Abstract
Recent studies suggest that some serous ovarian carcinomas arise from the fallopian tube epithelium rather than the ovarian surface epithelium. This hypothesis places emphasis on the fallopian tube secretory epithelial cell as a cell-of-origin. Herein we report the development of a novel ‘ex-vivo’ primary human fallopian tube epithelium culture system that faithfully recapitulates the in-vivo epithelium, as demonstrated by morphological, ultrastructural, and immunophenotypic analyses. Mass spectrometry-based proteomics reveals that these cultures secrete proteins previously identified as biomarkers for ovarian cancer. We also utilize this culture system to study the response of the fallopian tube epithelium to genotoxic stress and find that the secretory cells exhibit a distinct response to DNA damage when compared to neighboring ciliated cells. The secretory cells demonstrate a limited ability to resolve the damage over time, potentially leaving them more susceptible accumulation of additional mutagenic injury. This divergent response is confirmed with in-situ studies using tissue samples, further supporting the use of this ex-vivo culture system to investigate fallopian tube epithelial pathobiology. We anticipate that this novel culture system will facilitate the study of serous ovarian carcinoma pathogenesis, and propose that similar culture systems could be developed for other organ-site specific epithelia.
Keywords: ovarian cancer, fallopian tube, primary cell culture, cancer biomarkers, DNA damage and repair
Introduction
Epithelial ovarian cancer (EOC) is a leading cause of mortality in developed countries, with an incidence of about 190,000 new cases diagnosed annually worldwide, and 114,000 fatalities (Jemal et al., 2008). EOCs are generally subclassified into two types based on biological behavior and histology: low-grade tumors (also known as type I tumors), which typically present at an earlier stage and have an indolent natural history, and the high-grade or type II tumors, which typically have a more disseminated and aggressive behavior (Landen et al., 2008). Serous ovarian carcinoma (SOC) is a type II tumor and the most aggressive and most prevalent histological subtype of this disease (Cannistra, 2004). The poor prognosis of SOC is a direct consequence of advanced stage disease in a majority of newly diagnosed patients, and the eventual development of resistance to currently available chemotherapy.
Until recently, the ovarian surface epithelium (OSE) was considered the principal cell-of-origin for both type I and type II ovarian tumors. However, models depicting the transformation of the OSE have not been consistently corroborated by pathologic observations of transitions from OSE to malignancy. Recent studies raise a compelling hypothesis that the fallopian tube (FT) may harbor a cell-of-origin, the FT secretory epithelial cell (FTSEC), for serous tumors of the ovary and peritoneum (Jarboe et al., 2008). Evidence that supports this hypothesis includes: 1) 5–10% of BRCA+ women undergoing prophylactic salpingoophorectomy will have an early lesion, termed serous tubal intraepithelial carcinoma (STIC) and approximately 80% of these early cancers are found in the distal (fimbriated) end of the FT; 2) >50% of women with stage III/IV pelvic serous cancer also have a neoplastic lesion in their FT mucosa; 3) identical TP53 mutations have been identified in early FT neoplasia and the corresponding SOC (Kindelberger et al., 2007); 4) non-neoplastic FTSEC and SOC share similar morphologic and immunophenotypic features; and 5) a candidate precursor lesion (termed the ‘p53 signature’), composed of benign appearing FTSECs that harbor DNA damage and p53 mutations, has been described in the FT (Crum, 2009; Folkins et al., 2009; Kindelberger et al., 2007; Levanon et al., 2008). These observations suggest that the different pelvic serous carcinomas (PSC) previously assigned to different origins (ovary, FT, and peritoneum), share a common carcinogenic pathway not previously appreciated and originate in the secretory cell of the FTE (Levanon et al., 2008). Whether this model is correct remains to be determined.
With the identification of a candidate cell-of-origin for serous carcinomas comes the opportunity for basic and translational research aimed at deciphering the molecular mechanism linking the previously known risk factors and the actual serous carcinogenic process; developing new strategies for detection of the early lesions; and proposing biologically targeted therapy for PSC. At the same time, the need for tools to study the benign FTE becomes more obvious and urgent, as all of these studies require reproducible in-vitro and in-vivo model systems that involve human FTSECs. To accomplish this goal, we developed a reproducible ex-vivo culture system of primary benign cells that is capable of recapitulating the histology and function of the human FT fimbria epithelium. This model system has a number of advantages over previously reported cultures of fallopian tube epithelium (Kervancioglu et al., 194; Rapagopal et al., 2006). First, it represents a true co-culture of primary ciliated and secretory cells. Second, the cultures maintain polarity and morphology with each cell type expressing unique markers. Finally, it is a dynamic system as illustrated by the ability of the cultures to secrete factors unique to this epithelium and to response to injury by proliferating and healing a wound. Herein we report the use of this system to study the unique properties and differences between ciliated and secretory cells of the FT and how they respond to genotoxic stress. The unique features of this ex-vivo primary cell culture system lay the foundation for studies aimed at defining the molecular mechanism of serous tumorigenesis, and support the potential use of similar ex-vivo culture system in other organ systems.
Methods
Tissue samples
Fresh FT fimbria specimens were obtained from the Brigham and Women’s Hospital Department of Pathology with approval of the institutional review board. The fimbrial tissues used in this study are collected from surgical procedures for benign gynecological indications. Specifically, cases of inflammatory disease, infection, and extensive adhesions were excluded.
