Abstract
Whether precursors of the left-lateralization for human language can be found in the vocal and gestural communication systems of nonhuman primates remains a topic of intense research, particularly within theoretical discussions of the evolutionary origins of language. Although previous studies in chimpanzees have reported evidence of right-handedness for interspecies food beg gestures produced exclusively toward humans, some might question the generality of these results to intraspecies communicative signals. To address this issue, we recorded hand use in 70 captive chimpanzees for species-typical signals, that could be directed either toward conspecifics or humans. We found evidence of a predominance of right-handedness for species-typical gestures in captive chimpanzees when directed to both humans and conspecifics. Hand preferences during intraspecies communication were significantly positively correlated with gestures directed towards humans. By contrast, hand preferences for gestures did not significantly correlate with hand use for a non-communicative self-directed action. The collective results suggest (a) that evidence of predominance of right-handedness for human-directed gestures communication are not specific to this context and (b) the existence of a specific communicative system involving gestures constitute an ideal prerequisite for the cerebral substrates of human language and its typical left-lateralization.
Keywords: Handedness, gestural communication, hemispheric lateralization, language evolution, nonhuman primates
1. Introduction
Most language functions are under the control of the left cerebral hemisphere in the majority of humans (e.g., Knecht et al., 2000) and such a hemispheric asymmetry has been historically linked to right-handedness for manipulative actions. In fact, right-hand dominance in humans is revealed not only by actions associated with manipulation (almost 90% are right-handed: Annett, 1985), but are also expressed in manual gestural behaviors such as (a) signing in deaf people (Grossi et al., 1996; Vaid et al., 1989), (b) manual movements produced simultaneously when people are talking (Kimura, 1973a, 1973b), and (c) pointing gestures by infants during speech development (Blake et al., 1994). Moreover, using functional brain imaging (such as Positron emission tomography, PET), deaf people revealed an activation of Broca’s area in the left-hemisphere when producing signs (e.g., Corina et al., 2003; Emmorey et al., 2007). These findings collectively suggest that the production of such “language-related” gestures may involve left-lateralized language areas (see Kimura, 1993).
Many have previously argued that population-level behavioral and brain asymmetries are uniquely human and due, in part, to the emergence of language in humans (Crow, 2004; Ettlinger, 1988; Warren, 1980; Williams et al., 2006). This view has been challenged by numerous of studies in a host of vertebrates that have demonstrated behavioral and brain asymmetries at a population-level (Hopkins, 2007; Rogers and Andrew, 2002; Vallortigara and Rogers, 2005). Since nonhuman primates, particularly great apes, are our phylogenetically closest relative and communicate frequently by gestures with conspecifics in many social contexts (e.g., Call and Tomasello, 2007;Goodall, 1986; Pika et al., 2005a, for reviews), whether the left-lateralization for language in humans has its precursors in the communicative gestural or vocal system of nonhuman primates has been the topic of recent studies on behavioral asymmetries.
There remains considerable discussion, yet very little data on behavioral lateralization in the production of vocal and gestural communication in animals, notably primates (Hopkins and Fernandez-Carriba, 2002; Taglialatela, 2007; Vauclair and Meguerditchian, 2008), behaviors that would seemingly be critical for evaluating different theories of language origins as it relates to hemispheric specialization. Asymmetries in vocal production have largely been derived by quantifying oro-facial asymmetries during vocal communication. Marmosets, rhesus monkeys and chimpanzees all predominantly show, though not exclusively, oro-facial asymmetries in vocal production toward the left side of the mouth (i.e., right hemisphere dominance), which is consistent with the view that their vocalizations reflect emotional states (Fernandez-Carriba et al., 2002; Hauser, 1993; Hauser, 1999; Hook-Costigan and Rogers, 1998; but see Losin et al., 2008).
