Abstract
The aim of this article is to review the role of uric acid in the context of antioxidant effects of wine and its potential implication to human health. We described and discussed the mechanisms of increase in plasma antioxidant capacity after consumption of moderate amounts of wine. Because this effect is largely contributed by acute elevation in plasma uric acid, we paid special attention to wine constituents and metabolic processes that are likely to be involved in uric acid elevation.
The association between light-to-moderate wine consumption and risk reduction of cardiovascular diseases and/or reduction of all-cause mortality was confirmed by numerous epidemiological studies (1-7). Among other beneficial biological effects, it has been shown that wine can increase antioxidant capacity in humans (8-12) and reduce susceptibility of human plasma to lipid peroxidation (11,13). These effects of wine attracted significant research interest, as oxidative stress is implicated in the pathogenesis of various diseases such as cancer and cardiovascular and neurodegenerative diseases (14-19).
Wine and plasma antioxidant capacity
Early studies by St Leger et al (3) and Renaud et al (4) demonstrated an inverse relation between incidence of coronary heart disease and wine consumption in different developed countries, which prompted the efforts to discover the mechanisms underlying the observed effects. Soon, it was recognized that polyphenolic compounds highly contained in wine, especially in red wine, were responsible for various biological effects, including potent antioxidative activity.
Antioxidative activity of polyphenols is based on two mechanisms: chelation of free metal atoms such as iron and copper, which prevents biochemical reactions generating reactive oxygen species and scavenging of free radicals as effective hydrogen donors (20). Indeed, Frankel et al showed in 1993 that red wine phenolics inhibited oxidation of human low-density lipoprotein in vitro (21).
An increase in serum antioxidant activity following ingestion of red wine was first described in 1994 by Maxwell et al (8). In a similar study, Whitehead et al (9) showed that serum antioxidant capacity one hour after ingestion of 300 mL of red wine increased by 18%, which was comparable with 22% increase in serum antioxidant capacity after ingestion of 1 g of vitamin C.
Serafini et al (10,11), who studied the relationship between polyphenols content and antioxidant capacity of beverages with plasma levels of polyphenols and its antioxidant capacity, provided a deeper insight into this matter. Their studies with dealcoholized red and white wine and green and black tea demonstrated that total antioxidant capacity of plasma was associated with plasma total polyphenols concentration.
More recent analytical methods and kinetic studies, however, showed that polyphenols from food, including wine, were poorly absorbed in humans (22-24). The net increase in plasma polyphenols concentration after red wine consumption is insufficient to explain the observed elevation in plasma antioxidant capacity. Day and Stansbie (25) were first to propose an alternative explanation for the antioxidant effects of red wine. They reported a significant correlation between the increase in total serum antioxidant capacity and the increase in serum urate concentration after port wine consumption. This finding was later supported by Maxwell and Thorpe (8), who reanalyzed their results published in 1994 and reported that more than half of red wine-induced increase in antioxidant capacity in their study could be attributed to changes in serum urate (26). A recent study by our group demonstrated that acute elevation of plasma antioxidant capacity after red wine consumption was mediated by two separate factors, wine phenols and plasma urate (12).
It should be noticed that ethanol, as an important constituent of wine implicated in its various biological effects, does not contribute directly to the wine-related acute increase in plasma antioxidant capacity. On the contrary, there was a decrease in plasma antioxidant capacity after ethanol ingestion, indicating an ethanolic-mediated oxidative load (12,27). However, ethanol might be indirectly involved in plasma antioxidative activity after wine consumption. Even small changes in physical-chemical properties of hydroalcocholic solutions in which polyphenols are dissolved significantly influence their solubility, precipitation behavior, and their interactions with proteins, which in turn may influence their biochemical properties and bioavailability (11,28-30). Indeed, Duthie et al (27) showed that proportionally more phenolics were absorbed from whisky than from wine, which was partly ascribed to the greater ethanol content of whisky that aids phenolics absorption.
One more aspect of wine-related increase in plasma antioxidant activity should be mentioned, and that is the effect of unabsorbed polyphenols that remain in gastrointestinal system following the wine consumption. There they can scavenge free radicals locally, preventing lipid peroxidation and, at the same time, spare other antioxidants from oxidation. In such a way, although acting locally, the polyphenols could influence the whole organism and the plasma concentration of various antioxidants, which in turn could affect plasma antioxidant capacity (31-33). Moreover, red wine polyphenols have recently been attributed a new beneficial function: prevention of absorption of cytotoxic lipid peroxidation products (34).
