Abstract
Telomeres prevent chromosome ends from being repaired as double-strand breaks (DSBs). Telomere identity in Drosophila is determined epigenetically with no sequence either necessary or sufficient. To better understand this sequence-independent capping mechanism, we isolated proteins that interact with the HP1/ORC-associated protein (HOAP) capping protein, and identified HipHop as a subunit of the complex. Loss of one protein destabilizes the other and renders telomeres susceptible to fusion. Both HipHop and HOAP are enriched at telomeres, where they also interact with the conserved HP1 protein. We developed a model telomere lacking repetitive sequences to study the distribution of HipHop, HOAP and HP1 using chromatin immunoprecipitation (ChIP). We discovered that they occupy a broad region >10 kb from the chromosome end and their binding is independent of the underlying DNA sequence. HipHop and HOAP are both rapidly evolving proteins yet their telomeric deposition is under the control of the conserved ATM and Mre11–Rad50–Nbs (MRN) proteins that modulate DNA structures at telomeres and at DSBs. Our characterization of HipHop and HOAP reveals functional analogies between the Drosophila proteins and subunits of the yeast and mammalian capping complexes, implicating conservation in epigenetic capping mechanisms.
Keywords: capping complex, drosophila telomere, epigenetic mechanism, telomere maintenance
Introduction
Telomeres shield the ends of linear chromosomes from DNA repair activities. This capping function is essential for genome integrity, as uncapping can lead to chromosome fusions. Telomeres also facilitate the elongation of chromosome ends, a function performed by the telomerase enzyme in most eukaryotic organisms studied. Loss of telomerase function does not impair genome stability immediately, but only does so when telomeric repeats become critically short after several generations (Blasco et al, 1997; Riha et al, 2001; Meier et al, 2006). However, loss of the capping function can have immediate effects on genome integrity (van Steensel et al, 1998; Baumann and Cech, 2001; Ferreira and Cooper, 2001; Pardo and Marcand, 2005; Song et al, 2008), suggesting that the presence of telomeric repeats is not sufficient for maintaining telomere identity. Furthermore, specialized yeast and plant cells can be immortalized in the absence of telomeric repeats with protected telomeres, suggesting that the presence of the repeats is also not necessary for capping (Maringele and Lydall, 2004; Watson et al, 2005). These results suggest that sequence-independent capping might serve as a backup mechanism in telomerase-maintained organisms.
The understanding of this mechanism requires a clear picture of chromatin structure at telomeres. In lower eukaryotes, telomeric repeats are not packaged into regular nucleosomes (Wright et al, 1992), while the bulk of telomeric repeats in mammalian cells are packaged into nucleosome arrays (Makarov et al, 1993; Tommerup et al, 1994). Partly due to the repetitive nature of telomeric sequences, it has been difficult to study how duplex-binding proteins are distributed over telomeric chromatin in most organisms. The Rap1 protein from budding yeast binds telomeric repeats (Conrad et al, 1990) to serve its functions in telomere elongation and capping regulation (Marcand et al, 1997; Pardo and Marcand, 2005). Interestingly, Rap1 from budding and fission yeast and Taz1 from fission yeast have been localized to subtelomeric regions (Strahl-Bolsinger et al, 1997; Kanoh et al, 2005), suggesting that the binding of capping proteins need not be limited to the extreme end of a chromosome.
In Drosophila, telomere identity is determined epigenetically. Although telomeres are elongated by the transposition of telomere-specific retrotransposons, these elements are neither necessary nor sufficient for capping (reviewed in Rong, 2008a). In particular, terminally deleted chromosomes that lack telomeric retrotransposons are stable, hence capped, for many generations (Levis, 1989; Biessmann et al, 1992; Ahmad and Golic, 1998). In addition, population studies uncovered frequent occurrences of such terminally deleted chromosomes in natural populations (Kern and Begun, 2008).
Despite using a telomerase-independent mechanism for elongating chromosome ends, Drosophila use highly conserved factors to regulate capping. The ATM and ATR checkpoint kinases, along with the Mre11–Rad50–Nbs (MRN) complex and the ATRIP protein, respectively, control redundant pathways for capping regulation that are conserved in other organisms (Bi et al, 2005; Ciapponi et al, 2006; Oikemus et al, 2006). Several other proteins serving capping function in Drosophila have homologs in other organisms: HP1 (Fanti et al, 1998), UbcD1 (Cenci et al, 1997), Woc (Raffa et al, 2005) and the H2A.Z histone variant (Rong, 2008b). Epigenetic capping mechanisms that might be conserved in other organisms can be effectively studied in the unique system of Drosophila due to the natural uncoupling of the end capping function from the end elongation function.
Telomeres in yeast and mammals are capped by multi-subunit protein complexes that protect both the duplex and single-stranded regions of the telomere (Liu et al, 2004; Gao et al, 2007; Miyoshi et al, 2008). In Drosophila, the structural constituents of the ‘cap' remain poorly defined. The HP1/ORC-associated protein (HOAP) is cytologically present at telomeres, and loss of HOAP leads to telomere fusions (Shareef et al, 2001; Cenci et al, 2003). In this study, we isolated HOAP-interacting proteins by affinity immunoprecipitation, and identified the HP1-HOAP-interacting protein (HipHop) as a new component of the Drosophila capping complex. Using chromatin immunoprecipitation (ChIP) performed on a model telomere devoid of telomeric transposons, we discovered a large domain of telomeric chromatin enriched with HipHop, HOAP and HP1, suggesting that this capping complex prevents end fusion by maintaining a chromatin state that is independent of its underlying DNA sequence. Both HipHop and HOAP are fast-evolving proteins highlighting a common feature among telomeric-binding proteins in other organisms. On the basis of functional similarity and analogies in distribution patterns, we suggest that HipHop and HOAP serve similar function as subunits of the capping complex that bind the duplex region of telomeric DNA in other organisms.
Results
Isolation of HOAP-interacting proteins identified HipHop
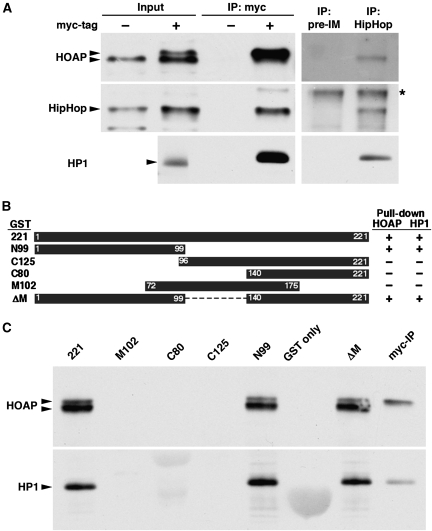
To isolate HOAP-interacting proteins by affinity purification, we tagged the endogenous HOAP-encoding caravaggio (cav) locus with a myc epitope tag at its N-terminus using gene targeting (Supplementary Figure S1). The myc-HOAP protein is functional in that flies with the myc-cav locus are viable and fertile whereas cav mutants are lethal (Cenci et al, 2003). We verified that a significant fraction of the cellular HOAP proteins are tightly associated with chromatin as reported earlier (Shareef et al, 2001), and generated a nuclease solublized chromatin extract from 0 to 12 h embryos for IP. Protein mixtures pulled down with an anti-myc antibody were analysed by mass spectrometry. As expected, peptides from HOAP were abundant in the ‘myc-tagged' but not the ‘untagged' extracts (Supplementary Figure S2). In anti-myc precipitants from the ‘tagged' extracts, we also identified peptides from HP1, a known HOAP-interacting protein, and peptides from a third protein encoded by the CG6874 gene (also known as l(3)neo26).