Primary human FT epithelium ex-vivo culture system
The FT tissue was kept in cold PBS in 4°C until processing. Once minced, the tissue is incubated in a dissociation medium composed of Eagle’s Minimum Essential Medium (EMEM, Cellgro, Manassas, VA, USA) supplemented with 3.5 mg/mL Pronase (Roche Diagnostics, Indianapolis, IN, USA), and 0.25 mg/mL DNase (SIGMA, St. Louis, MO, USA), for 48–72 hrs in 4°C with constant mild agitation. Human collagen IV (SIGMA) is dissolved in H2O to a concentration of 0.06 mg/mL. All cultureware used for FTE cell culture is coated with collagen prior to use and washed thoroughly with PBS before cell plating. The dissociated epithelial cells are harvested by centrifugation and re-suspended in growth medium (Dulbecco’s Modified Eagle’s Medium(DMEM)/Ham’s F12 1:1 (Cellgro), supplemented with 2% serum substitute Ultroser G (Pall France, St-Germain-en-Laye Cedex, France) and 1% penicillin/streptomycin (Invitrogen, Grand Island, NY, USA)). The number of cells from a single fimbria specimen is estimated to range between 105 and 5×106 epithelialcells. Cells are plated onto collagen IV-coated Costar polyester Transwell-clear inserts placed in 24-wells tissue culture plates (Corning, Corning, NY, USA) at a density of 1×104 cells per insert. Growth medium is then added to the lower compartment and the cells are incubated at 37°C in a humidified 5% CO2 incubator. In our experience using over 100 different FT samples, greater than 85% of the samples were viable and yielded pure epithelial cell cultures, with no evidence of other contaminant cell types. The medium is removed from the upper compartment 48 hours after plating of the cells.
Electron Microscopy
The cell cultures were prepared for electron microscopy using standard procedures. Briefly, the cell cultures were fixed in 2.5% glutaraldehyde in 0.1M cacodylate buffer followed by post fixation in 1% osmium tetroxide. The samples were then dehydrated in a graded series of ethanol. For scanning electron microscopy, the ex-vivo cultures were transitioned to hexamethyldisilizane and air dried overnight. The filters were cut from their support and mounted on aluminum stubs. After sputter coating with gold/palladium the sample were imaged in a Hitachi S-4800 scanning electron microscope (Pleasanton, CA, USA). For transmission electron microscopy the tissue is cleared in propylene oxide and infiltrated with Poly-Bed 812 (Polysciences, Warrington, PA, USA). Ultra-thin sections with silver to light golden interference color are stained with saturated uranyl acetate solution followed by lead citrate. The sections are examined in a JEM 1010 electron microscope (JEOL, Peabody, MA, USA) at 80KV acceleration voltage. Images are recorded with an AMT digital camera.
Wound healing assay
Confluent cultures on Transwell inserts were gently disrupted using a sterile plastic pipette tip and washed with PBS. The cultures were monitored until restoration of tissue confluence by serial imaging, using an OLYMPUS inverted microscope and the QCapture imaging software (QImaging). The cultures were then fixed, and IF staining for lineage markers of secretory vs. ciliated cells, Ki67 and 5-bromo-2-deoxyuridine (BrdU) labeling was performed. For BrdU labeling experiments, cells at different time points after plating were incubated with 32.5 μM BrdU (SIGMA) in growth medium for 2 hours, followed by fixation with 2% PFA and denaturation with 2M Hydrochloric acid (HCl) for 30 minutes. BrdU incorporation was detected by IF staining. These experiments were repeated thrice with cells from different patients.
Mass Spectometry
The upper compartment of confluent ex-vivo cultures was washed with PBS. Fresh PBS was then added to the upper compartment for 16–24 hours. The proteins present in the sample were reduced with 5mM DTT at 56°C for 1 hour, and digested with 10μg of trypsin (Promega, Madison, WI, USA) at 37°C overnight. Peptides were extracted using C18 cartridge (Waters, Milford, MA) and reconstituted with 200μl of incubation buffer (50mM HEPES, 50mM NaCl, pH 7.4). Cysteine-containing peptides were immobilized on activated thiol sepharose 4B resin (GE Healthcare, Piscataway, NJ, USA), eluted with 10mM DTT and alkylated with 55 mM iodoacetamide. Peptides were then analyzed by LC-MS/MS on an Orbitrap-XL mass spectrometer (Thermo Scientific, Waltham, MA, USA). MS/MS spectra were searched against Human database using Mascot (Matrix Science). Peptides with score over 25 (FDR < 1%) were accepted for protein identification.
DNA damage experiments
Confluent FTE ex-vivo cultures and whole FT tissues were treated with ionizing radiation in a Gammacell-1000 irradiator (Best Theratronics, Ottawa, Canada). Radiation dose was 500 rads for the ex-vivo cultures and 125 rad for the whole tissue pieces. Alternatively, cells or tissue were treated with 3 μM Mitomycin C (MMC, SIGMA) in growth medium over-night, 5 μM cisplatinum in growth medium for 4 hr (SIGMA, stock solution dissolved in DMF), or with 50μM H2O2 (SIGMA) for 10 minutes, followed by washing. DNA damage was detected by means of immunofluorescent or immunohistochemical staining for factors in the dsDNA repair pathway at different time points. The ex-vivo culture experiments have been repeated 5 times, with cells from 5 different donors. For quantitative analysis of each experiment at least 500 nuclei were evaluated for each time point. The experiments with whole tissue pieces have been repeated thrice, with tissues from different donors. The quantitative analysis was performed by counting of secretory and ciliated cells on 3 different fields per experiment on γH2A.X- and p53-stained slides.
