Abstract
Good and poor fifth-grade writers differed, after controlling for multiple comparisons, in 42 brain regions on group maps and then individual brain analyses for fMRI contrast between tapping adjacent fingers sequentially and same finger repeatedly. Of these, 11 regions were correlated with both handwriting and spelling (transcription). Gender differences on the fMRI contrast, with girls more activated, occurred only in left superior parietal, which was correlated with handwriting and spelling. Significance of serial-organization of fingers for handwriting and spelling is discussed.
Keywords: finger sequencing, serial organization of behavior, good and poor child writers, fMRI writing tasks, brain and writing development
Lashley (1950) called attention to the importance of the serial organization of human behavior and thus set the stage for the eventual paradigm shift from behaviorism to cognitive psychology in mainstream psychology. His point was that associations close in time could not account for all human learning and behavior.
Denckla (1973) and Wolf et al. (1983) introduced imitative finger tasks for assessing serial organization of finger movement. The number of taps (20) is held constant across two tasks, which vary as to whether the individual touches the thumb with each finger in sequence or touches the same finger to the thumb repeatedly. Both tasks, which are performed out of view without any visual cues, require execution of a motor act involving thumb and fingers but differ in whether planning sequential finger movements, that is serial behavior, is involved. The time difference between the two tasks captures this planning time.
These tasks are but two of the many that have been used to assess neurological soft signs in children, which reflect anomalies in neuropsychological functioning, but not brain lesions, and are associated with a variety of childhood disorders (e.g., Taylor , 1987; Tupper, 1998). However, the finger succession task—dominant hand— was shown to have excellent reliability and construct validity for assessment of writing problems in primary grade children (Berninger & Rutberg, 1992) and to explain unique variance in handwriting and composing in primary grade children (Berninger, Yates, Cartwright, Rutberg, Remy, & Abbott, 1992) and composing in intermediate grade children (Berninger, Cartwright, Yates, Swanson, & Abbott, 1994). Finger succession—non-dominant hand only—contributed uniquely to two spelling measures and a measure of composing in adults with dyslexia (Berninger & O'Donnell, 2004).
Many functional imaging studies have employed a finger tapping task in which the participant repeatedly taps the same finger, typically the index finger, either at a self-paced rate or auditorially or visually cued pace. In the current study this repeated tapping of the same finger was used as a control task. Others use bimanual tapping in which the participant alternates the hand used to tap repeatedly with the same finger. Others compare contrasting task conditions to identify brain regions involved in learning complex timing patterns underlying sequential finger movements, but fewer imaging studies have employed the sequential finger tapping task introduced by Denckla (1973) and Wolff et al. (1983), which requires only repeated sequential taps of thumb and each finger in succession without varying temporal requirements.
A functional imaging task that required the participant to tap the thumb with each finger in succession has been shown to activate the sensorimotor cortex, the cerebellum, and the supplementary motor area (Roberts, Disbrow, Roberts, & Rowley, 2000); Tegeler, Strother, Anderson, & Kim, S-G., 1999) in adults. The sensorimotor cortex controls motor movements and codes sensations for touch and kinesthesia, which registers sequential touches, that result from using fingers for motor output. The cerebellum may temporally coordinate the separate processes involved in planning and producing serial output, whereas the supplementary motor area may construct a precise timing plan for organizing forthcoming motor sequences.
This same fMRI task also showed that increased right superior parietal activation and decreased activation in contralateral motor regions differentiated children with and without attention deficit (Mostofsky, Rimrodt, Schafer, Boyce, Goldberg, Pekar, et al., 2006). In contrast, the left superior parietal region has been proposed as a writing center in the brain where internal codes for letter forms may be generated and stored for production (Basso, Taborelli, & Vignolo, 1978). Many children with attention problems may also have writing problems, depending on whether only right superior parietal or both right and left superior parietal regions differ from normal.
Far more neuroimaging research has been conducted on reading disabilities than writing disabilities (Butler, 2004). Neuroimaging studies of spelling in children (e.g., Booth, Cho, Burman, & Bitan, 2007; Richards, Aylward, Berninger, Field, Parsons, Richards et al., 2006) are appearing in the research literature, but research on handwriting disabilities has mainly involved adults (e.g., James & Gauthier, 2006; Longcamp, Anton, Roth, & Velay, 2003; Matsuo, Kato, Tanaka, Sugio, Matsuzawa, Inui et al., 2001). The purpose of the current research was to add to the neuroimaging research on writing in children by investigating the finger succession and finger repetition tasks adapted so that children could perform them during fMRI scanning.
Children who were and were not good writers at the end of fifth grade when they completed a five-year longitudinal study of writing were compared on a contrast between two fMRI tasks for (a) finger movement requiring planning for serial behavior and (b) finger movement not requiring planning for serial behavior. We tested two hypotheses related to transcription (handwriting and spelling) in cognitive models of writing (e.g., Alamargot, & Chanquoy, 2001; Hayes & Chenoweth, 2006; Hayes & Flower, 1980). The first hypothesis was that good and poor writers, based on their handwriting and spelling abilities, would differ in mean BOLD activation on the contrast between the two fMRI tasks that did and did not require serial finger movement. The second hypothesis was that behavioral measures of finger sequencing, handwriting, and spelling would predict activation on the fMRI contrast for sequential versus non-sequential finger movements. The rationale was that retrieving ordered alphabet letters, producing ordered strokes when writing letters, and writing ordered letters when spelling written words draw on serially organized finger motor movements.
Method
All methods, procedures, and consent forms used by this project were approved by the Institutional Internal Review Board (Approval no. 96-1872-D12). All persons who participated in this study gave their informed consent prior to their inclusion in the study, which was conducted in accordance with ethical and professional guidelines of the American Psychological Association for research with human participants.
Participants
All participants were right-handed children who did not wear non-removable metal. They were recruited from a just completed five-year longitudinal study of writing development in which they were first enrolled in first grade and studied annually thereafter. The children participated in the fMRI study in the summer between fifth and sixth grade. Children were included in the group of good writers if they were at or near or above the mean on the handwriting measure, dictated spelling measure, and written composition measure and were not significantly impaired in either of these writing skills. Children were included in the group of poor writers if they were below the population mean on one or more writing measure and/or at least one standard deviation below their Verbal IQ on one or more writing measure.
The resulting sample included one group of good writers (n= 12) and one group of poor writers (n=8). Of the good writers, 9 were females and 3 were males, and of the poor writers, 7 were males and 1 was female, consistent with cross-sectional (Berninger and Fuller, 1992) and longitudinal studies (Martin and Hoover, 1987) that showed more typically developing boys are impaired and are more severely impaired than typically developing girls in writing skills regardless of ethnicity at all developmental levels (Demie, 2001). Thus, it was not surprising that more boys would be in the group of poor writers and more girls in the group of good writers. However, increased incidence of writing disabilities in boys compared to girls does not mean that brain activation during the contrast investigated in the current study will necessarily show gender differences (see results section).
The Verbal IQs of the good (M=119.25, SD=17.00) and poor (M=116.63; SD=8.23) writers did not differ significantly, F(1,18)=0.16, p=.69. Comparison of means scaled as 0 (z-scores z) or 100 (standard scores ss) with standard deviations of 3 (z) or 15 (ss) showed that the good and poor writers differed significantly in the two transcription skills, handwriting and spelling, and text generation (composing), for which transcription skills are necessary but not sufficient. The good (M= .45 z; SD= .74) and poor (M= -.44 z; SD= .93) writers differed significantly on handwriting automaticity—number of legible letters printed in manuscript writing in correct alphabetic order in the first 15 seconds of an alphabet writing task from memory (Berninger et al., 1992, 1994, 2001), F(1,18)=5.66, p=.029. The good (M= 114.00; SD= 9.59) and poor (M= 93; SD= 6.46) writers differed significantly on Wechsler Individual Achievement Test, 2nd Edition (WIAT II) Spelling subtest (Psychological Corporation, 2002), F(1,18)=29.22, p= .001. The good (M= 113.25; SD= 8.78) and poor (M= 96.25; SD= 10.74) writers differed significantly on WIAT II Written Expression subtest, F(1, 18)=15.08, p=.001. Good (M= .42; SD= .52) and poor (M= -.20; SD= .99) writers differed marginally on a two-tail, non-directional test in accuracy of cursive letter writing, F(1,18)=3.30, p= .09, though significantly on a one-tail directional test, p <.05.