We verified the interaction between the CG6874 protein and HOAP using three different approaches. First, we generated an antibody against CG6874 and detected its presence in anti-myc IPs from myc-HOAP extracts but not from untagged extracts (left panels in Figure 1A). In a reciprocal experiment, anti-CG6874, but not its pre-immune serum, precipitated HOAP and HP1 from wild-type embryonic extracts (right panels in Figure 1A). Second, we purified GST-CG6874 fusion protein from bacteria and tested its ability to physically interact with HOAP in chromatin extracts from wild-type embryos. As shown in Figure 1C, GST-CG6874 produced from the ‘221' construct, but not the ‘GST only' control, precipitated both HOAP and HP1 from the extract. Using this assay, we mapped the domain in CG6874 that is responsible for interacting with HOAP and HP1 to the first 99 amino acids (‘N99' in Figure 1B and C). In the third approach, we demonstrated the ability of myc-tagged HOAP and FLAG-tagged CG6874 proteins to interact in co-transfection experiments using S2 cells (Supplementary Figure S3). On the basis of results from these approaches, we conclude that CG6874 strongly interacts with HOAP and HP1, and named CG6874 as the HP1-HOAP-interacting protein, or HipHop.
Figure 1.
HipHop interacts with HOAP and HP1. (A) Reciprocal IP between HipHop and HOAP. In the left three panels, embryonic extracts with (+) or without (−) myc-HOAP were subjected to anti-myc IP, followed by western blot analyses with antibodies against the antigens indicated to the left. Input samples were loaded as controls. The arrowheads mark the position of the proteins of interest. For HOAP, the two arrowheads mark myc-HOAP (upper) and HOAP (lower), respectively, as the extracts were from embryos having both myc-cav and cav alleles (see Supplementary data for explanation). In the right three panels, wild-type (untagged) extracts were subjected to IP with either a HipHop antibody (HipHop) or its corresponding pre-immune serum (pre-IM). In the HipHop western blot, a non-specific band is marked with *, which also served as a loading control. (B) GST-HipHop fusion proteins used to map the HOAP-interacting domain. The names of the construct are listed to the left of the black boxes, which indicate the regions of HipHop protein fused with GST. The right table summarizes the pull-down results (shown in C), with ‘+' indicating a positive interaction, and ‘−' for a lack of interaction. (C) GST-HipHop pull-down results. The names of the fusion constructs are listed on top of the membranes. The panels show GST pull down using embryonic extracts with both myc-HOAP and HOAP proteins, followed by western blot probed with antibodies listed to the left. Arrowheads mark the proteins of interest. In the last lane, a sample from anti-myc IP (from A) was loaded to indicate the running position of myc-HOAP.
HipHop protects telomeres from fusion
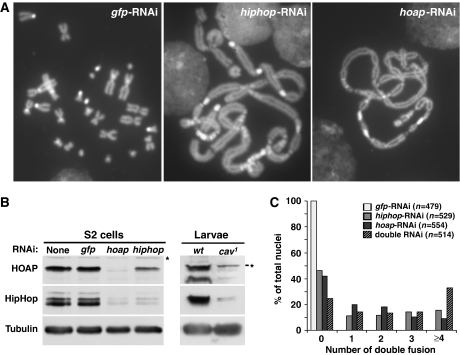
To establish HipHop's function in capping we set out to disrupt its function. The cav1 mutation in the gene encoding HOAP causes abundant telomere fusion in proliferating cells (Cenci et al, 2003). We expected a similar phenotype when HipHop function is disrupted. We used RNAi to reduce the level of HipHop in S2 cells, and performed mitotic chromosome spread to assay telomere uncapping. As a control, we treated cells with RNAi reagents that were highly effective in knocking down gfp expression (Wei et al, 2000). We observed telomere fusion in none of the gfp-RNAi-treated cells, but abundant fusion in 58% of the cav-RNAi-treated cells, and in 57% of the hiphop-RNAi-treated cells (Figure 2A). To quantify the degree of telomere uncapping in these RNAi-treated cells, we measured the frequency of double telomere fusions that involve two pairs of sister telomeres, as this type of fusion constitutes the majority class in cav1 larval neuroblasts (Cenci et al, 2003; Bi et al, 2005). As shown in Figure 2C, knocking down HOAP or HipHop caused similar degrees of uncapping. In addition, double knockdown of both HOAP and HipHop further enhanced uncapping, suggesting that each single knockdown resulted in a partial loss of function. This is consistent with western blot results showing partial reduction of the target proteins in RNAi-treated cells (Figure 2B).
Figure 2.
HipHop and HOAP protect telomere ends. (A) Pictures of DAPI-stained S2 cells in mitosis that have been treated with RNAi reagents against different genes (listed at the top of each picture). Telomere fusions are abundant in hiphop-RNAi and hoap-RNAi-treated cells with chromosomes forming ‘train-like' structures. (B) Western blot results showing the interdependence of HipHop and HOAP stability. The left panels show results using extracts from RNAi-treated S2 cells that were detected with antibodies indicated to the left. The right panels show results using extracts from wild-type (wt) or cav1 mutant larvae that were probed with the same set of antibodies. Non-specific bands are indicated with *. Our HOAP antibody identifies two bands in extracts from wild-type larvae, both of which are greatly reduced in the mutant. (C) Quantification of the severity of telomere uncapping in RNAi-treated cells. Only double telomere fusions were counted (see main text for definition). The ‘double RNAi' treatment involved using reagents against both hiphop and hoap. For statistical analyses of fusion events in different RNAi-treated cells, see Supplementary Table S4.
We observed an interesting interdependence of HOAP and HipHop protein stability as knocking down one protein led to a concomitant reduction of the other with knocking down HOAP having a larger effect on HipHop level (Figure 2B). We also observed a reduced level of HipHop in extracts for homozygous cav1 mutant larvae (Figure 2B), consistent with results from knocking down HOAP in S2 cells. This interdependence of protein stability is consistent with HipHop and HOAP forming a complex as suggested by our earlier biochemical results.
HipHop is a rapidly evolving protein
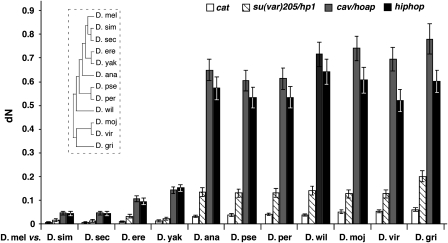
The predicted HipHop protein has 221 residues. Blast searches did not identify homologs in non-Drosophilidae organisms. Alignments among HipHop homologs from Drosophila species revealed large areas of sequence divergence (Supplementary Figure S4; Supplementary Table S1), a situation reminiscent of that for HOAP, which has been classified as one of the fastest evolving proteins in Drosophila (Schmid and Tautz, 1997). To provide evidence that HipHop is also rapidly evolving, we calculated the dN value for all pairwise comparisons between hiphop genes from Drosophila melanogaster and other Drosophila species. dN is the number of replacement mutations (non-synonymous substitutions) per non-synonymous site (codon sites that would result in an amino-acid change when altered). For comparisons, we also calculated dN values for the cav, su(var)205 and cat genes (Figure 3). su(var)205 encodes HP1, a conserved protein with a role in telomere capping in Drosophila (Fanti et al, 1998). cat encodes Catalase, an enzyme highly conserved in aerobically respiring organisms. It was chosen as a control locus because it abuts hiphop on the chromosome. The dN values for hiphop and cav increase dramatically as the evolutionary distance between a species and D. melanogaster increases. In contrast, only a moderate increase was observed for su(var)205, and the dN values for cat remain the lowest of the four genes. Therefore, HipHop seems to also evolve faster than typical proteins.
