Summary
Vascular involvement is frequent in systemic sclerosis, but the role of the lymphatic vasculature is poorly known. Our aim was to evaluate lymphatic vessels in systemic sclerosis skin lesions. We studied skin forearm biopsies of 9 patients with systemic sclerosis and 7 age-matched controls. Lymphatic vessels were labeled with the monoclonal antibody D2-40 and blood vessels with a polyclonal antibody to von Willebrand Factor. All blood and lymphatic vessels present in each section were counted and total area, inner luminal area, and shape factors were measured. The number of blood and lymphatic vessels in papillary dermis was greater and their diameter lower than in reticular dermis both in systemic sclerosis and controls. In the reticular dermis, the number of lymphatic vessels was markedly reduced in systemic sclerosis (4.9 ± 1.1 μm−2 versus 8.9 ± 1.2 μm−2P = .03), and a similar trend was observed in papillary dermis (8.4 ± 3.7 μm−2 versus 8.1 ± 5.3 μm−2). Interestingly, the number of periglandular lymphatics in systemic sclerosis was not different from controls. The inner luminal area (possibly indicating compensatory dilation) of lymphatic vessels, particularly the periglandular ones, was greater in systemic sclerosis than in controls. No differences were observed in the number of blood vessels, but the percentage of blood vessel profiles (without lumen) was significantly less in systemic sclerosis both in papillary and in reticular dermis. In conclusion, our data show that skin lesions in systemic sclerosis are characterized by a selective rarefaction of lymphatic vasculature that spares periglandular vessels and that might have a pathogenic role in the evolution and in the clinical manifestations of the disease.
Abbreviations: SSc, systemic sclerosis; vWF, von Willebrand Factor; VEGF, Vascular Endothelial Growth Factor
Keywords: Systemic sclerosis, Lymphatic vessels, Blood vessels, Skin, Immunohistochemistry
1. Introduction
Scleroderma or Systemic Sclerosis (SSc) is an autoimmune connective tissue disease of unknown etiology characterized by abnormalities of vasculature, immune system, and extracellular matrix that lead to fibrosis of the skin and internal organs (lungs, gastrointestinal tract, heart, and kidneys) [1,2].
Vascular injury and endothelial activation are among the earliest and potentially primary events in the pathogenesis of SSc [3-5]. The first symptom is Raynaud's phenomenon (RP), an episodic and reversible vasospasm of the fingers and toes. Under normal conditions or in primary (idiopathic) RP, nailfold capillaroscopy shows a regular disposition of the capillary loops along the nail bed whereas in secondary RP there are numerous capillaroscopic abnormalities, suggesting the presence of a connective tissue disease [6]. In the skin of SSc patients, nail fold videocapillaroscopy shows progressive changes in the capillary network [7] with the advancement of disease. At the early stages, capillary distribution is well preserved with few enlarged/giant capillaries and few capillary hemorrhages that become subsequently more frequent, with a mild disorganization of the capillary architecture and the presence of edema. The “late” nail fold video-capillaroscopy pattern is characterized by irregular capillary enlargement, hemorrhages, severe loss of capillaries with extensive avascular areas, disorganization of the normal capillary array, and appearance of ramified/bushy capillaries.
The abnormal function of the endothelium leads to edema, leukocyte and platelet adhesion, and perivascular inflammation. The earliest signs of fibrosis appear in the areas where vascular perturbation is most severe [4].
More recently, a reduction in capillary density has also been observed in forearm skin biopsies of SSc patients. Interestingly, the changes appeared to involve mostly capillaries without a visible lumen (“profiles”) and to be reversed by stem cell therapy [1].
The characteristic reduction of capillary density in SSc skin suggests a deregulated, insufficient angiogenic response. The term angiogenesis describes a complex process that leads to the formation of new vessels by sprouting of differentiated endothelial cells from pre-existing ones. In the skin of SSc patients, angiogenesis is insufficient despite severe hypoxia [8], a well-known major proangiogenic stimulus. Vascular endothelial growth factor (VEGF), a regulatory factor involved in several steps of physiological and pathological angiogenesis, is strongly overexpressed in the skin and serum of SSc patients [9] and serum levels of VEGF correlate with the development of fingertip ulcers [10]. Overexpression of VEGF occurring over a limited time has been shown to induce the formation of new functional vessels in adult organs. Prolonged exposure to VEGF leads, however, to formation of a chaotic vessel network with megacapillaries and reduced blood flow, resembling the disturbed vessel morphology of SSc patients [11]. Another vascular disorder, possibly related to growth factor stimulation of blood vessels, telangiectasias, is frequently seen in the limited form of SSc, most commonly on the face but also on the hand and internally [12].