For manual gestures, group-level right-handedness for gestural communication has been previously reported in small sample of captive bonobos and gorillas (Shafer, 1993; Hopkins and de Waal, 1995) and in 60 captive baboons for a hand slapping threat gesture directed either toward conspecifics or humans (Meguerditchian and Vauclair, 2006). Hopkins et al. (2005) examined hand use for food-begging manual gestures that were directed toward a human holding food and they found a pronounced right-hand bias in a sample of 227 captive chimpanzees. One potential limitation of this finding in chimpanzees is that the hand preferences were recorded on gestures exclusively produced to human experimenters in order to request food (extend hand or fingers through the cage, refer to “food beg gesture” or “pointing”). Such behaviors are considered a result of ritualization of motor processes exclusively associated to the particular environmental conditions of the chimpanzees (restrictive captivity and interactions with humans in an out of reach food context, Leavens et al., 2005). As such, it might be suggested that direct interactions with right-handed humans that shaped the ritualization of food-beg gestures may have influenced at least the pattern of right-handedness revealed in these studies (see McGrew and Marchant, 1997; Papademetriou et al., 2005). This leads to the empirical question of whether similar patterns of right-handedness are evident in chimpanzees for their species-typical repertoire when communicating with each other.
To investigate this question, in the current study we examined hand use for manual gestures used during intraspecies communicative exchanges between captive chimpanzees during everyday social interactions. In the present study, “communicative manual gestures” is defined as an expressive movement of limbs that is directed to another individual in order to influence its behavior and that is related to a request and/or a desired action/event (Pika et al., 2005b). In addition, for comparison to the data on hand use during intraspecies communication, we recorded hand use for gestures from the same species-typical repertoire that were directed towards humans. If being raised in captivity and having long histories of interacting with humans differentially influences handedness for gestural communication, then it might be hypothesized that significant differences in hand use may be present in these two contexts. Lastly, for comparison to the handedness data for species-typical gestural communication, we collected data on hand use for non-communicative self-directed touching action and replicated the measures of hand use for human-directed food beg gestures previously investigated in chimpanzees (Hopkins et al., 2005). Including the human-directed food beg gestures allowed for the assessment of consistency in hand use over time and to examine whether these patterns generalize to the species-typical gestural repertoire by contrast to non communicative actions.
2. Method
2.1. Subjects
Observational data were collected in 97 chimpanzees including 39 males and 58 females housed at the Yerkes National Research Primates Center of Atlanta (USA). This sample constituted the total number of possible subjects; however, the total number of apes included in the different analyses varied according to the frequency of occurrence of specific behaviors of interests. All the apes were living in social groups ranging from 2 to 12 individuals and ranged in age from 6 to 50 years (Mean = 23.76, S.E. = 1.05).
2.2. Procedure
Data were collected between 10:00 am and 6:00 pm each observational day from March 2007 to January 2008. During observational days, data were collected during two sessions, 2 hour observation periods, one in the morning and one in the afternoon. The social groups were randomly observed during an observation session and an all occurrences sampling procedure was used, in which responses of each individual was collected opportunistically when a behavior of interest occurred. When a given manual action was produced repetitively by an individual, a recorded response of hand use was distinguished from another when the subject returned to the initial manual position between the separated manual actions. In other words, if a sequence of repetitive gestures occurred without such an interruption, we considered the whole sequence as a single response of hand use.
2.2.1. Behavioral Ethogram for Species-Typical Gestures
During the observation periods, hand use data for several species-typical gestures (e.g., Nishida et al., 1999; Goodall, 1989) were recorded and included in the analyses. These behaviors included arm-threat gestures, extend arm and hand slap.
Arm threat gestures were defined by Goodall (1989) as the raise of the arm (whole or forearm only) in a quick jerky movement with the fingers flexed slightly.
Extend arm concerned all gestures that consisted of extending one arm or hand (wrist and/or fingers with palm up or down) towards a partner in various social contexts: reconciliation, submission, greeting, invitation of grooming, when shared excitation, reassurance-seeking after stress or aggressions, play… (Goodall, 1989; see for a review Nishida et al.,1999).