Figure 3.
hiphop is a fast-evolving gene. The mean dN values (shown with standard errors) for the four genes listed at the top were plotted for each pairwise comparison between Drosophila melanogaster (D. mel) and 11 other Drosophila species. The insert indicates the general evolutionary relationship among Drosophila species analysed. The lengths of the horizontal lines are not proportional to the evolutionary distances among the different species. D. sim, D. simulans; D. sec, D. sechellia; D. ere, D. erecta; D. yak, D. yakuba; D. ana, D. ananassae; D. pse, D. pseudoobscura; D. per, D. persimilis; D. wil, D. willistoni; D. moj, D. mojavensis; D. vir, D. virilis; D. gri, D. grimshawi.
HipHop is predominantly enriched at telomeres
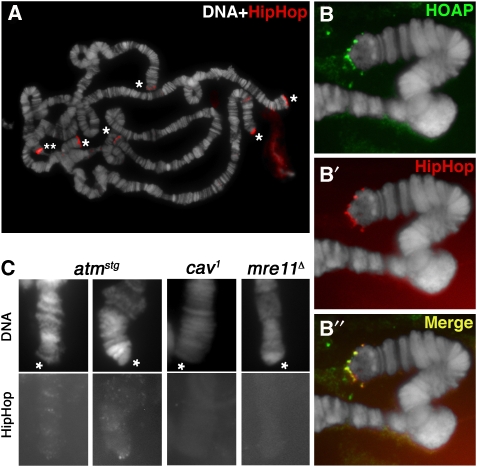
HipHop's intimate interaction with HOAP suggests that they may be similarly distributed, that is predominantly at telomeres. This was confirmed in immunostaining experiments performed first on the polytene chromosomes from larval salivary glands where we observed strong HipHop signals on all telomeres (Figure 4A). In double-staining experiments, HipHop co-localized with HOAP at telomeres (Figure 4B). We also studied HipHop localization in cycling cells from the larval brain and S2-cultured cells. In mitotic cells, HipHop is highly enriched at telomeres and co-localizes very well with HOAP (Figure 5A and B). During interphase, both HipHop and HOAP localize to multiple foci that possibly correspond to telomeres (Supplementary Figure S5), which is consistent with an earlier report on HOAP localization (Rashkova et al, 2002).
Figure 4.
HipHop is enriched at polytene telomeres. (A) Anti-HipHop staining on wild-type polytene chromosomes. DAPI-stained DNA in white, antibody signals in red. Telomeres are marked with * except that of the X chromosome, which was out of the picture. The band marked with ** is non-specific as it was not detected by a second anti-HipHop serum. (B, B', B'') HOAP and HipHop co-localization on a single wild-type telomere. Signals from anti-HOAP are in green (B), those from anti-HipHop in red (B') and merged signals are in yellow (B''). The extra-chromosomal signals in (B) are non-specific. (C) Genetic control of HipHop loading to polytene telomeres. Genotypes are shown on top of the pictures. DAPI-stained telomeres (marked by *) are labelled as ‘DNA' and shown in the top panels. Signals from anti-HipHop (HipHop) are shown in the bottom panels.
Figure 5.
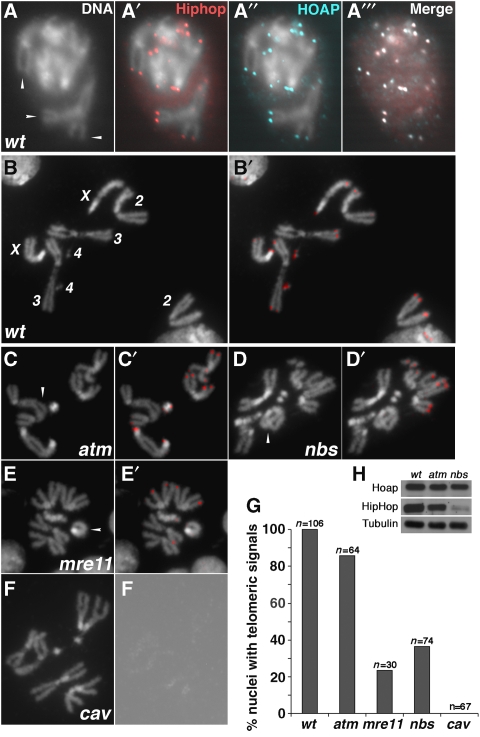
HipHop is enriched at mitotic telomeres. (A, A', A'', A''') HipHop and HOAP co-localization on mitotic telomeres of S2 cells. (A) Arrowheads indicate several examples of telomeric regions on condensed mitotic chromosomes that have HipHop signals (red in A') and HOAP signals (cyan in A''). DNA signals are not shown in the merged image of (A'''). (B, B') HipHop localization on telomeres from a wild-type larval neuroblast with all chromosomes labelled in (B). (C, C') HipHop localization on atm-mutant telomeres. (D, D') HipHop localization on nbs-mutant telomeres. (E, E') HipHop localization on mre11-mutant telomeres. (C, D, E) Arrowheads indicate examples of telomere fusion. (B', C', D', E') HipHop signals are in red. (F, F') HipHop is absent from cav-mutant telomeres. (F) A rare cav1 nucleus with no telomere fusion. (F') An over-exposed grey scale picture from anti-HipHop staining showing the lack of HipHop signals. (G) Quantification of HipHop localization on mitotic telomeres in different genetic backgrounds. (H) Western blot analyses of HipHop and HOAP levels in different genetic backgrounds.
HOAP localization to telomeres is controlled by a set of DNA damage checkpoint/repair proteins that include ATM and the MRN complex (reviewed in Rong, 2008a). We discovered that HipHop binding to telomeres is similarly regulated. On polytene chromosomes, HipHop was absent from mre11- or nbs-mutant telomeres and greatly reduced on atm-mutant telomeres (Figure 4C and data not shown). In larval neuroblasts, the frequency of telomeres with HipHop localization was greatly reduced by null mutations of either mre11 or nbs (Figure 5D and E). More specifically, we detected HipHop on at least two telomeres in 100% of the wild-type cells, but only in 23% of mre11-null cells and in 36% of nbs-null cells, a situation very similar to HOAP localization as reported earlier (Bi et al, 2004, 2005; Ciapponi et al, 2004, 2006). HipHop binding to atm-mutant telomeres was not severely affected in that 86% of the mutant cells displayed HipHop on multiple telomeres. Therefore, the presence of HipHop at telomeres depends on ATM and MRN functions, although there seems to be a stronger dependence in endo-replicating cells than in cycling cells, a phenomenon observed earlier, although the cause remains undetermined.