Mechanisms of wine-induced elevation in plasma uric acid
When it was determined that acute elevation of plasma uric acid after red wine consumption largely contributed to the observed increase in plasma antioxidant capacity, a new question was raised: Which wine component(s) is/are responsible for acute postprandial increase in plasma uric acid concentration and related antioxidant capacity?
Because polyphenols-stripped red wine caused an increase in plasma urate similar to that of the intact red wine (12,35), it could be concluded that the observed effect is polyphenols-independent. On the other hand, consumption of dealcoholized red wine did not cause an increase in plasma urate, pointing to the role of ethanol in the phenomenon (12,36). After ingestion of different combinations of non-phenolic constituents of wine (organic acids, sugars, glycerol), the increase in plasma urate and related antioxidant capacity was successfully replicated by a mixture of glycerol in ethanol-water solution. There was no glycerol effect if ethanol was removed from the solution (37).
The detailed biochemical mechanism responsible for this effect is yet to be revealed.
Nevertheless, by linking our results to the findings of Lundquist et al (38) and Thiden (39) on metabolic interactions of glycerol and ethanol in humans and in isolated liver slices, we propose credible explanation of the metabolic processes responsible for the described effect.
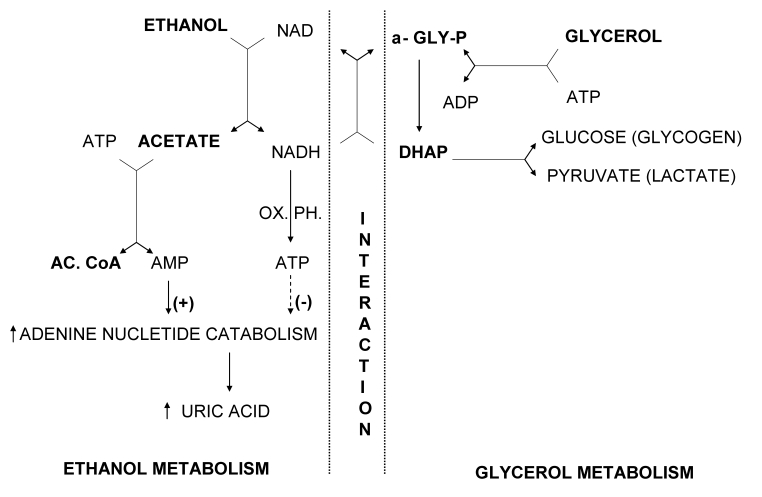
Almost 45 years ago, Lundquist et al (38) demonstrated that there was a significant reduction (up to 70%) in the uptake of glycerol in the splanchnic area in humans when glycerol and ethanol were infused together. On the other hand, no changes in the rate of ethanol metabolism or in the output of acetate from the liver were observed when ethanol was infused either alone or in combination with glycerol. Based on the available information at the time, an explanation for the observed inhibition of glycerol metabolism by ethanol was offered. It was proposed that dihydroxyaceton phosphate formed by oxidation of glycerophosphate might be reduced back to glycerophosphate by nicotinamide adenine dinucleotide (reduced form), which is produced in surplus by ethanol metabolism in liver tissue (Figure 1). Consequential accumulation of glycerophosphate could be the cause of the inhibitory effects of ethanol in the first step of glycerol metabolism, ie, inhibition of glycerol phosphorilation to glycerophosphate.
Figure 1.
Proposed biochemical mechanism of increased uric acid production induced by glycerol and ethanol (modified and updated after Lundquist et al (38). AMP – adenosine monophosphate; ADP – adenosine diphosphate; ATP – adenosine triphosphate; NAD – nicotinamide adenine dinucleotide; NADH – nicotinamide adenine dinucleotide (reduced form); AC. CoA – acetyl-coenzyme A; α-GLY-P – α-glycerophosphate; DHAP – dihydroxyacetone phosphate, OX. PH – oxidative phosphorylation. Ethanol is first oxidized to acetaldehyde, which is further oxidized to acetate. During these two oxidation steps, a parallel reduction of two molecules of NAD to NADH occurs. This generation of reducing equivalents in the liver is associated with ATP synthesis. Acetate is metabolized to acetyl-CoA via acetyl-AMP. During conversion of acetate to acetyl-AMP to acetyl-CoA, ATP is dephosphorylated to AMP; thus 2 mol of high-energy phosphate are consumed for each mol of ethanol metabolized. Although most of the AMP formed is resynthesized to ATP, a small amount of AMP may enter the pathway of adenine nucleotide degradation leading to uric acid production. ATP formed during ethanol oxidation to acetate is inhibitory to the adenine nucleotide degradation. The first step in glycerol metabolism is phosphorylation to α-glycerophosphate associated with dephosphorylation of ATP to ADP. The produced α-glycerophosphate is then oxidized to dihydroxyacetone phosphate (DHAP), which can be directed to production of glycogen (glucose) or pyruvate (lactate). In the presence of sufficient amounts of NADH, largely produced during ethanol metabolism, dihydroxyacetone phosphate can be reduced back to α-glycerophosphate. The net result of redirecting NADH to the production of α-glycerophosphate instead of ATP would be increased adenine nucleotide degradation and production of uric acid.
