Abstract
Substance dependence is associated with executive function deficits, but the nature of these executive defects and the effect that different drugs and sex have on these defects has not been fully clarified. Therefore, we compared the performance of alcohol- (n = 33; 18 women), cocaine- (n = 27; 14 women), and methamphetamine-dependent individuals (n = 38; 25 women) with sex-matched healthy comparisons (n = 36; 17 women) on complex decision-making as measured with the Iowa Gambling Task, working memory, cognitive flexibility, and response inhibition. Cocaine- and methamphetamine-dependent individuals were impaired on complex decision-making, working memory, and cognitive flexibility, but not on response inhibition. The deficits in working memory and cognitive flexibility were milder than the decision-making deficits and did not change as a function of memory load or task switching. Interestingly, decision-making was significantly more impaired in women addicted to cocaine or methamphetamine than men addicted to these drugs. Together, these findings suggest that drug of choice and sex have different effects on executive functioning, which, if replicated, may help tailor intervention.
Introduction
Substance dependent individuals (SDI) have been shown to have deficits in the domain of executive functioning (Verdejo-García & Pérez-García, 2007). Executive functioning involves the ability to plan, judge and weigh several options, to make complex decisions, to have an accurate perception of one's own abilities, and to organize, implement and control other cognitive functions such as memory (e.g., Oscar-Berman & Marinković, 2007; Tranel, Anderson & Benton, 1994). Neuropsychological and neuroimaging studies in humans, as well as lesion and electrophysiological studies in animals, suggest that executive functioning relies in large part on prefrontal cortex (PFC) and its interactions with other brain regions (Stuss & Benson, 1986). This observation, together with studies reporting PFC neuronal loss in SDI (Adams et al., 1993; Krill, Halliday, Svoboda & Cartwright, 1997), suggests that cognitive deficits observed in SDI may be associated with dysfunction of the PFC. The PFC is heterogeneous in its functions, and may subserve diverse abilities related to cognitive control functions such as working memory, cognitive flexibility and response inhibition. This raises the question whether the impairments in executive functioning reported in SDI can be explained by deficits in working memory and other cognitive domains that subserve executive functioning. The present study compared performance of SDI-Alcohol, SDI-Cocaine and SDI-Methamphetamine on various measures of executive functioning including complex decision-making, working memory, cognitive flexibility and response inhibition. In addition, it is not well understood whether men and women are affected differently by chronic drug abuse and this issue was addressed in the current study.
Executive functioning and substance dependence
Executive functioning is a complex construct which involves different cognitive domains. Decision-making is one domain of executive functioning that is impaired in heavy drug use. For example, SDI continue to use drugs despite negative health-related, psychological, social and legal consequences (Verdejo-García et al., 2007a). The Iowa Gambling Task (IGT) is an experimental decision-making task that requires the integration of different aspects of executive functioning in order for successful completion (see Dunn, Dalgleish & Lawrence, 2006). The key feature of the task is that participants have to forego high short-term rewards (i.e. facsimile money) for long-term profit (e.g. Bechara, Damasio & Damasio, 2000). Studies have consistently shown that SDI, including individuals addicted to alcohol, cocaine and methamphetamine pursue actions that bring immediate reward at the risk of incurring future negative consequences (Bechara & Damasio, 2002; Bechara & Martin, 2004; Scott et al., 2007; Verdejo-García et al., 2007a; Verdejo-García, Rivas-Pérez, Vilar-Lopez, & Pérez-García, 2007b), suggesting that these individuals have a “myopia for the future” (see also Rogers & Robins, 2001). An important region involved in processing many types of reward and punishment and making rapid changes in response to environmental change, is the orbitofrontal cortex (OFC), in particular the ventral and medial prefrontal cortex (VMPFC; e.g. Bechara et al., 2000). The OFC has been hypothesized to be dysfunctional in SDI (Adinoff et al., 2006; Breiter, Arahon, Kahneman, Dale & Shizgal, 2001; Knutson, Westdorp, Kaiser & Hommer, 2000; Tanabe et al., 2007). For example, using single photon emission computed tomography (SPECT), Adinoff and collegues (2006) showed that individuals addicted to cocaine had reduced activity in the OFC relative to healthy comparisons when performing the IGT.
The functional integrity of the OFC however, relies on neural systems that subserve memory, in particular working memory (Bechara, Tranel & Damasio, 2002). Working memory is defined as the process of storing and online manipulation of information (Baddeley, 1994), and includes short-term storage, rehearsal, and the executive processes that operate on the contents of memory (Smith, 2000). The dorsolateral prefrontal cortex (DLPFC) is thought to subserve working memory (Goldman-Rakic, 1992), and appears particularly important for the executive processor of mnemonic operations (Petrides, 2000). Studies with SDI have suggested that deficits in the working memory domain are not purely due to a mnemonic impairment. Rather; impairments may be due to an executive control problem. Specifically, Bechara and Martin (2004) found that individuals addicted to either alcohol, cocaine or methamphetamine performed below normal levels on the working memory task, but increasing the memory load did not influence performance. The task used in this study (i.e. the delayed non-match to sample; DNMS) includes a memory component and an executive component. Because performance of SDI was not influenced by increased memory load, the authors argued that the deficiency SDI displayed on this task must be related to the executive component of working memory as opposed to the storage component (Beachara & Martin, 2004). Thus according to these authors, problems in SDI arise when a strategy should be efficiently implemented to memorize items (Bechara & Martin, 2004; Woods et al., 2005; see also Scott et al., 2007). However, to clarify the nature of the working memory deficits in SDI, we need to evaluate performance on a task that specifically measures the mnemonic component of working memory. In this study we taxed the function of working memory with the Tic Tac Toe Test (Alting von Geusau, Stalenhoef, Huizinga, Snel & Ridderinkhof, 2004), a modified version of the Corsi-Milner paradigm (Milner, 1971). Participants have to decide whether a pattern matches with a pre-specified pattern, therefore the task taps specifically into the memory component of working memory. Furthermore, to directly elucidate the relationship between working memory and decision-making, we determined the contribution of the mnemonic working memory domain to decision-making performance. Since the executive domain of working memory is thought to contribute to decision-making (Bechara & Martin, 2004), we did not expect that the mnemonic domain would contribute to decision-making. Working memory most likely consists of separate modules, but in this study we focused on visuospatial working memory (Baddeley, 1994).
