line. Relative copy number dosage was normalized to endogenous murine TMPK (single copy gene) from WT.
Further verification/selection for the hTMPK was accomplished using PCR amplification of tail DNA using two specific primers that identify part of the α-MHC promoter in tandem with the hTMPK gene, thereby avoiding amplification of the endogenous murine TMPK (TMPK Fwd 5′- CAC ATA GAA GCC TAG CCC ACA -3′ and TMPK Rev 5′- TAT AGT CGA CTC ACT TCC ATA GCT CCC ACA GCG G -3′).
Antiretroviral treatment protocols
All procedures complied with Emory Institutional Animal Care and Use Committee and NIH guidelines. Wild-type (WT) and TG littermates (both genders) were age-matched (8-12 weeks) at the start of treatment protocols. Food and water were provided ad libitum in a 12h light/dark, humidity and temperature controlled environment at Emory. Antiretroviral drugs were from the manufacturers (compliments of Emory Center for AIDS Research Pharmacology Core). Dosing was by daily gavage (morning). Treatment regimens resembled those used in human therapy and included AZT (0.22 mg/day; 0.25 mL in 1% CMC), RCV (1.025 mg/day in saline) or respective vehicles alone. At day 35, animal weights and echocardiographic measurements were made (20) and animals were terminated. This termination date was chosen experimentally based upon our previous data that demonstrate measurable changes in cardiac function occur within 35 days of treatment with AZT (9, 10, 21-23). Heart samples were retrieved rapidly and frozen (−80°C storage) for subsequent DNA extraction and analysis, or were fixed for histology (10% neutral buffered formalin; Fisher Scientific, Pittsburgh PA) and electron microscopy (EM).
Echocardiography (ECHO) of TG mice
Left ventricular (LV) mass and LV end diastolic dimension (LVEDD) were determined as previously described using ECHO in age- and gender-matched (littermate) WT and TGs (23). Briefly, LV mass was calculated from LV wall thickness and dimension measurements using an established formula.
Mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) quantification in heart tissue using real time PCR
Methods employed were based on modifications of those used by others (24) and detailed by us (9, 25). Amplification was performed in a LightCycler 480 (Roche Diagnostics, Indianapolis, IN). Efficiency curves corresponding to mitochondrial and nuclear DNA were employed to determine the ratio of mitochondrial DNA to nuclear DNA in each sample. For mtDNA abundance, the resultant values were expressed as mean ± standard error (SEM), normalized to vehicle-treated WT mean (set at 1.0). A value of p<0.05 was considered statistically significant.
Fine structure pathological evaluations of mitochondrial changes with transmission electron microscopy (EM) of mitochondrial damage in hTMPK TGs
Mitochondrial ultrastructure was evaluated using transmission EM to support data from ECHO and mtDNA abundance using methods that resemble those employed regularly in the laboratory (26). Each EM photograph was reviewed independently by two investigators for the presence of structurally abnormal mitochondria, increased numbers of mitochondrial profiles per field, intramitochondrial lamellar bodies, abnormal cristae density, cristae reduplication, mitochondrial swelling, and intramitochondrial paracrystals as performed by others (27).
Histological analysis
Heart samples were processed, sectioned (6 μm), stained with Hematoxylin and Eosin (H&E), and examined microscopically as done by us previously (28). Images were stored electronically to compare histopathological features.
Experimental analysis and statistics
Values for LV mass, LVEDD, and mtDNA were compared in WT, TGs and NA-treated cohorts using ANOVA in GraphPad Prism 4 (GraphPad, San Diego, CA, USA). Post-hoc testing used Newman-Keul's and unpaired t-test. A value of P<0.05 (determined by student's unpaired t-test) was considered statistically significant.