Related changes in adenine nucleotides turnover and plasma uric acid, as a result of ethanol-glycerol metabolic interactions, were not envisioned at that time.
Four years later, in experiments using isolated liver slices, Thieden (39) showed that the addition of ethanol to the incubation medium containing glycerol caused a decrease in glycerol uptake by more than 40%, and the highest accumulation of glycerophosphate was observed in the slices incubated in the medium containing both glycerol and ethanol. However, this study resulted in another important finding – a significant depletion of adenosine triphosphate and total adenine nucleotides content only in the liver slices incubated with glycerol and ethanol. A possible meaning of this finding was not addressed in the article. Our finding that uric acid acutely rises after consumption of ethanol and glycerol indirectly confirms the validity of Thieden’s results, indicating that lost adenine nucleotides were metabolized to uric acid. Described metabolic interactions are shown in more detail in the modified and updated scheme after Lundquist et al (Figure 1).
A sharp distinction should be drawn between short-term effects of moderate amount of wine (ethanol and glycerol) consumption on plasma uric acid and ethanol-induced hypeuricemia.
It is rather well documented that ethanol by itself may induce hyperuricemia, which is mediated by both, a decreased renal excretion of uric acid as a result of increased blood lactate level (40,41) and an elevated production of urate that is secondary to enhanced turnover of adenine nucleotides (42-44).
The ethanol-induced hyperuricemia, however, occurs after the intake of much higher amounts of ethanol during prolonged period of time (40,42), and as such should not be confused with the acute elevation of plasma urate following the intake of moderate amounts of wine.
Indeed, oral intake of ethanol in amounts equivalent to those contained in 200-300 mL of red wine did not cause changes in plasma uric acid level for at least 3 hours (12). Moreover, two-hour intravenous administration of ethanol to human participants, at 0.25 to 0.35 g per kilogram per hour, did not cause substantial alterations in serum urate levels, urate clearance, and urinary uric acid excretion relative to the baseline values (42).
Acute hyperuricemia and cardiovascular effects
Uric acid is an end product of purine metabolism in humans. This is different than in most other mammals, which express urate oxidase, an enzyme responsible for further metabolism of uric acid to allantoin. During the course of human evolution, several mutational events have produced the loss of uricase activity (45). This, in addition to effective kidney re-absorption system for urate, has resulted in approximately 10 times higher plasma uric acid levels in humans than in most other mammals (46,47), indicating biological significance of uric acid in man.
Indeed, uric acid is the most abundant aqueous antioxidant, accounting for up to 60% of plasma antioxidative capacity (48). The antioxidative effect of uric acid is evidenced by its ability to directly scavenge free radicals (49) or to form stable complexes with transition-metal ions, such as iron, thereby preventing ascorbate oxidation and lipid peroxidation (50,51).
Consequently, administration of uric acid increased plasma antioxidant capacity (52) and reduced exercise-associated oxidative stress in healthy participants (53,54). Also, the acute elevation of plasma uric acid protected endothelial function in patients with type 1 diabetes and regular smokers (55), and protected from hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans (56). It is interesting that the beneficial effects in the mentioned studies were induced by uric acid elevation ranging from 15 to 100% relative to the control values, indicating the possibility of a threshold in the uric acid-mediated effects on vascular function.
Concluding implications
Acute, moderate elevation of plasma uric acid largely contributes to the increase in total plasma antioxidant capacity after red wine consumption (8,9,12). The elevation in plasma uric acid is mediated by two alcohols in wine, ethanol and glycerol (37).
Here, we propose an explanation of the metabolic processes that are likely to be involved in the phenomenon, although the exact underlying mechanism is yet to be proved.