Differences between the good and poor writers were also evaluated on other measures. They did not differ significantly on other handwriting measures involving (a) accuracy or total time for printing manuscript letters during alphabetic writing or sustained copying of a paragraph for 90 seconds, (b) cursive—automaticity or total time for the same alphabet writing task, or (c) keyboarding—automaticity, total accuracy, or total time for the same alphabet writing task except that the letter is selected by key press rather than formed by sequential strokes. Differences between good and poor writers were specific to automatic access, retrieval, and production of printed alphabet letters in order as found in prior research (Berninger et al., 1992, 1994). Although the good and poor writers did not differ significantly on the finger sequencing measure with the dominant hand, good writers (M= .74; SD=.83 ) and poor writers (M= -.25; SD= 1.12) did differ significantly on the finger sequencing measure with the nondominant hand, F(1,18)=5.22, p=.03, as was also the case with the affected adults in a family genetics study of dyslexia (Berninger & O'Donnell, 2004).
fMRI Tasks and Contrasts
The on and off tasks for Finger Tapping with Sequencing and Finger Tapping without Sequencing were taught and practiced outside the scanner before children performed them in the scanner. The tasks were practiced outside the scanner until children reached a criterion level of 100% accuracy (both tasks) and a steady pace of one finger tap every one second that was maintained for 30 seconds on both tasks. Children were timed and given feedback as to their accuracy and steady rate. To minimize motor artifact during practice, a book was placed on the child's chest at midline and the child used fingers of dominant hand to tap for both tasks. Note that in the scanner once the prompt to begin the on or off task began, children received no additional visual cues for performing either finger task. Thus, the contrast between them identified ability to remember and perform a serial finger maneuver shortly after it had been taught and learned to criterion.
To minimize motor artifact during scanning, a book was placed on child's chest. For Finger Sequencing the child tapped the thumb and each of the four fingers in order, beginning with the thumb and proceeding in sequence to the index finger, to the middle finger, to the ring finger, to the pinky, and then, repeated the sequence at the steady rate practiced as many times as possible within the 30 seconds-time limit. For Finger Non-Sequencing, the child tapped the index finger over and over on the book at the steady rate as practiced. This procedure, which stabilizes wrist movement, was piloted before the study proper to ensure that it minimized motor artifact on this task with children.
EPRIME timing: Fixation for 26 seconds, 5 cycles of on-30 seconds and off-30 seconds, and fixation for 26 seconds. On task: The child tapped the thumb and then one finger at a time in succession from index finger to pinky when this prompt appeared on the visual screen: “Start tapping the sequence of thumb and four fingers at a steady rate in the order we practiced.” Off task: The child tapped the same index finger repeatedly instead of tapping fingers in succession when this prompt appeared on the visual screen: “Start tapping your index finger at the steady rate as we practiced”.The Contrast comparing on and off tasks identified BOLD activation unique to motor planning for sequential finger movements that was unique to the on-task. Both tasks required motor execution of finger movements.
Imaging Protocol
Structural MR scans and fMRI scans for analysis of group maps were acquired on a Philips Achieva 3-T scanner (version 2.1, Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80 mT/m with a slew rate of 110 mT/m/s or 40 mT/m at a slew rate of 220 mT/m/s) using an 8 channel SENSE Head coil. An MPRAGE localizer was acquired in the sagittal plane for structural analysis and for fMRI co-registration of anatomy with parameters: TR/TE 7.0/3.2 msec, SENSE Factor = 1.5 in RL direction, 160 slices, 3D acquisition resolution matrix 224×221×160 with reconstructed resolution of 0.94×0.94×1.0 mm, flip angle = 8 degrees, field of view 240×240×160 mm, scan duration 449 seconds.
Functional MRIs were acquired using the following parameters: gradient echo (single shot) echo-planar pulse sequence (called epi field echo by Philips),TR/TE 3000/30 msecs, FOV 240 mm, slice thickness/gap 4.0/1.0, 32 slices covering the entire brain, 2D matrix 64×64, epifactor 63, SENSE factor = 1, number of dynamics 114, scan duration 349 seconds. A B0 map (Fast Field Echo, TR/TE 935/20 msec, echo difference time of 4 msec for B0map calculation, scan duration 123 seconds, 32 slices, 64×64 reconstructed matrix, FOV 240mm) was also acquired at exactly the same slice positions as the fMRI image with a B0 correction using FSL software.
fMRI Data Analysis
Pre-processing
FSL (FEAT Expert Analysis Tool Version 5.4 in FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) was used for the following pre-statistics processing: motion correction using MCFLIRT (Jenkinson, Bannister et al., 2002); non-brain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 5mm; mean-based intensity normalization of all volumes by the same factor; and highpass temporal filtering (Gaussian-weighted LSF straight line fitting, with sigma=50.0s). FEAT also has a feature for B0 correction, which was used for B0 phase, and magnitude maps, which Philips automatically produces as part of the B0 map image reconstruction (TE difference 4 milliseconds, dwell time = 0.655 microseconds, +y polarity, input parameters).
First level
Time-series statistical analysis was carried out using FMRIB's Improved Linear Model (FILM) (Woolrich, et al., 2001) in a block-design with local autocorrelation correction. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p=0.01 (Worsley et al., 1992). Registration to high resolution and/or standard images was carried out using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). ICA-based exploratory data analysis using Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) (Beckmann & Smith, 2004) was used to investigate the possible presence of unexpected artifacts or activation. The individual ICA/MELODIC output components were analyzed by custom software to find out which components had large amounts of activation rimness (greater than 0.65). Rimness is defined as the activation that occurs at boundaries of the brain surface or the ventricular walls. These ICA components were considered to be “artifact” that could arise in part from subject motion. The MELODIC filter option was used to filter out the “artifact” components that were identified in the previous step. The output 4D fMRI data were then re-run through FEAT individual-level analyses to find valid activation. Effects at each voxel were estimated; regionally specific effects were compared using linear contrasts.
Group level
The contrasts for the individual subjects were aggregated for the group in a random effects analysis. Higher-level analysis was carried out using FSL's FMRIB's Local Analysis of Mixed Effects (FLAME) stage 1 only (i.e., without the final MCMC-based stage) (Beckmann et al., 2003; Woolrich et al., 2001). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p=0.05 (Worsley et al., 1992). Effects at each voxel were estimated; and regionally-specific effects were compared using linear contrasts.
Individual brain analyses
Initial group analyses were followed by Region of Interest (ROI) FSL analyses in individual brains for regions where the difference between the good writers and poor writers was at least 200 voxels or larger. Such robust clusters of activation can be used in theory-driven interpretation of writing or writing-related processes.
Results
Contrast of Finger Tapping with and without Finger Sequencing
Group map differences between good and poor writers
The Contrast identified unique brain activation for all the processes involved in sequencing finger movements apart from motor production for each finger movement, the number of which was held constant across tasks. For the average BOLD activation of the good writers, see column in Table 1 labeled “z-score good writers.” For average BOLD activation of the poor writers, see column in Table 1 labeled “z-score poor writers.” Z-score differences in BOLD activation for group difference maps between the good and poor writers for the Contrast between Finger Tapping with and without Sequencing are also provided in Table 1 for 85 regions throughout the brain. These differences between good and poor writers in the group maps, even after corrections for multiple comparisons, were robust and are depicted in Figure 1.
Table 1.