RESULTS
hTMPK cardiac targeted transgenic design
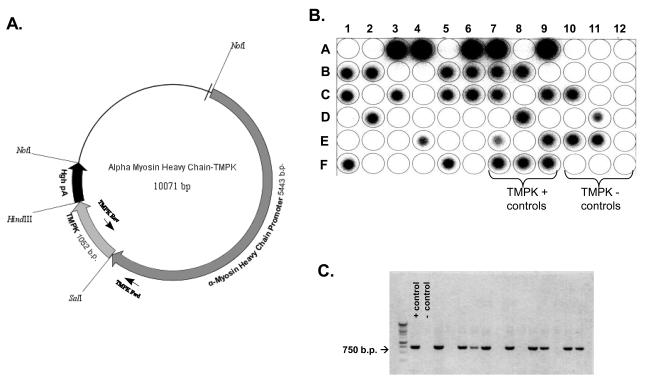
The mutant human TMPK clone as pGEX-RB plasmid was used (18). hTMPK was directionally inserted into a plasmid construct containing the alpha-myosin heavy chain promoter (α-MyHC) (16) using the nucleoside restriction sites SalI and HindIII (Figure 1A). This α-MyHC-hTMPK construct targeted overexpression of hTMPK in cardiac myocytes, based upon primary activation of the α-MyHC promoter activity exclusively in cardiomyocytes. Primers designed to amplify the region overlapping α-MyHC promoter and hTMPK gene (~750bp) provided a way to identify the transgene unambiguously while preventing amplification of the endogenous murine TMPK gene. Dot blot imaging was obtained routinely on DNA isolated from tail clippings for F1-F5 progeny. Signal for positive TGs was compared to WT progeny, TMPK positive (plasmid DNA, dark dots) and negative controls (no plasmid, white dots) as indicated (Figure 1B). Semi-quantitation of hTMPK copy number was accomplished from the signals on the dotblots (e.g., row A: position 3-4, 6-7, and 9, high copy) versus other positive dot blots (e.g., row B: position 1-2, 5-8, medium copy). Alternatively, all subsequent generations of progeny were routinely identified using PCR amplification with positive TGs identified by the production of a ~750bp band (Figure 1C).
Figure 1. Generation and verification of TMPK transgenesis.
A) Molecular map summarizes α-MyHC -TMPK construct with restriction sites and primer binding sites for positive identification of transgenes. Representative Dot blot (B) and agarose gel image of PCR amplification products (C) demonstrate selection of positive TGs from tail DNA of individual progeny from TG line(s) derived.
Murine TG characteristics
Cardiac targeted transgenic overexpression of hTMPK was accomplished in three lines (operationally labeled as line A, B and C). Animals bred true for five generations. In general, no gross phenotypic changes occurred in TGs or FVB/n WTs. No changes in behavior, growth, maturation, breeding behavior or Mendelian distribution of TGs were found in any of the three TG lines.
The relative hTMPK gene copy number in the lines was semiquantitatively determined (Table 1). All demonstrated multiple copy numbers of hTMPK. Lines A and B exhibited ~5 fold in excess of WT (FVB/n, single murine copy) while TG C line exhibited 25-fold excess. Line C was selected for these initial pharmacological studies based upon its high copy number. It was reasoned that functional impact of TMPK TG on mtDNA biogenesis and cardiac function with or without an NRTI (nucleoside analog) treatment would be most notable in a high copy number line.
Table 1.
hTMPK TG Gene Copy Number
| Line | TMPK Gene Dosage* | Copy Number |
|---|---|---|
| A B C |
5 5 25 |
Medium Medium High |
| FVB/n WT | 1 | Single copy |
Relative TMPK copy number normalized to FVB/n WT (single copy gene)
mtDNA abundance
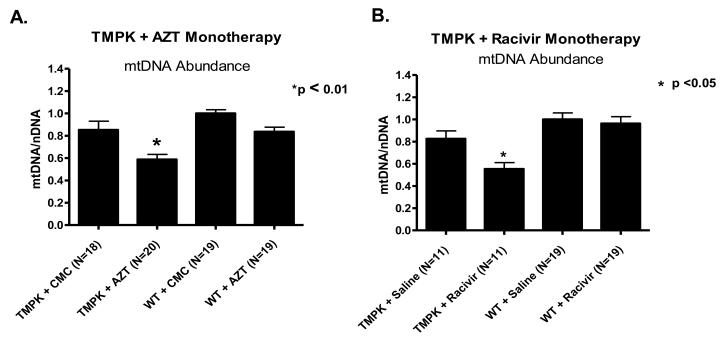
Age-matched cohorts of TGs and WTs were treated with AZT (CMC vehicle), RCV (saline vehicle) or the respective vehicle control for 35 days. At study termination, mice were sacrificed and extracts of total DNA were isolated from cardiac tissues from individual mice for each cohort. Both mtDNA and nDNA steady-state levels were determined using real-time PCR and resultant mtDNA/nDNA ratios (mtDNA abundance) were calculated and normalized as means ± SEM. Vehicle-treated TMPK TGs exhibited a small decrease (although not statistically significant, p>0.05) in mtDNA abundance compared to vehicle-treated WTs (Figure 2A and 2B).
