A systematic review and meta-analysis suggests that around 2% of scientists admit to have falsified research at least once (1). Up to 33% admit other questionable practices such as plagiarism, duplicate publication, undisclosed changes in pre-research protocols or dubious ethical behavior (1). There can be no doubt that discovered cases of research and publication misconduct represent a tip of an iceberg and many cases go unreported (2).
Experienced biomedical journal editors are aware of a “rogues’ gallery” of major fraudsters, such as Schoen, Hwang, Sudbo, Poehlman, Singh, and Chandra (3-8). Much more common are the less dramatic, because more subtle but probably more dangerous, examples; these are more dangerous because they remain undiscovered so may feed into meta-analyses and guidelines.
A seminar organized by the Esteve Foundation, held in Sitges in April 2009, concentrated on conflicts of interest (COI, sometimes also referred to as Competing Interests, CI), which underlie so much research and publication misconduct.
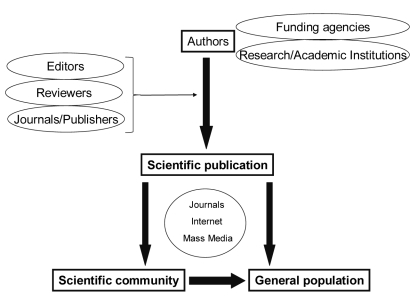
All attendants of the meeting agreed that there were many sources of COI in the general process of scientific communication (Figure 1). The meeting was mainly focused on non-financial COI. Three introductory presentations highlighted some of the topics related to COI in the contemporary scientific publishing enterprise.
Figure 1.
Main protagonists in the process of scientific communication among which conflict of interest may arise.
Dr Virginia Barbour, editor of PLoS Medicine, raised the following issues:
- Does past or present employment with industry prejudice authors and researchers? - Journal policies on competing interests are often unclear or ambiguous even though the problem is common and consumes a disproportionate amount of editors’ time. - It is difficult to persuade academic institutions and individual researchers to comply with accepted definitions of misconduct. - Editors and publishers have not got to grips with non-financial conflicts. - There is uncertainty and inconsistency about sanctions for misconduct.
Magne Nylenna, editor-in-chief of the Norwegian Electronic Health Library, raised the following issues:
- Good practice vs misconduct is not a matter of black and white. There are shades of gray.
- Of greatest significance is the researcher’s/author’s intent – and that may be difficult to determine. - Extreme cases are probably unpreventable so, as in preventive medicine generally, attention to the whole population is likely to have a better outcome than concentrating on outliers. - Simple guidelines are more useful than detailed, lengthy protocol - Everyone concerned merits training.
Ana Marušić, co-editor in chief of the Croatian Medical Journal, addressed the specificities of small journals:
- There are particular problems for small journals whose editors are likely to be academics, not full time medical journalists. - There is special risk from “scientific inbreeding” (the tradition of having tenure in the same institution where one trained). - In post-communist countries the concept that it is praiseworthy to cheat the government may be extended to other perceived authorities. - There needs to be a clear definition of when and how an article is retracted and what should be the consequences thereof.
Specific issues discussed
Generalizability
We all need to be aware of cultural differences between countries or even between academic disciplines in such matters as ethics and law, as they are applied to science. While science attempts to be universal and objective, issues which lead to research contamination, such as through conflicting interests, are essentially local and variable.
Whether or not it results from a conflict of interest, a prime example is plagiarism, which in certain cultures may represent respect for one’s seniors rather than theft of their work.
In any case, detecting plagiarism is easier for editors of large and well-resourced journals that can afford to employ staff to use software for detection. For the overwhelming majority of journals plagiarism detection is based more on chance than on forensic detection.
In whatever manner COI presents, we need internationally accepted definitions: thus, “corruption is the abuse of entrusted power for private gain” (9) should be axiomatic. There are three aspects to this definition, all culturally dependent –private gain (eg, journals and reprints, citations in deciding promotion); power (whom are you serving?); and abuse, such as cultural behavior deemed improper by others. At the symposium, an example was given of a principal investigator who, faced with possible misconduct by a researcher, sent a junior researcher to investigate with neither the knowledge nor the authority to be sufficiently critical. The choice of this researcher was, however, in line with local norms.