Significant Group Difference Brain Activation in Good (n=12) and Poor (n=8) Writers for fMRI Finger Succession Task
| Brain Region | Difference zscore | Diff Voxel | zscore good writers | SD | zscore poor writers | SD |
|---|---|---|---|---|---|---|
| 3Frontal_Sup_L | 2.713 | 121 | 1.103 | 0.311 | -2.656 | 0.338 |
| 4Frontal_Sup_R | 2.761 | 433 | 1.965 | 0.589 | -2.078 | 0.743 |
| 5Frontal_Sup_Orb_L | 2.796 | 163 | 1.205 | 0.763 | -2.749 | 0.591 |
| 6Frontal_Sup_Orb_R | 2.694 | 186 | 2.062 | 0.671 | -1.895 | 0.608 |
| 7Frontal_Mid_L | 2.799 | 734 | 1.64 | 0.445 | -2.437 | 0.572 |
| 8Frontal_Mid_R | 2.795 | 803 | 1.878 | 0.577 | -2.238 | 0.572 |
| 9Frontal_Mid_Orb_L | 2.646 | 131 | 1.552 | 0.473 | -2.289 | 0.465 |
| 10Frontal_Mid_Orb_R | 2.641 | 202 | 2.064 | 0.415 | -1.843 | 0.463 |
| 13Frontal_Inf_Tri_L | 2.598 | 140 | 1.592 | 0.716 | -2.161 | 0.609 |
| 14Frontal_Inf_Tri_R | 2.688 | 52 | 0.943 | 0.628 | -2.775 | 0.525 |
| 15Frontal_Inf_Orb_L | 2.499 | 150 | 1.088 | 0.464 | -2.401 | 0.408 |
| 16Frontal_Inf_Orb_R | 2.858 | 203 | 1.525 | 0.562 | -2.624 | 0.502 |
| 17Rolandic_Oper_L | 2.628 | 54 | 2.232 | 0.29 | -1.712 | 0.41 |
| 23Frontal_Sup_ Medial_L | 2.705 | 160 | 1.238 | 0.494 | -2.594 | 0.506 |
| 24Frontal_Sup_Medical_R | 2.563 | 78 | 1.375 | 0.545 | -2.309 | 0.479 |
| 26Frontal_Mid_Orb_R | 2.699 | 65 | 1.806 | 0.65 | -2.141 | 0.666 |
| 28Rectus_R | 2.653 | 150 | 2.204 | 0.726 | -1.777 | 0.535 |
| 29Insula_L | 2.764 | 198 | 1.543 | 0.505 | -2.449 | 0.512 |
| 30Insula_R | 2.491 | 102 | 1.392 | 0.294 | -2.189 | 0.29 |
| 31Cingulum_Ant_L | 2.692 | 486 | 0.88 | 0.671 | -2.812 | 0.462 |
| 32Cingulum_Ant_R | 2.784 | 384 | 1.312 | 0.631 | -2.603 | 0.533 |
| 33Cingulum_Mid_L | 2.686 | 536 | 2.311 | 0.945 | -1.622 | 0.899 |
| 34Cingulum_Mid_R | 2.666 | 520 | 1.482 | 0.786 | -2.295 | 0.755 |
| 37Hippocampus_L | 2.674 | 227 | 1.416 | 0.729 | -2.395 | 0.635 |
| 38Hippocampus_R | 2.49 | 127 | 1.682 | 0.598 | -1.953 | 0.48 |
| 39ParaHippocampal_L | 2.846 | 268 | 1.676 | 0.669 | -2.459 | 0.588 |
| 40ParaHippocampal_R | 2.642 | 125 | 1.288 | 0.504 | -2.457 | 0.320 |
| 43Calcarine_L | 2.542 | 185 | 3.613 | 0.804 | -0.007 | 0.895 |
| 44Calcarine_R | 2.524 | 235 | 2.451 | 0.77 | -1.331 | 0.702 |
| 45Cuneus_L | 2.449 | 113 | 1.915 | 0.643 | -1.659 | 0.529 |
| 46Cuneus_R | 2.46 | 106 | 1.17 | 0.472 | -2.292 | 0.375 |
| 47Lingual_L | 2.567 | 384 | 2.659 | 0.952 | -1.175 | 1.036 |
| 48Lingual_R | 2.687 | 630 | 2.621 | 0.857 | -1.412 | 0.966 |
| 50Occipital_Sup_R | 2.664 | 171 | 1.041 | 0.597 | -2.671 | 0.56 |
| 51Occipital_Mid_L | 2.751 | 994 | 1.122 | 0.516 | -2.719 | 0.519 |
| 53Occipital_Inf_L | 2.681 | 348 | 0.994 | 0.755 | -2.69 | 0.631 |
| 54Occipital_Inf_R | 2.815 | 312 | 1.377 | 0.768 | -2.561 | 0.614 |
| 55Fusiform_L | 2.736 | 621 | 1.444 | 0.757 | -2.45 | 0.604 |
| 56Fusiform_R | 2.664 | 390 | 1.458 | 1.007 | -2.338 | 0.735 |
| 57Postcentral_L | 2.602 | 299 | 3.874 | 0.76 | 0.561 | 1.119 |
| 58Postcentral_R | 2.511 | 73 | 2.106 | 0.376 | -1.611 | 0.414 |
| 59Parietal_Sup_L | 2.707 | 395 | 2.304 | 1.117 | -1.591 | 1.185 |
| 60Parietal_Sup_R | 2.726 | 843 | 2.016 | 0.673 | -1.977 | 0.608 |
| 61Parietal_Inf_L | 2.601 | 218 | 2.174 | 0.773 | -1.617 | 0.832 |
| 62Parietal_Inf_R | 2.665 | 279 | 2.445 | 0.682 | -1.495 | 0.761 |
| 63SupraMarginal_L | 2.723 | 352 | 1.82 | 0.721 | -2.161 | 0.757 |
| 64SupraMarginal_R | 2.64 | 497 | 1.907 | 0.635 | -1.97 | 0.582 |
| 66Angular_R | 2.599 | 80 | 1.561 | 0.461 | -2.195 | 0.524 |
| 67Precuneus_L | 2.672 | 921 | 2.025 | 0.594 | -1.903 | 0.535 |
| 68Precuneus_R | 2.702 | 1122 | 2.078 | 0.789 | -1.862 | 0.603 |
| 77Thalamus_L | 2.665 | 109 | 2.396 | 0.89 | -1.564 | 0.706 |
| 78Thalamus_R | 2.512 | 172 | 1.448 | 0.637 | -2.129 | 0.6 |
| 81Temporal_Sup_L | 2.658 | 843 | 2.155 | 0.597 | -1.822 | 0.58 |
| 82Temporal_Sup_R | 2.643 | 426 | 2.261 | 0.616 | -1.67 | 0.665 |
| 83Temporal_Pole_SuL | 2.523 | 126 | 1.529 | 0.595 | -2.105 | 0.49 |
| 85Temporal_Mid_L | 2.701 | 349 | 2.231 | 0.545 | -1.741 | 0.844 |
| 86Temporal_Mid_R | 2.586 | 285 | 2.667 | 0.519 | -1.169 | 0.558 |
| 89Temporal_Inf_L | 2.735 | 230 | 1.317 | 0.645 | -2.559 | 0.463 |
| 90Temporal_Inf_R | 2.627 | 275 | 1.276 | 0.869 | -2.371 | 0.652 |
| 91Cerebelum_Crus1_L | 2.76 | 832 | 2.518 | 0.557 | -1.543 | 0.84 |
| 92Cerebelum_Crus1_R | 2.727 | 522 | 2.178 | 1.106 | -1.761 | 1.015 |
| 93Cerebelum_Crus2_L | 2.573 | 87 | 2.615 | 0.386 | -1.122 | 0.412 |
| 97Cerebelum_4_5_L | 2.792 | 270 | 1.121 | 0.546 | -2.751 | 0.583 |
| 98Cerebelum_4_5_R | 2.663 | 222 | 1.434 | 1.009 | -2.308 | 0.846 |
| 99Cerebelum_6_L | 2.657 | 210 | 2.3 | 0.978 | -1.62 | 1.052 |
| 100Cerebelum_6_R | 2.561 | 134 | 2.444 | 0.98 | -1.351 | 1.014 |
| 103Cerebelum_8_L | 2.637 | 280 | 2.37 | 0.631 | -1.532 | 0.584 |
| 104Cerebelum_8_R | 2.608 | 211 | 2.403 | 0.896 | -1.368 | 0.935 |
| 105Cerebelum_9_L | 2.667 | 226 | 1.603 | 0.497 | -2.245 | 0.46 |
| 106Cerebelum_9_R | 2.584 | 99 | 2.049 | 0.761 | -1.705 | 0.64 |
| 110Vermis_3 | 2.862 | 97 | 2.043 | 0.431 | -2.223 | 0.467 |
