Abstract
Objectives
To provide an understanding of the principles and applications of pharmacogenetics in drug therapy optimization.
Design
An online learning session accessed using the hospital intranet and a live case-based session were offered to hospital pharmacists, residents, students, and interns.
Assessment
Knowledge was assessed with “check your knowledge” questions, case discussions, and a follow-up questionnaire. Pharmacists evaluated the instructor and the course using an anonymous survey tool.
Conclusion
This education provided pharmacists with a basic understanding of pharmacogenetics and the ability to apply pharmacogenetics to clinical practice.
Keywords: pharmacy practice, pharmacogenetics, pharmacogenomics, continuing education, professional development
INTRODUCTION
Variability in patient response to medications has been attributed largely to non-genetic variants, such as age, weight, renal function, and drug-drug interactions. However, these factors only partially explain inter-individual differences. Pharmacogenetics could be an additional determinant of drug variability. Pharmacogenetics is the study of the impact of genetic polymorphisms on drug response.1 The goals of pharmacogenetics are to optimize drug efficacy, limit drug toxicity, reduce overall costs, and thereby improve the quality of care. Pharmacogenetics holds the potential for drugs to be tailored to an individual's DNA, helping health care providers choose the right medication and dose that will be safe and effective. Pharmacists are likely to be asked questions regarding pharmacogenetic test results and their effects on drug response.2 The role the pharmacist will play in pharmacogenetics will continue to expand over time. Historically, there has been a lack of education committed to pharmacogenetics in the pharmacy curriculum.3 The need for increased knowledge in pharmacogenetics has been addressed in the Accreditation Council for Pharmacy Education (ACPE) Standards 2007.4 In addition, the National Coalition for Health Professional Education in Genetics (NCHPEG) has developed a core set of competencies that all health professionals should possess.5 Topics covered at professional meetings are changing to reflect the emergence of pharmacogenetics.6
Unfortunately, pharmacists who have already graduated may not have been educated in pharmacogenetics. To meet this need in the current workforce, pharmacists at the Children's Hospital of Wisconsin were educated directly at the jobsite. This education used both an online introductory module, as well as a live case-based scenario session. Key goals, standards, and guidelines from the ACPE report and competencies from the NCHPEG were used to develop the program. The program encouraged critical thinking and problem-solving skills. This model allowed pharmacists to take responsibility for their own learning by allowing them to schedule sessions according to their workload and to set their own pace. The learning sessions encouraged self-study, as well as group case-based scenario studies. The pharmacists were assessed using “check your knowledge” questions within the online module, during the group cases in the live session, and at 6 months using a follow-up questionnaire. The pharmacists were also given the opportunity to evaluate the online course and the instructor for the live session using an anonymous survey tool. Experts from the hospital's education services department were consulted to help in the design and delivery of the program. This paper describes the topics included in the module, assessment methods, and program evaluation.
DESIGN
A new program to introduce pharmacogenetics was offered to pharmacists, residents, interns, and students at the Children's Hospital of Wisconsin. The training was required for the pharmacists. The course was developed and administered by 1 pharmacist, a board-certified pharmacotherapy specialist who consulted in the area of pharmacogenetics. The general educational outcomes for the course were based on the core set of competencies developed by the National Coalition for Health Professional Education in Genetics. The key concepts pharmacists learned upon completion of the training included:
basic human genetics and pharmacogenetics terminology
how genetic variations contribute to differences in drug response between individuals
how genetic variations affect drug metabolism, transport, and target proteins
how genetic polymorphisms affect drug efficacy and toxicity
how to utilize various information resources to answer questions related to pharmacogenetic testing
how to apply pharmacogenetic test results to individualization of drug therapy regimens
This training was divided into 2 parts. The first part was an online training session that included topics shown in Table 1. Pharmacists accessed the training module through the hospital intranet. Recent pharmacy graduates, who had been exposed to at least some level of pharmacogenetics in their college education, were able to complete the online training in approximately 30 minutes. Others who required more in-depth information were able to spend more time on the session, which varied from person to person. In addition, individuals were able to access the program as often as desired. Pharmacists were able to set their own pace for completing this education, based on their workflow for the day and understanding of the material.
Table 1.