Lymphatic vessels are also extensively present in the skin and are known to participate in a variety of physiological and pathological processes that could be relevant in SSc, including lymphoedema, wound healing, and inflammatory reactions [13-15]. The investigation of lymphatic vessels has been long hampered by the difficulty in their identification and distinction from blood vessels, particularly venules. Moreover, when lymphatics are not filled with lymph, they tend to collapse and may become impossible to recognize. The recent discovery of D2-40 [16], a monoclonal antibody to podoplanin [17], that consistently and specifically reacts with lymphatic endothelium, has made histological study of lymphatic vessels feasible. In the present study we evaluated the distribution and morphology of lymphatic vessels stained with D2-40 and blood vessels stained with von Willebrand Factor (vWF) in the forearm affected skin of SSc patients compared to controls. The aim was to understand why SSc patients have no clinical evidence of lymphoedema in spite of the profound alterations of their skin that might potentially affect lymphatic circulation. We wondered whether lymphatic vessels might be increased in number as in inflammation [14,18] and wound healing [19], decreased in number due to inhibition of lymphangiogenesis by transforming growth factor beta1 overexpression [20,21], which is a hallmark of fibrotic diseases [2] and/or dilated as in conditions of chronic lymphostasis [13].
To the best of our knowledge, the only report on lymphatic vessels in the skin of SSc patients is a fluorescence microlymphography study by Leu et al. [22]. A solution of fluorescein isothiocyanate-dextran was injected into the subepidermal layer of the skin of fingers, hands and forearms. Lymphatic capillaries became visible in healthy controls. In SSc patients, the clinically affected areas showed a pattern of lymphatic microangiopathy, already described in lymphoedema and chronic venous insufficiency, characterized by increased length of the visualized lymphatic capillaries and cutaneous backflow or even the complete absence of stained microlymphatics. There was a correlation between disease duration and complete disappearance of the lymphatic network. Despite this evidence, to date, there are no histologic studies on lymphatic vessels in SSc.
2. Materials and methods
2.1. Patients
Biopsies of affected skin were obtained with informed consent, and ethical approval (RFH Ethics Committee), from the forearms of 9 patients affected by SSc classified according to LeRoy [23], of which 5 had limited and 4 had diffuse SSc (Table 1). No further information was available for one of the patients with the limited form, who was referred from another institution. Three patients had relatively early disease (<2 years), 5 had long standing disease (>10 years), and 1 had an intermediate disease length of 4 years. Clinical assessment of patients is summarized in Table 2. Site-matched normal skin samples were obtained from 7 sex- and age-matched volunteers.
Table 1.
Patients characteristics. Skin score was assessed with the modified Rodnan's skin score
| SSc (9) | Limited SSc (5) | Diffuse SSc (4) | |
|---|---|---|---|
| Age | 51.5 ± 4.3a | 58 ± 5a | 45.2 ± 6a |
| Disease duration | 10 ± 3a | 9.2 ± 5a | 11 ± 5a |
| Skin score | 13.1 ± 4a | 13.7 ± 6.5a | 12.5 ± 5.7a |
| Fingertip ulcers | 1 | 1 | 0 |
| Autoantibodies | |||
| ANA+b | 6 | 3 | 3 |
| RNAP+c | 1 | 0 | 1 |
| Scl-70+d | 5 | 4 | 3 |
| DLCO (%)e | 46.6 ± 12.8a | 59.5 ± 22.4a | 33.7 ± 11.7a |
| PAHf | 1 | 0 | 1 |
mean ± SEM.
ANAs, antinuclear antibodies.
RNAP, anti-RNA-polymerase antibodies.
Scl-70, anti- Scl-70 autoantibodies (anti-topoisomerase I).
DLCO, carbon monoxide diffusion capacity.
PAH, pulmonary hypertension.
Table 2.
Clinical characteristics of SSc patients
| Limited SSc (5) | Diffuse SSc (4) | |
|---|---|---|
| Mean duration of Raynaud's at time of biopsy | 11 years 9 months | 3 years 8 months |
| Muscle involvement | No | 1 polymyositis |
| Joint involvement | 2 | No |
| Renal involvement | No | No |
| Heart involvement | No | No |
| Lung involvement | 2 pulmonary fibrosis | 4 pulmonary fibrosis (1 minimal) |
| Edema in forearms | 2 | 2 |
| Teleangectasias | 3 | 4 |
2.2. Immunohistochemistry
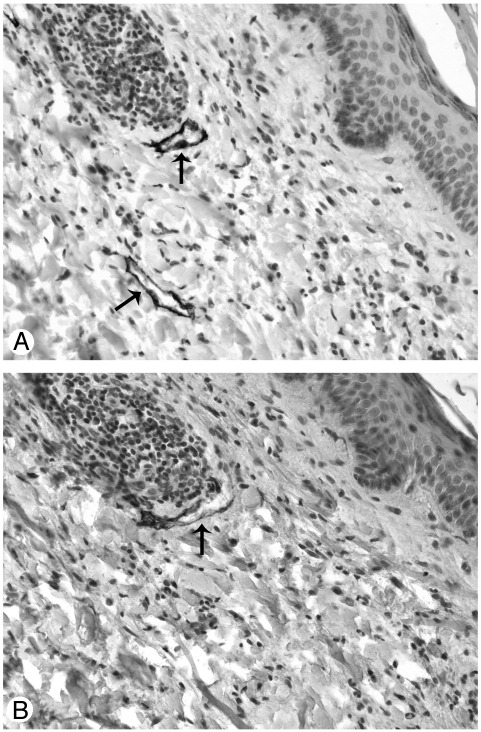