Epidemiological findings that the chronic hyperuricemia is a marker of increased cardiovascular risk (57-59) should not be confused with the cardiovascular effects of acute, transient increase in plasma uric acid. Acute hyperuricemia even at high concentrations and under different experimental conditions did not cause harmful cardiovascular effects in human participants. On the contrary, it reduced exercise-associated oxidative stress (53,54), protected endothelial function in patients with type 1 diabetes and regular smokers (55), and protected from hyperoxia-induced oxidative stress and increase in arterial stiffness (56), while lowering serum urate did not improve endothelial function in patients with type 2 diabetes (60). It should also be mentioned that acute actions of established cardiovascular risk factors, such as high-fat meals, hyperhomocysteinemia, or cigarette smoking, cause immediate impairment of vascular function (61-63), in contrast to acutely increased plasma uric acid.
Different mechanisms have been proposed as explanations of paradoxical associations of uric acid (64-68), but the role of uric acid as a causal, compensatory, or coincidental risk factor remains unclear.
Although chronic hyperuricemia is associated with gout (69) and ethanol intake is a well-established risk factor (44), recent epidemiological studies have shown that moderate consumption of wine is not associated with higher incidence of gout, in contrast to consumption of beer and spirits (70,71).
Taken together, we can conclude that acute plasma urate increase after wine consumption is not likely to cause detrimental effects to human health associated with chronic hyperuricemia. Quite the opposite, if wine is consumed with meals, the timed elevation of plasma uric acid may significantly contribute to the wine's protective effects against postprandial oxidative stress (13). Indeed, in the most recent review by Covas et al (72), who analyzed the studies on moderate wine consumption and oxidative damage in humans, protective effects of wine were most convincing during the meal-related postprandial oxidative stress. In line with this is the finding of a epidemiological study that showed that cardioprotective effects of wine intake were observed only in participants who consumed wine with meals, but not in those who consumed wine separate from the food intake (73).
Acknowledgment
This work was supported by grant No. 216-2160547-0537 from the Ministry of Science, Education, and Sports of the Republic of Croatia.
References
- 1.Athyros VG, Liberopoulos EN, Mikhailidis DP, Papageorgiou AA, Ganotakis ES, Tziomalos K, et al. Association of drinking pattern and alcohol beverage type with the prevalence of metabolic syndrome, diabetes, coronary heart disease, stroke, and peripheral arterial disease in a Mediterranean cohort. Angiology. 2007;58:689–97. doi: 10.1177/0003319707306146. [DOI] [PubMed] [Google Scholar]
- 2.Gronbaek M, Deis A, Sorensen TI, Becker U, Schnohr P, Jensen G. Mortality associated with moderate intakes of wine, beer, or spirits. BMJ. 1995;310:1165–9. doi: 10.1136/bmj.310.6988.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Leger AS, Cochrane AL, Moore F. Ischaemic heart-disease and wine. Lancet. 1979;1:1294. doi: 10.1016/S0140-6736(79)92250-5. [DOI] [PubMed] [Google Scholar]
- 4.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 5.Gronbaek M. Factors influencing the relation between alcohol and cardiovascular disease. Curr Opin Lipidol. 2006;17:17–21. doi: 10.1097/01.mol.0000203889.50138.98. [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Shaper AG. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am J Public Health. 1999;89:685–90. doi: 10.2105/AJPH.89.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theobald H, Johansson SE, Bygren LO, Engfeldt P. The effects of alcohol consumption on mortality and morbidity: a 26-year follow-up study. J Stud Alcohol. 2001;62:783–9. doi: 10.15288/jsa.2001.62.783. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell S, Cruickshank A, Thorpe G. Red wine and antioxidant activity in serum. Lancet. 1994;344:193–4. doi: 10.1016/S0140-6736(94)92795-2. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead TP, Robinson D, Allaway S, Syms J, Hale A. Effect of red wine ingestion on the antioxidant capacity of serum. Clin Chem. 1995;41:32–5. [PubMed] [Google Scholar]
- 10.Serafini M, Maiani G, Ferro-Luzzi A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J Nutr. 1998;128:1003–7. doi: 10.1093/jn/128.6.1003. [DOI] [PubMed] [Google Scholar]