Topics Included in Pharmacogenetics Education, Part 1
The first part of the online training included an introduction, the education plan, and the key concepts for the education session. The next section provided a definition of pharmacogenetics, as well as the goals of pharmacogenetics. The module stressed the importance of studying pharmacogenetics and the role of the pharmacist in helping to tailor medications to an individual's deoxyribonucleic acid (DNA). The next section informed the reader of the many factors that influence drug response (gender, body weight, organ function, concomitant drug use, diet, disease state), as well as genetic variability. Next, basic genetic and pharmacogenetic terms, such as genotype/phenotype, gene, allele, homozygous/heterozygous, reference, and variant were defined. The training included information about basic genetics, such as the human genome, the pairing of nucleotide bases, and a basic review of DNA and ribonucleic acid (RNA) structure. Forms of variations (deletions, insertions, substitutions, and duplication) and frequency of variations (rare mutations versus common mutations) were described, as well as the differing frequencies among ethnic varieties. The participant then learned about cytochrome P450 (CYP) nomenclature and was guided to an online site, www.cypalleles.ki.se, for a full listing of CYP alleles. The next portion of the module described the first phase of a pharmacogenetics implementation project specific to the hospital that was used to individualize antiepileptic medications for patients experiencing their first seizure. Basic treatment of epilepsy was then covered, as well as a list of commonly prescribed antiepileptic medications. Questions were assessed at this point regarding the treatment of epilepsy. The Epilepsy Gene Panel at our hospital was described, which included 3 drug metabolizing enzymes (CYP2C9, CYP2C19, EPHX1) and 1 drug transporter (ABCB1). The remainder of the training discussed in detail information regarding the metabolism, transport, and target sites of each of the commonly prescribed antiepileptic medications. Where available, information on pharmacogenetics studies was included. The training concluded with a summary, “check your knowledge” questions, and references. The “check your knowledge” questions were basic cases with multiple-choice responses drawn from information learned in the online module. These questions were designed to lead the participant into the next session, the live case-based scenarios.
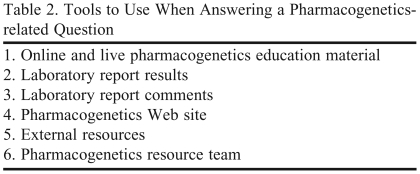
The live case-based scenario sessions lasted approximately 40 minutes and were offered in the pharmacy conference room during several lunch breaks. Two computers with intranet/Internet access were available. Each session had an average of 5 participants. Two handouts were available at these sessions with information on the tools available to answer a pharmacogenetics question (Table 2), the demonstration case, group cases, and a copy of the Epilepsy Gene Panel laboratory report.
Table 2.
Tools to Use When Answering a Pharmacogenetics-related Question
The live session opened with updates to the program since the online version was posted. The instructor also led a brief discussion about the history of the program at the hospital, as well as how pharmacists could be called upon for questions related to pharmacogenetics testing and how results of testing could affect medication therapy. There was a brief discussion about emergent situations, highlighting that emergent medication management should be completed based on standard therapeutic practices. While genotype could alter chronic dosing, the emergency is treated first. If the patient needs maintenance dosing, then pharmacogenetics could be considered at that time. Therefore, emphasis was placed on pharmacist involvement in non-emergent questions.
In the live session, the details of the Epilepsy Gene Panel laboratory report were discussed. A description of each of the enzymes and the transporter was given, along with information regarding active, partially active, and inactive alleles. The instructor gave examples of how genotypes with various combinations of alleles (ie, 1 active allele and 1 partially active allele versus 2 inactive alleles) could have varying degrees of enzyme function. A “check your knowledge” was done at this point. Questions included “what response to a drug would you expect from an individual with 2 inactive alleles” and “what response to a drug would you expect from an individual with 1 active allele and 1 inactive allele.”
Next, participants in the session logged on to the hospital intranet and accessed the pharmacogenetics Web site for a tour of the resources available. The pharmacogenetics Web site, which was developed by the pharmacogenetics implementation team, contains a variety of resources thought to be helpful when evaluating a patient who has had pharmacogenetic testing done. (See Table 3 for a list of resources posted on the pharmacogenetics Web site.)
Table 3.
References and Resources Available on the Pharmacogenetics Web site
For the remainder of the live session, pharmacists were involved in case studies to assess their knowledge of the material. The case studies focused on epilepsy patients who were also on other non-epilepsy medications. We anticipated that pharmacists would receive questions from physicians other than neurologists not as familiar with the test results.
The first 2 case studies were done together with the instructor. The pharmacists were informed that there were 3 categories of response to a pharmacogenetics-related drug question:
The medication is not metabolized or transported by any of the genes that have been reported for the patient. Therefore, no dosage adjustments are required for the medication in question.
The patient does have a variant allele and may have an increase or decrease in function, but the medication in question is metabolized using multiple pathways, so the variant genotype is not likely to affect dosing for the drug. The provider should proceed with standard dosing.
The patient has a variant allele that studies show may impact dosing. The provider may need to adjust the dose or choose an alternative medication, especially for a drug with a narrow therapeutic index.
Next, participants were divided into 2 groups. Participants in each group completed 2 case studies using the resources shown earlier in the session, then presented their findings to the other group.
EVALUATION AND ASSESSMENT
The live session was developed to encourage critical thinking and provide an opportunity to apply knowledge using problem-solving skills. Participants were assessed and given immediate feedback regarding their responses. Pharmacists anonymously evaluated the instructor, the online course, and the live session following completion of the course using a standard evaluation form provided by the hospital.