All samples were fixed in 10% formalin and embedded in paraffin. Before immunohistochemical labeling, sections were rehydrated via xylene and ethanol and placed for 10 minutes in boiling Antigen Unmasking Solution (VECTOR Laboratories, Burlingame, CA) for antigen retrieval. Endogenous peroxidase activity was quenched with 3% H2O2 for 15 minutes in the dark, and unspecific binding sites were blocked for 30 minutes with phosphate buffered saline containing 3% bovine serum albumin. Double labeling was performed by the sequential use of the monoclonal antibody D2-40 (Signet) that specifically stains lymphatic vessels [16] and a polyclonal antibody to vWF (DakoCytomation, Glostrup, Denmark), expressed by blood endothelium and, to a lesser extent, by lymphatic endothelium. Lymphatic vessels were stained by incubating sections for two hours at room temperature with D2-40 diluted 1:40 in phosphate-buffered saline containing 0.5% bovine serum albumin (hereafter referred to as buffer), followed by 30-minute incubation with antimouse IgG (Dako) diluted 1:25 in buffer and, eventually, 30 minutes with peroxidase-antiperoxidase (PAP-mouse, Dako) diluted 1:100 at room temperature. The reaction was revealed with 3,3′-diaminobenzidine Substrate Kit for Peroxidase (VECTOR), which contains a nickel solution that converts the brown color characteristic of 3,3′-diaminobenzidine in black. Lymphatic vessels were rendered easily recognizable by their black staining that was not covered by the subsequent red staining with the polyclonal antibody to vWF. Blood vessels were then stained by overnight incubation at 4°C with the polyclonal antibody to vWF (1:50) followed by a 45-minute incubation at room temperature with anti-rabbit/alkaline phosphatase (Dako) (1:50). The reaction was visualized with Fuchsine Substrate-Chromogen System (Dako) so that blood vessels were stained in red. Sections were counterstained with hematoxylin. In preliminary experiments aimed at selecting the most reliable lymphatic marker, the same vessels that stained strongly with D2-40 (Fig. 1A) were stained, not completely and less intensely by LYVE-1 (Lifespan Biosciences, Seattle, WA, 1:20) (Fig. 1B). D2-40 was thereafter used as lymphatic marker.
Fig. 1.
Consecutive sections of human control skin stained with two different lymphatic markers: the endothelium of the two lymphatic vessels indicated by arrows is intensely and completely stained by D2-40 (A), faintly and incompletely stained by LYVE–1 (B). Original magnification ×20.
Some sections were also stained with a polyclonal antibody to VEGF-C (Invitrogen, diluted 1:50 in buffer) followed by an alkaline phosphatase conjugated goat antirabbit secondary antibody (Dako, diluted 1:50 in buffer). The reaction was revealed by BCIP/NBT Substrate System (Dako).
Samples were viewed under a Nikon Eclipse E600 light microscope equipped with a digital camera. The entire section was photographed with 4× and 20× magnification objective.
2.3. Morphometric analysis
The area of papillary and reticular dermis was obtained from the total section area computed with a 4× objective after subtracting the area occupied by hypodermic fat and sebaceous glands. The whole area of each section was then examined with a 20× objective and all the lymphatic (stained in black by D2-40), and blood (stained in red by vWF) vessels in each section were counted. Total area, outer perimeter and inner luminal area were measured using the morphometric software “Nis Elements” by Nikon. Inner luminal area was automatically calculated by the software tracing the inner luminal profile and was expressed as a percentage of total vessel area measured tracing the outer profile of the vessel. Roundness factor was computed as (4π area)/perimeter2, and ellipse shape factor, as the ratio between the shorter and the longer radium of an ellipse having the same area and perimeter of each vessel. Both parameters can have a maximum value of 1 in the case of a perfectly circular vessel, with decreasing values as the shape of the vessel departs from circularity. Vessel density was expressed as the total number of vessels identified in each patient divided by the tissue area in mm2, and vessel area, as the percentage of the sum of the areas of all vessels over tissue area.
2.4. Statistical analysis
Vessel density and areas were compared using Student t test. Mean vessel area, percentage of inner luminal area, roundness, and ellipse shape factor were compared using linear mixed models with diagnosis (control or SSc), layer (papillary or reticular), and their interaction as fixed effects and the patient as random effects. Changes in the percentage of vessels with or without a visible lumen (profiles) were analyzed using similar logistic generalized mixed models. Mean estimates were obtained in the appropriate subsamples as the intercept and SE of the constant for fixed effect in models where no factors with fixed effects were included. Unless otherwise specified, data are presented as M ± SE. A value of P < .05 for a two-tail distribution was considered significant. All the analyses were performed using R version 2.8.1, http://www.r-project.org [24].