Lastly, cognitive flexibility and response inhibition are important domains of executive functioning. There is evidence to suggest that alcohol abusers (Errico, King, Lovallo & Parsons, 2002) and cocaine abusers (Klüber, Murphy& Garavan, 2005) show poorer performance on measures of cognitive flexibility and response inhibition (see also Verdejo-García, Bechara, Recknor & Pérez-García, 2006). To measure the performance of SDI on each of these specific cognitive domains, we included task-switching paradigms (Rogers & Monsell, 1995) and a response inhibition paradigm that are specifically designed to tap into these domains of executive functioning. In the switching task, participants rapidly switch between two or more reaction-time (RT) tasks that are typically performed to the same set of stimuli (e.g., local and global figures, Miyake et al., 2002). Switching between tasks is associated with a sizeable decrement in response speed and accuracy (Rogers & Monsell, 1995). We also included the Wisconsin Card Sorting Task (WCST) as a measure of cognitive flexibility, because it requires flexible adjustment of actions following performance feedback (Stuss & Levine, 2002). Response inhibition was assessed using the stop-signal task (Logan & Cowan, 1984; Aron, Fletcher, Bullmore, Sahakian & Robbins, 2003). The stop-signal paradigm has been developed to investigate the covert cognitive processes that constitute inhibitory control (Logan & Cowan, 1984). The task is unique because of its direct assessment of the ability to inhibit a pre-potent action, especially at the cognitive or attentional level, as opposed to the motor response level.
Thus, the first aim of this study was to describe the pattern of executive functioning deficits in SDI by evaluating their performance on complex decision-making, working memory, cognitive flexibility and response inhibition. The interpretation of executive deficits in substance dependence however, is constrained by the possibility that different drugs may have different effects on brain functioning. Also, there is more and more evidence for sex-dependent brain-behavior relationships (Tranel, Damasio, Denburg & Bechara, 2005). Therefore, in an attempt to fully understand patterns of executive functioning in SDI, it is important to examine the effects of different types of drugs and sex.
Drug type
There is mounting evidence to suggest that substance abuse affects an individual's executive functioning, however the type of substance used may influence this particular relationship (Selby & Azrin, 1998). Gonzalez, Bechara and Martin (2007) directly compared performance of alcoholics and methamphetamine addicted individuals on the IGT and the delayed non-matched to sample working memory task. They found that the effects of alcohol on these measures were milder than the effects of methamphetamine (see also Bechara & Martin, 2004; Verdejo-García et al., 2007a). On the same note, chronic alcohol abuse seems to have less of an effect on executive functioning relative to cocaine abuse (Easton & Bauer, 1997). In addition, nearly half of the 18 million alcoholics in the USA do not show any signs of cognitive, sensory or motor impairments. When signs are present, deficits in tests of memory, fluency, cognitive flexibility and perseverative responding are generally only mild (Oscar-Berman & Marinković, 2007). In contrast, chronic methamphetamine use for example, has been associated with persistent cognitive impairments in various domains of executive functioning (Barr et al., 2006). Based on these findings, it seems reasonable to expect that different drug types may have differential effects on executive functioning. Specifically, we expected that chronic cocaine and methamphetamine abuse would be associated with more severe neurotoxic effects and hence more severe impairments on various measures of executive functioning relative to chronic alcohol abuse.
Sex
Another variable that may explain differences in performance on various measures of executive functioning may be sex. Interestingly, sex differences in healthy comparisons have been reported for the IGT. That is, men tend perform slightly better than women (Bechara & Martin, 2004; Bolla, Eldreth, Matochick & Cadet, 2004; Overman, 2004; Overman et al., 2006). For example, in a study reviewed by Overman (2004) men selected the advantageous decks 79% of the time by the third block, whereas women chose the advantageous decks 68% of the time at that time point. Sex-related differences in frontal-lobe metabolism rate during resting state as well as structural differences in the frontal lobes have been reported and may underlie the differences in performance between men and women on the IGT (Overman, 2004). Whether sex affects decision-making in SDI as well is not well understood, partly because women tend to be underrepresented in studies on drug abuse and decision-making. Although findings on sex-related differences in SDI are still considered controversial (Oscar-Berman & Marinković, 2007), molecular (reviewed in Barr et al.2006) as well as neuroimaging studies (e.g. Adinoff et al., 2006) have shown that methamphetamine and cocaine affect the central nervous system different in men and women. This study aimed to contribute to the current knowledge on sex and drug abuse by exploring whether sex has an effect on executive functioning at a behavioral level.
Although sex may influence the relationship between executive functioning and substance abuse, it is important to keep in mind that not every experimental task is sensitive enough for potential differences between men and women. That is, except for mental rotation (e.g. Peters, Manning & Reimers, 2007) and fine motor tasks (e.g. Nicholson & Kimura, 1996), differences between men and women in terms of cognitive control tend to be subtle. The IGT has been shown to be sensitive to sex differences in some studies (e.g. Overman, 2004), however most studies with “simple” cognitive control tasks similar to those used in the current study, do not tend to find sex differences at the behavioral level (see Bell, Wilsson, Wilman, Dave & Silverstone, 2006; Heaton, 1981; Ray Li, Huang, Constable & Sinha, 2006; Mulvihill et al., 1997; Overman, 2004;Yehene & Meiran, 2007). Therefore, we expected to find sex differences on the IGT only, with men outperforming women on this particular task (see Overman, 2004).
Summary
The goal of this study was to examine patterns of executive functioning deficits in SDI in different cognitive domains with a particular interest in exploring the effects of different types of substances used and the influence of sex. We expected decision-making as measured with IGT to be deficient in SDI-Cocaine and SDI-Methamphetamine only (Gonzalez et al., 2007). In addition, it is hypothesized that working memory deficiencies in SDI are due to the executive component of working memory (Bechara & Martin, 2004), suggesting that the mnemonic component is still intact. Based on this hypothesis, we expected that SDI would perform normally on our measure of working memory (see also Bechara and Martin, 2004). In terms of the other executive functioning tasks; there is evidence to suggest that chronic substance abuse is associated with impaired performance on tasks that tap into cognitive flexibility and response inhibition (e.g. Klüber et al., 2005). We therefore hypothesized that SDI-Cocaine and SDI-Methamphetamine would show poorer performance on those measures of executive functioning as well.
The second goal was to further elucidate the relationship between decision-making and other cognitive control functions. It has been suggested in the literature that the executive component of working memory (Bechara & Martin, 2004), cognitive flexibility and response inhibition (Dunn et al., 2006) contribute to decision-making. Based on these hypotheses, we predicted that performance on the shifting task, WCST and the response inhibition task would make significant contributions to the combined regression model of decision-making. Because our working memory task specifically taps into the mnemonic domain of working memory (Bechara & Martin, 2004) we did not expect working memory performance to contribute to decision-making.
Methods
Participants
In total, 133 participants from the broader Iowa area participated in the study. Healthy, drug-free comparison participants (n=36) were selected from a registry of such individuals maintained in the University of Iowa's Division of Behavioral Neurology and Cognitive Neuroscience. These participants were initially recruited through advertisement at the rehabilitation center. The selection criteria for the comparison participants included the absence of a history of mental retardation, learning disability, psychiatric disorder, substance abuse, neurological disorder, or systemic disease that may affect the central nervous system, based on clinical interviews conducted with these subjects before their induction. The healthy comparisons in the current sample had not undergone neuropsychological assessment prior to this study. In contrast to the substance dependent participants, no structured clinical interview was administered in the healthy group.