Results
The ex-vivo culture system is a phenocopy of the FTE
The FTE is composed of two cell populations: the secretory cells and the ciliated cells (Crow et al., 1994). Previous efforts to recapitulate primary human FTE in culture have produced suboptimal results due to the rapid dedifferentiation and loss of the polarized morphology and the biological behavior of the two sub-populations of epithelium (Ando et al., 2000; Comer et al., 1998; Henriksen et al., 1990; Kervancioglu et al., 1994).
As described in Methods, epithelial cells are dissociated from fresh surgical samples of FT fimbria and grown at the air-liquid interface in the upper compartment of a porous polyester membrane of a Transwell insert (Fig. 1A). Our experience with over 100 different subjects’ samples demonstrates that we can obtain highly reproducible and viable cultures that can be maintained for over 28 days. Since the cells are derived from primary tissue, they will senesce upon subsequent passage or when the cells are plated at a very low concentration (data not shown). Importantly, our specific dissociation and culturing conditions result in cultures that are highly enriched for epithelial cells (data not shown).
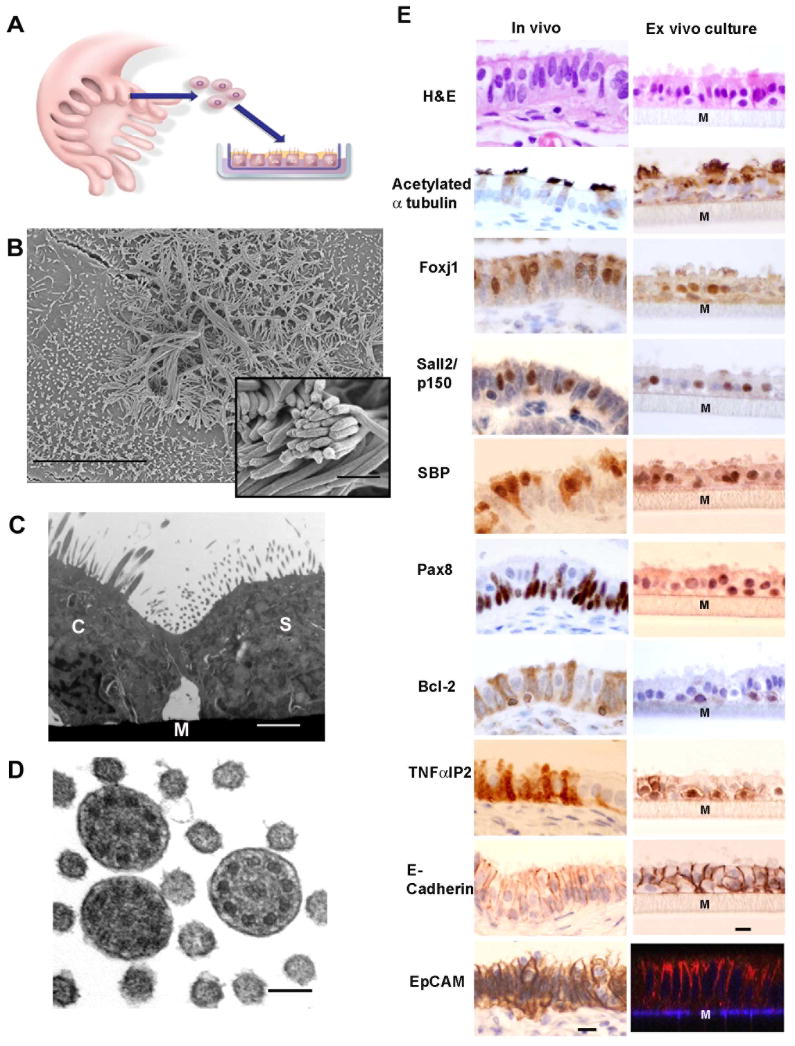
Figure 1.
The FTE ex-vivo culture system displays morphology and immunomarkers of both FTSECs and ciliated cells. (A) A schematic illustration of the ex-vivo co-culture system. The co-culture is composed of primary human FTE cells grown in the air-liquid interface on Transwell inserts. The cells form a polarized monolayer. (B) Scanning electron microscope image of a confluent culture showing both ciliated cells and secretory cells with microvilli (bar 10μm). Inset shows cilia in higher power (bar 1 μm). (C) Transmission electron microscope image showing a ciliated cell (C) and a secretory cell (S) and the Transwell membrane (M) (bar 1μm). (D) transmission electron microscopy cross section through cilia demonstrating the 9+2 arrangement of axial microtubules (bar 100nm). (E) H&E and immunohistochemistry of normal human fallopian tube fimbria and cross sections of formalin-fixed-paraffin-embedded ex-vivo cultures with the ciliated lineage markers: Acetylated α-tubulin, FoxJ1, p150/Sall2, SELENBP1 (SBP); secretory lineage markers: Pax-8, Bcl-2 and TNFαIP2; pan-epithelial surface markers: E-Cadherin and Ep-CAM (immunofluorescence, Z-stack reconstitution of confocal microscopy images). The gray matrix beneath the cell layer represents the Transwell membrane (Marked by M). Bar 10 μm.
Our first goal was to characterize the ex-vivo cultures by analyzing their composition, immunoprofile, and ultrastructural components. We examined the cultures using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). These techniques revealed that the ex-vivo cultures are composed of true ciliated cells (Fig. 1B,C) with the classical arrangement of 9+2 microtubules typically seen in motile cilia (Fig. 1D) (Satir and Christensen, 2008), as well as secretory cells which are characterized by abundant microvilli (Fig. 1C).