- 11.Serafini M, Laranjinha JA, Almeida LM, Maiani G. Inhibition of human LDL lipid peroxidation by phenol-rich beverages and their impact on plasma total antioxidant capacity in humans. J Nutr Biochem. 2000;11:585–90. doi: 10.1016/S0955-2863(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 12.Modun D, Music I, Vukovic J, Brizic I, Katalinic V, Obad A, et al. The increase in human plasma antioxidant capacity after red wine consumption is due to both plasma urate and wine polyphenols. Atherosclerosis. 2008;197:250–6. doi: 10.1016/j.atherosclerosis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman B, Lavy A, Aviram M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am J Clin Nutr. 1995;61:549–54. doi: 10.1093/ajcn/61.3.549. [DOI] [PubMed] [Google Scholar]
- 14.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 15.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 16.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Visconti R, Grieco D. New insights on oxidative stress in cancer. Current Opinion in Drug Discovery and Development. 2009;12:240–5. [PubMed] [Google Scholar]
- 18.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 21.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–7. doi: 10.1016/0140-6736(93)90206-V. [DOI] [PubMed] [Google Scholar]
- 22.Bell JR, Donovan JL, Wong R, Waterhouse AL, German JB, Walzem RL, et al. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am J Clin Nutr. 2000;71:103–8. doi: 10.1093/ajcn/71.1.103. [DOI] [PubMed] [Google Scholar]
- 23.Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 25.Day A, Stansbie D. Cardioprotective effect of red wine may be mediated by urate. Clin Chem. 1995;41:1319–20. [PubMed] [Google Scholar]
- 26.Maxwell S, Thorpe G. Impact of red wine on antioxidant status in vivo. Eur Heart J. 2000;21:1482–3. doi: 10.1053/euhj.2000.2235. [DOI] [PubMed] [Google Scholar]
- 27.Duthie GG, Pedersen MW, Gardner PT, Morrice PC, Jenkinson AM, McPhail DB, et al. The effect of whisky and wine consumption on total phenol content and antioxidant capacity of plasma from healthy volunteers. Eur J Clin Nutr. 1998;52:733–6. doi: 10.1038/sj.ejcn.1600635. [DOI] [PubMed] [Google Scholar]
- 28.Serafini M, Maiani G, Ferro-Luzzi A. Effect of ethanol on red wine tannin – protein (BSA) interactions. J Agric Food Chem. 1997;45:3148–51. doi: 10.1021/jf960864x. [DOI] [Google Scholar]
- 29.Zanchi D, Vernhet A, Poncet-Legrand C, Cartalade D, Tribet C, Schweins R, et al. Colloidal dispersions of tannins in water-ethanol solutions. Langmuir. 2007;23:9949–59. doi: 10.1021/la700694b. [DOI] [PubMed] [Google Scholar]
- 30.Zanchi D, Poulain C, Konarev P, Tribet C, Svergun DI. Colloidal stability of tannins: astringency, wine tasting and beyond. J Phys Condens Matter. 2008;20:494224–9. doi: 10.1088/0953-8984/20/49/494224. [DOI] [Google Scholar]
- 31.Kanner J, Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31:1388–95. doi: 10.1016/S0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 32.Gorelik S, Lapidot T, Shaham I, Granit R, Ligumsky M, Kohen R, et al. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: health implications. J Agric Food Chem. 2005;53:3397–402. doi: 10.1021/jf040401o. [DOI] [PubMed] [Google Scholar]
- 33.Gorelik S, Ligumsky M, Kohen R, Kanner J. The stomach as a “bioreactor”: when red meat meets red wine. J Agric Food Chem. 2008;56:5002–7. doi: 10.1021/jf703700d. [DOI] [PubMed] [Google Scholar]
- 34.Gorelik S, Ligumsky M, Kohen R, Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008;22:41–6. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 35.Caccetta RA, Croft KD, Beilin LJ, Puddey IB. Ingestion of red wine significantly increases plasma phenolic acid concentrations but does not acutely affect ex vivo lipoprotein oxidizability. Am J Clin Nutr. 2000;71:67–74. doi: 10.1093/ajcn/71.1.67. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto M, Kim S, Eto M, Iijima K, Ako J, Yoshizumi M, et al. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am J Cardiol. 2001;88:1457-60, A9. [DOI] [PubMed]
- 37.Modun D, Music I, Katalinic V, Dujic Z, Boban M. Glycerol and ethanol in red wine are responsible for urate-related increases in plasma antioxidant capacity. Clin Chem. 2006;52:785–7. doi: 10.1373/clinchem.2005.065656. [DOI] [PubMed] [Google Scholar]
- 38.Lundquist F, Tygstrup N, Winkler K, Jensen KB. Glycerol metabolism in the human liver: inhibition by ethanol. Science. 1965;150:616–7. doi: 10.1126/science.150.3696.616. [DOI] [PubMed] [Google Scholar]
- 39.Thieden HI. The influence of ethanol on glycerol metabolism in liver slices from fed and fasted rats. Acta Chem Scand. 1969;23:237–43. doi: 10.3891/acta.chem.scand.23-0237. [DOI] [PubMed] [Google Scholar]