Figure 2. Cardiac mtDNA abundance in TMPK TGs and WTs following NRTI treatment.
Total DNA were extracted from cardiac tissues isolated from TMPK TG and WT cohorts treated with zidovudine (AZT in CMC vehicle), racivir (RCV in saline vehicle) or vehicle alone for 35 days. mtDNA abundance was assessed using a ratio of mtDNA/nDNA as determined by real time PCR amplification. Cardiac mtDNA abundance was significantly decreased in TMPK TGs treated with AZT (A) or RCV (B) compared to WT littermates.
Likewise, AZT had only a moderate effect on mtDNA abundance in WTs (Figure 2A). This effect can be attributed to the endogenous activity of mouse TMPK. Mouse TMPK is more active than its human counterpart with AZTMP (29). Therefore, in mice more AZT-triphosphate is generated relative to the condition in human cells. It is this activation of AZT by endogenous mouse TMPK that moderately decreases the mtDNA-abundance. In the case of RCV and WT animals, no decrease in mtDNA is observed (Figure 2B).
In contrast, AZT-treated TGs exhibited a significant decrease in cardiac mtDNA abundance (p<0.001 and p<0.01, respectively) (Figure 2A), suggesting disruption of mitochondrial biogenesis. With RCV treatment, TMPK TGs exhibited a significant decrease in mtDNA abundance only when compared to WTs (Figure 2B).
Ultrastructural (EM) features of mitochondria in TG hearts
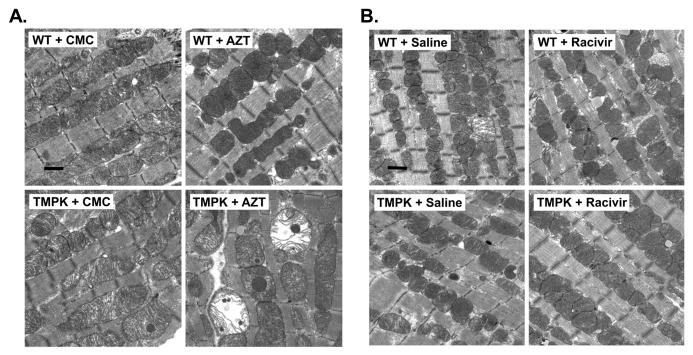
EM profile of cardiac myocytes from hTMPK TGs and WTs treated with AZT, RCV, or respective vehicle were assessed from formalin-fixed cardiac tissues. Ultrastructural features in mitochondrial structure from vehicle-treated TGs (both CMC and saline) were essentially unchanged compared to WT littermates (Figure 3A and B). Likewise, mitochondria from AZT- or RCV-treated WTs appeared unremarkable when compared to their vehicle-treated littermates. Mitochondria of cardiac myocytes from AZT-treated TGs, however, exhibited disruption of sarcomeres and tubules, mitochondrial swelling, and decreased cristae density compared to all other cohorts (Figure 3A, lower right panel). These findings correlate with decreased mtDNA abundance in cardiac samples from AZT-treated TGs and together suggest disruption of mitochondrial biogenesis. In contrast, mitochondria from RCV-treated TGs exhibited essentially no ultrastructural changes (Figure 3B, lower right panel) and appeared identical to WT littermates.
Figure 3. Electron photomicrographs of mitochondria from cardiac myocytes of TMPK TG and WT treated with AZT or RCV.
Representative TEM profiles of cardiac tissues from “2×2” studies of TMPK TGs and WTs treated with AZT (A), RCV (B), and their respective vehicle controls are shown. Cardiac myocytes from TMPK TGs treated with AZT demonstrate disrupted sarcomeres and tubules with mitochondrial swelling and reduced cristae density (A, lower right panel) compared to vehicle-treated WTs. (B) Changes following treatment with RCV in TGs and WTs were unremarkable. (original magnification: X 22,300, Marker indicates 1μm).