We used western blot to investigate whether the reduced presence of HipHop and HOAP at ATM or MRN-deficient telomeres is associated with a reduced level of the target proteins. Although we did not observe significant changes of HOAP level in both mutants and of HipHop level in the atm mutant, we did observe a significant reduction of HipHop in the nbs mutant (Figure 4H). This is consistent with earlier immunostaining results showing fewer HipHop-occupied telomeres in mrn mutants than in atm mutants. It is possible that inefficient loading of HipHop to mrn-mutant telomeres leads to it destabilization. However, we cannot rule out the possibility that MRN directly regulates HipHop protein stability.
We observed essentially background staining of HipHop on cav1 mutant chromatin in interphase (not shown), and on mutant telomeres of polytene or mitotic chromosomes (Figures 4C and 5F). This is consistent with that HipHop level is greatly reduced in cav1 mutants (Figure 2B).
A large domain of HipHop and HOAP binding at a model telomere
Results from immunostaining experiments clearly showed a predominant enrichment of the HipHop–HOAP complex at telomeres, although at a limited resolution. To gain mechanistic insights into how the two proteins fulfill their capping functions, we investigated their binding to telomere chromatin at a higher resolution using ChIP and real-time PCR to measure enrichment. As loss of HipHop or HOAP leads to telomere fusion in cycling cells, we focused our analyses on those cells in larvae by performing ChIP on dissected tissues of brains and imaginal discs.
The repetitive nature of the telomeric retrotransposons makes it difficult to study protein distribution in relationship to the chromosome ends. To sidestep this problem, we established a model telomere that has unique sequences suitable for designing ChIP primers, by taking advantage of the fact that terminally deleted chromosomes are functionally capped even in the absence of retrotransposons. We constructed a P-element insertion, D4A, at the tip of chromosome 2R, about 200 kb from the nearest natural telomere (Supplementary Figure S6). The D4A insertion carries a mini-white reporter gene with a direction of transcription pointing towards the centromere. This gene gives rise to eye pigmentation in an otherwise white-null background. Next to its promoter, we placed a recognition site for the rare-cutting I-SceI endonuclease from yeast (Rong and Golic, 2003). When I-SceI was expressed from a heat-inducible transgene in the germline of D4A heterozygous flies, I-SceI cutting at D4A sometimes led to the loss of the distal-most 200 kb fragment, giving rise to a terminal deletion (TD) of 2R. Such a TD placed the white promoter at the very end of the chromosome, resulting in a variegated eye pigmentation pattern, distinct from the uniform pattern produced by the original D4A insertion (Supplementary Figure S6B). We named this TD as D4ATD. The non-repetitive nature of the sequences at D4ATD ends allowed us to design primers for real-time PCR that covers an 11-kb region from the new telomere. As a control, we used samples from the original D4A flies in which mini-white has a non-telomeric location.
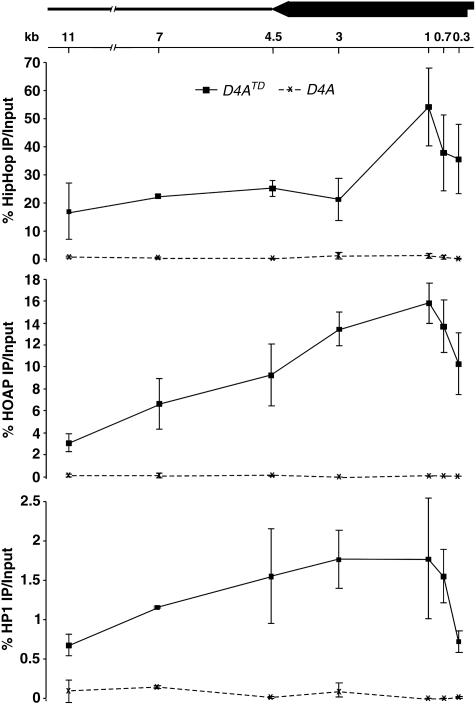
For the original D4A chromosome, HipHop and HOAP binding to the P-element and its surrounding region were at the background level (Figure 6). For D4ATD, we detected highly significant binding for both proteins within the 11-kb region at the telomere. The binding seems very strong as we observed as much as 320-fold enrichment for HipHop and 270-fold enrichment for HOAP over their binding to the same region on the original D4A chromosome (Supplementary Table S2.1). In contrast, Histone H3 occupancy at the D4A region did not show enrichment towards the telomere (Supplementary Figure S7). Therefore, HipHop and HOAP are highly enriched at the telomeric region of D4ATD, and cover a large duplex region of the telomere. More importantly, this broad domain of HipHop and HOAP binding is independent of the presence of telomeric retrotransposons.
Figure 6.
HipHop, HOAP and HP1 enrichment over a large telomeric domain. Average ‘% IP over input' values with standard deviations are shown for ChIP experiments using HipHop (top panel), HOAP (middle panel) and HP1 (bottom panel) antibodies. The solid lines connect data points derived from D4ATD samples, and the dashed lines connect those derived from D4A samples. On the top is a schematic diagram of the mini-white (block arrow) region in D4ATD, with the telomere at the right. The left-facing arrow depicts the direction of white transcription. The thick line depicts the genomic region centromere proximal to the insertion site of D4A. The locations for each primer pairs are indicated under the diagram with the distance from the PCR amplified region to the telomere in D4ATD shown in kb.
The HP1 protein has a ‘spreading' behaviour that allows it to occupy large heterochromatic domains in many different organisms (reviewed in Grewal and Jia, 2007). We studied its distribution at the model telomere and discovered a large HP1-binding domain with a distribution pattern essentially the same as those of HipHop and HOAP (Figure 6). In particular, there is a prominent reduction of HP1 binding at the ‘0.3-kb' position when compared with its peak of binding at the ‘1-kb' position. A similar reduction, but to a lesser degree, was observed for both HipHop and HOAP. Therefore, the binding of HipHop, HOAP and HP1 are not limited to the extreme end of the telomere but cover >10 kb of the double-stranded telomere region.
Sequence-independent binding of HipHop–HOAP to natural telomeres
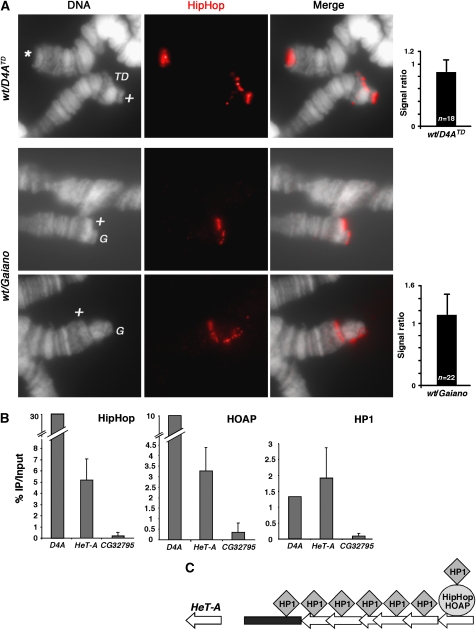
Our ChIP results showed that HipHop and HOAP are capable of binding to telomeres without the presence of retrotransposons. However, it is possible that this mode of binding is different from that to the natural, transposon-capped telomeres in that HipHop and HOAP might have developed higher binding affinity for certain regions of the retro-elements. If that were the case, one would predict that the level of HipHop and HOAP binding might be proportional to the abundance of telomeric transposons, a behaviour similar to that of the Prod protein in Drosophila (Török et al, 2007). To test this prediction, we performed immunostaining on polytene nuclei that have homologous chromosomes bearing different amounts of telomeric transposons. In the first case, we compared HipHop binding to D4ATD with its binding to the natural end of 2R on the homologous chromosome and discovered that the intensities of antibody signal on those ends were very similar (Figure 7A). Second, we used the Gaiano strain in which the retrotransposon arrays on all telomeres are several times longer than those in typical laboratory strains (Siriaco et al, 2002). We generated flies in which all bivalents were heterozygous for a wild type and a Gaiano chromosome. In polytene cells, the Gaiano chromosomes were visibly longer than their wild-type homologs, allowing us to directly compare HipHop binding to the two ends bearing different levels of retrotransposons. Again, the degrees of HipHop binding were very similar for all bivalents (Figure 7A). These results suggest that HipHop and possibly HOAP binding to natural telomeres are not affected by the amount of telomeric transposons, but are likely sequence independent and limited to the vicinity of the ends.