We found that FTSECs, both in-vivo and in the ex-vivo cultures, uniquely expressed several lineage specific markers: Pax-8, which is a paired-box transcription factor that is involved in lineage determination of the thyroid and the reproductive system (Bowen et al., 2007), Bcl-2, an anti-apoptotic mitochondrial protein (Piek et al., 2001), and TNFαIP2, a poorly characterized protein which is induced by tumor necrosis factor-α (TNF-α) and retinoic acid (Rusiniak et al., 2000)(Fig. 1E). We identified TNFαIP2 by mining the Human Protein Atlas database for factors that are expressed by only one of the two cell types in the FTE (Ponten et al., 2008). To our knowledge, this is the first study showing that TNFαIP2 is a FTSEC marker, and that this specificity is also preserved in our ex-vivo cultures. Conversely, the motile cilia stain positive for acetylated α-tubulin (Seeley et al., 2009). Additionally, ciliated cells expressed Foxj1, a member of the Forkhead transcription factors family implicated in programming ciliogenesis (Okada et al., 2004; You et al., 2004), Sall2, a zinc finger transcription factor involved in the cell senescence program (Li et al., 2004; Liu et al., 2007), and SELENBP1, selenium binding protein (Huang et al., 2006), which was also identified by searching the archives of the Human Protein Atlas (Fig. 1E) (Ponten et al., 2008). The two cell lineages share several common pan-epithelial markers, such as Ep-CAM and E-Cadherin (Fig. 1E); E-Cadherin being critical for the proper formation and maintenance of adherent junctions and polarity in areas of cell-cell contact. Based on these observations, we conclude that the ex-vivo system mirrors the morphologic and immunophenotypic properties of FTE in-vivo.
The ex-vivo culture is a viable and responsive model system
We next set out to characterize the dynamic behavior of the culture system. Ideally, we want our cultures to be responsive to biologically relevant stimuli and suitable for manipulation over an extended period of time, as an essential prerequisite of a model system to study factors that lead to carcinogenesis. Towards this end, we examined the proliferative and restorative capacity and the trans-differentiation potential of the cells in the ex-vivo cultures by performing tissue wounding assays. Specifically, we wanted to know whether disruption of the tissue would lead to restoration of integrity through migration, proliferation, or a combination, and most importantly, which cell type, FTSECs or ciliated cells, participates in the healing of the ‘wound’. Confluent ex-vivo cultures were disrupted using a sterile pipette tip and washed with PBS (Fig. 2A). The cultures were allowed to recover for 48 hours (Fig. 2A–C). At the end of the recovery period, the wound had healed (Fig. 2B, C). This wound repair was at least in part due to proliferation of cells at the edges of the disrupted tissue, as demonstrated by Ki67 staining of the healing cultures on day 2 post wounding (Fig. 2D). This was confirmed by BrdU labeling (Fig. 2E). The cells that occupied the gap were secretory cells, as determined by co-staining for the lineage markers, Pax-8 and Foxj1 (Fig. 2F and Fig. S1). As a control, we fully scraped a matched ex-vivo culture and returned the cells back to an identical Transwell insert, but in this case, where the architecture of the tissue was completely disrupted, none of the cells adhered to the collagen-coated insert after 7 days (data not shown). It is noteworthy that when pure FTSECs were derived from the mixed FTE cells, and then plated onto the Transwell inserts, we did not observe trans-differentiation into ciliated cells (data not shown). Consistent with our observation in-vivo, the FTSECs in our ex-vivo cultures retain their proliferative capacity (see Supplementary Information (SI) and Fig. S2), and this property is preserved over 15 days in culture (SI text and Fig. S3). The proportion of secretory vs. ciliated cells is variable between specimens but remains constant during the 15 days in culture (SI text and Fig. S4). These findings suggest that the ex-vivo culture system, which is composed of primary cells, is a dynamic model, which does not senesce after becoming confluent, but rather retains a proliferative potential.
Figure 2. Wound healing in the FT ex-vivo culture system.
(A–C) Bright field images of the healing of a scratch in a confluent ex-vivo culture, at day 0 (A), day 1 (B) and day 2 (C) post scratching (red lines mark the borders of the scratch). Proliferating cells on the perimeter of the healing wound, as demonstrated by IF staining for either the proliferation factor Ki67 (D, green) or BrdU (E, green) which co-localize with nuclei of secretory cells (Pax-8 positive nuclei, red) giving a yellow signal. (F) While both cell types exist in the culture, he wound is ‘healed’ by predominantly FT secretory cells (right of the white line), as demonstrated by co-staining for the lineage markers for secretory cells (Pax-8, red) and for ciliated cells (Foxj1, green).