ECHO evalution of LV mass in hearts of TMPK TGs with and without antiretroviral therapy
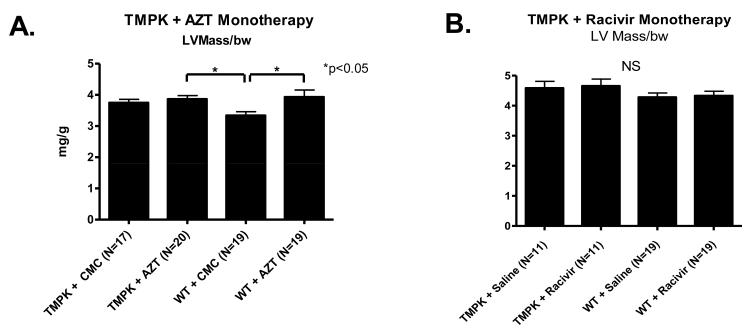
Both LV mass and LVEDD offer direct assessments of cardiac function and mass. An increase in either parameter is characteristic of cardiac dysfunction and suggestive of cardiac hypertrophy (increased LV mass) and/or dilation (increased LVEDD). ECHO data were recorded at termination of studies for individual mice from each cohort of hTMPK TGs and WTs with and without AZT or RCV treatments (35 days). LV mass for vehicle-treated (CMC or saline) TGs remained unchanged compared to WT littermates (Figure 4A and B, p>0.05). AZT treatment led to increased LV mass in both WTs and TGs compared to vehicle (CMC)-treated WT (Figure 4A left, p <0.05), suggesting LV hypertrophy. No significant change in LV mass was found following RCV treatment compared to vehicle (saline)-treated WTs (Figure 4B, right). LV cavity volume, reflected in LVEDD, remained unchanged for all cohorts, including AZT-treated TGs (data not shown), suggesting no left ventricular dilation.
Figure 4. Quantitative analysis of ECHO images.
LV mass was determined from ECHO images captured just prior to termination. Data were normalized to body weight (mg/g) and plotted as mean±SEM. A) AZT treatment increased LV mass in WT and TGs, compared to vehicle-treated WTs (p<0.05). B) LV mass remained unchanged in TGs and WTs following RCV treatment (right graph).
Histological analysis
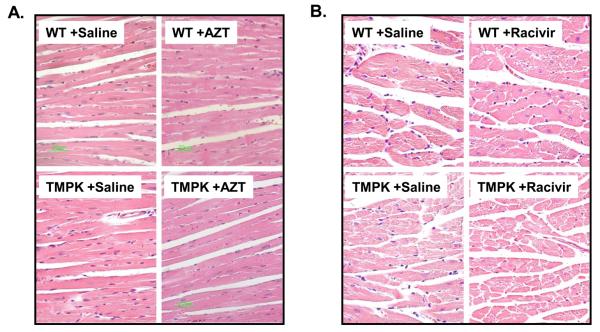
H&E-stained microscopic sections of hearts from TGs and WTs with and without AZT or RCV treatment were analyzed comparatively (original amplification 40X). Representative images from each cohort demonstrate no significant myocytolytic changes in any of the heart tissues including treatment with AZT (Figure 5A) or RCV (Figure 5B).
Figure 5. Histological analysis of cardiac tissues from TMPK TGs and WTs treated with AZT or RCV.
Parallel H&E –stained slides were made from gender-matched pairs of cardiac tissues after treatment (35 days). All tissues from TMPK TGs and WTs treated with AZT (A) or RCV (B) showed intact cardiomyocytes with comparable nuclei (original magnification x 40).
Discussion
Transgenic mice with cardiac-targeted gene expression are useful and powerful tools to define features of cardiac dysfunction (30), antiretroviral-associated cardiomyopathy (31), genetic changes related to mitochondrial function (32), and defects in mitochondrial biogenesis relate to drug toxicity (33). We report here a novel in vivo murine model with cardiac-targeted overexpression of human TMPK. TMPK is a cell-cycle regulated enzyme, expressed in the S-phase (34). Three individual TG lines were generated. Each encoded multiple copies of the human TMPK gene in addition to the native murine TMPK gene. It was imperative to select a screening method that differentiates hTMPK from the native murine TMPK. The successful approach employed specific primers to target tandem regions of α-MyHC promoter and hTMPK (Figure 1A) and a set of probes that would bind to the amplified product. Our method was reproducible, accurate, and resulted in the amplification and detection only of the hTMPK gene (Figure 1B and C). TG line C (possessing the highest gene copy number) was selected because robust overexpression of hTMPK in cardiac muscle was expected to increase phosphorylation of dTMP to dTDP most effectively, and impact mtDNA replication and cardiac function.