Substance Dependent Individuals (SDI) consisted of inpatients who had been admitted to Mid-Eastern Center for Chemical Abuse (MECCA), an inpatient facility for detoxification and treatment. Of the 113 SDI who were included, 33 participants were primarily alcohol dependent, 38 participants were primarily cocaine dependent, 27 participants were primarily methamphetamine dependent as indicated by the participants' reports. Drug of choice was defined as substance used >80% of the time prior to treatment, but some subjects used other drugs occasionally in the past 30 days prior to treatment, especially cannabis (see Table 2). Note that the non-acute effects on neuropsychological performance of cannabis tend to be mild (Gonzalez, 2007). All SDI had experienced serious substance abuse problems in the past that had required professional intervention, which was the reason for their treatment. The selection criteria for SDI were 1) meeting the DSM-IV criteria for substance dependence; 2) absence of psychosis; 3) no documented head injury or seizure disorder. Each SDI was tested at the end-stage of their treatment shortly before their discharge. The duration of abstinence from substance use was known in these participants based on their length of stay at MECCA. The time varied among individuals, but the minimum period of abstinence from any substance use was 15 days. Thus at the time of their testing, the SDI were no longer in acute withdrawal or taking any medications to control withdrawal (e.g., benzodiazepines). Urine toxicology screening for opiates, stimulants, marijuana, and breathalyzers tests were conducted on these SDI immediately before testing. SDI were also routinely checked at MECCA, including the day before they were brought for testing. Therefore, not only very recent substance use can be ruled out, but also it is reasonable to rule out the use of substances during the entire period of abstinence. The Structured Clinical Interview for DSM-IV (SCID-IV) was used to assign Axis I diagnoses (including alcohol and other drug abuse and/or dependence). SDI whose preponderant use was alcohol or stimulant drugs were identified through verbal report. The results showing primary drug of choice are presented in Table 1. The areas of co-morbid psychopathologies that we probed with the SCID were psychoses, major depressive disorder, and anxiety disorders. The sum of scores on each psychopathology was obtained and participants who had a score greater than 3 were excluded from the study. SDI who met the criteria for psychoses or a current major depressive disorder were excluded.
Table 2. Drug histories.
Drug histories of subjects who participated in the study.
Drug Use Histories of SDI*
| N | Occasional Drug Use in Past 30 days | ||
|---|---|---|---|
| Drug type | Number of participants | ||
| SDI-Alcohol | 33 | Amphetamine | 2 |
| Cannabis | 4 | ||
| Cocaine | 1 (used in combination with cannabis) | ||
| SDI-Cocaine | 27 | Alcohol | 7 |
| Amphetamine | 1 | ||
| Cannabis | 9 | ||
| Heroin | 1 (used in combination with cannabis) | ||
| Opiates/Analgsics | 1 | ||
| Sedatives/Hypnotics/Anti-Anxiety | 1 (used in combination with cannabis) | ||
| SDI-Methamphetamine | 38 | Alcohol | 4 (used in combination with cannabis in 3 participants) |
| Cannabis | 12 | ||
| Cocaine | 5 (used in combination with cannabis in 4 participants) | ||
| Opiates/Analgsics | 2 (used in combination with cannabis in 1 participant) | ||
| Sedatives/Hypnotics/Anti-Anxiety | 3 (used in combination with cannabis in 2 participants) | ||
Substance-Dependent Individuals
Table 1. Demographics.
Demographics of subjects who participated in the study
Drug of Choice (used > 80% of the time)
| Healthy Comparisons | SDI-Alcohol | SDI-Cocaine | SDI-Methamphetamine | |
|---|---|---|---|---|
| Total N | 36 | 33 | 27 | 38 |
| Age (years): Mean ± SD | 28.9 ± 9.8 | 37.9 ± 9.9* | 34.5 ± 8.9 | 30.7 ± 7.0 |
| Gender (M/F) | 17F/19M | 18F/15M | 14F/13M | 25F/13M |
| Handedness | 35 right-handed, 1 mixed | 28 right-handed, 4 left-handed, 1 unknown | 34 right-handed, 3 left-handed, 1 unknown | 23 right-handed, 3 left-handed, 1 mixed |
| Education (years): Mean ± SD | 16.2 ± 2.4 | 13.8 ± 2.4* | 11.4 ± 2.2* | 11.7 ± 1.4* |
| Times used in past 30 days: Mean ± SD | N.A. | 2.7 ± 3.5 | 2.9 ± 4.5 | 3.8 ± 5.0 |
| Years of Abuse: Mean ± SD | N.A. | 19.5 ± 10.4 | 10.1 ± 7.5 | 7.8 ± 4.9 |
Note. SDI = Substance Dependent Individuals; N.A. = Not Applicable
significantly different from healthy comparison group at p < .05.
All participants were adults and provided informed consent that was approved by the appropriate human subject committees at the University of Iowa. All participants were paid for their participation in gift certificates at an hourly rate. The demographic data of the groups are presented in Table 1. An ANOVA for mean age revealed a significant group difference, F (3, 133) = 5.33, p < .01. Post hoc comparisons showed that the alcohol-SDI were older than the four other groups. The methamphetamine SDI, cocaine SDI, and the comparison group did not differ from each other in age.
A chi-square comparison revealed that there was no difference between groups in sex distribution. An ANOVA revealed that groups differed significantly in years of education, F (3, 133) = 35.77, p < .001, and post hoc comparisons revealed that the comparison group was significantly more educated compared to all the SDI groups. The differences in age and years of education may be a concern when interpreting group difference in cognitive control, because both demographic variables have been found to influence performance on tasks that tap into executive functioning (Fals-Stewart & Bates, 2003). To take these demographic factors into account, we entered age and years of education as covariates in all our analyses.
Decision-Making (Bechara et al., 1994)
Decision-making was tested with a computerized version of the Iowa Gambling Task (IGT).The task involves 4 decks of cards called A′, B′, C′, and D′. In two decks (A′&B′), choosing a card is followed by a high gain of facsimile money, but at unpredictable points, the selection of a card is followed by a high penalty, so that in the long run, these decks are disadvantageous. In the other two decks (C′&D′), the immediate gain is smaller but the future loss is also smaller, so that in the long run, these decks are advantageous. The total number of trials was set at 100 card selections. Subjects were told that the goal of the game is to win as much money as possible, but that some of the decks are worse than others, and that they should try to stay away from those decks in order to win the game.
To score the performance of the subject on the IGT, the number of cards picked from decks A′ and B′ are added in each block of 20 cards, and the number of cards picked from decks C′ and D′ are added separately in each block of 20 cards. A net score is then obtained by subtracting the total number of cards selected from advantageous minus disadvantageous decks ((C′+D′)-(A′+B′)) for each block of 20 cards (Bechara & Martin, 2004).