The ex-vivo culture system recapitulates the secretome of the human FT epithelium
Finally, we wanted to confirm the functional similarity of our system to FTE in-vivo. For this purpose we probed conditioned medium from the cultured cells for the presence of proteins that are known to be secreted by human FT, including Oviductin (MUC9) and Glycodelin A (PP14) (Briton-Jones et al., 2002; Saridogan et al., 1997). We detected both Oviductin and Glycodelin A in serum-free conditioned medium collected after 24–48 hours of incubation with confluent ex-vivo cultures (Fig. 3A). These proteins were not detected when medium was collected after 2 hours of incubation, suggesting that they did not originate from the medium. We also did not detect these proteins when the cells were grown in standard submerged cultures on plastic (Fig. 3A). Interestingly, the cultures also secreted HE4 (Fig. 3B), a recently approved serum biomarker of ovarian carcinoma (Drapkin et al., 2005; Hellstrom et al., 2003; Moore et al., 2009). Since the ex-vivo culture system is a polarized cell layer, we wanted to test our ability to detect secreted proteins that are present in the apical (lumen-facing) side of the cells. We noted that once totally confluent, the cultures formed a leak-proof fluid barrier, and that none of the serum-containing feeding media in the lower compartment of the culture vessel could permeate the upper compartment. We exploited this property to isolate fluid from the apical aspect of the culture. To obtain the conditioned media, the upper chamber of the filters was washed with PBS which was collected 24 hours later. We profiled 11 different culture samples using liquid chromatography tandem mass spectrometry and found several established ovarian cancer serum biomarkers in the secretions of normal FTE (Table 1). Several of these biomarkers were further validated by immunohistochemical staining of normal FT fimbria sections (Fig. S5). Moreover, secreted proteins that were collected and analyzed in an identical manner from two different OSE cell lines grown under the same conditions did not include the major ovarian cancer biomarkers (Table 1 and Table S2). Although the number of FTE and OSE cultures tested in this manner is small, these data further support the notion that the FTE may be the tissue-of-origin for a significant subset of patients with serous carcinoma. This data also suggests that the ex-vivo culture system can be uniquely interrogated to yield potentially valuable information regarding biomarkers for this disease.
Figure 3. FT ex-vivo culture secretes known FT and ovarian carcinoma proteins.
(A) Detection of oviductin (MUC9, ~150KD) and Glycodelin A (PP14, ~28KD) by western blot in conditioned medium of FTE from seven different donors grown in ex-vivo cultures, while there is no detectable protein secretion when grown in regular culture conditions. (B) Detection of HE4 (~28KD), an ovarian cancer serum biomarker, in some established ovarian cancer cell lines and some FTE ex-vivo cultures.
Table 1.
Selected serous carcinoma biomarkers in the FT ex-vivo culture secretome. A representative subset of proteins that are secreted by FTE cells in the ex-vivo cultures, and were previously reported to be detectable in ovarian cancer or FT secretions. The incidence of is calculated out of 11 different patients’ samples. Proteins secreted by at least one of two OSE samples analyzed in an identical manner are marked.
| Accession Number | Protein Description | Incidence* | Expressed by OSE |
|---|---|---|---|
| gi|115298678|ref|NP_000055.2| | complement component 3 precursor [Homo sapiens] | 8 | |
| gi|4557871|ref|NP_001054.1| | transferrin [Homo sapiens] | 8 | |
| gi|5032057|ref|NP_005611.1| | S100 calcium binding protein A11 [Homo sapiens] | 7 | |
| gi|22208982|ref|NP_002767.2| | kallikrein-related peptidase 10 preproprotein [Homo sapiens] | 6 | |
| gi|4507509|ref|NP_003245.1| | tissue inhibitor of metalloproteinase 1 precursor [Homo sapiens] | 6 | + |
| gi|83367077|ref|NP_078966.2| | mucin 16 [Homo sapiens] | 6 | |
| gi|4504919|ref|NP_002264.1| | keratin 8 [Homo sapiens] | 5 | |
| gi|62414289|ref|NP_003371.2| | vimentin [Homo sapiens] | 5 | + |
| gi|4505185|ref|NP_002406.1| | macrophage migration inhibitory factor (glycosylation-inhibiting factor) [Homo sapiens] | 5 | + |
| gi|4505773|ref|NP_002625.1| | prohibitin [Homo sapiens] | 5 | |
| gi|53988378|ref|NP_005814.2| | mesothelin isoform 1 preproprotein [Homo sapiens] | 5 | |
| gi|4502693|ref|NP_001760.1| | CD9 antigen [Homo sapiens] | 5 | |
| gi|4557485|ref|NP_000087.1| | ceruloplasmin precursor [Homo sapiens] | 4 | |
| gi|56699495|ref|NP_006094.3| | WAP four-disulfide core domain 2 precursor [Homo sapiens] | 4 | |
| gi|48255935|ref|NP_000601.3| | CD44 antigen isoform 1 precursor [Homo sapiens] | 4 | |
| gi|5031701|ref|NP_005851.1| | follistatin-like 3 glycoprotein precursor [Homo sapiens] | 4 | |
| gi|91984773|ref|NP_658985.2| | apolipoprotein A-I binding protein precursor [Homo sapiens] | 3 | |
| gi|67782365|ref|NP_005547.3| | keratin 7 [Homo sapiens] | 3 | |
| gi|5453678|ref|NP_006423.1| | epididymal secretory protein E1 precursor [Homo sapiens] | 3 | |
| gi|21237748|ref|NP_004348.2| | CD151 antigen [Homo sapiens] | 3 | |
| gi|4507065|ref|NP_003055.1| | secretory leukocyte peptidase inhibitor precursor [Homo sapiens] | 3 | |
| gi|4505787|ref|NP_002629.1| | elafin preproprotein [Homo sapiens] | 3 | |
| gi|5453541|ref|NP_006399.1| | anterior gradient 2 homolog [Homo sapiens] | 2 | |
| gi|65301117|ref|NP_002447.4| | mucin 1 isoform 1 precursor [Homo sapiens] | 2 | |
| gi|65507501|ref|NP_001018059.1| | * glycodelin precursor [Homo sapiens] | 2 | |
| gi|4505583 | * progestagen-associated endometrial protein (placental protein 14; pregnancy-associated endometrial tumor-associated calcium signal transducer 1 precursor[Homo sapiens] | 2 | |
| gi|4505059|ref|NP_002345.1| | oviductal glycoprotein 1 precursor [Homo sapiens] | 2 | |
| gi|58386720|ref|NP_002548.3| | * the same protein | 1 |
A total of eleven (11) fallopian tube culture secretions were analyzed using liquid chromatography tandem mass spectrometry (MS). This column represent the number of samples (out of 11) in which this particular protein was identified by MS.