Figure 7.
HipHop and HOAP binding at natural telomeres. (A) HipHop binding to telomeres is independent of the abundance of telomeric retrotransposons. The top three panels show anti-HipHop staining on polytene chromosomes from larvae that were heterozygous for D4ATD (wt/D4ATD), with antibody signals in red. ‘*' marks normal 2L telomeres. ‘TD' marks the D4ATD chromosome, which is shorter than its 2R homolog (marked with ‘+'). The graph to the right shows the average ratio of fluorescence intensity (with standard deviation) from anti-HipHop staining on ‘+' telomeres over that on ‘TD' telomeres. The lower two sets of three panels show polytene chromosomes stained with anti-HipHop, which were from larvae heterozygous for the Gaiano chromosomes (wt/Gaiano). The Gaiano chromosome 2R (in the upper set) and 2L (in the lower set) (both marked with ‘G') are visibly longer than their wild-type homologs (marked with ‘+'). The right graph shows quantification of fluorescence intensity expressed as the ratio of signals on ‘+' telomeres over those on ‘G' telomeres. (B) Average ‘% IP over input' values are shown for HipHop ChIP (left chart), HOAP ChIP (middle chart) and HP1 ChIP (right chart) experiments using primers from HeT-A element (HeT-A) and a non-telomeric control locus (CG32795). The values for the D4ATD region are averages obtained from the seven pairs of D4A primers in Figure 6. (C) A model for how HipHop and HOAP distributions differ from that of HP1 at natural telomeres. HipHop–HOAP complexes (collectively represented by the oval) bind only to distal retro-element(s) (left-facing block arrow), whereas two classes of HP1 molecules (diamonds) occupy the telomeric region. One associates with HipHop–HOAP. The other binds multiple retro-elements as well as subtelomeric repetitive regions (black rectangle).
We performed ChIP to study HipHop and HOAP binding to natural telomeres in cycling cells, using a primer pair from the conserved GAG region of HeT-A, the most abundant telomeric retrotransposon in Drosophila (reviewed in Pardue and Debaryshe, 2008). We observed significant enrichments for both HipHop and HOAP when compared with for the non-telomeric CG32795 locus (Figure 7B, 5.19% versus 0.22% IP/input for HipHop (a 23-fold enrichment) and 3.28% versus 0.36% for HOAP (a 9-fold enrichment)). However, these values from HeT-A primers are much lower than the values generated with the seven D4A primers (30.45% on average for HipHop (a 138-fold enrichment) and 10.32% on average for HOAP (a 28-fold enrichment), from Figure 6; Supplementary Table S2.1). Because of the repetitive nature of telomeric transposons, the enrichments derived from HeT-A primers could represent moderate HipHop and HOAP binding to all or most HeT-A elements. Alternatively, we favour that these moderate enrichments reflect strong protein binding to a selected few HeT-A elements but averaged over the entire transposon array as the PCR primers would anneal to all HeT-A elements, but only some of which bind HipHop and HOAP (see the model in Figure 7C). We suggest that these HeT-A elements that HipHop–HOAP binds are at the very ends of the chromosomes as supported by our earlier immunostaining results (Figure 7A).
HP1 was also enriched at telomeric HeT-A elements (Figure 7B), but it behaves differently from either HipHop or HOAP. The ‘% IP over input' value for HP1 derived from the HeT-A primers was similar to the average value derived from D4A primers (an average of 1.31% for the seven D4A primers versus 1.9% for HeT-A primers). This is different from what we observed in HipHop or HOAP ChIP experiments, suggesting that HP1 binding is not limited to distal most HeT-A elements. We suggest the existence of two classes of HP1 molecules on telomeres (see the model in Figure 7C): one associates with the HipHop–HOAP complex and serves capping function, the other associates with multiple retrotransposons and possibly other repetitive elements such as TAS and serves functions in transcriptional regulation as suggested earlier (Perrini et al, 2004; Frydrychova et al, 2008). Kanoh et al (2005) reported strikingly similar distribution patterns of the Swi6 protein (HP1 homolog) and the Taz1 capping protein in Schizosaccharomyces pombe as those of Drosophila HP1 and HipHop–HOAP.
Discussion
In this study, we identified the HipHop protein as a novel component of a Drosophila capping complex. HipHop localizes to all telomeres in both proliferating and endo-replicating cells. Loss of HipHop leads to one of the strongest uncapping defect observed so far.
We identified HipHop based on its ability to associate with HOAP through biochemical purification. Such an approach could be useful for future studies in Drosophila telomere biology as similar ones have been very productive in other systems (Liu et al, 2004; Miyoshi et al, 2008). Our biochemical approach was aided by our ability to epitope-tag the endogenous cav locus, eliminating potential artifacts associated with the overproduction of bait proteins. With the recent development of the SIRT targeting method in Drosophila (Gao et al, 2008), biochemical purification using endogenous tags could be efficiently applied in the study of other biological processes in Drosophila.
The HipHop–HOAP complex
Several lines of evidence suggest that HipHop and HOAP likely function as a complex. First, HipHop was abundantly present in HOAP IPs, suggesting a strong interaction between the two proteins (Supplementary Figure S2). Second, bacteria expressed HipHop was able to interact with HOAP in fly extracts (Figure 1). Third, the changes of HOAP and HipHop levels showed inter-dependency (Figure 2). Fourth, the loading of both HipHop and HOAP to telomeres was under the same genetic controls of MRN and ATM (Figures 4 and 5). Finally, the two proteins had very similar distribution patterns on our model telomere and co-localized precisely in immunostaining experiments (Figures 5 and 6). On the basis of some of the same criteria, HP1 is likely to be a part of the complex. The Modigliani(Moi)/DTL protein was recently identified as another capping protein that is enriched at telomeres and interacts with both HOAP and HP1 (Komonyi et al, 2009; Raffa et al, 2009). We did not detect Moi/DTL peptides in HOAP IPs.