Hand Slap was recorded when a chimpanzee slapped repeatedly the ground, the cage or the wall (with their whole hand or the back of his wrist) in direction of a recipient (a human or a conspecific) in order to invite them to play, to threat them, or to attract their attention (Goodall, 1989; Nishida et al., 1999).
2.2.2. Comparison measures of hand preferences
For evaluating whether or not the patterns of hand preference for gestures were solely related to its communicative property, we recorded hand use for two other manual actions, serving as comparison behaviors to the species-typical gestures: These actions included (a) self-directed nose wipe, a non-communicative face touching action, and (b) a replication of the human-directed food beg gestures (Hopkins and Cantero, 2003; Hopkins et al., 2005).
The “nose wipe” consists of a quick passage of the hand across the bridge of the nose (e.g., Marchant and McGrew, 1996). Such self-directed behaviors are not considered in the literature as communicative signals but rather as external signals of edginess, motivational ambivalence or frustration in primates including chimpanzees (Aureli and de Waal, 1997; Leavens et al., 2004).
Human-directed food beg gesture consists of extending fingers through the wire mesh of the chimpanzee’s enclosure toward the human recipient or toward an out-of-reach piece of food in order to request food. This behavior occurred frequently in presence of a human observer and was the behavior previously recorded by Hopkins et al. (2005) in this same sample of chimpanzees.
2.3. Data analysis
For all manual behaviors, we did not include in the final analysis the subjects that produced less than 6 responses. In accordance with the literature on nonhuman primate handedness (see Hopkins, 1999), an individual z-score was calculated on the basis of the total left and right-hand responses in order to determine the direction of hand preference for each subject and manual actions (specific-typical gestures, nose wipe, human-directed food begs). The chimpanzees were then classified as left-handed (z ≤ −1.96), right-handed (z ≥ 1. 96) or ambiguously handed (−1. 96< z < 1. 96) for each category of manual action on the basis of a level of significance at p < .05. In addition, for each subject, the degree of manual asymmetry was evaluated by calculating an individual handedness index score (HI) using the formula HI=(#R−#L)/(#R + #L), where R and L represent the total number of right and left hand responses, respectively. The HI values varied on a continuum from −1.0 to 1.0 with the sign indicating direction of hand preferences (positive = right-hand preference, negative = left-hand preference). The absolute value of the HI scores (ABS-HI) reflect the strength of individual hand preference.
3. Results
3.1. Descriptive Data
Because of the unequal distribution in the number of observations across the three gestural categories (threat, extend arm, slap) due to natural variation of the frequencies between each behavior (see Table 1a for distribution of the data per category of gesture), all the hand use data for these gestures were combined together for each individual and constituted a single category of gestures referred to “species-typical”. Combining the data allowed for a larger sample of individual responses per subject concerning the species-typical gestures. Among the 3127 responses collected on 89 individuals (1241 intra-specific gestures, 1886 gestures directed to humans), 3083 were included in the final analysis from 70 chimpanzees (24 males, 46 females), each of whom performed a minimum of 6 responses (observations per subject varied from 6 to 175 responses, M = 44.04, S.E. = 4.50).
Table 1.