Science’s attitudes to COI
Participants considered that the scientific community is largely skeptical of the frequency and adverse impact of COI. There is a commonly held myth that science is “self-correcting” and that, by default, scientists behave ethically. In addition many scientists fail to recognize that they have competing interests so, unsurprisingly, do not declare them.
Editors and publishers may be more cynical but are still less than properly alert to COI, especially where it is not financial. Industry connections raise suspicions but should not government appointments do the same? It was pointed out that in certain disciplines, for example toxicology, there is a regular flow of personnel between government regulators, industry, and academia. Of course, publishers and editors might also stand to gain from having COI, in terms of enhancing circulation, obtaining finance for reprints or just improving the impact factor
Motives
The “Publish or perish” principle dominates researchers’ and authors’ behavior (10). Modifying this behavior will require widely accepted policies and adequate training and mentoring and incentives other than just publication as an academic reward.
The need for standards
Those journals which screen for image manipulation (eg, PLoS journals) tend to quantify it as about 1% of accepted papers. What is especially surprising is that when authors are asked to provide original data to further investigate potential manipulation, some 25% of authors respond that they do not have the original data. Indeed there is no standard approach to data storage in academic institutions (compared to industry where there are much more strict standards). We need to have agreements about such matters for example how to maintain and enter record of experiments and how to archive them. One difficulty is that the accumulation of data during a project does not follow the neat process of IMRAD (Introduction, Methods, Results and Discussion) but rather tends to be random in time.
Authorship
It is well recognized that there is poor agreement between editors and researchers as to the precise definitions of authorship or contributorship. This leads to disputes about “guest” and “ghost” authors.
Reviewers
Reviewers are often unaware that they have potential COI outside the obvious (ie, financial) – for example, personal friendship (or enmity), religious beliefs or nationalism may get in the way. A reviewer may be over-enthusiastic or unnecessarily hostile about a topic, without realizing he or she is an outlier in that respect. In small subspecialties or communities, finding a genuinely independent reviewer may be difficult.
In many journals, reviewers’ COI is not addressed. Esteve Fernández pointed out that in small scientific communities, which may have only 2 local journals, the reviewers know the authors personally and sometimes do research in the same topic, which is in itself COI, which has to be declared and managed. In his experience, only some journals allow exclusion of researchers with competing interests from reviewing the submitted manuscript. Finally, editors may also have their own conflicts of interest (11).
Financial conflicts and other links with industry
In a Lancet commentary in 2000 (12), Professor David Weatherall from the University of Oxford discussed the public apology issued by the New England Journal of Medicine, having discovered that in nearly half the articles on drug therapy published in the journal between 1997 and 1999, authors had an undisclosed financial link to the product manufacturer (13). Weatherall pointed out that governments worldwide were pushing universities to link up with industry and that “reduced support for clinical research is driving investigators towards industry as a source of funding.” In 2009, Marcia Angell, ex-editor of the New England Journal, quoted US Senator Charles Grassley, ranking Republican on the Senate Finance Committee, as revealing that a researcher at Harvard Medical School had received US $1.6 million (€1.1.million) in consulting and speaking fees between 2000 and 2007 from pharmaceutical companies (13). These companies included those which manufactured drugs which he had advocated in numerous journal publications. Another US academic was stated by Senator Grassley to control more than US$6 million (€4.2 million) of stock in a company he co-founded which was testing a drug used in psychotic depression; at the same time he was a principal investigator on a US National Institute of Mental Health grant that included research on the drug, about which he had co-authored three papers. Angell estimated that, in the US, pharmaceutical companies pay tens of billions of dollars annually to physicians.