Specific cognitive control tasks
Working Memory (Tic Tac Toe; Alting von Geusau et al., 2004)
Participants sat in front of a computer screen on which a 3×3 grid was continuously presented. The task consisted of two phases; a presentation phase and a recognition phase. During the presentation phase, a combination of Xs and Os was presented within the grid, containing a pattern of either 3 figures (low memory load) or 4 figures (high memory load). The recognition phase could be initiated by pressing the space bar. During the recognition phase Xs and Os were presented 600 ms each, one after another at different positions in the grid. The length of the series varied between 4 to 7 presentations for low load and 4 to 9 presentations for high load. When the combination of presented Xs and Os matched the pre-specified pattern indicated by the presentation phase, participants were required to press a left (z) or right (/) button at the keyboard (counterbalanced across participants). The task was presented in blocked order, starting with a practice block of 3 trials, followed by 2 blocks including 15 trials for load 3 and 15 trials for load 4 separately. The order of blocks was counterbalanced across participants to control for effects of time-on-task. The main dependent variables were the proportion correct responses and median RT in blocks of load 3 (low) versus load 4 (high).
Cognitive Flexibiliy (see also Rogers & Monsell, 1995)
A cue was presented on the computer screen, consisting of a small or large figure, followed by a 400 ms delay, followed by a geometric figure. In this figure, lines of a global figure (square or rectangle) were composed of smaller local figures (squares or rectangles). Depending on the cue, participants were instructed to respond with a left hand response to squares and a right hand response to rectangles (counterbalanced across participants). Thus, when the cue indicated a local figure, participants responded to the shape of the local figure by giving a left or right hand response. When the cue was a global figure, participants responded to the shape of the global figure by giving a left or right hand response. The cue changed in predictable order following four consecutive rules. The task consisted of two practice blocks for local and global figures separately and an experimental block of 160 trials. The main dependent variables were median RT and percentage errors on cue alternation and cue repetition trials.
The Wisconsin Card Sorting Test (Stuss & Levine, 2002)
The WCST is originally designed to measure rule-shifting and abstraction ability. Participants were presented with 2 decks of 64 cards each.
Subjects were told that each time they sort a card the computer will tell them whether they were right or wrong, depending on the criterion at stake (i.e. sorting based on color, shape or number). Subsequently, subjects were told that once the rule was correctly applied for a (pre-specified) number of trials, the matching principles changed unannounced and participants were to adapt their strategy based on feedback from the computer. We included ‘perseverative error scores’ and the ‘number of categories completed’ as dependent variables.
Response Inhibition (Aron et al., 2003)
Participants responded to a left or right pointing arrow on the screen by pressing the corresponding keys on the keyboard (‘z’ and ‘/’ respectively) with the left or right index finger. Arrows were presented in green against a black background screen and the arrow direction varied randomly and with equal probability. On 25% of the trials, a stop-signal was presented consisting of a color change of the arrow – from green to red. The timing of the color change was dynamically adjusted and targeted at 50% correct inhibits using a tracking algorithm. Upon successful stopping, the stop-signal delay on the next stop trial was increased by 50 ms. Failures to inhibit were followed by a 50 ms decrease in stop-signal delay. Subjects were instructed to respond fast and accurate to the go signals, and not to delay their go responses awaiting the possible occurrence of a stop signal. The task consisted of a practice block of 50 trials, and two experimental blocks of 100 trials each. The dependent variables were RTs to go signals and stop-signal RT as an index of the speed of response inhibition. Stop-signal RT was estimated using the horse-race model (for details, see Logan & Cowan, 1984).
Results
The main goal of this study was to examine executive control functioning in SDI, taking into account drug type and sex. To this end, we performed analyses of covariance (ANCOVA) which allow for estimating the variance explained by drug type and sex for performance on each individual cognitive task after controlling for confounding variables (i.e. age and education).
The second goal was to clarify the relationship between decision-making and other cognitive domains, such as working memory. We addressed this question by performing a stepwise regression analysis. By using this type of regression we could determine whether individual variables contribute significantly to decision-making. Variables will be entered in the combined regression model based on whether they individually correlate with decision-making (i.e. significant Pearson correlations).
ANCOVAs
Decision-Making
The number of advantageous choices on the IGT was submitted to a Sex (2) × Group (Healthy comparisons, SDI-alcohol, SDI-cocaine, and SDI-methamphetamine) × Task Block (5) Repeated Measures ANOVA with age and years of education as covariates. This resulted in a main effect for Group, F(3, 112) = 3.10, p < .05, Sex, F (1, 112) = 4. 54, p < .05 Block, F (4, 448) = 3.10, p < .05, a significant Block × Group interaction, F (4, 448) = 2.13, p < .05, and a marginally significant Block × Group × Sex interaction, F(10, 448) = 1.8, p < .055 (Greenhouse-Geisser). The Block × Group interaction suggests that different groups learn at different rates and this was examined by group-wise comparisons. For each group comparison, it was determined whether the groups differed in learning rate, which was statistically verified by Block × Group interactions. As predicted, this resulted in significant interactions between comparisons and SDI-Cocaine, F (3, 184) = 4.04, p < .01, and between comparisons and SDI-Methamphetamine, F (4, 220) = 7.82, p < .001, but the interaction between comparisons and SDI-Alcohol was not significant (p = .09). Comparisons between SDI groups revealed that these groups did not differ significantly from each other.
Separate one-way ANCOVAs identified group differences for block 2 through 5, with Tukey post-hoc comparisons suggesting that the healthy comparisons choose more advantageously than SDI-Cocaine and SDI-Methamphetamine for block 2 through 5 (all ps < .05). Healthy comparisons choose also more advantageously than SDI-Alcohol in block 4 and block 5 (ps < .05).
Against expectations, no significant sex differences were found for the normal comparisons and SDI-Alcohol, but interestingly, the Block × Group × Sex trend suggests that the women addicted to cocaine or methamphetamine perform worse on the IGT than men addicted to the same drug phenotypes (see figure 1).
Figure 1.
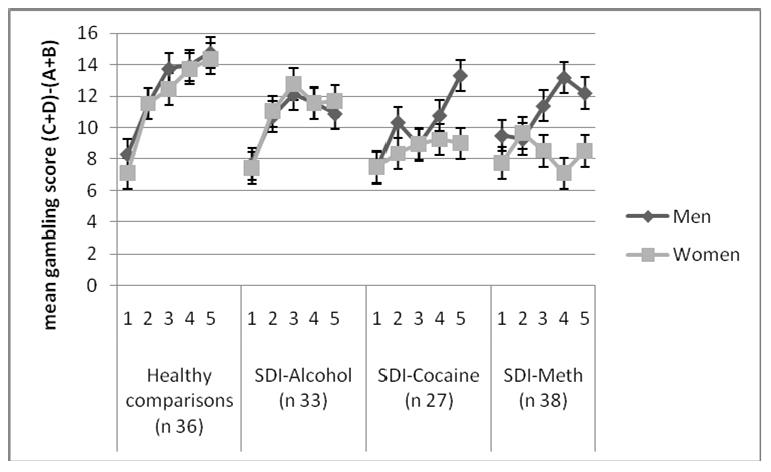
Iowa Gambling Task performance
Performance of comparison participants, alcohol dependent individuals, cocaine dependent individuals and methamphetamine (meth) dependent individuals on the Iowa Gambling Task (IGT).