| 111Vermis_4_5 | 2.568 | 66 | 2.469 | 0.286 | -1.326 | 0.46 |
| MNI coordinates and Brodmann area definition | |||||
|---|---|---|---|---|---|
| Brain region | ave. Z | MNI X | MNI Y | MNI Z | Brodmann |
| Frontal_Sup_L | 2.713 | -24 | 54 | 10 | 10 |
| Frontal_Sup_R | 2.761 | 24 | 60 | 16 | 10 |
| Frontal_Sup_Orb_L | 2.796 | -18 | 58 | -10 | 11 |
| Frontal_Sup_Orb_R | 2.694 | 16 | 64 | -6 | 11 |
| Frontal_Mid_L | 2.799 | -30 | 38 | 26 | 46 |
| Frontal_Mid_R | 2.795 | 32 | 48 | 26 | 46 |
| Frontal_Mid_Orb_L | 2.646 | -20 | 58 | -10 | 11 |
| Frontal_Mid_Orb_R | 2.641 | 20 | 58 | -10 | 11 |
| Frontal_Inf_Oper_L | 2.491 | -50 | 18 | 32 | 44 |
| Frontal_Inf_Tri_L | 2.598 | -48 | 20 | 30 | 44 |
| Frontal_Inf_Tri_R | 2.688 | 40 | 36 | 2 | 47 |
| Frontal_Inf_Orb_L | 2.499 | -34 | 38 | -6 | 47 |
| Frontal_Inf_Orb_R | 2.858 | 42 | 44 | -10 | 47 |
| Rolandic_Oper_L | 2.628 | -46 | -28 | 20 | 48 |
| Rolandic_Oper_R | 2.553 | 62 | -20 | 16 | 42 |
| Supp_Motor_Area_L | 2.49 | -12 | -4 | 46 | 0 |
| Supp_Motor_Area_R | 2.514 | 12 | -8 | 46 | 0 |
| Frontal_Sup_Medial_L | 2.705 | 2 | 34 | 32 | 32 |
| Frontal_Sup_Medial_R | 2.563 | 12 | 28 | 54 | 8 |
| Frontal_Mid_Orb_L | 2.518 | -12 | 58 | -4 | 10 |
| Frontal_Mid_Orb_R | 2.699 | 14 | 64 | -6 | 11 |
| Rectus_R | 2.653 | 6 | 48 | -20 | 11 |
| Insula_L | 2.764 | -38 | 8 | -12 | 48 |
| Insula_R | 2.491 | 40 | -4 | 0 | 48 |
| Cingulum_Ant_L | 2.692 | -10 | 16 | 30 | 0 |
| Cingulum_Ant_R | 2.784 | 6 | 10 | 28 | 0 |
| Cingulum_Mid_L | 2.686 | -10 | -2 | 36 | 0 |
| Cingulum_Mid_R | 2.666 | 6 | 10 | 30 | 24 |
| Cingulum_Post_L | 2.474 | -4 | -42 | 12 | 29 |
| Cingulum_Post_R | 2.706 | 2 | -42 | 14 | 29 |
| Hippocampus_L | 2.674 | -16 | -32 | -6 | 27 |
| Hippocampus_R | 2.49 | 16 | -6 | -14 | 34 |
| ParaHippocampal_L | 2.846 | -16 | -34 | -8 | 27 |
| ParaHippocampal_R | 2.642 | 16 | -32 | -12 | 30 |
| Calcarine_L | 2.542 | 2 | -92 | -12 | 17 |
| Calcarine_R | 2.524 | 20 | -86 | -2 | 18 |
| Cuneus_L | 2.449 | 2 | -88 | 34 | 0 |
| Cuneus_R | 2.46 | 6 | -82 | 42 | 0 |
| Lingual_L | 2.567 | -14 | -34 | -8 | 27 |
| Lingual_R | 2.687 | 12 | -30 | -10 | 30 |
| Occipital_Sup_L | 2.512 | -26 | -78 | 38 | 19 |
| Occipital_Sup_R | 2.664 | 28 | -80 | 46 | 7 |
| Occipital_Mid_L | 2.751 | -40 | -80 | 14 | 19 |
| Occipital_Inf_L | 2.681 | -32 | -82 | -4 | 19 |
| Occipital_Inf_R | 2.815 | 36 | -78 | -14 | 19 |
| Fusiform_L | 2.736 | -38 | -62 | -22 | 37 |
| Fusiform_R | 2.664 | 36 | -76 | -14 | 19 |
| Postcentral_L | 2.602 | -34 | -42 | 70 | 0 |
| Postcentral_R | 2.511 | 16 | -44 | 80 | 1 |
| Parietal_Sup_L | 2.707 | -34 | -46 | 68 | 0 |
| Parietal_Sup_R | 2.726 | 28 | -80 | 48 | 7 |
| Parietal_Inf_L | 2.601 | -56 | -36 | 46 | 40 |
| Parietal_Inf_R | 2.665 | 46 | -44 | 40 | 40 |
| SupraMarginal_L | 2.723 | -54 | -34 | 26 | 48 |
| SupraMarginal_R | 2.64 | 50 | -38 | 32 | 48 |
| Angular_L | 2.401 | -44 | -54 | 28 | 39 |
| Angular_R | 2.599 | 46 | -48 | 36 | 48 |
| Precuneus_L | 2.672 | -16 | -60 | 72 | 0 |
| Precuneus_R | 2.702 | 4 | -70 | 52 | 7 |
| Paracentral_Lobule_R | 2.456 | 0 | -40 | 70 | 4 |
| Pallidum_R | 2.427 | 26 | -12 | -2 | 0 |
| Thalamus_L | 2.665 | -16 | -28 | -2 | 0 |
| Thalamus_R | 2.512 | 6 | -28 | 2 | 0 |
| Heschl_L | 2.513 | -44 | -22 | 8 | 48 |
| Heschl_R | 2.475 | 62 | 0 | 6 | 48 |
| Temporal_Sup_L | 2.658 | -42 | -12 | -8 | 48 |
| Temporal_Sup_R | 2.643 | 50 | -8 | -8 | 22 |
| Temporal_Pole_Sup_L | 2.523 | -28 | 16 | -28 | 38 |
| Temporal_Mid_L | 2.701 | -48 | -58 | -6 | 37 |
| Temporal_Mid_R | 2.586 | 54 | -24 | -6 | 21 |
| Temporal_Pole_Mid_L | 2.564 | -34 | 12 | -36 | 20 |
| Temporal_Inf_L | 2.735 | -54 | -54 | -8 | 37 |
| Temporal_Inf_R | 2.627 | 40 | -66 | -10 | 19 |
| Cerebelum_Crus1_L | 2.76 | -30 | -82 | -24 | 0 |
| Cerebelum_Crus1_R | 2.727 | 42 | -50 | -32 | 0 |
| Cerebelum_Crus2_L | 2.573 | -30 | -66 | -40 | 0 |
| Cerebelum_Crus2_R | 2.627 | 40 | -46 | -40 | 0 |
| Cerebelum_3_R | 2.933 | 8 | -40 | -10 | 0 |
| Cerebelum_4_5_L | 2.792 | -20 | -44 | -20 | 0 |
| Cerebelum_4_5_R | 2.663 | 6 | -42 | -6 | 0 |
| Cerebelum_6_L | 2.657 | -38 | -64 | -22 | 0 |
| Cerebelum_6_R | 2.561 | 26 | -70 | -20 | 0 |
| Cerebelum_7b_L | 2.771 | -30 | -62 | -44 | 0 |
| Cerebelum_7b_R | 2.779 | 38 | -48 | -42 | 0 |
| Cerebelum_8_L | 2.637 | -28 | -60 | -44 | 0 |
| Cerebelum_8_R | 2.608 | 36 | -48 | -44 | 0 |
| Cerebelum_9_L | 2.667 | -12 | -46 | -54 | 0 |
| Cerebelum_9_R | 2.584 | 16 | -56 | -46 | 0 |
| Vermis_3 | 2.862 | 6 | -38 | -10 | 0 |
| Vermis_4_5 | 2.568 | 6 | -44 | -6 | 0 |
Note: Column 2 is the average group difference zscore comparing good writers to poor writers within the significant cluster within the specified brain region. Column 3 is the number of voxels within the significant cluster within the specified brain region. Column 4 is the average zscore for the good writers within the exact same location as defined in column 2. Column 5 is the average zscore for the poor writers within the exact same location as defined in column 2.