Distribution of the number of observations per category of manual actions
| a. Species-typical gestures | ||||
|---|---|---|---|---|
| n responses | N subjects | Mean | S.E. | |
| 1. Extend arm | 405 (13%) | 50 | 8.02 | 1.06 |
| - Intra-specific | 397 | 50 | 7.94 | 1.05 |
| - Human-directed | 4 | 3 | 1.33 | 0.33 |
| 2. Arm threat | 156 (5%) | 32 | 4.88 | 1.06 |
| - Intra-specific | 103 | 30 | 3.43 | 0.59 |
| - Human-directed | 53 | 12 | 4.42 | 1.44 |
| 3. Hand slap | 2570 (82%) | 83 | 30.96 | 3.58 |
| - Intra-specific | 741 | 52 | 14.25 | 1.76 |
| - Human-directed | 1829 | 78 | 23.45 | 2.89 |
| TOTAL Gestures | 3127 (100%) | 89 | 35.13 | 3.98 |
| - Total intra-specific | 1241 (40%) | 64 | 19.39 | 2.14 |
| - Total human-directed | 1886 (60%) | 78 | 24.18 | 2.99 |
| b. Comparative behaviors | ||||
|---|---|---|---|---|
| n responses | N subjects | Mean | S.E. | |
| Food beg gestures | 1549 | 94 | 16.48 | 1.36 |
| Self-directed nose wipe | 1287 | 71 | 18.13 | 2.23 |
With respect to nose-wipes, 1287 responses were recorded from 71 subjects (see Table 1b). A total of 1212 were included in the final analysis and 43 chimpanzees (13 males, 30 females) which performed a minimum of 6 responses contributed to this sample (the number of observations per subject varied from 6 to 85 responses, M = 28.19, S.E. = 2.75).
Lastly, for food begs, 1549 responses recorded from 94 subjects (see Table 1b). Seventy four chimpanzees (28 males, 46 females) that produced a minimum a 6 responses were included in the final analysis, which included 1488 responses (the number of observations per subject varied from 6 to 68 responses, M = 20.11, S.E. = 1.46).
3.2. Direction of hand preferences
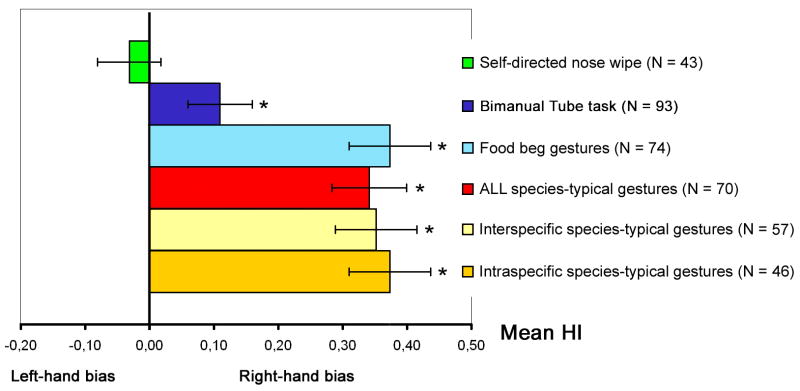
The mean HI scores as well as the distribution of handedness for each of the three manual actions are shown in Table 2. Initially, we evaluated whether the mean HI values for each manual action was skewed to the right or left and differed significantly from 0 using a one-sample t-tests (see Fig. 2). Significant population-level right-handedness was found for species-typical gestures, t(69) = 6.59, p < .001 and human-directed food begs, t(73) = 6.54, p < .001, but not for nose wipes, t(42) = .53, p > .60.
Table 2.
Distribution of hand preferences and degree of group-level manual bias for each investigated manual behaviors.
| Manual behaviors | # L | # R | # A | N | M.HI | S.E. | t | p |
|---|---|---|---|---|---|---|---|---|
| Species-typical gesture | 3 | 34 | 33 | 70 | 0.34 | 0.05 | 6.59 | < 0.001, s. |
| - Intra-specific | 1 | 23 | 22 | 46 | 0.37 | 0.06 | 6.20 | < 0.001, s. |
| - Human-directed | 4 | 26 | 27 | 57 | 0.35 | 0.06 | 6.01 | < 0.001, s. |
| - Males | 1 | 9 | 14 | 24 | 0.22 | 0.09 | 2.48 | = 0.02, s. |
| - Females | 2 | 25 | 19 | 46 | 0.41 | 0.06 | 6.48 | < 0.001, s. |
| Self-directed nose wipe | 5 | 5 | 33 | 43 | −0.03 | 0.05 | 0.53 | > 0.60, ns |
| - Males | 2 | 2 | 9 | 13 | −0.08 | 0.11 | 0.75 | > 0.40, ns |
| - Females | 3 | 3 | 24 | 30 | −0.01 | 0.05 | 0.10 | > 0.90, ns |
| Food beg gestures | 6 | 32 | 36 | 74 | 0.37 | 0.06 | 6.54 | < 0.001, s. |
| - Males | 2 | 12 | 14 | 28 | 0.41 | 0.09 | 4.81 | < 0.001, s. |
| - Females | 4 | 20 | 22 | 46 | 0.35 | 0.08 | 4.59 | < 0.001, s. |
# L: number of left-handed subjects; # R: number of right-handed subjects; # A: number of ambiguous handed subjects; N: sample of subjects; M.HI: Mean Handedness Index score of N individuals that corresponds to degree of population-level handedness, the sign indicates the direction of the manual bias (negative value: left-hand bias, positive value: right-hand bias); S.E. : Standard Error of the mean; t: value of the t resulting from a t-test; p: significance of p; ns: non significant; s.: significant.