Another area of research where COI arguments have become prominent is in toxicology. In an overview, Claxton, from the US Environmental Protection Agency, has rehearsed the debate (14). For example, there has been criticism that health assessments of some potential carcinogens were carried out partly by scientists employed by industrial organizations which produced or distributed the materials in question. There is, of course, a counter-argument: namely that industrial scientists’ work is highly regulated, meets high standards and unfavorable results are not suppressed (15). The subject is complicated by the presence, both in research teams and in those designing regulation or guidelines, of scientists employed by government – which might itself have a vested interest in the data eventually published. Moreover, in many fields scientists move freely between academia, industry, and government, sometimes holding down posts funded by more than one institution.
Claxton (14), in a non-systematic review of toxicological studies where conflict of interest was discussed, acknowledged cases where COI proved to have “negatively impacted the designing, conducting, analysis and use of research efforts.” But he also uncovered evidence that such outside influence and collaboration “can be beneficial because scientists receive additional financial support, there are increased opportunities for collaboration and they might obtain otherwise unobtainable materials to study.”
While readers of published papers might be suspicious of bias resulting from COI, especially when undeclared, they can also be misled by the simple suppression of data or even of full papers. For example, Lexchin et al (15) showed that company-sponsored research was less likely to be published than that sponsored by other institutions or individuals. It was more likely to show a favorable result toward the company’s product. Company sponsored research was of a generally high standard. The question is begged, therefore, of where are the negative studies? Fault may lie with sponsors, authors or editors – in that the last may be prejudiced against accepting “negative” studies. The opposite side of the coin is exemplified by the meta-analysis of a systematic review by Tramčr et al into studies of the use of ondansetron in gastro-esophageal reflux disease (16): these authors found that, using the number needed to treat (NNT) as an outcome measure, studies whose data had been published more than once (that is duplicated or redundant publication) suggested greater potency than those which had not been duplicated. Thus, including the duplicated studies (even singly) into the meta-analysis reduced the NNT. One does not know the motivation of authors in submitting their papers more than once; of course, collecting citations is itself a potential COI in that it enhances career progression – and therefore likely income. But, given the “positivity” of the findings from these duplicate publications, it is possible that “sponsorship bias” was also at work. Using the text matching search engine eTBLAST, Errami & Garner found 421 duplicates in a sample of 62 000 Medline abstracts (17): extrapolation to the full Medline database suggests there may be as many as 117 500 duplicate publications. The potential bias these may have introduced is clear.
More recently, a review of 1534 original oncology papers published in eight leading journals showed that 29% had conflicts of interest and 17% declared industrial funding. Where a COI was present, survival outcomes of studies focusing on treatment were more positive (18).
Finally, it has to be kept in mind that research is always performed in the social and economical context, which very often determines the topic of research. This is the reason, for example, for more research in clinical medicine then in public health or health services (19).
Non-financial conflicts
These have been reviewed in an editorial by the PLoS Medicine editors (20) and include such issues as political, ideological or religious beliefs, personal friendships or enmity, academic favoritism or jealousy and personal ambition. The authors quote scenarios including a reviewer whose religious affiliation makes her morally opposed to fetal cell research being asked to referee a study of a project using cell lines derived from an aborted fetus; and an editor sending to known sympathetic reviewers a paper from an author who had supervised his postdoctoral fellowship and who remained a personal friend. Data are lacking on whether such non-financial conflicts prejudice the scientific record in a similar way to financial, industrial or governmental links.
One area of particular concern is the role of consulting firms employed by producers of potentially toxic chemicals (including tobacco) in questioning studies that have identified hazards (21). Such “strategic reviews” may be given undeserved credibility by being published in peer-reviewed indexed journals. As Michaels states (22): “Strategic data synthesis exercises, whether literature reviews or meta-analyses, are often little more than advocacy briefs made to resemble scientific papers.”
Research teams, journal editors, and publishers have their own unique COIs. For example, they increasingly use the medium of press releases to trumpet success. Partly this may be self-promotion but it might also represent a disguised plea for funding, especially from charities (non-profit organizations). Astute editors know that wide dissemination through the lay media of papers published in their journals can increase citations and so enhance their impact factor (23). In some circumstances, a COI can seem to be totally beneficial, for example campaigning by patient self-help groups: yet many are tied up financially with manufacturers of devices or drugs (24).