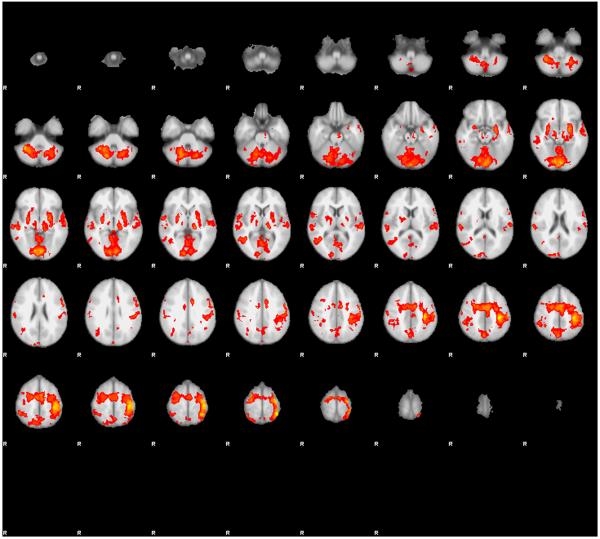
Figure 1.
Group difference map of fMRI activation comparing good (n=12) and poor writers (n=8) during contrast of sequential finger tapping (on task) versus repetitive finger tapping (off task).
Differences were found in the following homologous structures bilaterally: superior frontal, superior orbital, middle frontal, inferior frontal triangularis and orbital, frontal superior medial, anterior and middle cingulate, insula, hippocampus, parahippocampus, calcarine, cuneus, lingual, inferior occipital, fusiform, postcentral, superior parietal, inferior parietal, precuneus, supramarginal, thalamus, superior temporal, middle temporal, inferior temporal regions, and five cerebellar regions (crus 1, 4_5, 6, 8, and 9). In addition, these regions were identified on the left only— rolandic operculum, temporal pole, middle occipital, and cerebellar (crus 2 and vermis 3 and 4_5)—or on the right only— middle orbital, rectus, and angular gyrus. To summarize, the brain differences between good and poor writers in planning serial finger movements show that many more processes than mere motor output are involved in planning serial finger movements.
As can be seen in Table 1, good writers showed consistent positive brain activation and the poor writers showed consistent negative brain activation in regions that replicated across group map and individual brain analyses. Negative brain activation in this case means that there was more activation for the finger tapping task versus the finger succession task. The poor writers showed levels of brain activation substantially below the normal levels shown by good writers in the initial group analyses of the brain scans for the contrast (see Methods). This subnormal level of activation compared to normal levels suggests that the poor writers are not engaging the brain regions that good writers do in planning sequential finger movements.
Differences between females and males who were good writers were statistically evaluated for each significant difference in the group maps. Only ONE region of the 85 regions showed a significant difference in fMRI activation between females and males who were good writers and that was in the left superior parietal region. See Discussion for the significance of this finding.
Individual brain differences between good and poor writers
To ensure reliability of findings so widespread across the brain, the group map analyses were followed by individual brain analyses performed only in regions where significant fMRI activation occurred in the group analyses. A region of interest (ROI) approach was taken in which clusters with spatial extent of 200 voxels or more were considered to show substantial significant activation. The individual brain analyses reduced the regions of significant fMRI activation from 85 to 42. The results, z-scores for individual brains in each of those 42 regions, are too lengthy to report in a journal article, but are available from the first author upon request. To summarize, the regions that met both the group and individual brain criteria for significant activation included nine frontal or cingulate regions (right superior frontal, left and right middle frontal, right middle frontal orbital, right inferior frontal orbital, left and right anterior cingulate, and left and right middle cingulate), twelve temporal regions (left hippocampus, left parahippocampus, left and right fusiform, left and right lingual, left and right inferior temporal, left and right middle temporal, left and right superior temporal), four occipital regions (right calcarine, left middle occipital, left and right inferior occipital), nine parietal regions (left postcentral, left and right superior parietal, left and right inferior parietal, left and right supramarginal, left and right precuneus), and eight cerebellar regions (left and right cerebellum crus 1, cerebellum 4_5, cerebellum 8, and left cerebellum 6 and 9). Correlations between behavioral measures of finger sequencing and transcription skills (handwriting and spelling) and each of the 42 regions of significant activation on the fMRI contrast for finger sequencing were then computed. Significant correlations were deemed worthy of theoretical interpretation related to writing.
Validation of Contrast for Transcription
Handwriting
Instead of correcting for multiple comparisons statistically, pattern analyses were conducted across the correlations between behavioral measures and activation in regions associated with the fMRI contrast that were significant at p ≤ .05 or .01. The behavioral measures for finger tasks were given on average one year and nine months prior to the brain imaging when children were in fourth grade and the behavioral measures for handwriting and spelling were given on average nine months prior to the fMRI brain imaging. This longitudinal approach to prediction is justified because the patterns of shared and unique correlations between writing behaviors and activation on the fMRI contrast were grounded in current cognitive and neuropsychological theory related to writing acquisition. Longitudinal correlations across longer time periods than in the past studies that reported concurrent correlations increase confidence in stability of the behavior-brain relationships. Also, resulting patterns can be used to generate hypotheses to be tested in future research.
The behavioral measures of finger succession (sequential finger movement) and finger repetition (non-sequential finger movement) (Berninger, 2001) were first taught to the child and then the child was timed while performing these tasks without being able to look at his or her hands. These measures, given on average one year and nine months earlier than the imaging, longitudinally predicted unique fMRI activation on the contrast in 3 regions for the dominant hand and in 16 regions for the non-dominant hand, as shown in Table 2. The significance of why more brain regions were associated with the non-dominant than dominant hand and of which brain regions were associated with each hand is considered in the Discussion.
Table 2.
Patterns of Longitudinal Correlations between Regions of Unique BOLD Activation in Individual Brains on Finger Sequencing Contrast Differentiating Good and Poor Writers at End of 5th Grade and Behavioral Measure of Finger Sequencing in 4th Grade
| Brain Regions | Finger Sequencing Tasksk Dominant Hand | Finger Sequencing Tasksk Non-Dominant Hand |
|---|---|---|
| L Superior Parietal | r=.51* | r=.64** |
| L Precuneus | r= -.48* | r= -.44* |
| R Cerebellum 4 5 5 | r= -.47* | |
| L Middle Cingulate | r= -.45* | |
| L Lingual | r= -.44* | |
| L Inferior Occipital | r= -.50* | |
| L Fusiform | r= -.48* | |
| R Fusiform | r= -.47* | |
| L Postcentral | r=-.61** | |
| R Superior Parietal | r= -.49* | |
| L Inferior Parietal | r= -.60** | |
| L Supramarginal | r= -.64** | |
| L Superior Temporal | r= -.49* | |
| L MiddleTemporal | r= -.45* | |
| L Inferior Temporal | r= .50* | |
| R Cerebellum Crus 1 | r= -.45* | |
| L Cerebellum 4 5 | r= -.52* |
p ≤.05
p ≤.01
R=right; L=left
Unique fMRI activation was also predicted longitudinally, as shown in Table 3, from each of the behavioral measures of writing1:
for alphabet task, automatic manuscript letter writing in 15 regions; accuracy of cursive writing in fourteen regions, ten of which overlapped with automatic printing; and automatic keyboarding in six regions, which overlapped with one region for printing; and
for spelling, in 34 regions, eleven of which overlapped with automatic alphabet letter printing.
Table 3.