Secretory cells and ciliated cells exhibit different DNA repair kinetics
Having established the structural and functional validity of our FTE model system, we wanted to ask whether the system could be used to start deciphering the mechanisms underlying the apparent predisposition of the secretory cell to neoplastic transformation. We chose to address the effect of genotoxic stress on our cultures. Ex-vivo cultures were exposed to ionizing radiation (IR) at a dose of 500 rad (5 Gy) and the activation of the dsDNA repair pathway was monitored by co-staining for repair foci containing either phosphorylated ATM (pS1981) (Branzei and Foiani, 2008), a protein kinase that is activated in response to dsDNA breaks, or γH2A.X (pS139), a histone isoform that is phosphorylated by activated ATM and recruits DNA repair factors, and for lineage-specific markers (Branzei and Foiani, 2008), either Pax8 (for secretory cells) or FoxJ1 (for ciliated cells) (Fig. 4A and Fig. S6–9). Analysis of a time-course revealed an early activation of the dsDNA breakage repair pathway in the secretory cells 1–2 hours after induction with IR, in contrast to late response of the ciliated cells which peaked after 8–10 hours. While the dsDNA breaks in the ciliated cells were almost completely repaired by 24 hours, as measured by resolution of the γH2A.X foci, these foci persisted in FTSECs even after 48 hours (Fig. 4B). No increase in apoptosis or necrotic cell death, or change in the proportions of secretory vs. ciliated cells, was encountered during the course of these experiments (data not shown). Importantly, when the DNA damage was induced by other agents, including Mitomycin C (MMC, 3 μM for 16 hours), cisplatinum (5 μM for 4 hr), or H2O2 (50μM for 10 minutes) similar results were observed (Fig. S9). Therefore, the DNA damage response (DDR) kinetics of FTSECs is characterized by an early and rapid activation that persists beyond the time in which ciliated cells resolve the damage. These results suggest that FTSECs may be more prone to accumulating DNA lesions that could potentially lead to mutations and the genesis of p53 signatures.
Figure 4. Differential dsDNA break response in the fallopian tube epithelium ex-vivo.
(A) Immunofluorescence (en-face images) of representative cells in the ex-vivo culture system, stained for the secretory lineage marker Pax8 (red) and γH2A.X (green), and the nuclei are outlined with DAPI (blue). The formation of γH2A.X foci was recorded 2, 4, 10, 24 and 48 hours after exposure of the co-cultures to ionizing radiation (500 rad). γH2A.X foci are preferentially detectable in FTSECs 2 hours after irradiation and persist for at least 48 hours. Original magnification X60. (B) The kinetics of dsDNA break pathway activation in FTSECs vs. ciliated cells ex-vivo.γH2A.X foci form in FTSECs early after exposure to ionizing radiation, and persist in 70% of the cells for at least 48 hours. Under the same conditions, γH2A.X foci formation in ciliated cells peaks after 10 hours and returns to baseline within 24 hours. The graph represents integration of 4 experiments.
In order to validate these ex-vivo findings, we asked whether fresh FT tissue exhibits a similar differential kinetics in the DDR between the FTSECs and the ciliated cells. Fresh FT tissue fragments were exposed to low doses of IR (125 rad). We recorded the response shortly after the exposure (1 hour) and again 24 hours later, during which time the tissue was maintained in culture medium. This time point represents a complete DNA repair cycle in many cell types (Keogh et al., 2006). While higher doses of ionizing radiation (500 rad, as used for the ex-vivo cultures) resulted in dsDNA breaks in all epithelial cells, as determined by staining for γ-H2A.X, exposure to low doses (125–250 rad), resulted in a markedly different response: early activation of DDR in the secretory cells, with sustained signaling even after 24 hours, while the ciliated cells had recovered by the later time point (Fig. 5A and B). The activation of the DDR is displayed as preferential phosphorylation of ATM, histone H2A.X, and Chk2, and accumulation of p53 in FTSECs (Fig. 5). The general morphology of the tissue was preserved over the 24 hour incubation period (H&E staining in Fig. 5). While an average of 53% of the secretory cells in these experiments displayed γ-H2A.X foci 24 hours after exposure to IR, only 22% of the ciliated cells still retained such foci (Fig. 5B). The difference between the two cell lineages became more evident when p53 was looked at: 71% of the secretory cells and only 4% of the ciliated cells had accumulation of p53 at the 24 hours time-point (Fig. 5B). These results further corroborate the validity of the ex-vivo culture system as a tool for the characterization and manipulation of the FTE and suggest that persistent DNA damage in FTSECs may underlie its susceptibility to genomic instability.
Figure 5. Preferential activation of the dsDNA break checkpoint pathway in FTSEC in-situ.