Fig. 2.
Degrees of group-level handedness (M.HI) for self-directed “nose wipe”, replicated food beg gestures and species-typical gestures in both inter- and intraspecific communication. Mean handedness index scores (M.HI)±S.E. The error bar represents the standard error around the MHI score. Asterisk indicates that the MHI score differed significantly from zero. *p < .05. The sign of the M.HI values indicates the direction of the group-level handedness (positive = group-level right-handedness, negative = group-level left-handedness). The absolute values of the M.HI scores reflect the strength of group-level manual bias.
Chi-square analyses of the categorical handedness data largely confirmed the one sample t-tests results. Significant differences in the number of left and right-handed chimpanzees was not found for nose wipe χ2 (1, N = 10) = 00.00, p = 1, but only for species-typical gestures χ2 (1, N = 37) = 25.97, p < .001 and human-directed food begs χ2 (1, N = 38) = 17.79, p < .001. For both communicative behaviors, there was significantly more right- than left-handed individuals.
The effects of sex on handedness were assessed for each behavioral action using an analysis of variance (ANOVA) with the HI score serving as the dependent measure while sex was the between group factor. There were no significant differences between males and females for species-typical gestures, F(1,70) = 2.89, p = .10, self-directed nose wipes, F(1,43) = .50, p > .40, or human-directed food beg gestures, F(1,74) = .32, p > .50 (See Table 2).
3.3. Potential effects of the human experimenter on asymmetries of species-typical gestures
Recall that species-typical gestures could be directed toward both humans and conspecifics. In this analysis, we evaluated whether hand use varied as a function of whether the gestures were directed toward humans or conspecifics. Among the total of 1886 species-typical gestures directed to humans, 1823 were included in the final analysis from the 57 chimpanzees which performed a minimum of 6 interspecies responses (from 6 to 134 responses, M = 31.98, S.E. = 3.57). Concerning intraspecies gestures, among the 1241 observations, 1189 were included in the final analysis from the 46 chimpanzees which performed a minimum of 6 intra-specific responses (from 6 to 78 responses, M = 25.85, S.E. = 2.37). The mean handedness index scores for these two sub-samples were nearly identical (see Table 2) and did not differ significantly based on a paired-samples t-test, t(34) = .53, p > .50. A Pearson product-moment correlation indicated that the HI scores for intraspecies gestures significantly correlated with human-directed species-typical gestures, within the 34 individuals who produced at least 6 intra- and inter-species-typical gestures, r(34) = .51, p < .01. Moreover, the strength of manual biases (i.e., mean of absolute values of handedness index scores, M.ABS-HI) were similar between intra-specific gestures (M.ABS-HI = .44, S.E. = .05) and inter-specific gestures (M.ABS-HI = .48, S.E. = .04) and the ABS-HI do not significantly differ according to a paired-sample t-test, t(34) = .07, p > .90.