Working Memory
The Repeated Measures analysis for % correct with Load (low versus high) as within-subject factor, and Group (Healthy Comparisons, SDI-Alcohol, SDI-Cocaine, and SDI-Methamphetamine) and Sex (2) as between-subject factors resulted in a main effect for Group, F(1, 106) = 5.15, p < 001. Age explained a significant proportion of the variance in working memory performance and the effect for Load did no longer reach significance after entering this variable as covariate (see also Bechara & Martin, 2004). Separate group comparisons identified significant group effects for healthy comparisons and SDI-Cocaine, F(1, 62) = 6.81, p < .05, and for healthy comparisons and SDI-Methamphetamine, F(1, 52) = 13.05, p < .01. Healthy comparisons made fewer errors the SDI groups, but the performance of the SDI did not worsen as a function of memory load (i.e. no significant Group × Memory Load interaction). Interestingly, the SDI-Methamphetamine made more errors than the SDI-Alcohol, F (1, 42) = 4.26, p < .05) and SDI-Cocaine, F(1, 48) = 6.50, p < .05. Men and women in all groups performed comparable on this task as indicated by the non-significant effect for Sex (see figure 2).
Figure 2.
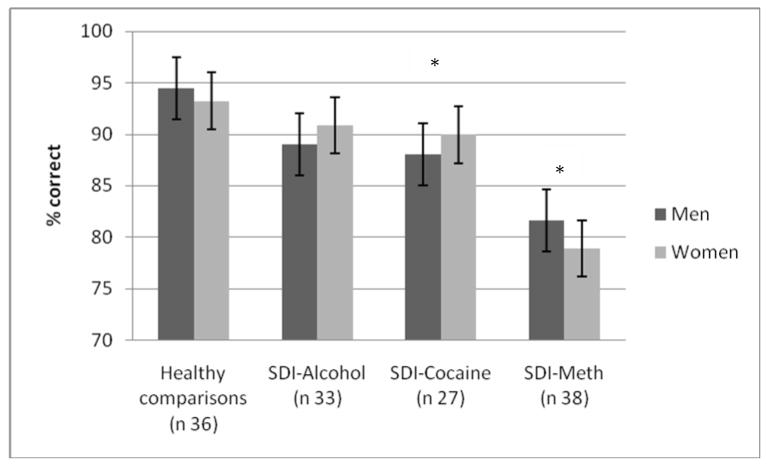
Working memory performance
Performance of comparison participants, alcohol dependent individuals, cocaine dependent individuals and methamphetamine (meth) dependent individuals on the working memory task. Men and women are plotted separately. Note: * SDI-Cocaine and SDI-Meth differ significantly from healthy comparisons.
Cognitive Flexibility
Median RTs for correct responses were submitted to a Rule (local and focal) × Switch (repetition, switch) × Group (Healthy Comparisons, SDI-Alcohol, SDI-Cocaine and SDI-Methamphetamine) × Sex (2) Repeated Measures Analysis. Rule represents here the RTs for local and focal rules, whereas Switch is representative for the RTs on repetition trials and switch trials. This analysis resulted in a main effect for Group, F (3, 112) = 2.73, p < .05 and Sex, F(1, 112) = 8.20, p < .01. Separate group comparisons revealed that the healthy comparisons were faster than the SDI-Cocaine, F (1, 66) = 5.24, p < .05, and the SDI-Methamphetamine, F(1, 53) = 10.52, p < .01. There was no differential decrement in response speed for the various drug groups as indicated by the non-significant Group × Switch interaction (see figure 3). Against predictions, regardless of group membership women were slower than men, as indicated by the main effect for Sex.
Figure 3.
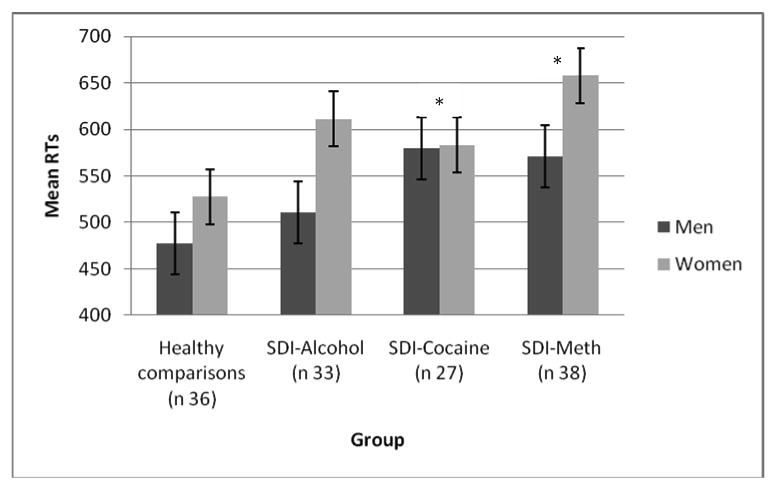
Switch task performance
Performance of comparison participants, alcohol dependent individuals, cocaine dependent individuals and methamphetamine (meth) dependent individuals on the local-global task switch task, showing that SDI-Cocaine and SDI-Meth groups are slower on this paradigm than the healthy comparisons. Men and Women are plotted separately. Note: * SDI-Cocaine and SDI-Meth differ significantly from healthy comparisons.
Furthermore a significant Rule × Group interaction, F (3, 112) = 4.61, p < .01 was found. This interaction was followed up groupwise comparisons. Results showed that SDI-Alcohol and SDI-Cocaine were faster on global rule trials than on local rule trials, whereas healthy comparisons were faster on local rule trials than on global rule trials (F(1, 55) = 5.84, p < .05, and F(1, 66) = 12.41, p < .001 respectively; see figure 4). The Group × Rule interaction was only marginally significant for SDI-Methamphetamine and healthy comparisons, p < .06. Unexpectedly, a significant Rule × Switch × Sex interaction, F(1, 112) = 3.93, p < .05 was found that indicated that men were somewhat faster to switch on the global rule trials as compared to local rule trials. In contrast, for women switching was associated with slower RTs independent of the rule. In addition, there was a significant Switch × Age interaction, F(1, 112) = 12.20, p < .001, which suggested that younger participants were faster than the older participants. Finally, neither the effects for Rule type and Switch, nor an ANCOVA for % of errors were significant. Age most likely explained a substantial part of the variance (see also Yehene & Meiran, 2007).
Figure 4.