TMPK TGs here (vehicle-treated) showed a small yet measurable decrease in mtDNA abundance (Figure 2, p>0.05). While overexpression of TMPK may cause increased TDP, dNTP pools are tightly regulated and rely on steady state of a reaction. Increasing the abundance of enzyme alone does not necessarily increase flux through a pathway unless substrate is unlimited. Factors such as availability of substrate (dTMP), demand for product (dTTP), and activities of other enzymes in the phosphorylation and de-phosphorylation pathways also play a role.
It could be argued that any potential increase in cardiac mitochondrial dTTP abundance caused by TMPK TGs per se has only moderate impact, as mentioned earlier, via the effects on other nucleoside kinases and on ribonucleotide reductase. Specifically, in vitro studies found dTTP is a feedback inhibitor of human thymidine kinase 1 (35, 36) and is a regulator of ribonucleotide reductase (37). It remains unclear whether these inhibitory mechanisms can occur in quiescent cells, such as in cardiac myocytes.
In contrast, significant mtDNA depletion, mitochondrial ultrastructural damage and increased LV mass were found in TMPK TGs treated with AZT. Cardiac overexpression of TMPK, the rate limiting enzyme in the pathway converting AZT to AZTTP, may have resulted in increased AZTTP levels which can directly inhibit pol γ (33). However, these experiments offer no direct evidence (i.e. measurement of dTTP pools) to support this conclusion.
RCV had no impact on TG (or WT) LV mass or mitochondrial ultrastructure. Because TMPK kinase is relatively substrate specific for thymidine, the cytidine analog, RCV is not phosphorylated by it. However, RCV treatment of TGs significantly decreased mtDNA abundance (Figure 2B). This latter finding supports (albeit indirectly) the notion of the absence of a universal mechanism of mitochondrial toxicity of NAs (38). While AZT led to decreased mtDNA abundance and mitochondrial ultrastructural changes that ultimately impacted cardiac function, the effect of RCV was limited to decreased mtDNA abundance alone.
Overexpression of TMPK may also impact dNTP pool balance. A direct assessment of dNTP pools would help define the role of TMPK on mitochondrial dNTPs. Such measurements were not made in these studies. Future studies utilizing TMPK TGs will directly determine dNTP pools.
Overall, initial studies suggest enhanced TMPK activity by genetically engineered overexpression (TG) renders detectable changes in the mtDNA replication with potentially significant subcellular consequences.
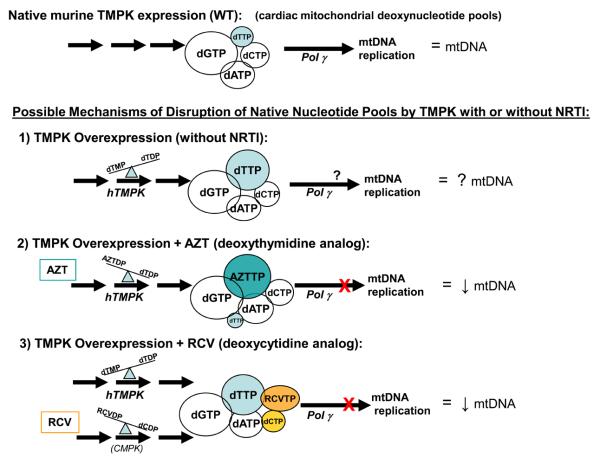
Figure 6.
Acknowledgements
We thank the Department of Pathology Experimental Core Laboratory for their support. We also thank Sarah Lewis for her assistance. The studies were supported by R01 HL79867 and HL59798 to WL. JK is is a recipient of K01 DK78513. QY is a recipient of R01 HL085499.