Patterns of Correlations between Regions of Unique fMRI BOLD Activation in Individual Brains Differentiating Good and Poor Writers on Finger Sequencing Contrast at End of 5th Grade and Behavioral Measures for Specific Writing Tasks in Early 5th Grade
| Brain Region on | Alph 1515 Print | AlphAcccy Cursive | Alph15 Keyboard | WIAT2 Spelling |
|---|---|---|---|---|
| R Superior Frontal | r=.53* | r=.61** | ||
| R Middle Frontal | r=.50* | r=.56* | r=.44* | |
| R Inferior Frontal Orbital | r=.44* | r=.44* | r=.46* | |
| L Middle Cingulate | r=.53* | r=.55* | r=.45* | |
| L Superior Parietal | r=.59* | r=.55* | ||
| L Inferior Parietal | r=.48* | |||
| L Supramarginal | r=.58** | r=.47* | r=.46* | |
| R Supramarginal | r=.50* | r=.44* | ||
| L Precuneus | r=.49* | r=.55* | r=.54* | |
| R Precuneus | r=.50* | r=.44* | r=.48* | r=.50* |
| L Middle Temporal | r=.46* | r=.53* | ||
| R Middle Temporal | r=.48* | r=.52* | ||
| L Inferior Temporal | r=.51* | r=.56* | ||
| R Inferior Temporal | r=.44* | r=.46* | r=.61** | |
| L Cerebellum 8 | r=.44* | r=.55** | ||
| L Anterior Cingulate | r=.45* | r=.45* | ||
| L Postcentral | r=.47* | r=.53* | ||
| L Superior Temporal | r=.49* | r=.51* | ||
| R Superior Temporal | r=.45* | |||
| R Calcarine | r=.50* | r=.48* | ||
| R Inferior Occipital | r=.47* | r=.56* | ||
| R Fusiform | r=.45* | r=.51* | ||
| R Cerebellum Crus 1 | r=.48* | |||
| L Cerebellum 9 | r=.50* | |||
| L Middle Frontal | r=.64** | |||
| R Middle Frontal Orbital | r=.50* | |||
| R Anterior Cingulate | r=.47* | |||
| R Middle Cingulate | r=.45* | |||
| L Lingual | r=.47* | |||
| R Lingual | r=.55** | |||
| L Middle Occipital | r=.62** | |||
| L Inferior Occipital | r=.59* | |||
| L Fusiform | r=.50* | |||
| R Superior Parietal | r=.49* | |||
| L Cerebellum Crus 1 | r=.60** | |||
| L Cerebellum Crus 2 | r=.57** | |||
| R Cerebellum 4 5 | r=.54** | |||
| L Cerebellum 6 | r=.49* | |||
| R Cerebellum 8 | r=.67*** | |||
| L Cerebellum 9 | r=. .54 ** |
Notes.
1. Alph15 is automatic writing—number of legible letters in correct order in first 15 seconds. Alphacc is accurate writing—number of total legible letters in correct order regardless of time taken.
2. No brain region activated during the fMRI Finger Sequence Contrast correlated significantly with total time for writing alphabet in printing, or by keyboard or with sustained copying (printing) over 90 seconds. Only one region correlated significantly with automatic alphabet letter writing in cursive, left superior parietal, r=.44*, which also correlated with automatic alphabet letter printing. Only one region correlated significantly with automatic alphabet letter writing by keyboard—right cerebellum 8, r=.48*.
p≤.05
p<.01
p<.001
R=right; L=left; also see Notes at end of table.
No regions of unique activation on this contrast correlated significantly with total accuracy or total time in printing letters or keyboarding, automatic cursive letter writing, or total time for cursive on the alphabet task. Four findings showed that longitudinal correlations between behavioral measures of transcription skills and fMRI contrast varied as a function of the specific writing skill assessed. Each finding is supported by results displayed in Table 3.
First, more correlations were found between brain regions on the fMRI contrast and on the automatic letter printing on the alphabet task than on any other handwriting task. Good and poor writers had also differed significantly in mean performance on this handwriting task, but had not on the other handwriting measures (see Participants in Methods).
Second, correlations for accuracy of cursive writing on the alphabet writing task, on which the good and poor writers had only differed marginally (see Participants in Methods), were significant for ten of the same regions as for printing manuscript letters on the alphabet writing task, suggesting considerable overlap in brain-behavior relationships underlying the two modes of letter writing. However, four regions correlated with accurate cursive writing were not correlated with printing manuscript letters, indicating cursive writing mode may also draw on distinct neural networks supporting the serial organization of fingers.
Third, automaticity of writing letters by keyboard on the alphabet writing task was correlated with different regions of brain activation than was the case for the same task when letters were formed by pen or pencil, for either manuscript or cursive letters. Overall the pattern analysis showed that mode of letter production can influence observed brain-behavior relationships in writing the alphabet from memory, although some of these are common across modes of letter production.
Fourth, spelling real words from dictation was correlated longitudinally with activation in 32 brain regions on the fMRI contrast. Of these, eleven also correlated longitudinally with automatic letter printing on the alphabet task, showing that spelling words in writing may be associated with some of the same brain regions as handwriting; but 21 of the regions associated with spelling were not associated with handwriting, showing that spelling may involve more than producing letters in sequence. Elsewhere we compare the brain regions that were correlated only with spelling on the fMRI finger sequence contrast (this study) and that activated on the fMRI contrast for real-word spelling recognition (no handwriting requirements) in another study with children of the same age (Richards, Berninger, & Fayol, in press). The goal of these comparisons is to identify the brain regions not related to handwriting that are involved in spelling (associated with cognitive, metacognitive, and linguistic functions) (Richards & Berninger, 2009).
Discussion
Relationship of Serial Organization of Finger Movement to Transcription
Many of the activated brain regions that differentiated good and poor writers on the contrast for serial organization of fingers also activated when adults performed similar tasks (Katanoda et al., 2001; Van Mier et al., 1998). The clinical version of finger sequencing, the Finger Succession Task, has long been known to differentiate children with and without learning disabilities at the behavioral level (e.g., Denckla, 1973; Taylor, 2004; Tupper, 1998; Wolff et al., 1983) and recent evidence suggests that it also differentiates children with and without attention deficit (Mostofsky et al., 2006). The poor and good writers differed in left and right parietal regions in the current study and the children with and without attention deficit differed only in right parietal regions in the Mostofsky study. Future research might compare correlations between clinical measures of finger sequencing and brain activation on the fMRI finger sequencing contrast in bilateral superior parietal regions for groups of children carefully selected for writing disability only, ADHD only, comorbid writing disability and ADHD, and no evidence of either writing disability or ADHD.
In contrast to other brain imaging studies using non-sequential finger tapping tasks that cued the participant visually or auditorially during the execution of the task and did not train participants to criterion before a brain scan, the two fMRI tasks used in the current study required the participant to first learn to criterion the finger tasks and then to self-regulate in performing these previously taught sequential or non-sequential finger movements. Unlike other fMRI finger tapping tasks that require the participant to learn sequences that vary in timing or rhythm, the current finger succession task required only a constant sequence that repeated at the same steady rate. Also, in contrast to other fMRI finger tasks that do not control for motor execution to identify the planning for the serial organization of the motor output, the current contrast did.
Lashley (1951) proposed that such planning of serial organization underlies human cognitive and executive functions. His view is supported by these findings: First, good and poor writers differed significantly in fMRI activation in many frontal brain regions on the finger sequencing contrast used in the current study (see Table 1 for group map findings, results section for individual brains, and Table 3 for longitudinal correlations with behavioral measure of finger sequencing). Second, behavioral measures of handwriting and spelling also showed longitudinal correlations with four frontal or cingulate regions that differentiated good and poor writers on the fMRI finger sequencing contrast—right superior frontal, right middle frontal, right inferior frontal orbital, and left middle cingulate (see Table 3). In addition, left anterior cingulate and left middle frontal gyrus correlated with spelling (see Table 3). Exner (1881) proposed that left middle frontal gyrus was a writing center in the brain. More recently,Anderson, Damasio, and Damasio (1990) reported supporting evidence for the role of Exner's area in coactivation of movement sequences needed to generate letters.
Relationships with Dominant and Non-Dominant Hands
Of interest, the behavioral measure for the non-dominant hand performing the behavioral measure of Finger Succession had longitudinal correlations with the fMRI contrast in more brain regions than did the dominant hand. That is probably because the dominant hand has more practiced connections with serial finger movements than does the non-dominant hand that has to engage more cognitive resources (i.e. more brain regions) than the dominant hand to program the novel, non-automatic sequential finger movement task (see Table 2). However, the regions that were significant for the dominant hand suggest that self-regulating while performing a previously taught and practiced finger sequencing task with one's usual hand for writing activates brain regions involved in orthographic (left precuneus), working memory (left superior parietal), and timing coordination (right cerebellum). These brain regions may underly the orthographic loop of working memory, which supports orthographic-hand connections used in writing (Berninger, Nielsen, Abbott, Wijsman, & Raskind, 2008). The additional brain regions associated with performing a taught task with the non-dominant hand suggest that planning novel, sequential finger movements and executing them with the hand rarely, if ever, used for writing is associated with activation of additional brain regions: mainly on left in frontal, parietal, temporal, occipital, and cerebellar regions, but also on right in fusiform, superior parietal, and cerebellar regions. See Table 2.