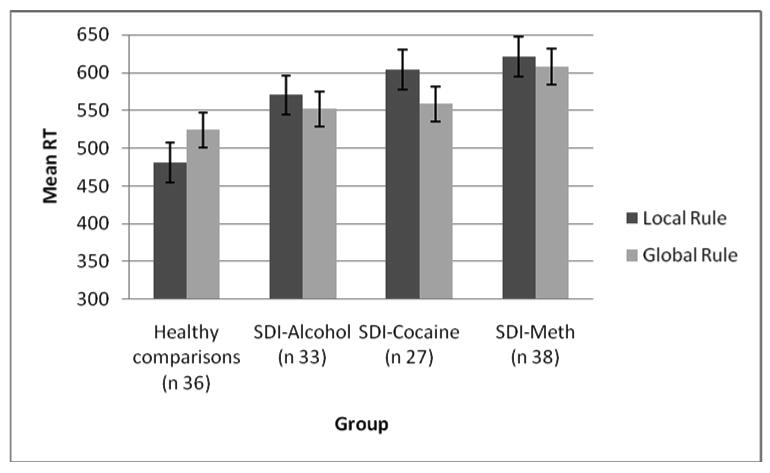
switch task performance, rule × group interaction
Performance of comparison participants, alcohol dependent individuals, cocaine dependent individuals and methamphetamine (meth) dependent individuals on the local-global task switch task, showing that healthy comparisons are faster on local trials, whereas the SDI-groups are faster on the global trials.
Wisconsin Card Sorting Test
A group (Healthy Comparisons, SDI-alcohol, SDI-methamphetamine, and SDI-cocaine) and Sex (2) ANOVA for preservative errors with age and education as covariates resulted in a significant effect for age only, F(1, 121) = 4.17, p < .05, indicating that younger age is associated with less preservative errors. No main effects for group or Sex were found (all ps > .2).
Response Inhibition
Participants who failed to inhibit responses on more than 80% of the stop trials were excluded from the analysis (n = 30, 24% of our sample), because this would impair the estimation of the stop-signal reaction time. Mean RTs to go-signal trials (i.e., trials where no stop-signal was presented) and stop-signal RTs (SSRTs) were computed for each participant. First, it should be noted that all participants included in this analysis were able to inhibit their responses on about half of the trials on which a stop signal instructed them to stop, so the tracking algorithm worked well for all groups (50%, 53%, 52% and 51% for control, SDI-alcohol, SDI-methamphetamine, SDI-cocaine, respectively, F <1).
Against expectations, the Group (Healthy Comparisons, SDI-Alcohol, SDI-Methamphetamine, and SDI-Cocaine) and Sex (2) ANOVA for go-signal RTs with age and years of education as covariates did not result in a significant effect (all ps > .10) The same holds for stop-signal RTs (SSRTs).
Stepwise regression analyses
We performed a stepwise regression analysis to clarify the relationship between decision-making performance (total advantageous – total disadvantageous), drug status, sex and performance on various executive functioning tasks. With this method variables are entered in the model based on the semi-partial correlation with the dependent variable, i.e. based on the relationship between the individual variable and the dependent variable while controlling for the effect of the other variables on the dependent variable. For each new variable added, it is tested whether the variance explained (i.e. R2) was significantly increased and whether both variables were needed. First we explored simple correlations between decision-making and all other individual variables to determine which variables to include in the combined model. Decision-making was significantly correlated with education (r = .38), drug status (r = -.40) sex (r = -.23), % correct on working memory (r = .23), total switching reaction time (r = -.23) and total correct on WCST (r = -.24), all ps < .02. Subsequently, these variables were used in the regression model. All the collinearity statistics were in the normal range, suggesting the predictor variables were not highly correlated with each other. The first variable entered was drug status, which accounted for 17% of the variability in decision-making (F (1, 82) = 16.82, p < .001; see table 3). Sex was entered in the second step, which accounted for an additional 14% of the variability in decision-making (Fchange (1, 81) = 16.54, p < .001). Lastly, total correct on WCST contributed significantly to decision-making and accounted for an additional 4.3% of the variability (Fchange (1, 80) = 5.34, p < .05). Thus a total of 35% of the variability in decision-making was explained by these three variables (see table 3). The other variables, including % correct on working memory, total switch rt and age did not meet the criteria to be included in the model. Even though collinearity cutoffs were not violated, significant intercorrelations between working memory and switching probably explained why these indices did not reach significance in the regression. We decided to drop switching to estimate the effect of working memory on decision-making. Again, total % correct on the working memory task did not contribute significantly to performance on the IGT (p = .45).
Table 3. Hierarchial regression analysis.
| B | Std. Error | β | |
|---|---|---|---|
| Step 1a | |||
| Constant | 24.66 | 4.61 | |
| Drug status | -10.98 | 2.68 | -.41** |
| Step 2b | |||
| Constant | 56.81 | 8.97 | |
| Drug status | -10.23 | 2.46 | -.38** |
| Sex | -22.11 | 5.44 | -.38** |
| Step 3c | |||
| Constant | 102.15 | 21.47 | |
| Drug status | -8.97 | 2.46 | -.34** |
| Sex | -23.08 | 5.31 | -.39** |
| Total correct WCST | -.63 | .27 | -.21* |
R2 = .17 for step 1
ΔR2 = .14 for step 2
ΔR2 = .04 (all ps < .05).
p < .001
p < .05
Discussion
SDI with cocaine as their primary drug of choice and SDI with methamphetamine as their primary drug of choice performed below normal levels on all measures of decision-making, working memory and cognitive flexibility, as assessed with the IGT, the Tic Tac Toe working memory test and the task-switching paradigm respectively. The deficits of cocaine- and methamphetamine addicted individuals on working memory and cognitive flexibility were relatively mild, and the impairments did not increase as a function of delay (Bechara & Martin, 2004; Woods et al., 2005) or as a function of task switching. In addition, only drug status and sex, and to some extend also WCST performance, accounted for the variability in decision-making performance as modeled in the stepwise regression. Although performance on the working memory task and switching task correlated individually with decision-making, performance on neither of the tasks did significantly contribute to decision-making when modeled in combination with the other predictor variables.
Bechara and Martin (2004) speculated that impairment in working memory reported in SDI may lie in the executive component (including shifting and response inhibition). Furthermore, this type of working memory dysfunction as opposed to the mnemonic component of working memory is thought to interfere with decision-making. Our results are partly compatible with this notion, given that the memory component of working memory did indeed not contribute to decision-making. The WCST as a measure of shifting contributed to decision-making, indicating that shifting may play a role in IGT performance. However, our results also show that SDI-Cocaine and SDI-Methamphetamine performed below normal levels on our measure of working memory that explicitly tapped into mnemonic aspects of working memory, indicating that even this component of working memory may be mildly affected in SDI.