3. Results
3.1. General morphology
Under light microscopy, as expected, there were many tissue changes between the skin of SSc patients and controls: altered thickness of the epidermis, loss of dermal-epidermal ridges, and flattening of papillae (Fig. 2). These modifications became more severe with duration of disease. The epidermis of SSc patients with a duration of disease of more than 2 years had a mean thickness of 65.2 ± 6.7 μm (mean ± SE) versus 49.0 ± 4.0 μm of early SSc (first 2 years of disease) and 42.6 ± 3.4 μm of controls (P < .05). All the 3 patients with a duration of disease lower than 2 years had forearm edema. Forearm edema was also present in 1 of the 5 patients with a long duration of disease (23 years). Sebaceous and sweat glands and hair follicles appeared to be preserved also in SSc patients.
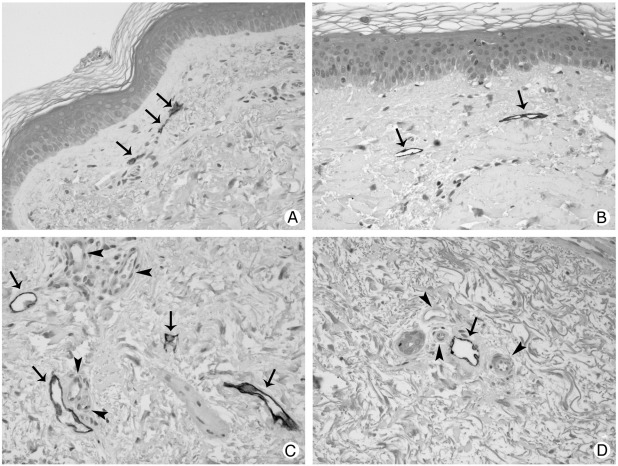
Fig. 2.
Double staining of lymphatic vessels with D2-40 (arrows) and blood vessels (arrowheads) with vWF in the papillary (A and B) and reticular (C and D) dermis of controls (A and C) and SSc patients (B and D). In SSc affected skin (B) papillae are flattened and the epidermis is thicker. Original magnification ×20.
3.2. Immunohistochemistry
Lymphatic vessels were consistently and intensely stained by D2-40. Cross-reactivity, even if of lower intensity, was only found in some epithelial cells particularly in the basal layer of sebaceous glands. No reactivity was ever found in blood vessels. Most of the lymphatic vessels had a patent lumen delineated by a tortuous and irregular profile (Fig. 1A). Their wall was made solely by endothelial cells which were stained in dark brown by the immunohistochemical reaction. Some lymphatic vessels were completely or partially collapsed. As to blood vessels, the endothelium and the subendothelial layer were stained in red by vWF (Fig. 2). The reaction was particularly pronounced in smaller vessels (capillaries and venules).
3.3. Morphometric evaluation
No differences were observed between sections from controls and SSc patients in the mean area of papillary dermis (0.15 ± 0.01 versus 0.16 ± 0.3 mm2) or of reticular dermis tissue (1.8 ± 0.2 versus 2.5 ± 0.6 mm2) present in each section. A total of 1365 blood vessels (527 in controls and 838 in SSc) and 215 lymphatic vessels (111 in controls and 104 in SSc) were identified and measured.
Both in controls and SSc biopsies, the density of lymphatic and blood vessels in the papillary dermis resulted markedly greater, and their mean area conversely smaller, than in the reticular dermis (Table 3 and Fig. 2).
Table 3.
Morphometric data of lymphatic and blood vessels
| Lymphatic vessels |
Blood vessels |
||||
|---|---|---|---|---|---|
| Papillary | Reticular | Papillary | Reticular | ||
| Vessel densitya | Controls | 18.1 ± 5.3 | 8.9 ± 1.2 | 139.5 ± 39 | 36.4 ± 6.7 |
| Ssc | 8.4 ± 3.7 | 4.9 ± 1.1⁎ | 113.6 ± 29.7 | 35.7 ± 4.6 | |
| Percentage vessel areab | Controls | 0.3 ± 0.1 | 0.4 ± 0.1 | 4.4 ± 0.9 | 2.2 ± 0.4 |
| Ssc | 0.1 ± 0.1 | 0.2 ± 0.04⁎ | 2.6 ± 0.5 | 2.1 ± 0.4 | |
| Mean vessel areac | Controls | 186 ± 53 | 511 ± 114 | 347 ± 58 | 657 ± 112 |
| Ssc | 290 ± 347 | 490 ± 111 | 263 ± 28 | 597 ± 85 | |
| Mean percentage luminal aread | Controls | 14.7 ± 6.8 | 15.9 ± 3.9 | 7.9 ± 1.6 | 11 ± 0.5 |
| Ssc | 15.6 ± 11.9 | 24 ± 4.9⁎ | 12.6 ± 1.1 | 12.7 ± 1.1 | |
| Ellipse shape factor | Controls | 0.21 ± 0.03 | 0.17 ± 0.02 | 0.5 ± 0.02 | 0.51 ± 0.01 |
| Ssc | 0.3 ± 0.04 | 0.22 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.01 | |
| Roundness | Controls | 0.39 ± 0.06 | 0.32 ± 0.03 | 0.77 ± 0.02 | 0.77 ± 0.01 |
| Ssc | 0.47 ± 0.09 | 0.4 ± 0.03 | 0.75 ± 0.02 | 0.73 ± 0.01 | |
| % Profiles | Controls | 41.3 ± 15.6 | 32.9 ± 8.1 | 19.9 ± 6.2 | 7.9 ± 2.6 |
| Ssc | 56.3 ± 12.8 | 27.5 ± 8.6 | 5.2 ± 3.2⁎ | 3.0 ± 1.4⁎ | |
Vessels/mm2.