HipHop–HOAP is part of the telomeric chromatin
The model telomere D4ATD has allowed us an unprecedented view of the chromatin landscape in the vicinity of a Drosophila telomere. We located HipHop, HOAP and HP1 essentially at the very end of a chromosome, strengthening earlier results from immunolocalization experiments. Remarkably, HipHop, HOAP and HP1 seem to bind to a much larger region than the immediate vicinity of the chromosome end. We envision one possible mechanism that could lead to such a binding pattern. After the initial recruitment of the HipHop–HOAP complex to the chromosome end, they ‘spread' internally to cover a larger region. It is tempting to speculate that this ‘spreading' might be mediated by HP1 as we observed a binding pattern of HP1 essentially identical to those of HipHop and HOAP on D4ATD. However, results from ChIP experiments using HeT-A primers suggest that HP1 occupies a larger region than HipHop or HOAP on transposon-capped telomeres (Figure 7B and C), which implies that the mere presence of HP1 on chromatin is not sufficient for HipHop or HOAP binding. In addition, HOAP can be localized to telomeres in su(var)205/hp1 mutants (Cenci et al, 2003), suggesting that HP1 is not necessary for HOAP and possibly HipHop binding to telomeres. Whether HP1 affects the extent of HipHop–HOAP spreading requires ChIP localization of HipHop and HOAP on our model telomere in a su(var)205 mutant background.
We suggest that the binding patterns of HipHop and HOAP on our model telomere is a qualitative reflection of their patterns on natural telomeres, as we observed very similar binding intensity of HipHop on D4ATD versus its homologous telomere in immunostaining experiments. Similar observations were documented for HP1 on polytene and HOAP on mitotic telomeres using TDs (Fanti et al, 1998; Cenci et al, 2003).
Potential functional homologs of HipHop and HOAP in other organisms
HipHop and HOAP share functional characteristics with capping proteins in other eukaryotes. First, they bind to the double-stranded region of the telomere in vivo. Second, they occupy a large domain on telomeric chromatin. Third, they are continuously present at the telomeres. Finally, the loss of these proteins leads to frequent telomere fusions. We suggest that HipHop and HOAP behave similarly and might serve similar functions as the Rap1 protein in S. cerevisiae, Taz1 in S. pombe, and TRF2 in mammals. Further dissection of HipHop and HOAP's molecule function would be needed to confirm our proposition.
The telomere loading of HipHop and HOAP is under the control of ATM and MRN. The same set of proteins mediate the loading of various telomeric factors including telomerase activity, and the Cdc13 capping protein in yeast (Diede and Gottschling, 2001; Goudsouzian et al, 2006; Negrini et al, 2007). This high degree of functional conservation suggest that it is unlikely that these factors directly act on capping proteins, which are generally divergent at the sequence level. It is more likely that these proteins modulate a common DNA/chromatin structure at telomeres of eukaryotic cells. One conceivable candidate for this ‘universal' structure is the terminal 3′ overhang (reviewed in Lydall, 2009). The reduced occupancy of HipHop, HOAP and HP1 at the extreme end of our model telomere (Figure 6), suggests that Drosophila chromosomes might also terminate as a 3′ overhang.
HipHop and HOAP are representatives of fast-evolving telomeric proteins
HipHop and HOAP seem to evolve faster than typical proteins. An interesting proposition is that this faster rate of evolution is driven by the fast-evolving telomeric retrotransposons (Villasante et al, 2008), to which the HipHop–HOAP complex binds. HOAP was implicated in binding DNA (Shareef et al, 2001). Whether HipHop is capable of binding DNA directly is currently under investigation. Under the limited resolution of immunostaining, we did not detect any change in HipHop–HOAP binding efficiency to telomeres with different levels of retrotransposons (Figure 7). Nor did we or others observe any phenotypic effects of having a ‘retrotransposon-free' telomere. Although TDs can be efficiently maintained under laboratory conditions, it remains undetermined whether there is any fitness cost for animals with a TD irrespective of the loss of essential genes. Therefore, further studies are required to identify the driving force for the fast evolution of HipHop and HOAP.
Interestingly, telomeric proteins from other systems are generally less conserved at the sequence level and show signs of fast evolution (Li et al, 2000; Martín et al, 2007; Shakirov et al, 2009). Further investigation into the functional relationship between HipHop–HOAP and the telomeric retrotransposons in Drosophila might reveal the significance for this fast evolution of telomeric proteins in general.
Materials and methods
Primer sequences are listed in Supplementary Table S3. The sequences for primers used in sequencing are available on request. Detail protocols for affinity purification of HOAP-interacting proteins, generation of D4ATD and ChIP are provided in Supplementary data.
Drosophila stocks
The cav1 mutant was described in Cenci et al (2003), atmstg and mre11Δ35K1 in Bi et al (2004) and nbsΔ in Gao et al (2008). The construction of a myc-tagged cav allele is described in Supplementary data.
Antibodies
A guinea pig (GP) HOAP antibody was raised against the full-length protein as described earlier (Shareef et al, 2001). The antibody was used at 1:1000 for western blot analyses, and 1:100 in immunostaining experiments. It was also used in ChIP.
Rabbit and GP anti-HipHop antibodies were raised against an antigen consisting of residues 72–175 of HipHop, and affinity purified using the same antigen. The coding region for this antigen was amplified with primer pair: FP6874 and RP6874 and cloned into the BamHI and XhoI sites in the pGEX-6p-1 vector. The GST-fusion protein was purified and the GST tag removed as per the manufacturer's (Novagen) instructions. The serum was used at 1:2000 for western blot analyses and 1:200 in immunostaining experiments. The GP antibody was used in ChIP.
A rabbit anti-HP1 antibody from Covance and a rabbit anti-Histone H3 from Abcam were used in ChIP.
GST-fusion proteins and GST pull-down assays
The GST-fusion proteins described in Figure 1B were expressed from the pGEX-6P-1 vector with coding regions of the different fragments cloned between BamHI and XhoI sites. The coding regions for ‘221' was amplified with primers F-6874fl and R-6874fl; ‘N99' with primers F-TCP001 and R-TCP099; ‘C125' with F-TCP096 and R-TCP221; ‘C80' with F-TCP140 and R-TCP221; M102 with FP6874 and RP6874. For the ΔM construct, the ‘221' plasmid containing GST fused to full-length HipHop was used as a template in PCR using 5′ phosphorylated primers pF-TCP141 and pR-TCP100. The PCR products were self-ligated generating a plasmid with an internal deletion of HipHop, which was verified by sequencing.
GST pull-down assays were performed using standard protocols. Briefly, purified GST-fusion proteins were incubated at 4°C for 1 h with 1 ml of chromatin extracts from myc-tagged embryos in binding buffer (25 mM Tris-HCl at pH 7.6, 150 mM NaCl, 2 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.1% NP-40, 20% glycerol and 100 μg/ml BSA supplemented with protease inhibitors from Roche). About 20 μl of glutathione agarose beads were added and the mixture was incubated for 1 h at 4°C. The beads were washed five times each with 1 ml of binding buffer. The bound proteins were eluted with SDS sample buffer and resolved by SDS–PAGE for western blot analyses.
RNAi-mediated knockdown of HipHop and HOAP in S2 cells
To produce dsRNA for RNAi, individual DNA fragments either 200 or 500 bp in length, containing sequences from the coding regions for the proteins to be knocked down were amplified by PCR. For hiphop, two pairs of primers were used: dHiphopT7F with dHiphopT7220R and dHiphopT7F70 with dHiphopT7520R. For hoap, two primer pairs were used: dHoapT7F with dHoapT7220R and dHoapT7F with dHoapT7525R. For the gfp control, the primers T7-EGFP-F and T7-EGFP-R were used. Each primer contains a sequence for the T7 promoter followed by sequences specific for the targeted genes. Single-stranded RNAs were synthesized from purified PCR products by in vitro transcription using the MEGAscript RNAi kit (Ambion). To promote dsRNA formation, RNA mixtures were first denatured at 90°C for 1 min, then re-annealed by slow cooling to room temperature.