In the end, open disclosure provides extra information with which readers, consumers, and providers of grants can decide on how much credence to place on the data presented. While the large majority of researchers, institutions, and companies are honest, secrecy – whether deliberate or accidental – can breed only suspicion.
Shades of gray
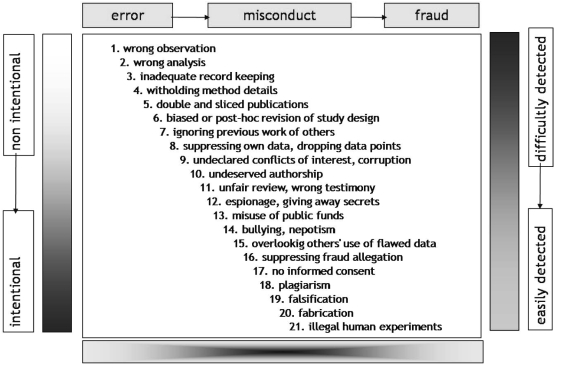
From what has been stated above, it is clear that the simple presence of COI does not imply fraud or even any misconduct at all. COI of some sort, especially non-financial, may be inevitable, so not necessarily culpable. Nylenna and Simonsen postulated that there is no distinct line between ethical and unethical behavior; rather there is a continuum from honest error to outright fraud and what matters is intention, a quality which may be impenetrable to the observer (25). Nylenna has updated and amplified an illustration from that paper which is shown as the Figure 2.
Figure 2.
The continuum from honest error, over misconduct to outright fraud (top horizontal axis). The vertical axis shows the continuum from non-intentional to intentional nature of the publication behavior, as well as from their easy to difficult detection.
COI may be part of the problem at any step in the progression shown in the Figure 2, perhaps becoming more likely as one proceeds steadily toward the more serious transgressions. In deciding how to prevent or deal with corruption of the scientific record, “shades of grey” need to be borne in mind. Marušić pointed out at the symposium that there may be an additional category in post-Communist countries. Previously it was widely regarded that cheating the government was praiseworthy; in the post-communist era, this mindset may have been extended to other perceived authorities – including employing institutions, funders, and editors.
Journal policies
When an editor informs a submitting author that he has concerns about a possible undeclared COI, a frequent reply is that he or she did not realize that they might have such a problem; sometimes this may be because they have not read the journal’s instructions to authors or advice to contributors. Alternatively it may be because the journal’s requirements are not stated, ambiguous or incomplete. Thus, some journals simply require a general statement that the author knows of no significant competing interest. Others ask for specific information on funding. A minority demand completion of more comprehensive checklists, enquiring into the precise role of any supporting institution in design, analysis or preparation for publication. Currently, many of the larger journals are conducting a trial of a standard unified declaration available from the International Committee of Medical Journal Editors (ICMJE) (26) This declaration (available from http://www.icmje.org/coi_disclosure.pdf) requires all authors to complete a 4 page questionnaire which seeks information about any resources received by the author or his or her institution to enable them to complete the work; it also asks for all sources of revenue relevant to the submitted work received over the previous 3 years – not just from the entity which sponsored the research. Similar information is required of any similar financial arrangement with the spouse, partner or children under 18 years of all authors. They are also required to report any personal, professional, political, institutional, religious or other associations “that a reasonable reader would want to know about in relation to the submitted work.” Tick boxes are included specifically relating to gifts, grants, honoraria, payment for manuscript preparation, patents, royalties, payments for educational activities, stock options, travel and accommodation reimbursement, board membership, consultancy, employment, and fees for expert testimony.