- 40.Lieber CS, Jones DP, Losowsky MS, Davidson CS. Interrelation of uric acid and ethanol metabolism in man. J Clin Invest. 1962;41:1863–70. doi: 10.1172/JCI104643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieber CS. Hyperuricemia induced by alcohol. Arthritis Rheum. 1965;8:786–98. doi: 10.1002/art.1780080442. [DOI] [PubMed] [Google Scholar]
- 42.Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. 1982;307:1598–602. doi: 10.1056/NEJM198212233072602. [DOI] [PubMed] [Google Scholar]
- 43.Puig JG, Fox IH. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. 1984;74:936–41. doi: 10.1172/JCI111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T, Moriwaki Y, Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta. 2005;356:35–57. doi: 10.1016/j.cccn.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 46.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–31. doi: 10.1016/0891-5849(93)90143-I. [DOI] [PubMed] [Google Scholar]
- 48.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988;263:1709–12. [PubMed] [Google Scholar]
- 50.Sevanian A, Davies KJ, Hochstein P. Conservation of vitamin C by uric acid in blood. J Free Radic Biol Med. 1985;1:117–24. doi: 10.1016/0748-5514(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 51.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–54. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–71. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003;105:425–30. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 54.Mikami T, Yoshino Y, Ito A. Does a relationship exist between the urate pool in the body and lipid peroxidation during exercise? Free Radic Res. 2000;32:31–9. doi: 10.1080/10715760000300041. [DOI] [PubMed] [Google Scholar]
- 55.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–32. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 56.Vukovic J, Modun D, Budimir D, Sutlovic D, Salamunic I, Zaja I, et al. Acute, food-induced moderate elevation of plasma uric acid protects against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis. 2009;207:255–60. doi: 10.1016/j.atherosclerosis.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Feig DI, Mazzali M, Kang DH, Nakagawa T, Price K, Kannelis J, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol. 2006;17:S69–73. doi: 10.1681/ASN.2005121331. [DOI] [PubMed] [Google Scholar]
- 58.Gagliardi AC, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–7. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50:2572–9. doi: 10.1007/s00125-007-0817-7. [DOI] [PubMed] [Google Scholar]
- 61.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. J Am Coll Cardiol. 1999;33:1050–5. doi: 10.1016/S0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 62.Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation. 1999;99:1156–60. doi: 10.1161/01.cir.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 63.Papamichael C, Karatzis E, Karatzi K, Aznaouridis K, Papaioannou T, Protogerou A, et al. Red wine's antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am Heart J. 2004;147:E5. doi: 10.1016/S0002-8703(03)00468-X. [DOI] [PubMed] [Google Scholar]
- 64.Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 65.Waring WS, Webb DJ, Maxwell SR. Uric acid as a risk factor for cardiovascular disease. QJM. 2000;93:707–13. doi: 10.1093/qjmed/93.11.707. [DOI] [PubMed] [Google Scholar]
- 66.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–14. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Ghirlanda G. Serum uric acid, mortality and glucose control in patients with Type 2 diabetes mellitus: a PreCIS database study. Diabet Med. 2008;25:508. doi: 10.1111/j.1464-5491.2008.02392.x. [DOI] [PubMed] [Google Scholar]
- 69.Choi HK, Mount DB, Reginato AM, American College of Physicians American Physiological Society. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 70.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–81. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 71.Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341–5. [PubMed] [Google Scholar]
- 72.Covas MI, Gambert P, Fitó M, de la Torre R. Wine and oxidative stress: up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis. 2010;208:297–304. doi: 10.1016/j.atherosclerosis.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 73.Trevisan M, Schisterman E, Mennotti A, Farchi G, Conti S, Risk Factor And Life Expectancy Research Group. Drinking pattern and mortality: the Italian Risk Factor and Life Expectancy pooling project. Ann Epidemiol. 2001;11:312–9. doi: 10.1016/S1047-2797(00)00183-6. [DOI] [PubMed] [Google Scholar]