RNAi experiments were performed as described earlier (Rogers and Rogers, 2008) with slight modifications. Briefly, S2 cells at about 2.0 × 106 cells/ml were diluted 1:1 in culture medium and cultured for 16–20 h. Cells were transfected with dsRNAs using Effectene reagents from Qiagen. Four days after transfection, cells were washed in PBS, diluted 1:1 with culture medium and transfected again as above. The transfected cells were incubated for an additional 4 days at 24°C, and then processed for cytology or western blot analyses.
Calculation of dN values for evolutionary study of HipHop
We used pairwise and multiple sequence alignment-based methods to study the evolutionary history of HipHop. We compare hiphop with three other genes cav, su(var)205 and cat. Orthologs of these genes in Drosophila species were identified by homology searches (Supplementary Table S1). Multiple sequence alignments of orthologous amino-acid sequences were created (Supplementary Figure S4). Pairwise estimates of the number of non-synonymous substitutions per non-synonymous site (dN) are based on calculations using a maximum likelihood approach (Yang and Nielsen, 2000) and were conducted using the yn00 program as part of the PAML package v4.1 (Yang, 2007).
Cytology
Immunostaining on larval polytene and larval neuroblasts were performed as described in Fanti et al (1998) and Cenci et al (2003), respectively. Using the AxioVision software from Zeiss, the following procedure was used to quantify fluorescent intensity from antibody-stained polytene telomeres (Figure 7). For a particular pair of homologous telomeres, the average fluorescent intensity (AFtelomere) and the total area (TAtelomere) for each telomeric region were determined as well as the average fluorescent intensity from a non-telomeric region on the same chromosome (AFbackground). A fluorescence index (FI) was calculated for each telomere using the formula: FI=(AFtelomere−AFbackground) × TAtelomere. The ratios between FI values from multiple pairs of homologous telomeres were averaged and plotted in Figure 7A.
DAPI staining on mitotic chromosomes from RNAi-treated cells was performed as described (Somma et al, 2008) with slight modification. Briefly, S2 cells were first treated with 0.4 μg/ml of colchicine for 2 h. Cells were centrifuged at 1500 r.p.m. for 3 min. Pelleted cells were washed in PBS, spun down and re-suspended in 1 ml of 0.5 M sodium citrate for 10 min. Cells were spun down, fixed in 1 ml of fixative solution (methanol:acetic acid=3:1) for 10 min, spun down briefly and re-suspended in a small volume of the fixative solution. Cells were dropped onto a microscope slide, which was first air dried and then mounted in a DAPI-containing medium from Vector laboratories.
Immunostaining of S2 cells was performed as described earlier with slight modification (Somma et al, 2008). Briefly, cells were fixed for 20 min in 2% paraformaldehyde and permeabilized with 100% cold methanol for 10 min. Cells were washed with PBS twice and centrifuged onto a micro slide from VWR (1500 r.p.m. for 3 min). Slides were frozen in liquid nitrogen and processed for immunostaining as slides prepared from larval neuroblasts.
Supplementary Material
Supplemental Materials
Review Process File
Acknowledgments
We thank Germana Colazzo for constructing the myc-cav targeting construct, Xiao-feng Zheng for characterizing myc-cav reduction events and the initial characterization of terminal deficiencies, Giovanni Cenci at University of L'Aquila for comments and supporting PM; Gaku Mizuguchi for extensive advice on biochemical purification, Qin Wei and Bruce Paterson for assistance on S2 cell culture experiments, Jim Mason at NIEHS for the Gaiano stock, Michael Lichten and Carl Wu at NCI for comments on the paper. The intramural research program of the National Cancer Institute supported this research.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahmad K, Golic KG (1998) The transmission of fragmented chromosomes in Drosophila melanogaster. Genetics 148: 775–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Bi X, Srikanta D, Fanti L, Pimpinelli S, Badugu R, Kellum R, Rong YS (2005) Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc Natl Acad Sci USA 102: 15167–15172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Wei SC, Rong YS (2004) Telomere protection without a telomerase; the role of ATM and Mre11 in Drosophila telomere maintenance. Curr Biol 14: 1348–1353 [DOI] [PubMed] [Google Scholar]
- Biessmann H, Champion LE, O'Hair M, Ikenaga K, Kasravi B, Mason JM (1992) Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. EMBO J 11: 4459–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34 [DOI] [PubMed] [Google Scholar]
- Cenci G, Rawson RB, Belloni G, Castrillon DH, Tudor M, Petrucci R, Goldberg ML, Wasserman SA, Gatti M (1997) UbcD1, a Drosophila ubiquitin-conjugating enzyme required for proper telomere behavior. Genes Dev 11: 863–875 [DOI] [PubMed] [Google Scholar]
- Cenci G, Siriaco G, Raffa GD, Kellum R, Gatti M (2003) The Drosophila HOAP protein is required for telomere capping. Nat Cell Biol 5: 82–84 [DOI] [PubMed] [Google Scholar]
- Ciapponi L, Cenci G, Ducau J, Flores C, Johnson-Schlitz D, Gorski MM, Engels WR, Gatti M (2004) The Drosophila Mre11/Rad50 complex is required to prevent both telomeric fusion and chromosome breakage. Curr Biol 14: 1360–1366 [DOI] [PubMed] [Google Scholar]
- Ciapponi L, Cenci G, Gatti M (2006) The Drosophila Nbs protein functions in multiple pathways for the maintenance of genome stability. Genetics 173: 1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad MN, Wright JH, Wolf AJ, Zakian VA (1990) RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell 63: 739–750 [DOI] [PubMed] [Google Scholar]
- Diede SJ, Gottschling DE (2001) Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr Biol 11: 1336–1340 [DOI] [PubMed] [Google Scholar]
- Fanti L, Giovinazzo G, Berloco M, Pimpinelli S (1998) The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol Cell 2: 527–538 [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2001) The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol Cell 7: 55–63 [DOI] [PubMed] [Google Scholar]
- Frydrychova RC, Mason JM, Archer TK (2008) HP1 is distributed within distinct chromatin domains at Drosophila telomeres. Genetics 180: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Cervantes RB, Mandell EK, Otero JH, Lundblad V (2007) RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol 14: 208–214 [DOI] [PubMed] [Google Scholar]
- Gao G, McMahon C, Chen J, Rong YS (2008) A powerful method combining homologous recombination and site-specific recombination for targeted mutagenesis in Drosophila. Proc Natl Acad Sci USA 105: 13999–14004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsouzian LK, Tuzon CT, Zakian VA (2006) S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol Cell 24: 603–610 [DOI] [PubMed] [Google Scholar]
- Grewal SI, Jia S (2007) Heterochromatin revisited. Nat Rev Genet 8: 35–46 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Sadaie M, Urano T, Ishikawa F (2005) Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol 15: 1808–1819 [DOI] [PubMed] [Google Scholar]