3.4. Consistency of food begs data
Since the replicated measures of hand preferences for human-directed food beg gestures has been collected by a second observer 3 years after the collection of the data by Hopkins et al. (2005), it provided an opportunity to assess consistency in hand use across time of this behavior. The two sets of data revealed the same patterns of handedness insofar as (a) they exhibited similar degree of group-level right-hand bias (first session 2004 in 227 subjects: M.HI = .34; second session 2007 in 74 subjects: M.HI = .37) and (b) the measures of hand preferences (HI) significantly correlated between the two session within 58 subjects tested on both occasions, r(58) = .68, p < .001.
3.5. Comparison of handedness indexes between different behaviors
Using paired-sample t-tests, a comparison in the HI values did not differ between species-typical gestures and replication of human-directed food begs, t(59) = −.50, p > .60 but did differ significantly between species-typical gestures and nose wipe, t(41) = 5.94, p < .001, and between food begs and nose wipe, t(39) = −3.38, p < .003. The Pearson product-moment correlation confirmed the paired-sample t-test results. The measures of hand preferences (HI) for species-typical gestures statistically correlated with the measures for food beg gestures within the 59 common individuals, r(59) = .53, p < .001, but did not statistically correlate with the measures for non-communicative self-directed nose wipe within the 41 common individuals, r(41) = .26, p = .09. Moreover, the measures of hand preferences for nose wipe did not correlate either with the measures for food beg gestures within the 39 common individuals, r(39) = .11, p > .10.
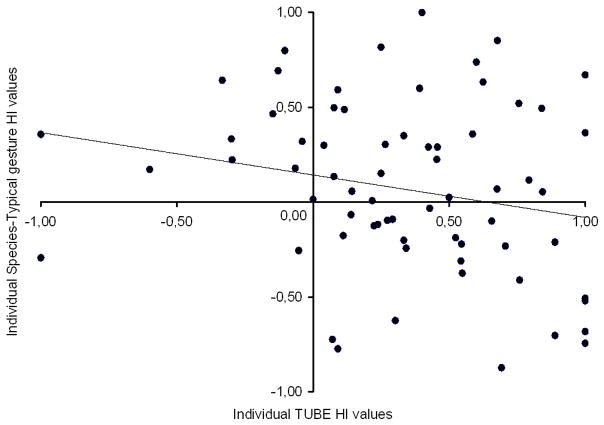
Predominance of right-handedness has been previously reported in captive chimpanzees for non communicative motors actions, particularly for bimanual coordinated actions referred to the TUBE tasks (e.g. Hopkins, 1995, Hopkins et al., 2004). The TUBE task consists of removing food with fingers of one hand from inside a PVC tube while holding it with the opposite hand. It has been demonstrated that the pattern of right-handedness differ between food beg gestures and the measures of the TUBE task in captive chimpanzees. Indeed the hand preferences for food beg gestures not only revealed a more pronounced degree of population-level right-handedness than the TUBE task measures but also did not correlated with the individual hand preferences assessed with the TUBE task in the same subjects (Hopkins et al., 2005; Hopkins and Wesley, 2002). Here, we compared our measures of hand preferences (HI) for species-typical gestures with the measures of the TUBE task published in the literature (e.g. Hopkins et al., 2004). Among the 97 chimpanzees observed in the present study, 93 have performed the TUBE task (M.HI = 0.11, S.E. = 0.05) including 67 subjects that produced also species-typical gestures. First, as can be seen in the Fig. 3, the HI score for species-typical gestures did not statistically correlate with the HI values for the TUBE task within the 67 common individuals, r(67) = −.22, p = .075. Moreover, a paired sample t-test showed a significant difference of HI values between these two manual actions, t(67) = 3.00, p < .005. The degree of group-level right-handedness for species-typical gestures (M.HI = .34) were significantly higher than for the TUBE task (M.HI = .11), see Fig. 2.
Fig. 3.