Contrary to our expectations, response inhibition as assessed with the stop-signal task as well as performance on the WCST was not affected in our sample of SDI. Some studies suggest that SDI are impaired on these tasks (e.g., Errico et al., 2002), but deficits do not seem to be robust enough when covariates such as age and education are taken into account especially with small sample sizes (see also Fals-Stewart & Bates, 2003). Convergent evidence has been reported for both the stop-signal task and the WCST in poly drug users, cocaine users and methamphetamine abusers (Grant et al., 2000; Hoffman et al., 2006; Verdejo-García et al., 2006; Verdejo-García & Pérez-García, 2007; Bartzokiz et al., 2000). In addition, duration of abstinence may be linked with performance improvements on these executive functioning measurements. Longer duration of abstinence from alcohol for example, is associated with better performance on short-term memory task (Brandt, Butters, Ryan & Bayog, 1983 in Medina, Shear, Schafer, Gangopadhyay & Dyer, 2001). Similar results have been reported in abstinent cocaine abusers, in whom duration of abstinence was correlated with improved attention scores (O'Malley, Adamse, Heaton & Gawin, 1992) and IGT performance (Bartzokis et al., 2000). Importantly, chronic cocaine abuse was still related with mild, but definite neuropsychological deficiencies (O'Malley et al., 1992; Verdejo-García et al., 2006). In addition, although IGT performance was better in abstinent cocaine abusers (n = 6) than non-abstinent abusers (n = 6) in the study of Bartzokis et al. (2007), individual scores of the former group were still mostly in the impaired range. Thus, SDI-Cocaine and SDI-Methamphetamine do not seem to be impaired on some domains of executive functioning which is probably related to duration of abstinence. In addition, Fals-Stewart and Bates (2003) have suggested that the brain may have the capacity to compensate for the effects of a single drug. Nevertheless, significant impairments in various domains of executive functioning (e.g. decision making) persist in people addicted to cocaine or methamphetamine. This suggests that the neurotoxicity of cocaine and methamphetamine may be associated with more severe and even permanent structural and functional damage to the frontal lobe.
On the other hand, individuals who identified alcohol as their drug of choice in our sample showed no evidence of deficits in decision-making, working memory, cognitive flexibility or response inhibition, indicating that the effects of alcohol in non-Korsakoff alcoholics on executive functioning are milder if present at all (Gonzalez et al., 2007; Oscar-Berman & Marinković, 2007). Nevertheless, we need to interpret this finding with caution, since it is difficult to isolate ‘pure’ users. We only included SDI who used their drug of choice over 80% of the time prior to their treatment, but drug use is often not constant over their lifetime (Gonzalez et al., 2007) and especially the subjects addicted to either cocaine or methamphetamine tended to use other drugs occasionally. Another complication is that substance dependence tends to be associated with mood disorders (see Bechara & Martin, 2004), and it is difficult to interpret the differential effects of drug phenotypes. However, from a treatment point of view, our findings may be helpful in tailoring intervention programs.
This study also made an attempt to address sex differences in SDI. Contrary to our expectations, no sex differences on the IGT were found in the healthy comparisons and the alcohol dependent group. However, women addicted to either cocaine or methamphetamine showed more severe impairments in decision-making than men addicted to cocaine or methamphetamine. Interestingly, the possibility is raised that the OFC may be affected differently by cocaine or methamphetamine in men and women (Adinoff et al., 2003; Barr et al., 2006; Chang, Ernst, Strickland, Mehringer, 1999; Levin, Holman, Mendelson, et al., 1994; Scott et al., 2007). For example, Adinoff et al. (2006) showed that women addicted to cocaine showed deficient perfusion bilaterally in the medial OFC compared to healthy women, whereas men addicted to cocaine showed deficient perfusion on the both sides of the lateral OFC compared to healthy men. The significance of differences in activation patterns is not well understood, but might be associated with reward versus punishment processing (O'Doherty, Kringelbach & Rolls, et al., 2001). Also at a behavioral level there is evidence that substance abuse may impact men and women differently. Most notably is the notion of “telescoping” in women (i.e. faster progression to drug dependence after a relatively late onset), which is hypothesized to result from a sex specific biological vulnerability to psychoactive substances (Becker & Hu, 2008; Zilberman, Tavares & el-Guebaly, 2003). Taken together, there is emerging evidence for sex-specific effects of drugs on the central nervous and this issue needs further exploration.
Sex differences reported for IGT performance are not modulated by differences in the ability to reverse responses (Overman et al., 2006). Therefore, we believe that in spite of our finding that women in our sample were generally slower to switch, deficient switching is not the sole cause of sex differences in SDI. That women were slower to switch was unexpected and somewhat puzzling though. Although speculative, our results may be explained by the properties of the switching paradigm, which include fast and accurate left/right distinction. Women are more likely to rate themselves as having a poorer ability to make left/right distinctions and there is some evidence that such self-ratings influence actual performance (Jordan, Wüstenberg, Jaspers-Feyer, Fellbrich & Peters, 2004). Also, young girls are somewhat more reluctant to shift their responding to a newly positive stimulus, which has been called “checking” behavior (Overman, Bachevalier, Schumann & Ryan, 1994). However, self-ratings about left/right distinctions presumably do not influence simple switching (Jordan et al., 2004) and it is only in the early years of development that subtle sex differences in switching can be observed (Overman et al., 1994). Therefore, we need to evaluate this interpretation with cautiousness and replication is warranted before firm conclusions about potential sex differences with regard to switching can be made. A limitation of this study was the difference in age and education between healthy comparison participants and SDI. However, we took such differences into account by using ANCOVA. Age and education did explain a fair amount of the variance, but most group differences were still significant after age and education effects were partialled out. We are therefore confident that our group differences are not confounded by the differences in these attribute variables, and we would cautiously infer that the differences are, instead, due to drug abuse. The present study evaluated current executive functioning in SDI and did not address the question whether certain people have a biological predisposition (i.e. impulsivity) that may be a risk factor for the initiation of substance use. This scenario could be true; however, chronic substance use in addition to biological susceptibility may have an additive effect on pre-existing vulnerabilities.
Another important issue to keep in mind is that in our version of the IGT, facsimile monetary rewards were used. Although it has been shown that IGT performance did not differ between groups that were either playing for facsimile money or real money, there was more variation in the facsimile group (Bowman & Turnball, 2003). Larger variation creates a greater possibility of false positive and false negative classification; however, our results replicate earlier findings reported in the literature (e.g. Verdejo-García et al., 2007a) which strengthens our notion that decision-making may be impaired in cocaine and methamphetamine abusers.
To conclude, SDI appear to have poor working memory and shifting skills, probably due to a deficient executive component in both cognitive domains. The impairments in executive functioning appear most pronounced in the domain of effective decision-making, thus in situations when there is no easy answer. In a review, Volkow and Fowler (2000) presented an interesting theory, demonstrating that addiction is the result of the interplay between different neural circuits associated with reward processing, motivation, memory and learning, and cognitive/executive control. Volkow and Fowler (2000) argued that the disruption of the PFC leads to loss of self-directed behavior in favor of more automatic sensory-driven behavior. An interesting avenue for future research would be to examine how manipulating reward values can alter SDI's performance on PFC functions. If performance in different domains of executive functioning can be influenced by reward values, then this might provide important insight for treatment programs. Future research is necessary to replicate our findings and assess the relevance of sex-specific differences in people addicted to substances.