Bibliography
- 1.Clayton DA. Mitochondrial DNA replication: what we know. IUBMB Life. 2003;55:213–217. doi: 10.1080/1521654031000134824. [DOI] [PubMed] [Google Scholar]
- 2.Bogenhagen D, Clayton DA. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977;11:719–727. doi: 10.1016/0092-8674(77)90286-0. [DOI] [PubMed] [Google Scholar]
- 3.Mathews CK. DNA precursor metabolism and genomic stability. Faseb J. 2006;20:1300–1314. doi: 10.1096/fj.06-5730rev. [DOI] [PubMed] [Google Scholar]
- 4.Mathews CK, Song S. Maintaining precursor pools for mitochondrial DNA replication. FASEB J. 2007;21:2294–2303. doi: 10.1096/fj.06-7977rev. [DOI] [PubMed] [Google Scholar]
- 5.Spinazzola MNGIE: an autosomal rcessive disorder due to thymidine phosphorylase mutations. Ann Neuruol. 2000;47:792–800. [PubMed] [Google Scholar]
- 6.Ferraro P, Nicolosi L, Bernardi P, et al. Mitochondrial deoxynucleotide pool sizes in mouse liver and evidence for a transport mechanism for thymidine monophosphate. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18586–18591. doi: 10.1073/pnas.0609020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraro P, Bianchi V, Biasin MR, et al. Deoxynucleotide pools and DNA synthesis in resting and PHA-stimulated human lymphocytes treated with mutagens. Experimental Cell Research. 1992;199:349–354. doi: 10.1016/0014-4827(92)90444-d. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Munch-Petersen B, Kierdaszuk B, et al. Expression and substrate specificities of human thymidine kinase 1, thymidine kinase 2 and deoxycytidine kinase. Adv Exp Med Biol. 1991;309B:239–243. doi: 10.1007/978-1-4615-7703-4_53. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini SH, Kohler JJ, Haase CP, et al. Targeted Transgenic Overexpression of Mitochondrial Thymidine Kinase (TK2) Alters Mitochondrial DNA (mtDNA) and Mitochondrial Polypeptide Abundance: Transgenic TK2, mtDNA, and Antiretrovirals. Am J Pathol. 2007;170:865–874. doi: 10.2353/ajpath.2007.060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler JJ, Hosseini SH, Cucoranu I, et al. Murine cardiac mtDNA: effects of transgenic manipulation of nucleoside phosphorylation. Lab Invest. 2009;89:122–130. doi: 10.1038/labinvest.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther. 2000;87:189–198. doi: 10.1016/s0163-7258(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447:689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- 13.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3:415–424. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 14.Lavie A, Schlichting I, Vetter IR, et al. The bottleneck in AZT activation. Nat Med. 1997;3:922–924. doi: 10.1038/nm0897-922. [DOI] [PubMed] [Google Scholar]
- 15.Lavie A, Konrad M. Structural requirements for efficient phosphorylation of nucleotide analogs by human thymidylate kinase. Mini Rev Med Chem. 2004;4:351–359. doi: 10.2174/1389557043403981. [DOI] [PubMed] [Google Scholar]
- 16.Robbins J, Palermo J, Rindt H. In vivo definition of a cardiac specific promoter and its potential utility in remodeling the heart. Ann N Y Acad Sci. 1995;752:492–505. doi: 10.1111/j.1749-6632.1995.tb17458.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis W, Kohler JJ, Hosseini SH, et al. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: evidence supporting the DNA pol gamma hypothesis. Aids. 2006;20:675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brundiers R, Lavie A, Veit T, et al. Modifying human thymidylate kinase to potentiate azidothymidine activation. J Biol Chem. 1999;274:35289–35292. doi: 10.1074/jbc.274.50.35289. [DOI] [PubMed] [Google Scholar]
- 19.Ostermann N, Schlichting I, Brundiers R, et al. Insights into the phosphoryltransfer mechanism of human thymidylate kinase gained from crystal structures of enzyme complexes along the reaction coordinate. Structure Fold Des. 2000;8:629–642. doi: 10.1016/s0969-2126(00)00149-0. [DOI] [PubMed] [Google Scholar]
- 20.Hoit BD, Walsh RA. Kluwer Academic; Norwell: 2002. p. 403. [Google Scholar]