Two regions of fMRI activation were significantly correlated across dominant and dominant hands: left superior parietal and right precuneus. Basso et al. (1998) proposed that left superior parietal is a writing center for letter form coding (see introduction). Right precuneus is associated with orthographic processing (Temple, Poldrack, Salidis, Deutsch, Tallal, Merzenich et al., 2001), which also involves letters. Together these findings show that serial finger movements on both hands activate brain regions involved in orthographic processing: The behavioral measure that requires serial organization of finger movement, but not orthographic processing directly, was correlated with an fMRI contrast for finger sequencing that also required serial organization of fingers but not orthographic processing. Thus, even when a task does not involve processing written language, evidence of the brain's neural circuitry for connecting letter forms with serial finger movements is observed.
Longitudinal Brain-Behavior Relationships
Longitudinal correlations of behavioral measures of writing at an earlier time in development are considered for their implications for transcription—letter writing by hand and by keyboard and word spelling.
Automatic retrieval and formation of legible printed letters by hand on alphabet task (first 15 seconds)
Automatic legible printing of the alphabet in manuscript format in alphabetic order from long-term memory was correlated with four frontal regions, six parietal, four temporal, and one cerebellar region but no occipital regions. Thus, handwriting is not merely a visual process any more than it is merely a motor process. The behavioral measures of automatic letter writing (both for manuscript printing by hand and by keyboard) were correlated with activation on the fMRI finger sequencing contrast in homologous regions in both hemispheres in supramarginal, precuneus, inferior temporal, and middle temporal regions. Superior, middle, and inferior frontal regions in right hemisphere and middle cingulate in left hemisphere may support the executive functions regulating automatic letter retrieval and production. Temporal regions bilaterally may support letter form retrieval on basis of language cues such as name codes. Parietal regions bilaterally may support the role of phonological codes (supramarginal) and orthographic codes (precuneus) in maintaining letters in working memory during letter production. Left cerebellum may support the temporal coordination in automatic letter writing. Clearly, many brain regions may participate in automatic retrieval and production of letter forms. Please note that significant correlations were observed only for automatic letter writing—not for total accuracy (all 26 letters) or letter writing speed (total time for 26 letters) on this alphabet writing task. See Table 3.
Legible formation of cursive letters by hand
Legible cursive writing on the same alphabet task (total accuracy for 26 letters) was correlated with activation in left anterior cingulate (executive functions), left postentral (somatosensory feedback), and bilateral superior temporal (language) regions. See Table 3.
Automatic correct selection of letters by keyboard (first 15 seconds)
Only automatic letter production when writing the alphabet by keyboard was correlated with fMRI activation in occipital regions (2), suggesting that visual processes play a bigger role in writing by keyboard than by pen or pencil. In addition, the alphabet writing task by keyboard was correlated with activation in left fusiform, a region where letter and word forms are processed, and two cerebellar regions, but different cerebellar regions than those associated with manuscript letter printing. Keyboarding, which is done bi-manually, may pose different timing regulation requirements than handwriting performed uni-manually. See Table 3.
Spelling written words
The results for spelling are, on one hand, remarkable because of the sheer number of brain regions associated with planning serial finger movement that were correlated with spelling (see Table 3). These regions were in bilateral middle frontal and right inferior frontal, cingulate (anterior and middle cingulate bilaterally), left parietal (superior parietal, supramarginal, precuneus, postcentral), right parietal (precuneus, superior parietal), left temporal (fusiform, lingual, inferior, superior), right temporal (fusiform, lingual, inferior, middle), left occipital (inferior and middle occipital), right occipital (calcarine and inferior occipital), left cerebellum (regions crus 1, crus 2, 6, and 8), and right cerebellum (4 5, 8). This widespread involvement of brain in the serial organization of motor output by fingers predicted from spelling nine months earlier, on average, suggests that spelling draws on planning processes for serial planning of finger movements in producing letters in sequence as well as language processes. See Richards, Berninger, and Fayol (in press) for brain activation associated with recognition of real word spellings in long-term memory that included many language regions. Good spellers but not poor spellers activated in the primary motor regions on the spelling recognition task that had no motor output requirements, suggesting that recognizing correctly spelled real words, even if no handwriting is involved, depends on motor patterns for writing the letters in written words (Richards et al., in press).
Common and Unique Brain Activation across Handwriting and Spelling
On the one hand, common brain regions may support automatic letter writing by hand and written spelling. Longitudinal correlations between behavioral measures earlier in time and the fMRI finger sequencing contrast on average nine months later were consistently significant in eleven brain regions for automatic letter printing by hand and written spelling —right middle frontal, right inferior frontal orbital, left middle cingulate, left superior parietal, left supramarginal, left and right precuneus, right middle temporal, left and right inferior temporal, and left cerebellum 8 (see Table 3). These eleven brain regions may comprise a neural network that supports transcription processes in general during writing. On the other hand, as shown in Table 3 four activated regions on the fMRI contrast for finger sequencing were correlated exclusively with the behavioral measure of handwriting and sixteen correlated exclusively with spelling, showing unique neural correlates with specific writing skills as well. . Exner's area in middle frontal gyrus (Exner, 1991; Anderson et al., 1990) was correlated with spelling rather than letter writing. Richards and Berninger (2009) report the longitudinal correlations between a composition measure and the same fMRI finger sequence contrast and describe a neural network of five brain regions that are correlated consistently with behavioral measures of both transcription skills (automatic letter formation by hand and dictated spelling) and composition. Considering that four of the five are correlated with transcription skills, transcription appears to be an important process in written expression of ideas.
Gender Differences
Of significance for future research was the finding that, of the 85 brain regions in the group map analyses for the fMRI contrast for finger sequencing, only one—left superior parietal—showed gender differences, with boys showing less activation on the contrast. This same brain region was significantly correlated to all the behavioral measures—finger sequencing on the dominant and non-dominant hands, automatic letter printing, and spelling. Left superior parietal regions, proposed by Basso et al. (1978) may be a brain center, if not the only brain center, involved in writing as it does appear to be involved across skills and to be a region of gender differences that could explain the higher incidence of males with writing problems (see introduction). Certainly additional research to test this hypothesis based on the current study's findings is warranted.
Limitations of the Current Study and Future Research
Although studying brain activation on tasks that are trained outside the scanner and then performed while the brain is scanned has value, some tasks cannot be monitored during scanning as is possible with tasks that require a button press. Admittedly, exact rates at which children performed the finger taps with and without sequencing during scanning could not be recorded, but observation of children during scanning through the one-way mirror indicated that they were keeping up with the pacing of the task. Also children did not appear to have any difficulty during training outside the scanner in learning to perform the task at a steady rate.
Nevertheless robust differences were found between good and poor writers in many brain regions on the contrast for planning serial finger movement, and these were predicted longitudinally from behavioral measures of writing given on average nine months earlier. These longitudinal correlations identified patterns of convergent and discriminant validity across handwriting (automatic letter writing), and spelling. Of course the findings of the current study may only generalize to good and poor writers of the age studied.
Even though more girls than boys were found to be good writers in this sample drawn from a longitudinal study of typical writing development, differences between good and poor writers on the fMRI contrast for sequential finger movements were never significantly different for boys and girls in 84 of the 85 regions in the group map analyses. Future research might study groups of good and poor writers selected so that each group has equal numbers of boys and girls. The one region where gender differences occurred on the contrast was left superior parietal. Future studies might investigate whether gender differences in this brain region are related to serial organization of writing behavior and explain the reported poorer writing of boys in prior cross-sectional and longitudinal studies (see introduction). Future research should also try to replicate the correlations between the behavioral measures of specific writing skills and the fMRI contrast for finger sequencing versus non-sequencing.
Summary and Conclusions
In the current study longitudinal correlations between behavioral measures of writing and brain activation in the fMRI finger sequencing contrast showed patterns of discriminant and convergent validity. Longitudinal correlations were significant in four brain regions exclusively on automatic letter writing by hand, sixteen brain regions exclusively on dictated spelling and eleven common brain regions across automatic letter writing by hand and dictated spelling (see Table 3). The last eleven regions may collectively serve as a common neural network for the transcription processes of the writing brain. The brain's ability to program fingers for serial movement through activation in frontal, parietal, and temporal regions, which are associated with handwriting and spelling, may enable writing development, but not cause it apart from quality writing instruction and many opportunities to apply transcription skills to meaningful writing activities (Berninger & Richards, 2002).
Acknowledgements
The authors thank Jeff Stevenson for his technical help in developing the MR imaging protocols used on the Philips Achieva 3T Scanner.
Grants HD 25858 and P50 33812 from the National Institute of Child Health and Human Development (NICHD) supported this research.