- Kern AD, Begun DJ (2008) Recurrent deletion and gene presence/absence polymorphism: telomere dynamics dominate evolution at the tip of 3L in Drosophila melanogaster and D. simulans. Genetics 179: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komonyi O, Schauer T, Papai G, Deak P, Boros IM (2009) A product of the bicistronic Drosophila melanogaster gene CG31241, which also encodes a trimethylguanosine synthase, plays a role in telomere protection. J Cell Sci 122: 769–774 [DOI] [PubMed] [Google Scholar]
- Levis RW (1989) Viable deletions of a telomere from a Drosophila chromosome. Cell 58: 791–801 [DOI] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T (2000) Identification of human Rap1: implications for telomere evolution. Cell 101: 471–483 [DOI] [PubMed] [Google Scholar]
- Liu D, O'Connor MS, Qin J, Songyang Z (2004) Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J Biol Chem 279: 51338–51342 [DOI] [PubMed] [Google Scholar]
- Lydall D (2009) Taming the tiger by the tail: modulation of DNA damage responses by telomeres. EMBO J 28: 2174–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Gilson E, Shore D (1997) A protein-counting mechanism for telomere length regulation in yeast. Science 275: 986–990 [DOI] [PubMed] [Google Scholar]
- Makarov VL, Lejnine S, Bedoyan J, Langmore JP (1993) Nucleosomal organization of telomere-specific chromatin in rat. Cell 73: 775–787 [DOI] [PubMed] [Google Scholar]
- Maringele L, Lydall D (2004) Telomerase- and recombination-independent immortalization of budding yeast. Genes Dev 18: 2663–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V, Du LL, Rozenzhak S, Russell P (2007) Protection of telomeres by a conserved Stn1-Ten1 complex. Proc Natl Acad Sci USA 104: 14038–14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B, Clejan I, Liu Y, Lowden M, Gartner A, Hodgkin J, Ahmed S (2006) trt-1 is the Caenorhabditis elegans catalytic subunit of telomerase. PLoS Genet 2: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Kanoh J, Saito M, Ishikawa F (2008) Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320: 1341–1344 [DOI] [PubMed] [Google Scholar]
- Negrini S, Ribaud V, Bianchi A, Shore D (2007) DNA breaks are masked by multiple Rap1 binding in yeast: implications for telomere capping and telomerase regulation. Genes Dev 21: 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikemus SR, Queiroz-Machado J, Lai K, McGinnis N, Sunkel C, Brodsky MH (2006) Epigenetic telomere protection by Drosophila DNA damage response pathways. PLoS Genet 2: e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Marcand S (2005) Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J 24: 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue ML, Debaryshe PG (2008) Drosophila telomeres: a variation on the telomerase theme. Fly 2: 101–110 [DOI] [PubMed] [Google Scholar]
- Perrini B, Piacentini L, Fanti L, Altieri F, Chichiarelli S, Berloco M, Turano C, Ferraro A, Pimpinelli S (2004) HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol Cell 15: 467–476 [DOI] [PubMed] [Google Scholar]
- Raffa GD, Cenci G, Siriaco G, Goldberg ML, Gatti M (2005) The putative Drosophila transcription factor woc is required to prevent telomeric fusions. Mol Cell 20: 821–831 [DOI] [PubMed] [Google Scholar]
- Raffa GD, Siriaco G, Cugusi S, Ciapponi L, Cenci G, Wojcik E, Gatti M (2009) The Drosophila modigliani (moi) gene encodes a HOAP-interacting protein required for telomere protection. Proc Natl Acad Sci USA 106: 2271–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashkova S, Karam SE, Kellum R, Pardue ML (2002) Gag proteins of the two Drosophila telomeric retrotransposons are targeted to chromosome ends. J Cell Biol 159: 397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K, McKnight TD, Griffing LR, Shippen DE (2001) Living with genome instability: plant responses to telomere dysfunction. Science 291: 1797–1800 [DOI] [PubMed] [Google Scholar]
- Rogers SL, Rogers GC (2008) Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat Protoc 3: 606–611 [DOI] [PubMed] [Google Scholar]
- Rong YS, Golic KG (2003) The homologous chromosome is an effective template for the repair of mitotic DNA double-strand breaks in Drosophila. Genetics 165: 1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS (2008b) Loss of the histone variant H2A.Z restores capping to checkpoint-defective telomeres in Drosophila. Genetics 180: 1869–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS (2008a) Telomere capping in Drosophila: dealing with chromosome ends that most resemble DNA breaks. Chromosoma 117: 235–242 [DOI] [PubMed] [Google Scholar]
- Schmid KJ, Tautz D (1997) A screen for fast evolving genes from Drosophila. Proc Natl Acad Sci USA 94: 9746–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirov EV, McKnight TD, Shippen DE (2009) POT1-independent single-strand telomeric DNA-binding activities in Brassicaceae. Plant J 58: 1004–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareef MM, King C, Damaj M, Badagu R, Huang DW, Kellum R (2001) Drosophila heterochromatin protein 1 (HP1)/origin recognition complex (ORC) protein is associated with HP1 and ORC and functions in heterochromatin-induced silencing. Mol Biol Cell 12: 1671–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriaco GM, Cenci G, Haoudi A, Champion LE, Zhou C, Gatti M, Mason JM (2002) Telomere elongation (Tel), a new mutation in Drosophila melanogaster that produces long telomeres. Genetics 160: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma MP, Ceprani F, Bucciarelli E, Naim V, De Arcangelis V, Piergentili R, Palena A, Ciapponi L, Giansanti MG, Pellacani C, Petrucci R, Cenci G, Vernì F, Fasulo B, Goldberg ML, Di Cunto F, Gatti M (2008) Identification of Drosophila mitotic genes by combining co-expression analysis and RNA interference. PLoS Genet 4: e1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Leehy K, Warrington RT, Lamb JC, Surovtseva YV, Shippen DE (2008) STN1 protects chromosome ends in Arabidopsis thaliana. Proc Natl Acad Sci USA 105: 19815–19820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M (1997) SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev 11: 83–93 [DOI] [PubMed] [Google Scholar]
- Tommerup H, Dousmanis A, de Lange T (1994) Unusual chromatin in human telomeres. Mol Cell Biol 14: 5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török T, Benitez C, Takács S, Biessmann H (2007) The protein encoded by the gene proliferation disrupter (prod) is associated with the telomeric retrotransposon array in Drosophila melanogaster. Chromosoma 116: 185–195 [DOI] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413 [DOI] [PubMed] [Google Scholar]
- Villasante A, de Pablos B, Méndez-Lago M, Abad JP (2008) Telomere maintenance in Drosophila: rapid transposon evolution at chromosome ends. Cell Cycle 7: 2134–2138 [DOI] [PubMed] [Google Scholar]
- Watson JM, Bulankova P, Riha K, Shippen DE, Vyskot B (2005) Telomerase-independent cell survival in Arabidopsis thaliana. Plant J 43: 662–674 [DOI] [PubMed] [Google Scholar]
- Wei Q, Marchler G, Edington K, Karsch-Mizrachi I, Paterson BM (2000) RNA interference demonstrates a role for nautilus in the myogenic conversion of Schneider cells by daughterless. Dev Biol 228: 239–255 [DOI] [PubMed] [Google Scholar]
- Wright JH, Gottschling DE, Zakian VA (1992) Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev 6: 197–210 [DOI] [PubMed] [Google Scholar]
- Yang Z, Nielsen R (2000) Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol 17: 32–43 [DOI] [PubMed] [Google Scholar]
- Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials
Review Process File