Total vessel area/total tissue area × 100.
μm2.
Luminal area/vessel area × 100.
P < .05 versus controls.
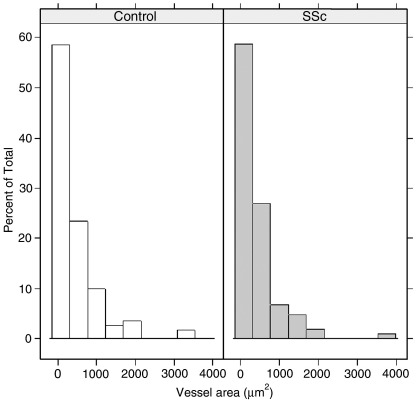
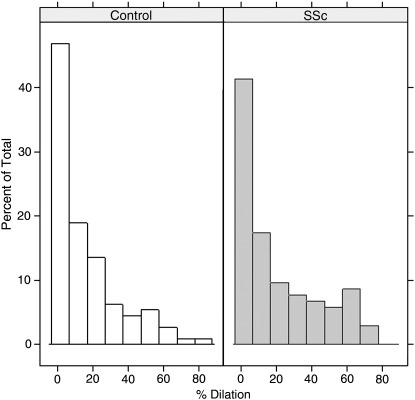
In SSc, the density of lymphatic vessels and their total area was significantly lower than in controls in the reticular dermis, and a similar trend (although not reaching statistical significance) was observed in the papillary layer. Furthermore, although the mean outer area was similar in the two groups (Table 3 and Fig. 3), in the reticular dermis the percentage of inner luminal area, which can be considered a sign of dilation (Table 3 and Fig. 4), was significantly greater in SSc with respect to controls (P < .05). This difference was mainly due to the dilation of periglandular lymphatics. As to profiles (vessels without visible lumen) there was no difference in lymphatic profiles number in SSc skin compared to controls (Table 3), whereas there was a significant (P < .05) reduction of blood profiles in SSc versus controls.
Fig. 3.
Distribution of lymphatic vessels in relation to their individual area (outer vessel area in μ2) does not differ in SSc versus controls.
Fig. 4.
Distribution of the percentage of dilation of lymphatic vessels evaluated as inner luminal area in relation to total area. Increased percentage of vessels with dilated lumen in SSc versus controls.
No differences were observed between the two groups in the density, total and mean area or inner luminal area of blood vessels either in the papillary or in the reticular dermis. However, the percentage of blood vessels without a visible lumen was significantly lower in SSc than in controls (P < .05), both in the papillary and in the reticular dermis (Table 3).
As expected, blood vessels showed a higher degree of circularity with respect to lymphatic vessels according to both the morphological form factors investigated. However, no statistical differences in these form factors were observed between the two groups. No statistical effects were observed when the age of patients and duration of disease were added to the statistical models.
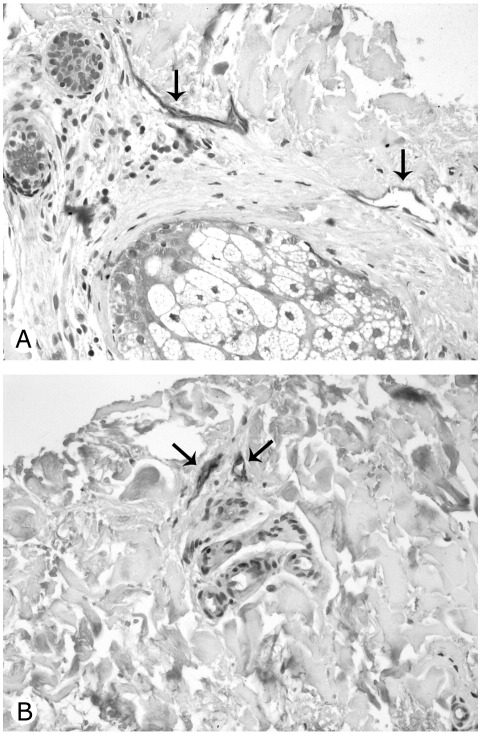
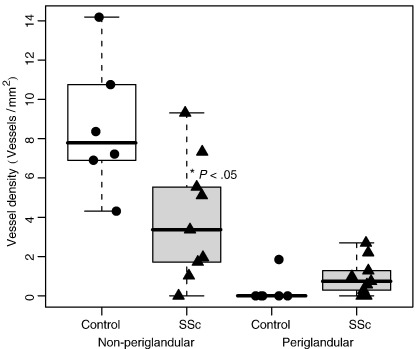
Because while scoring we had the impression that lymphatic vessels in connection with glands were relatively preserved in patients with SSc with respect to controls (Fig. 5A and B), we conducted a post hoc analysis comparing the density of lymphatic vessels connected or not connected with glands (<100 μm from the closest sebaceous or sweat gland). No differences were observed between the two groups in the area occupied by sebaceous glands or the density of sweat glands. The density of lymphatic vessels not connected to glands was reduced in SSc with respect to controls (3.9. ± 1.0 versus 8.6 ± 1.4 mm−2, P = .02), whereas an opposite trend, without a significant difference, was observed among lymphatic vessels close to glands (0.3 ± 0.3 versus 1.0 ± 0.3 mm−2) (Fig. 6). No differences between the two groups according to proximity to glands were observed among blood vessels.