Acknowledgments
Supported by NIDA R01 DA022549 and R01 DA11779. We thank Cynthia Cutshall and Jill Arnold for their invaluable assistance in testing the participants of this study and collecting the data. The authors also like to thank Mariette Huizinga for providing the computerized tasks and Rupa Gupta for helpful comments during the process of writing the manuscript. Finally, we thank the anonymous reviewers for their constructive remarks on earlier versions.
Contributor Information
Ellen A. A. van der Plas, Department of Neurology, University of Iowa, Iowa City, IA, USA
Eveline A. Crone, University of Leiden, Leiden, The Netherlands
Wery P. M. van den Wildenberg, University of Amsterdam, Amsterdam, The Netherlands
Daniel Tranel, Department of Neurology, University of Iowa, Iowa City, IA, USA.
Antoine Bechara, Brain and Creativity Institute and Department of Psychology, University of Southern California, Los Angeles, CA, USA.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KF, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clinical Experimental Research. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Best SS, Harris TS, Chandler P, Frock SD, Williams MJ. Regional cerebral blood flow in female cocaine-addicted subjects following limbic activation. Drug and Alcohol Dependence. 2003;71:255–268. doi: 10.1016/s0376-8716(03)00138-8. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD. Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-depedent men and women. Gend Med. 2006;3(3):206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alting von Geusau NJ, Stalenhoef P, Huizinga M, Snel J, Ridderinkhof KR. Impaired executive function in male MDMA (“ecstasy”) users. Psychopharmacology. 2004;173:331–341. doi: 10.1007/s00213-004-1832-8. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. In: Gazzaniga MS, editor. The Cognitive neurosciences. Cambridge, Mass.: MIT Press; 1994. pp. 755–764. [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DL, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31(5):301–313. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Beckson M, Rapoport R, Grant S, Wiseman EJ, London ED. Abstinence from cocaine reduces high-risk responses on a gambling task. Neuropsychopharmacology. 2000;22:102–103. doi: 10.1016/S0893-133X(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky Business: Emotion, Decision-Making and Addiction. Journal of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-Making And Addiction (Part I): Impaired Activation of Somatic States in Substance Dependent Individuals when Pondering Decisions with Negative Future Consequences. Neuropsychologia. 2002;40(10):675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision-making, and the orbitofronta cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired Decision-Making Related to Working Memory Deficits in Individuals with Substance Addictions. Neuropsychology. 2004;18(1):152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio AR. The somatic marker hypothesis and decision-making. In: Grafman J, editor. Handbook of neurpsychology: Frontal lobes. 2nd. Vol. 7. Amsterdam: Elsevier; 2002. pp. 117–143. [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. NeuroImage. 2006;30:529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochick JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bowman CH, Turnbull OH. Real versus facsimile reinforcers on the Iowa Gambling Task. Brain and Cognition. 2003;53:207–210. doi: 10.1016/s0278-2626(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatr. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Easton C, Bauer L. Neuropsychological differences between cocaine-dependent, alcohol dependent, and dually-dependent patients. Psychiatry Res. 1997;71:97–103. doi: 10.1016/s0165-1781(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairments in abstinent male alcoholics. Alcoholism: Clinical and Experimental Research. 2002;26(8):1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Bates ME. The neuropsychological test performance of drug-abusing patients: An examination of latent cognitive abilities and associated risk factors. Experimental and Clinical Psychopharmacology. 2003;11(1):34–45. doi: 10.1037//1064-1297.11.1.34. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychological Review. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive fuctions among individuals with methamphetamine or alcohol as drug of choice: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory and the mind. Scientific American. 1992;267:111–117. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision-making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay-discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wüstenberg T, Jaspers-Feyer F, Fellbrich A, Peters M. Sex differences in Left/Right confusion. Cortex. 2006;42:69–78. doi: 10.1016/s0010-9452(08)70323-x. [DOI] [PubMed] [Google Scholar]
- Klüber A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. European Journal of Neuroscience. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Krill JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Levin JM, Holman BL, Mendelson JH, Teoh SK, Garada B, Johnson KA, Springer S. Gender differences in cerebral perfusion in cocaine abuse: Technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med. 1994;35:1902–1909. [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and actions: A theory of act and control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemipheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Miyake A, Frieman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2002;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Schafer, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Archives of Clinical Neuropsychology. 2004;19:245–258. doi: 10.1016/S0887-6177(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Kimura D. Sex differences for speech and manual skill. Percept Mot Skills. 1996;82:3–13. doi: 10.2466/pms.1996.82.1.3. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. American Journal of Drug and Alcohol Abuse. 1992;18(2):131–145. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Overman WH, Bachevalier J, Schuhmann E, Ryan P. Cognitive gender differences in very young children parallel biologically based cognitive gender differences in monkeys. behavioral neuroscience. 1996;110(4):673–684. doi: 10.1037//0735-7044.110.4.673. [DOI] [PubMed] [Google Scholar]
- Overman WH, Graham L, Redmon A, Eubank R, Boettcher L, Samplawski O, Walsh K. Contemplation of moral dilemmas eliminates sex differences on the Iowa Gambling Task. Behavioral Neuroscience. 2006;120(4):817–825. doi: 10.1037/0735-7044.120.4.817. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Manning JT, Reimers S. The effects of sex, sexual Orientation, and digit ratio (2D:4D) on mental rotation performance. Arch Sex Behav. 2007;36:251–260. doi: 10.1007/s10508-006-9166-8. [DOI] [PubMed] [Google Scholar]
- Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. Journal of Neuroscience. 2000;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Li C, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124(2):207–231. [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Azrin RL. Neuropsycholgical functioning in drug abusers. Drug and Alcohol Dependence. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Smith EE. Neural basis of human working memory. Current Directions in Psychological. 2000;9:45–49. [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Stuss DT, Levine B. Adult clinical Neuropsychology: Lessons from studies of the frontal lobes. Annual Reviews in Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontacortex activity is reduced in gambling and non-gambling substance users during decision-making. Human Brain Mapping. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Anderson SW, Benton A. Development of the concept of ‘executive functioning’ and its relation to the frontal lobes. In: Boller F, Spinnler H, Handler JA, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. pp. 125–148. [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex. Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Verejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. Journal of International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Pérez-García M. Ecological assessment of executive functions in substance depedent individuals. Drug and Alcohol Dependence. 2007;90:48–55. doi: 10.1016/j.drugalcdep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing witht the Iowa Gambling Task. Drug and Alcohol Dependence. 2007a;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Rivas-Pérez C, Vilar-Lopez R, Pérez-García M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug and Alcohol Dependence. 2007b;86:139–146. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependece. Neurpsychology. 2005;19(1):35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Yehene E, Meiran N. Is there a general switching ability? Acta Psychologica. 2007;126:169–195. doi: 10.1016/j.actpsy.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Zilberman M, Taveres H, el-Guebualy N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22(4):61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]