(A) H&E and immunohistochemistry of normal human fallopian tube fimbria following exposure to ionizing radiation, demonstrating preferential activation of the dsDNA break signaling pathway in secretory cells (marked by arrowheads), compared to ciliated cells (marked by arrows). Original magnification ×60. (B) Quantitative analysis of number of adjacent secretory cells and ciliated cells that stained positive for γH2A.X foci and p53 24 hours after exposure to IR in-situ (125–250 rad). No staining was seen in non-irradiated cell.
Discussion
Developing a reliable model system for studying the early events in SOC pathogenesis has been particularly challenging, largely because of the uncertainty regarding the cell-of-origin of this disease. Given recent data implicating the FTSEC in this process, it became important to develop an in-vitro model system that would recapitulate the morphology and biology of these cells. Our first goal was to culture normal human primary FTE in a way that would preserve the composition of both secretory and ciliated cells, as well as the degree of differentiation and tissue polarity. We also expected the system to be able to overcome inherent problems of working with benign primary cells, such as early senescence, narrow time window for manipulation and experimentation, lack of reproducibility, and loss of plasticity over time. The model that we have developed permits the isolation and growth of both secretory and ciliated epithelial cells, which retain their phenotypic properties over an extended period of in-vitro culture. The cultures are viable over a period of several weeks, and retain a proliferative capacity. The cells also function in a way that recapitulates the complex biology of the FT, by both secreting specific proteins and by responding to stimuli, such as cell injury and DNA damage, in a manner that is observed in-vivo.
In addition to validating our culture system as a faithful replicate of FTE, our data also lends support to the notion that the FTE is a field-of-origin for serous carcinomas. The identification of ‘cancer’ biomarkers (Bast et al., 2005; Drapkin et al., 2005; Hellstrom et al., 2003; Kozak et al., 2005) in the secretome of benign FTE supports the hypothesis that the cell-of-origin of ovarian tumors is likely Müllerian (Drapkin et al., 2005). Furthermore, the fact that these biomarkers are below detectable levels in normal subjects does not necessarily suggest that they are expressed de novo as part of the carcinogenic process, but rather reflect the increase in mass of the tissue that normally secretes them. Importantly, as a control for specificity, we show that the profile of proteins secreted by the FT ex-vivo cultures is divergent from that of OSE cultured under identical conditions. Although the significance of these findings is compromised by the small number of samples, it is tempting to postulate that manipulating this system to undergo changes that are typical of serous carcinoma, such as loss of BRCA1 or of TP53, may result in genomic instability and the development of serous precursor lesions that could alter the signature of secreted proteins. We intend to use this model to study the early events in serous oncogenesis, focusing on identification of candidate targets for drug development and secreted biomarkers for early detection.
Secretory and ciliated epithelial cells are intimately mixed within the FT inner lining. Although they may run two different transcriptional and translational programs, they are essentially exposed to the same micro-environment. We utilized the ex-vivo culture system to study how these two cell lineages response to DNA damage. DNA damage plays a major role in malignant transformation and the DDR is not only committed to repairing the DNA, but also serves as a barrier to transformation by inducing cell cycle arrest, apoptosis or senescence. This general pattern is observed in multiple tumor types (Bartkova et al., 2005; Halazonetis et al., 2008). We focused our study on the dsDNA repair pathway because it is a common pathway to several other repair mechanisms, and as such, provides an insight into the genotoxic stress in a cell or a tissue regardless of the cause. We found that the FTSECs display a rapid yet prolong activation of the DDR, following minimal damage. In contrast, the ciliated cells appear to activate the DDR more slowly but resolve the injury within the time frame of our studies. This phenomenon may reflect the mere fact that the ciliated cells are terminally differentiated and that most of these cells are quiescent and in G0/G1. We currently do not know whether the mechanism by which ciliated cells and FTSECs repair dsDNA breaks is different due to their proliferative capacities but one could imagine that non-homologous end-joining (NHEJ) and homologous recombination (HR), may be preferentially used in each cell type (Branzei and Foiani, 2008). In this regard, the recent report indicating that the interaction between CtIP and BRCA1 acts as a molecular switch to shift the balance of DNA break repair from error-prone NHEJ to error-free HR in cell transitioning out of G0/G1 (Yun and Hiom, 2009) is compelling in light of the role that BRCA1 plays in genome integrity control and in hereditary forms of serous ovarian cancer. More importantly, our in-situ studies confirmed the findings in the ex-vivo cultures and lend support to biological integrity of the culture system.
We believe that the ex-vivo culture system will enable us to begin testing the hypothesis that the FTSEC is a cell-of-origin for serous carcinomas. Exposure of FTE in these cultures to hormonal changes and inflammatory mediators released during ovulation may provide insights into the genetic aberrations that culminate in PSC. These discoveries can in turn be utilized to develop early detection biomarkers, preventive approaches, and novel drugs. It is also likely that a similar system can be broadly applied to studying the normal biology of other epithelia where precursor lesions and malignant counterparts are not entirely understood. For example, similar systems have been described to study airway biology and disease (Karp et al., 2002; Vermeer et al., 2003) and a system in monkeys has been described to study reproductive biology (Rajagopal et al., 2006). Using such model systems to study the tumorigenic process may greatly impact our understanding of epithelial biology and oncogenesis.
Supplementary Material
Acknowledgments
We thank Phil Karp, Thomas Moninger and Drs. Paola Vermeer and Joseph Zabner (University of Iowa) for their enthusiastic support and assistance in establishing the FT ex-vivo culture system, Drs. Steve Cannistra, Glenn Dranoff, and David Livingston for thoughtful suggestions and comments on the manuscript, and Drs. Tom Benjamin and Dawei Li for the Sall2 antibody. Special thanks goes to the faculty, technicians, residents and fellows of the division of Women’s and Perinatal Pathology in the Department of Pathology at the Brigham and Women’s Hospital, Boston, MA, for the allocation of tissues.