Fig. 5.
The lymphatic vessels (arrows) around sebaceous (A) and sweat (B) glands are preserved in SSc patients. Original magnification ×20.
Fig. 6.
Box plot graph of the density of periglandular and not periglandular lymphatic vessels of reticular dermis in SSc patients compared with controls. In SSc patients there is a significant (P = .02) reduction of not periglandular lymphatic vessels; whereas periglandular lymphatic vessels are preserved. The line in the box represents the median, the box margins the upper and lower quartiles, and the whiskers the range.
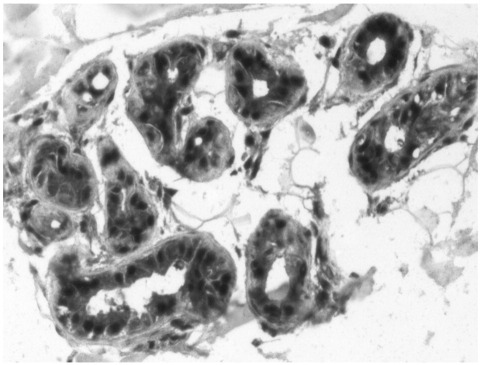
To assess whether periglandular lymphatic vessel preservation could be due to local production of lymphangiogenic factors, some sections were stained with a polyclonal antibody to VEGF-C. The epithelial cells of glands were strongly immunoreactive for VEGF-C (Fig. 7). As expected, lymphatic endothelium was also immunoreactive for VEGF-C (data not shown).
Fig. 7.
The epithelium of sweat glands is strongly immunoreactive for VEGF-C. Original magnification ×20.
4. Discussion
While the role of blood vessel and vessel dysfunction in the pathogenesis of SSc has been the object of much investigation, only a microlymphography study by Leu et al. [22] focuses on lymphatic vessels in this disease. The present immunohistochemical study is, to the best of our knowledge, the first detailed expression and morphometric analysis of lymphatic vessels in the skin of patients affected by SSc.
The affected skin of patients was altered in comparison to control skin: the epidermis thickness increased with duration of disease. Loss of dermal-epidermal ridges and flattening of papillae with effacement of rete reminded of the changes that has been described in aging skin in association with failure of vasculature [25].
A marked, significant decrease of lymphatic vessel number and an increase in the inner luminal area (implying dilation) of the remaining ones was observed in the reticular dermis of SSc patients compared with controls. A similar trend was seen in the papillary layer, possibly failing to reach statistical significance only because of the reduced statistical power, due to the relatively smaller area of the papillary as compared to the reticular dermis. Dilation of lymphatic vessels may be interpreted as a compensatory mechanism for the reduction in their number. Since skin lymphatic vessel are usually collapsed, the increased percentage of dilated lymphatic vessels may also indicate the presence of a certain degree of lymphostasis. Dilation of lymphatic vessels has been sporadically reported in other autoimmune diseases: in the skin in cutaneous histiocytic lymphangitis, an unusual manifestation of rheumatoid arthritis [26,27] and in the intestine in a case of protein losing enteropathy in systemic lupus erythematosus [28].
In contrast with the decrease in density of reticular dermis lymphatic vessels, we found that periglandular lymphatics are well preserved and dilated in the skin of SSc patients, suggesting that the microenvironment in the proximity of the glands is somewhat protective for lymphatics. The glands and the other skin appendages have been shown to be preserved, although reduced in number, even in the advanced skin pattern of the disease [29]. VEGF-C is expressed in the immature cells of sebaceous glands in an animal model of cutaneous wound healing [19]. In this study, we report that sweat glands are also positive for the lymphatic specific angiogenic factor VEGF-C. Based on these results we hypothesize that local production of angiogenic factors by sweat and sebaceous glands may be responsible for periglandular lymphatic preservation.
Unlike others [1], we failed to detect significant differences in the density of blood vessels between SSc and controls. This could be explained by differences in the patient population or in the methods used. Notably, Fleming et al [30] did not report separate data for papillary and reticular dermis, while we found that vessel density is very different in these regions. It is plausible that Fleming's data were biased by an imbalance in the percentage of papillary and reticular tissue present in the biopsies of the two groups that they examined. Nevertheless, we confirm their finding that the number of “profiles” (blood vessels without a visible lumen) is reduced in SSc.