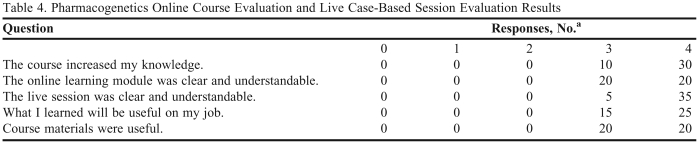
Over 50 pharmacists, residents, students, and interns completed the pharmacogenetics training sessions. Forty pharmacists filled out the course evaluations, results of which are shown in Table 4. Participants in the sessions were generally pleased with the program. One hundred percent of the pharmacists returning evaluations agreed or strongly agreed the course increased their knowledge, the course was clear and understandable, and the course materials were useful. Most participants who wrote additional free text commented that the live class was more helpful than the online module. After the program was live for 6 months, a follow-up questionnaire was given to the pharmacists. This questionnaire assessed how well the pharmacists recalled the information that was taught 6 months prior, and also assessed how well they were applying what they learned to patient cases. Pharmacists were allowed to use any resources which were introduced in the previous sessions to aid in answering the questionnaire.
Table 4.
Pharmacogenetics Online Course Evaluation and Live Case-Based Session Evaluation Results
aScale definition: 1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree, 0 = does not apply
All pharmacists completing the questionnaire were able to correctly answer questions regarding the definition and goals of pharmacogenetics, which factors influence drug response, and basic genetic terminology. All pharmacists completing the questionnaire were able to correctly identify the 4 genes included on the hospital's Epilepsy Gene Panel. Although some of the pharmacists had different opinions regarding drug-level monitoring, all agreed on dosing adjustments based on genotype and were able to provide appropriate responses with justification to the cases presented in the follow-up questionnaire. (Examples of the “check your knowledge” questions, case studies, or follow-up questionnaire mentioned in this article can be obtained from the author.)
DISCUSSION
Participants in the training were excited to see the profession of pharmacy moving forward to adapt extended roles and address pharmaceutical needs beyond preparation and supply of medications. This training allowed practicing pharmacists to make preparations to enable them to take on greater involvement in pharmacogenetics. The pharmacogenetics program is expanding at the Children's Hospital of Wisconsin and now is the time for pharmacists to embrace it. Participants in the sessions were eager to develop new roles. In fact, some individuals expressed that they would be disappointed if they learned this material and then were not given the opportunity to use this new skill. Feedback from the follow-up questionnaire at 6 months showed that pharmacists were able to retain the information and use the resources introduced at the sessions appropriately.
One challenge to teaching this course was the number of sessions (for the live portion) that were required to reach all of the pharmacists, as many of the them worked part-time and were not working on the days the training was initially offered. In addition, the live session was offered during the lunch break, as dedicated time was not provided by the department. This limited the time available per live session to approximately 40 minutes. The session required active participation, therefore, a common complaint was that there was no time to eat during the session. Also, reserving space in the pharmacy conference room, where computers with intranet/Internet access were available, was challenging, as the space was frequently occupied.
CONCLUSIONS
An online-learning module and a live case-based session were offered to hospital pharmacists to provide them with an understanding of the principles and applications of pharmacogenetics in drug therapy optimization. Evidence that objectives of the training were being met included comments from pharmacists that they had the confidence to answer pharmacogenetics-related questions from health care providers, and favorable results from the 6-month follow-up questionnaire.
This training will continue to be offered to new pharmacists as a required competency. Training material will continue to be updated as more studies become available or more genes are added to the panel. The program will continue to emphasize ways in which pharmacists can be a resource to the health care provider to help individualize medication therapy based on the patient's genotype.
REFERENCES
- 1.Rogers JF, Nafziger AN, Bertino JS., Jr Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am J Med. 2002;113(9):746–750. doi: 10.1016/s0002-9343(02)01363-3. [DOI] [PubMed] [Google Scholar]
- 2.Clemerson JP, Payne K, Bissell P, Anderson C. Pharmacogenetics, the next challenge for pharmacy? Pharm World Sci. 2006;28(3):126–130. doi: 10.1007/s11096-006-9029-3. [DOI] [PubMed] [Google Scholar]
- 3.Latif DA, McKay AB. Pharmacogenetics and pharmacogenomics instruction in colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2005;69(2):152–156. [Google Scholar]
- 4. Accreditation Council for Pharmacy Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree. Adopted January 15, 2006. http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed December 2, 2009.
- 5. National Coalition for Health Professional Education in Genetics. Core competencies in genetics for health professionals. Third edition, September 2007. http://www.nchpeg.org/core/Core_Comps_English_2007.pdf. Accessed December 2, 2009.
- 6.Streetman DS. Emergence and evolution of pharmacogenetics and pharmacogenomics in clinical pharmacy over the past 40 years. Ann Pharmacother. 2007;41(12):2038–2041. doi: 10.1345/aph.1K273. [DOI] [PubMed] [Google Scholar]