It remains to be seen whether authors (and reviewers) comply with these stringent requirements and whether journal editors police them adequately. An important criterion is that the corresponding author is expected to seek and confirm information for co-authors at the time of submission. This is important given the results of a prospective study on the journal disclosure form for authorship required by the Croatian Medical Journal. Ilakovac et al interrogated 919 authors of 200 papers (27). The corresponding author of the papers completed checklists for all 919 authors and a blank form was subsequently sent to each individual author. The main outcome measure was test-retest differences between the corresponding authors’ self-declarations. Reliability of answers between the two requests was low to moderate both for corresponding authors themselves and when acting as proxies for co-authors.
Not only authors, but reviewers and editors have potential COI. How seriously the latter two groups take their policing role will be crucial. Again, the evidence is a matter for concern. In a survey of 37 journals, 19/30 respondents considered it important or very important that they declare their editors’ COI; 13/30 gave the same answer about their editorial board and 11/30 those of other editorial advisers. Only half of those who considered the matter important had a policy to deal with the issue, which was “internal” and “often vague.” Few had mechanisms for updating declarations (28).
More recently, Wager et al received responses from 231 editors-in-chief of medical, health care, life sciences, and social science journals published by Wiley-Blackwell. Offered a perceived severity score for their journal of 0 (not a problem) to 3 (a very serious problem), the mean rating for undisclosed authorial COI was 0.73. Asked how frequently they encountered the problem (on a 0-4 scale for increasing frequency) the mean result was 0.90. The authors concluded that at least some editors of science journals may be unaware of many of the ethical problems that they might be facing (29).
The vast majority of biomedical journal editors are “amateurs” in the sense that their editorial role is subsidiary to their research or clinical employment and is often unpaid. It is hardly surprising that they are sometimes blind to problems that are all too evident to their “professional” colleagues from major journals. One small comfort, of course, is that many small specialty journals publish few studies, such as drug trials, where significant financial interests may be at stake, so may be less prone to authorial misconduct.
Editors cannot claim to be without resources. Advice on recognizing and dealing with COI is available from the Web sites of ICMJE, the World Association of Medical Editors (WAME) (www.wame.org), COPE (www.publicationethics.org) and the Council of Science Editors (www.councilscienceeditors.org) among others. Many publishers, including Elsevier and Wiley-Blackwell also provide ethical advice for their editors. The latter’s position is described in detail in its Best Practice Guidelines (30) which makes it clear that editors, authors and peer reviewers all have a responsibility to disclose interests “that might appear to affect their ability to present or review data objectively.” The guideline authors state that the existence of a COI does not prevent someone being listed as an author although “editors may prefer not to commission subjective articles (for example editorials or non-systematic reviews) from author with a COI” but remind editors that adapting such a policy runs the risk of encouraging authors to conceal relevant interests so may be counter-productive.
Institutional responses
Systematic data are not available. However, qualitative data from the COPE suggests that institutions vary greatly in their response to allegations of misconduct by an employee, including that of undeclared COI. Frequently, editors who have been advised by COPE to request an institution to investigate a matter of concern find that the response is tardy or no action results. Nonetheless, many institutions have clearly defined and publicized policies for handling misconduct.
In general, research institutions or funders are responsible for dealing with misconduct by their employees or those who have contracted with them. In certain countries, review and appellate functions reside in governmental bodies – particularly in regard to government funded studies. The most developed national means of investigating misconduct are in the USA, Canada, Scandinavia, Australia, and Germany with fledgling operations in the UK, Croatia, China, India, and Japan (31).
Regulatory bodies also vary in their responses: in the UK, the General Medical Council, with whom all practicing physicians must register, declares in its guidance to researchers that: “You must ensure that your judgment about the research is not influenced, or seen by others to be influenced, by financial, personal, political or other external interest at any stage of the process. You should always declare any conflicts that may arise to an appropriate person, authority or organization.” The guidance goes on to specify to whom researchers must declare any financial matters – including research ethics committees, research participants, nurses and non-clinical staff in the research team. The GMC can investigate complaints that a registered doctor has not complied with these requirements and can apply sanctions, after due process of investigation, which might include a ban from conducting research, suspension or even erasure from the register (32).