Footnotes
Longitudinal correlations were also computed between behavioral measures of composition and the fMRI contrast and are reported in Richards and Berninger (2009).
References
- Abbott R, Berninger V. Structural equation modeling of relationships among developmental skills and writing skills in primary and intermediate grade writers. Journal of Educational Psychology. 1993;85:478–508. [Google Scholar]
- Alamargot D, Chanquoy L. Through the models of writing. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. [Google Scholar]
- Anderson S, Damasio A, Damasio H. Troubled letters but not numbers. Domain specific cognitive impairments following focal damage in frontal cortex. Brain. 1990;113:749–760. doi: 10.1093/brain/113.3.749. [DOI] [PubMed] [Google Scholar]
- Basso A, Taborelli A, Vignolo L. Dissociated disorders of speaking and writing in aphasia. Journal of Neurology, Neurosurgery, and Psychiatry. 1978;41:556–563. doi: 10.1136/jnnp.41.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith S. General multi-level linear modeling for group analysis in fmri. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berninger V. Development of language by hand and its connections to language by ear, mouth, and eye. Topics in Language Disorders. 2000;20:65–84. [Google Scholar]
- Berninger V. Process Assessment of the Learner (PAL) Test Battery for Reading and Writing. The Psychological Corporation; San Antonio, TX: 2001. Revised in 2007 Process Assessment of the Learner, 2ndEdition. Diagnostic for Reading and Writing (PAL-II RW) [Google Scholar]
- Berninger V, Cartwright A, Yates C, Swanson HL, Abbott R. Developmental skills related to writing and reading acquisition in the intermediate grades: Shared and unique variance. Reading and Writing: An Interdisciplinary Journal. 1994;6:161–196. [Google Scholar]
- Berninger V, Fuller F. Gender differences in orthographic, verbal, and compositional fluency: Implications for diagnosis of writing disabilities in primary grade children. Journal of School Psychology. 1992;30:363–382. [Google Scholar]
- Berninger V, Nielsen K, Abbott R, Wijsman E, Raskind W. Writing problems in developmental dyslexia: Under-recognized and under-treated. Journal of School Psychology. 2008;46:1–21. doi: 10.1016/j.jsp.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger V, O'Donnell L. Research-supported differential diagnosis of specific learning disabilities. In: Prifitera A, Saklofske D, Weiss L, Rolfhus E, editors. WISCIV Clinical use and interpretation. Academic Press; San Diego, CA: 2004. pp. 189–233. [Google Scholar]
- Berninger V, Richards T. Brain literacy for educators and psychologists. Academic Press; New York: 2002. [Google Scholar]
- Berninger V, Rutberg J. Relationship of finger function to beginning writing: Application to diagnosis of writing disabilities. Developmental Medicine & Child Neurology. 1992;34:198–215. doi: 10.1111/j.1469-8749.1992.tb14993.x. [DOI] [PubMed] [Google Scholar]
- Berninger V, Yates C, Cartwright A, Rutberg J, Remy E, Abbott R. Lowerlevel developmental skills in beginning writing. Reading and Writing. An Interdisciplinary Journal. 1992;4:257–280. [Google Scholar]
- Booth J, Cho S, Burman D, Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Developmental Science. 2007;10:441–451. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina D, Richards T, Serafini S, Richards A, Steury K, Abbott R, et al. fMRI auditory language differences between dyslexic and able reading children. Neuroreport. 2001;12:1195–1201. doi: 10.1097/00001756-200105080-00029. [DOI] [PubMed] [Google Scholar]
- Demie F. Ethnic and gender differences in educational achievement and implications for school improvement strategies. Educational Research. 2001;43:91–106. [Google Scholar]
- Denckla M. Development of speed in repetitive and successive finger movements in normal children. Developmental Medicine and Child Neurology. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Dewey D, Bottos S. Neuroimaging of developmental motor disorders. In: Dewey D, Tupper D, editors. Developmental motor disorders. A neuropsychological perspective. Guilford; New York: 2004. pp. 26–43. [Google Scholar]
- Exner S. Untersuchungen űber die Lokalisation der Funktionen in der Grossshirnrinde des Menschen. Wilhelm Braumuller; Vienna: 1881. [Google Scholar]
- Hayes JR, Chenoweth N. Is working memory involved in the transcribing and editing of texts? Written Communication. 2006;23:135–149. [Google Scholar]
- Hayes JR, Flower LS. Identifying the organization of writing processes. In: Gregg L, Steinberg E, editors. Cognitive processes in writing: An interdisciplinary approach. Erlbaum; Hillsdale, NJ: 1980. pp. 3–30. [Google Scholar]
- James KH, Gauthier I. Letter processing automatically recruits a sensory-motor brain network. Neuropsychologia. 2006;44:2937–2949. doi: 10.1016/j.neuropsychologia.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yashikawa K, Sugishita M. A functional mri study on the neural substrates for writing. Human Brain Mapping. 2001;13:34–42. doi: 10.1002/hbm.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jeffries LA, editor. Cerebral mechanisms in behavior. John Wiley & Sons; New York: 1951. pp. 112–136. [Google Scholar]
- Liberman AT. The reading researcher and the reading teacher need the right theory of speech. Scientific Studies of Reading. 1999;3:95–111. [Google Scholar]
- Longcamp M, Anton JL, Roth M, Velay JL. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage. 2003;19:1492–1500. doi: 10.1016/s1053-8119(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Luria AR. The working brain. Basic Books; New York: 1973. [Google Scholar]
- Martin D, Hoover H. Sex differences in educational achievement: A longitudinal study. Journal of Early Adolescence. 1987;7:65–83. [Google Scholar]
- Matsuo K, Kato C, Tanaka S, Sugio T, Matsuzawa M, Inui T, et al. Visual language and handwriting movement: Functional magnetic resonance imaging at 3 tesla during generation of ideographic characters. Brain Research Bulletin. 2001;55:549–554. doi: 10.1016/s0361-9230(01)00564-0. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . Wechsler Individual Achievement Test--Second Edition. Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Richards T, Aylward E, Berninger V, Field K, Parsons A, Richards A, et al. Individual fMRI activation in orthographic mapping and morpheme mapping after orthographic or morphological spelling treatment in child dyslexics. Journal of Neurolinguistics. 2006;19:56–86. [Google Scholar]
- Richards T, Berninger V. The writing brain within a working memory architecture. In: Grigorenko E, Mambrino E, Preiss D, editors. Handbook of Writing: A mosaic of perspectives and views. Psychology Press; New York: 2009. [Google Scholar]
- Richards T, Berninger V, Fayol M. FMRI activation differences between 11- year-old good and poor spellers' access in working memory to temporary and long-term orthographic representations. Journal of Neurolinguistics. 2009 [Google Scholar]
- Roberts T, Disbrow E, Roberts H, Rowley H. Quantification and reproducibility of tracking cortical extent of activation by use of functional mr imaging and magnetoencephalography. American Journal of Neuroradiology. 2000;21:1377–1387. [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler C, Strother S, Anderson J, Kim S-G. Reproducibility of bold-based functional mri obtained at 4 t. Human Brain Mapping. 1999;7:267–283. doi: 10.1002/(SICI)1097-0193(1999)7:4<267::AID-HBM5>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG. The meaning and value of soft signs. In: Tupper D, editor. Soft neurological signs. Grune & Stratton; New York: 1987. pp. 297–335. [Google Scholar]
- Temple E, Poldrack R, Salidis J, Deutsch G, Tallal P, Merzenich M, et al. Disrupted neural response s to phonological and orthographic processing in dyslexic children: an fMRI study. NeuroReport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Tupper D. The issues of soft signs. In: Tupper D, editor. Soft neurological signs. Grune & Stratton; Orlando, FL: 1998. pp. 1–16. [Google Scholar]
- Van Mier H, Temple L, Perlmutter J, Raichle M, Petersen S. Changes in brain activity during motor learning measured with pet: Effects of hand performance and practice. Journal of Neurophysiology. 1998;80:2177–2199. doi: 10.1152/jn.1998.80.4.2177. [DOI] [PubMed] [Google Scholar]
- Wolff P, Gunnoe C, Cohen C. Associated movements as a measure of developmental age. Developmental Medicine and Child Neurology. 1983;25:417–429. doi: 10.1111/j.1469-8749.1983.tb13786.x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fmri data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for cbf activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]