Whatever the cause of the discrepancy with previous data, our data indicate that the lymphatic microcirculation is altered earlier and more consistently than its blood counterpart. It is difficult to speculate on the possible mechanisms of this phenomenon. Extensive studies on blood vessels in scleroderma suggests that vascular loss is due to a combination of vessel destruction by a variety of mechanisms [31], including defective angiogenesis [32], defective vasculogenesis (a reduction in number, and function of circulating endothelial stem cells has been found in SSc patients) [33-35], and auto-antibodies (particularly antiendothelial cell antibodies, which are commonly found in SSc patients) [31]. It is unknown whether the same antibodies may also act on lymphatic endothelium and/or whether autoantibodies specific for lymphatic endothelium exist. Although no information is available about lymphatic vessels in SSc, all these mechanisms could be involved in the decrease in lymphatic vessel density. Lymphatic vessels could be more sensitive to damage than blood vessels because of the more fragile structure of their wall [36], which is made by a thin layer of endothelial cells without smooth muscle cells or pericytes. As for angiogenesis, studies in the last decade have led to a better understanding of the cell and molecular mechanisms involved, showing that lymphangiogenesis and angiogenesis are partially characterized by a distinctive dependence from different ligands and receptors. Thus, angiogenesis is mostly dependent on the linkage of VEGF-A and B on receptors VEGF receptor-1 and VEGF receptor-2, while lymphangiogenesis is more dependent on VEGF-C and D binding to VEGF receptor-3 [13,37]. VEGF and VEGF receptors are by no means the only molecules involved, and many more have been identified. While several studies in SSc have investigated a variety of molecules involved in angiogenesis, sometimes with conflicting results (reviewed in Refs. [1,31,32]), much less information is available about mediators of lymphangiogenesis, and there are currently no data to support or explain a defect in lymphangiogenesis in these patients. In fact, both circulating levels of VEGF-C [38] and local expression of its lymphatic receptor VEGF receptor-3 in the skin [39] have been reported to be increased in patients with scleroderma, and no data are available on other molecules potentially involved. Bone-marrow-derived circulating endothelial precursors are thought to be recruited for the formation and repair of blood vessels in adults [40] and have also been found in newly formed lymphatic vessels in a corneal lymphangiogenesis model in mice [41]. Bone-marrow-derived circulating endothelial precursors are significantly reduced in the serum of SSc compared to rheumatoid arthritis patients and healthy subjects [33,34], but no data are yet available on circulating lymphatic endothelial precursors in these patients. Much more data are needed to investigate the distribution of a larger spectrum of molecules involved in lymphangiogenesis in scleroderma and to elucidate whether the reported overexpression of VEGF-C and VEGF receptor-3 despite apparent decrease in lymphatics constitutes an exaggerated response to a block of lymphangiogenesis in some downstream mechanism, such as a defect in circulating lymphatic precursors. Furthermore, it would be important to investigate whether lymphangiogenesis occurs in earlier stages of the disease.
Regardless of the mechanism and the timing, the disappearance of lymphatic vessels could have a pathogenic role in the evolution of the disease. Once lymphatic vessels are damaged and are no longer functional, fluids and macromolecules derived from blood microvessels may accumulate in the interstitial fluid causing edema at first and subsequently fibrosis. This is frequently observed in SSc patients in which an early edematous phase is followed with time by progressive fibrosis. Indeed, we found that the lumen of the lymphatic vessels was enlarged with respect to controls, suggesting that these vessels are relatively overloaded, possibly as a result of an increase in interstitial fluid due to the pathologic process and/or to the overall reduced capacity of lymph drainage caused by the decreased number of vessels.
In conclusion, we showed, for the first time, that lymphatic vessels are markedly affected in the skin lesions of scleroderma. This observation could lead to a better understanding of the pathogenic mechanisms of the early phases of the disease process, and open new avenues for therapeutic advantage.
Footnotes
This work was supported by PAR grants of the University of Siena, and the Arthritis Research Campaign, UK.