Sanctions are also available to (and used by) the US Office of Research Integrity in regard to misconduct in research or in grant applications that have been federally funded. There have been calls for regulators to require authors who are required to submit their work for approval to share underlying data collected in a study so that others can assess reliability; this could include agencies posting comprehensive conflict disclosures on their Web sites along with submitted studies (33).
Sanctions
In general, sanctions are available only to regulatory bodies, employers, and sponsors in relationship with researchers and authors. It can be very frustrating to editors if none of these are able or willing to take action. Some editors have proposed their own sanctions – such as banning authors from submitting papers for a certain length of time or warning editors of related journals. But there are potential legal pitfalls, depending on the particular jurisdiction, of what might be alleged as restraint of trade or defamation. In consequence, COPE advises its members that, beyond rejecting a paper or publishing an expression of concern or retraction once a serious allegation has been raised or proved, they should go no further than asking the study sponsor or employing institution to investigate.
Retractions
The National Library of Medicine (NLM) states that an article can be retracted or withdrawn by its authors, academic or institutional sponsor, editor or publisher, because of pervasive error or unsubstantiated or irreproducible data. NLM does not differentiate between retraction due to honest error and that due to misconduct. The citation for the article is not removed from the database but is updated to indicate retraction and links the original citation to the published retraction notice (34). Thus, there is no specific reference to COI, although COI may be the prime cause of misconduct or error.
Elsevier advises its authors that they can withdraw an article in press or retract one already published for various reasons, including infringements of professional ethical codes, such as multiple submission or duplication, bogus authorship claims, plagiarism, fraudulent data “or the like” but do not refer specifically to undeclared COI.
Before due process by a responsible body has made a finding of misconduct but where the case appears strong, it is open to editors to publish a “notice of concern” or even an editorial explaining the issues involved – but short of any formal retraction.
Conclusions of the discussion
The existence of a COI does not necessarily imply misconduct or harm but is well recognized to have the potential to lead to feelings of obligation or reciprocity by the individual concerned (35). Lack of transparency at the time of submission and publication, when followed by subsequent discovery of an undeclared interest, is likely to lead to a perception by readers and editors that the research is tainted, even possibly fraudulent. Neither concept is helpful to scientific advance or the public interest in probity.
The contrary argument is that editors habitually conflate serious misconduct by academics without corporate ties with far less heinous cases where authors have not responded appropriately to unjustifiably over-elaborate requirements for disclosure (36). In an invited polemic, Stossel claims that “adverse outcomes objectively ascribed to financial COI are almost non-existent” and that “no evidence supports that corporate detailing and gifting adversely affects patient care” and that COI strictures actually inhibit medical advances.
One way of dealing with the controversy might be to follow the suggestions of Nylenna and Simonsen (25) by taking a population rather than an individual approach to COI. These authors based their argument on the concept proposed a quarter of a century ago by Rose (37) in relation to disease prevention. Rose defined the prevention paradox – namely, a measure that brings large benefit to the community but offers little to every participant means that minor changes in the right direction by most of the population is more effective than major changes by the few. The Gaussian distribution curve is shifted to the left. Nylenna and Simonsen conceptualize scientific misconduct, which would include deliberate lack of transparency by non-disclosure, as an unhealthy condition diffused throughout the science community to different degrees of seriousness. By moving the whole research community in one direction, the number of serious cases can be reduced. This approach requires clear definitions and simple and readily available guidelines at all levels of research and practice, including entry, with appropriate mentoring and audit; national independent bodies should be permitted to investigate serious cases and whistleblowers encouraged and safeguarded. As Marušić pointed out at the symposium, any such action is even more cogent in cultures where “scientific inbreeding” (38) has led to academics routinely enjoying tenure in the institution where they were trained.
Does it matter?
Editors should not underestimate readers. Many may bring the requisite level of skepticism in considering the validity or impact of published research but those who are naive would benefit from training in appraising scientific papers.