- 21.Lewis W, Grupp IL, Grupp G, et al. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest. 2000;80:187–197. doi: 10.1038/labinvest.3780022. [DOI] [PubMed] [Google Scholar]
- 22.Lewis W, Haase CP, Miller YK, et al. Transgenic expression of the deoxynucleotide carrier causes mitochondrial damage that is enhanced by NRTIs for AIDS. Laboratory Investigation. 2005;85:972–981. doi: 10.1038/labinvest.3700301. [DOI] [PubMed] [Google Scholar]
- 23.Kohler JJ, Hosseini SH, Green E, et al. Cardiac-targeted transgenic mutant mitochondrial enzymes: mtDNA defects, antiretroviral toxicity and cardiomyopathy. Cardiovasc Toxicol. 2008;8:57–69. doi: 10.1007/s12012-008-9015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote HC, Yip B, Asselin JJ, et al. Mitochondrial:nuclear DNA ratios in peripheral blood cells from human immunodeficiency virus (HIV)-infected patients who received selected HIV antiretroviral drug regimens. J Infect Dis. 2003;187:1972–1976. doi: 10.1086/375353. [DOI] [PubMed] [Google Scholar]
- 25.Lewis W, Day BJ, Kohler JJ, et al. Decreased mtDNA, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab Invest. 2007;87:326–335. doi: 10.1038/labinvest.3700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis W, Haase CP, Raidel SM, et al. Combined antiretroviral therapy causes cardiomyopathy and elevates plasma lactate in transgenic AIDS mice. Laboratory Investigation. 2001;81:1527–1536. doi: 10.1038/labinvest.3780366. [DOI] [PubMed] [Google Scholar]
- 27.Dalakas MC, Illa I, Pezeshkpour GH, et al. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322:1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- 28.Kohler JJ, Cucoranu I, Fields E, et al. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest. 2009 doi: 10.1038/labinvest.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balzarini J, Pauwels R, Baba M, et al. The in vitro and in vivo anti-retrovirus activity, and intracellular metabolism of 3′-azido-2′,3′-dideoxythymidine and 2′,3′-dideoxycytidine are highly dependent on the cell species. Biochemical Pharmacology. 1988;37:897–903. doi: 10.1016/0006-2952(88)90178-5. [DOI] [PubMed] [Google Scholar]
- 30.Robbins J. Remodeling the cardiac sarcomere using transgenesis. Annu Rev Physiol. 2000;62:261–287. doi: 10.1146/annurev.physiol.62.1.261. [DOI] [PubMed] [Google Scholar]
- 31.Lewis W. Defective mitochondrial DNA replication and NRTIs: pathophysiological implications in AIDS cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003;284:H1–9. doi: 10.1152/ajpheart.00814.2002. [DOI] [PubMed] [Google Scholar]
- 32.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. Journal of Molecular & Cellular Cardiology. 2005;38:81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of nrti antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 34.Huang SH, Tang A, Drisco B, et al. Human dTMP kinase: gene expression and enzymatic activity coinciding with cell cycle progression and cell growth. DNA Cell Biol. 1994;13:461–471. doi: 10.1089/dna.1994.13.461. [DOI] [PubMed] [Google Scholar]
- 35.Bresnick E, Thompson UB, Morris HP, et al. Inhibition of thymidine kinase activity in liver and hepatomas by TTP and d-CTP. Biochem Biophys Res Commun. 1964;16:278–284. doi: 10.1016/0006-291x(64)90340-7. [DOI] [PubMed] [Google Scholar]
- 36.Barrie SE, Paine RM, Stock JA, et al. Enzyme inhibition by phosphonate analogues of dTTP. Adv Exp Med Biol. 1984;165(Pt B):371–374. doi: 10.1007/978-1-4757-0390-0_70. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Faber C, Uchiki T, et al. Structures of eukaryotic ribonucleotide reductase I provide insights into dNTP regulation. Proc Natl Acad Sci U S A. 2006;103:4022–4027. doi: 10.1073/pnas.0600443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lund KC, Peterson LL, Wallace KB. Absence of a universal mechanism of mitochondrial toxicity by nucleoside analogs. Antimicrob Agents Chemother. 2007;51:2531–2539. doi: 10.1128/AAC.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]