References
- 1.Fleming J.N., Schwartz S.M. The pathology of scleroderma vascular disease. Rheum Dis Clin North Am. 2008;34:41–55. doi: 10.1016/j.rdc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Varga J., Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claman H.N., Giorno R.C., Seibold J.R. Endothelial and fibroblastic activation in scleroderma. The myth of the “uninvolved skin”. Arthritis Rheum. 1991;34:1495–1501. doi: 10.1002/art.1780341204. [DOI] [PubMed] [Google Scholar]
- 4.LeRoy E.C. Systemic sclerosis. A vascular perspective. Rheum Dis Clin North Am. 1996;22:675–694. doi: 10.1016/s0889-857x(05)70295-7. [DOI] [PubMed] [Google Scholar]
- 5.Abraham D., Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. 2007;9:S2. doi: 10.1186/ar2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallenberg C.G. Early detection of connective tissue disease in patients with Raynaud's phenomenon. Rheum Dis Clin North Am. 1990;16:11–30. [PubMed] [Google Scholar]
- 7.Cutolo M., Pizzorni C., Tuccio M. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology. 2004;43:719–726. doi: 10.1093/rheumatology/keh156. [DOI] [PubMed] [Google Scholar]
- 8.Distler O., Distler J.H., Scheid A. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 9.Choi J.J., Min D.J., Cho M.L. Elevated vascular endothelial growth factor in Systemic Sclerosis. J Rheumatol. 2003;30:1529–1533. [PubMed] [Google Scholar]
- 10.Distler O., Del Rosso A., Giacomelli R. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4:R11. doi: 10.1186/ar596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dor Y., Djonov V., Abramovitch R. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahaleh B., Meyer O., Scorza R. Assessment of vascular involvement. Clin Exp Rheumatol. 2003;21:S9–S14. [PubMed] [Google Scholar]
- 13.Cueni L.N., Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 14.Jackowski S., Janusch M., Fiedler E. Radiogenic lymphangiogenesis in the skin. Am J Pathol. 2007;171:338–348. doi: 10.2353/ajpath.2007.060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajiya K., Kunstfeld R., Detmar M., Chung J.H. Reduction of lymphatic vessels in photodamaged human skin. J Dermatol Sci. 2007;47:241–243. doi: 10.1016/j.jdermsci.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn H.J., Marks A.A. new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 17.Schacht V., Dadras S.S., Johnson L.A., Jackson D.G., Hong Y.K., Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunstfeld R., Hirakawa S., Hong Y.K. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 19.Ji R.C., Miura M., Qu P., Kato S. Expression of VEGFR-3 and 5′-Nase in regenerating lymphatic vessels of the cutaneous wound healing. Microsc Res Tech. 2004;64:279–286. doi: 10.1002/jemt.20082. [DOI] [PubMed] [Google Scholar]
- 20.Oka M., Iwata C., Suzuki H.I. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 21.Clavin N.W., Avraham T., Fernandez J. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 22.Leu A.J., Gretener S.B., Enderlin S. Lymphatic microangiopathy of the skin in systemic sclerosis. Rheumatology (Oxford) 1999;38:221–227. doi: 10.1093/rheumatology/38.3.221. [DOI] [PubMed] [Google Scholar]
- 23.LeRoy E.C., Black C., Fleischmajer R. Scleroderma (systemic sclerosis) classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 24.West B.T., Welch B.T., Gatecki A.T., editors. Linear mixed models. A practical guide using statistical software. Chapman and Hall/CRC; Boca Raton (Fla): 2007. p. 353. [Google Scholar]
- 25.Ryan T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron. 2004;35:161–171. doi: 10.1016/j.micron.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Pruim B., Strutton G., Congdon S., Whitehead K., Donaldson E. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101–105. doi: 10.1046/j.1440-0960.2000.00404.x. [DOI] [PubMed] [Google Scholar]
- 27.Takiwaki H., Adachi A., Kohno H., Ogawa Y. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases. J Am Acad Dermatol. 2004;50:585–590. doi: 10.1016/j.jaad.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T., Damião A.O., Gabriel Júnior A. Protein-losing enteropathy in systemic lupus erythematosus. Rev Hosp Clin Fac Med Sao Paulo. 1991;46:34–37. [PubMed] [Google Scholar]
- 29.Ibba-Manneschi L., Niissalo S., Milia A.F. Variation of neuronal nitric oxide synthase in systemic sclerosis skin. Arthritis Rheum. 2006;54:202–213. doi: 10.1002/art.21543. [DOI] [PubMed] [Google Scholar]
- 30.Fleming J.N., Nash R.A., McLeod D.O. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE. 2008;e1452:3. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahaleh B. Vascular disease in scleroderma: mechanisms of vascular injury. Rheum Dis Clin North Am. 2008;34:57–71. doi: 10.1016/j.rdc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan-Kehoe M.J., Simons M. Vascular disease in scleroderma: angiogenesis and vascular repair. Rheum Dis Clin North Am. 2008;34:73–79. doi: 10.1016/j.rdc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Kuwana M., Okazaki Y., Yasuoka H., Kawakami Y., Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364:603–610. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- 34.Del Papa N., Quirici N., Soligo D. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54:2605–2615. doi: 10.1002/art.22035. [DOI] [PubMed] [Google Scholar]
- 35.Gomer R.H. Circulating progenitor cells and scleroderma. Curr Rheumatol Rep. 2008;10:183–188. doi: 10.1007/s11926-008-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan T.J. Structure and functions of lymphatics. J Invest Dermatol. 1989;93:18S–24S. doi: 10.1111/1523-1747.ep12580899. [DOI] [PubMed] [Google Scholar]
- 37.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 38.Chitale S., Al-Mowallad A.F., Wang Q., Kumar S., Herrick A. High circulating levels of VEGF-C suggest abnormal lymphangiogenesis in systemic sclerosis. Rheumatology (Oxford) 2008;47:1727–1728. doi: 10.1093/rheumatology/ken372. [DOI] [PubMed] [Google Scholar]
- 39.Mackiewicz Z., Sukura A., Povilenaité D. Increased but imbalanced expression of VEGF and its receptors has no positive effect on angiogenesis in systemic sclerosis skin. Clin Exp Rheumatol. 2002;20:641–646. [PubMed] [Google Scholar]
- 40.Asahara T., Masuda H., Takahashi T. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 41.Religa P., Cao R., Bjorndahl M. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]