Financial support: This work was supported by the National Cancer Institute [P50 CA105009, K08 CA108748, R21 CA124688], Ovarian Cancer Research Fund (Individual Investigator Award and Program Project Development Award), Phi Beta Psi Sorority Charitable Trust, Fannie E. Ripple Foundation, Robert and Deborah First Fund, Randi and Joel Cutler Ovarian Cancer Research Fund, the Columbia Hospital for Women Research Foundation, Marsha Rivkin Foundation Scientific Scholar Award, AACR-George and Patricia Sehl Fellowship for Cancer Genetics Research, and the American Physicians Fellowship for Medicine in Israel- Claire and Emmanuel G. Rosenblatt Foundation Grant.
Footnotes
Supplementary information is available at Oncogene’s website.
References
- Ando H, Kobayashi M, Toda S, Kikkawa F, Masahashi T, Mizutani S. Establishment of a ciliated epithelial cell line from human Fallopian tube. Hum Reprod. 2000;15:1597–603. doi: 10.1093/humrep/15.7.1597. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–7. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- Briton-Jones C, Lok IH, Yuen PM, Chiu TT, Cheung LP, Haines C. Human oviductin mRNA expression is not maintained in oviduct mucosal cell culture. Fertil Steril. 2002;77:576–80. doi: 10.1016/s0015-0282(01)03216-2. [DOI] [PubMed] [Google Scholar]
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998;13:3114–20. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- Crow J, Amso NN, Lewin J, Shaw RW. Morphology and ultrastructure of fallopian tube epithelium at different stages of the menstrual cycle and menopause. Hum Reprod. 1994;9:2224–33. doi: 10.1093/oxfordjournals.humrep.a138428. [DOI] [PubMed] [Google Scholar]
- Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–70. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–9. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- Folkins AK, Jarboe EA, Roh MH, Crum CP. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009 doi: 10.1016/j.ygyno.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–5. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–700. [PubMed] [Google Scholar]
- Henriksen T, Tanbo T, Abyholm T, Oppedal BR, Claussen OP, Hovig T. Epithelial cells from human fallopian tube in culture. Hum Reprod. 1990;5:25–31. doi: 10.1093/oxfordjournals.humrep.a137034. [DOI] [PubMed] [Google Scholar]
- Huang KC, Park DC, Ng SK, Lee JY, Ni X, Ng WC, et al. Selenium binding protein 1 in ovarian cancer. Int J Cancer. 2006;118:2433–40. doi: 10.1002/ijc.21671. [DOI] [PubMed] [Google Scholar]
- Jarboe EA, Folkins AK, Drapkin R, Ince TA, Agoston ES, Crum CP. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology. 2008;53:127–38. doi: 10.1111/j.1365-2559.2007.02938.x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–37. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature. 2006;439:497–501. doi: 10.1038/nature04384. [DOI] [PubMed] [Google Scholar]
- Kervancioglu ME, Saridogan E, Martin JE, Maguiness SD, Djahanbakhch O. A simple technique for the long-term non-polarised and polarised culture of human fallopian tube epithelial cells. Biol Cell. 1994;82:103–7. doi: 10.1016/s0248-4900(94)80012-x. [DOI] [PubMed] [Google Scholar]
- Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–96. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tian Y, Ma Y, Benjamin T. p150(Sal2) is a p53-independent regulator of p21(WAF1/CIP) Mol Cell Biol. 2004;24:3885–93. doi: 10.1128/MCB.24.9.3885-3893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Adler AS, Segal E, Chang HY. A transcriptional program mediating entry into cellular quiescence. PLoS Genet. 2007;3:e91. doi: 10.1371/journal.pgen.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–6. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Ohta Y, Brody SL, Watanabe H, Krust A, Chambon P, et al. Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J Mol Endocrinol. 2004;32:615–25. doi: 10.1677/jme.0.0320615. [DOI] [PubMed] [Google Scholar]
- Piek JM, van Diest PJ, Verheijen RH, Kenemans P. Cell cycle-related proteins p21 and bcl-2: markers of differentiation in the human fallopian tube. Histopathology. 2001;38:481–2. doi: 10.1046/j.1365-2559.2001.1163c.x. [DOI] [PubMed] [Google Scholar]
- Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol. 2008;216:387–93. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- Rajagopal M, Tollner TL, Finkbeiner WE, Cherr GN, Widdicombe JH. Differentiated structure and function of primary cultures of monkey oviductal epithelium. In Vitro Cell Dev Biol Anim. 2006;42:248–54. doi: 10.1290/0602015.1. [DOI] [PubMed] [Google Scholar]
- Rusiniak ME, Yu M, Ross DT, Tolhurst EC, Slack JL. Identification of B94 (TNFAIP2) as a potential retinoic acid target gene in acute promyelocytic leukemia. Cancer Res. 2000;60:1824–9. [PubMed] [Google Scholar]
- Saridogan E, Djahanbakhch O, Kervancioglu ME, Kahyaoglu F, Shrimanker K, Grudzinskas JG. Placental protein 14 production by human Fallopian tube epithelial cells in vitro. Hum Reprod. 1997;12:1500–7. doi: 10.1093/humrep/12.7.1500. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129:687–93. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–30. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–6. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, et al. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L650–7. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009 doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.