They should also be aware of the veneration for science that inhabits the popular mind. “It’s in a peer-reviewed journal, so it must be true” – whereas scientists know that all published work is merely part of work in progress, so may well be contradicted eventually. In this respect, researchers, their institutions, and editors should understand how they might themselves display a COI when they involve the media, for example with press releases. They may be demonstrating an “abuse of entrusted power.”
Acknowledgment
The meeting of the discussion groups was organized and financially supported by the Esteve Foundation (http://www.esteve.org). The opinions of the participants do not reflect the views of the sponsor.
References
- 1.Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta-analysis of survey data. PLoS One. 2009;4:e5738. doi: 10.1371/journal.pone.0005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titus SL, Wells JA, Rhoades LJ. Repairing research integrity. Nature. 2008;453:980–2. doi: 10.1038/453980a. [DOI] [PubMed] [Google Scholar]
- 3.Retractions’ realities. Nature. 2003;422:1. doi: 10.1038/422001a. [DOI] [PubMed] [Google Scholar]
- 4.Cheers for disgraced clone scientist Hwang-Woo Suk who misused Ł400,000. Times OnLine Oct 27 2009. Available from: http://www.timesonline.co.uk/tol/news/science/article6890229.ece#cid=OTC-RSS&attr=797093 Accessed: February 11, 2010.
- 5.Horton R. Retraction – Non-steroidal anti-inflammatory drugs and the risk of oral cancer: a nested case-control study. Lancet. 2006;367:382. doi: 10.1016/S0140-6736(06)68120-8. [DOI] [PubMed] [Google Scholar]
- 6.Poehlman ET. Letter of apology for falsification of data. Am J Physiol Endocrinol Metab. 2005;289:e357. doi: 10.1152/ajpendo.00218.2005. [DOI] [PubMed] [Google Scholar]
- 7.White C. Suspected research fraud: difficulties at getting at the truth. BMJ. 2005;331:281–8. doi: 10.1136/bmj.331.7511.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R. Investigating the previous studies of a fraudulent author. BMJ. 2005;331:288–91. doi: 10.1136/bmj.331.7511.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Transparency International. Global Corruption Report 2007: Corruption in Judicial Systems. Cambridge: University Press, 2007. Available from: http://www.transparency.org/news_room/faq/corruption_faq Accessed: February 11, 2010.
- 10.Relman AS. Publish or perish-or both. N Engl J Med. 1977;297:724–5. doi: 10.1056/NEJM197709292971313. [DOI] [PubMed] [Google Scholar]
- 11.Marusic A, Katavic V, Marusic M. bRole of editors and journals in detecting and preventing scientific misconduct: strengths, weaknesses, opportunities, and threats. Med Law. 2007;26:545–66. [PubMed] [Google Scholar]
- 12.Weatherall D. Academia and industry: increasingly uneasy bedfellows. Lancet. 2000;355:1574. doi: 10.1016/S0140-6736(00)02211-X. [DOI] [PubMed] [Google Scholar]
- 13.Angell M. Drug companies and doctors: a story of corruption. New York Review of Books 2009; Jan 15. Available from: http://www.nybooks.com/articles/22237 Accessed: February 11, 2010.
- 14.Claxton LD. A review of conflict of interest, competing interest and bias for toxicologists. Toxicol Ind Health. 2007;23:557–71. doi: 10.1177/0748233708089046. [DOI] [PubMed] [Google Scholar]
- 15.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: a systematic review. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tramer MR, Reynolds DJ, Moore RA. L. Impact of covert duplicate publication on meta-analysis: a case study. BMJ. 1997;315:635–40. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Errami M, Hicks JM, Fisher W, Trusty D, Wren J, Long TC, et al. Deja vu – a study of duplicate citations in Medline. Bioinformatics. 2008;24:243–9. doi: 10.1093/bioinformatics/btm574. [DOI] [PubMed] [Google Scholar]
- 18.Jagsi R, Sheets N, Jankovic A, Motomura AR, Amamath S, Ubel PA. Frequency, nature and correlates of conflicts of interest in published clinical cancer research. Cancer. 2009;115:2783–92. doi: 10.1002/cncr.24315. [DOI] [PubMed] [Google Scholar]
- 19.Moses H, III, Dorsey ER, Matheson DH, Thier SO. Financial anatomy of biomedical research. JAMA. 2005;294:1333–42. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 20.The PLoS Medicine Editors Making sense of non-financial competing interests. PLoS Med. 2008;5:e199. doi: 10.1371/journal.pmed.0050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaels D. Doubt is their product: how industry’s assault on science threatens your health. New York (NY): Oxford University Press, 2008. [Google Scholar]
- 22.Michaels D. Addressing conflict in strategic literature reviews: disclosure is not enough. J Epidemiol Community Health. 2009;63:599–600. doi: 10.1136/jech.2009.089524. [DOI] [PubMed] [Google Scholar]
- 23.Chapman S, Nguyen TN, White C. Press-released papers are more downloaded and cited. Tob Control. 2007;16:71. doi: 10.1136/tc.2006.019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcovitch H. How do potential conflicts of interest confuse medicine and public health. J Epidemiol Community Health. 2009;63:608–9. doi: 10.1136/jech.2008.086132. [DOI] [PubMed] [Google Scholar]
- 25.Nylenna M, Simonsen S. Scientific misconduct: a new approach to prevention. Lancet. 2006;367:1882–3. doi: 10.1016/S0140-6736(06)68821-1. [DOI] [PubMed] [Google Scholar]
- 26.Drazen JM, Van der Weyden MB, Sahni P, Rosenberg J, Marusic A, Laine C, et al. Uniform format for disclosure of competing interests in ICMJE journals. Croat Med J. 2009;50:427–8. doi: 10.3325/cmj.2009.50.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilakovac V, Fister K, Marusic M, Marusic A. Reliability of disclosure forms of authors’ contributions. CMAJ. 2007;176:41–5. doi: 10.1503/cmaj.060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haivas I, Schroter S, Waechter F, Smith R. Editors’ declaration of their own conflicts of interest. CMAJ. 2004;171:475–6. doi: 10.1503/cmaj.1031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wager E, Fiack S, Graf C, Robinson A, Rowlands I. Science journal editors’ views on publication ethics: results of an international survey. J Med Ethics. 2009;35:348–53. doi: 10.1136/jme.2008.028324. [DOI] [PubMed] [Google Scholar]
- 30.Graf C, Wager E, Bowman A, Fiack S, Scott-Lichter D, Robinson A. Best Practice Guidelines on Publication Ethics: a publisher's perspective. Int J Clin Pract Suppl. 2007;(152):1–26. doi: 10.1111/j.1742-1241.2006.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott-Lichter D; Editorial Policy Committee, Council of Science Editors. CSE’s white paper promoting integrity in science journal publications, 2009 Update. Reston,Va, 2009. Available from: www.councilscienceeditors.org/editorial_policies/white_paper.cfm Accessed: February 11, 2010.
- 32.General Medical Council. Research: the role and responsibilities of doctors. London: GMC, 2002. Available from: http://www.gmc-uk.org/guidance/ethical_guidance/research.asp Accessed: February 11, 2010.
- 33.Wagner W, McGarity T. Regulatory reinforcement of journal conflict of interest disclosures. J Epidemiol Community Health. 2009;63:606–7. doi: 10.1136/jech.2008.085001. [DOI] [PubMed] [Google Scholar]
- 34.National Library of Medicine. NLM policy on retractions. Available at: http://www.nlm.nih.gov/pubs/factsheets/errata.html Accessed: February 11, 2010.
- 35.Lee K. Has the hunt for conflicts of interest gone too far? No. BMJ. 2008;336:477. doi: 10.1136/bmj.39491.391215.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stossel TP. Has the hunt for conflicts of interest gone too far? Yes. BMJ. 2008;336:476. doi: 10.1136/bmj.39493.489213.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–8. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 38.Navarro A, Rivero A. High rate of inbreeding in Spanish universities. Nature. 2001;410:14. doi: 10.1038/35065259. [DOI] [PubMed] [Google Scholar]