Scatterplot that relates the HI of the non-communicative bimanual tube task with the HI of the communicative species-typical gestures within the same 67 subjects who performed these both types of manual actions. Each of these individuals is represented by a black point with HI values in both manual actions (bimanual tube task on the X-Axis, communicative species-typical gestures on the Y-Axis). The sign of the HI values indicates the direction of hand preferences (positive = right-hand preference, negative = left-hand preference). The absolute values of the HI scores reflect the strength of individual hand preferences. As can be seen, the line that crosses the figure shows that there is no correlation of individual hand preferences (HI) between the tube task and the communicative species-typical gestures, r(67)= −.22, p = .075.
3.6. Potential effect of the number of responses per subject
Palmer (2002) has suggested that some studies of handedness in nonhuman primates are problematic due to differences in the number of observations that derive a given HI value. Specifically, it has been suggested that when sample size is not uniform, there is a need to demonstrate that the distribution of left and right-handedness follow predictable sampling patterns. That is, as sample size increases, there should be fewer non-lateralized subjects with higher sample sizes because you are increasing the probability of measuring a statistically significant behavioral trait. Because the number responses per subject varied between 6 and 175 responses for the species-typical gestures in this study, we addressed this issue in two different ways.
First, we tested the robustness of the handedness evidence of the lateralized subjects for species-typical gestures. We compared the respective degrees of group-level handedness (M.HI) between the 15 lateralized chimpanzees who performed less than 25 gestures (M.HI = .58, S.E. = .17) and the 22 lateralized chimpanzees who performed more than 25 gestures (M.HI = .53, S.E. = .05). In both groups, the M.HI scores were significantly different from 0, t(15) = 3.38, p < .005; t(22) = 9.41, p < .001, respectively. The small difference observed in the degree of group-level right-handedness (see M.HI) between the two groups is not significant using an analysis of variance with the HI score serving as the dependent measure, F(1, 37) = .12, p > .10.
Second, whether the measures of hand preferences (z-scores) were potentially skewed by the variation of the number of responses per subject was assessed by creating a funnel plot. The number of responses was plotted against the individual z-scores. As can be seen on Fig. 4, as the number of responses per subject increased, we noticed fewer ambiguously handed individuals (that could be seen in the center of the funnel between the two lines drawn by the white points and the black triangles). This suggests that our hand preference classification data were not biased due to differences in sample sizes.
Fig. 4.
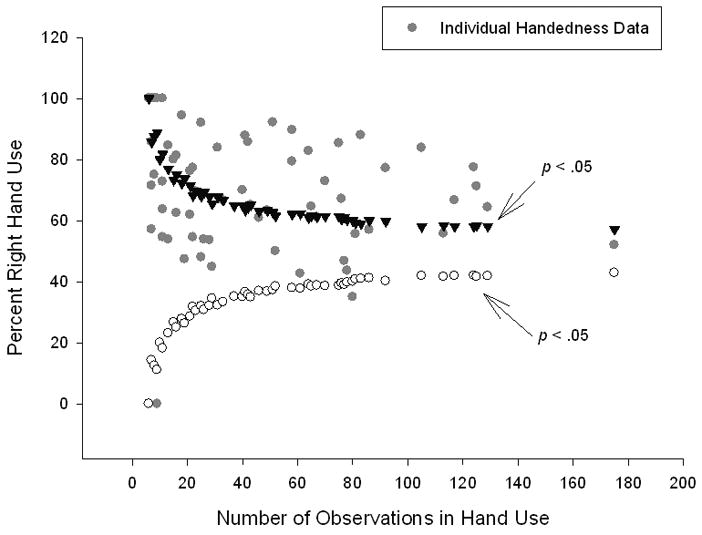
Funnel plot of the percentage right hand for each subject plotted against the number of observations in hand use for gestures. The black triangles and white circles represent the critical z-score value at p < .05 for hypothetical different numbers of observations in each individual subject. The gray points are the individual subjects’ z-scores based on the number of individual observations of hand use for manual gestures. Gray data points that lie above or below the theoretical z-score distribution represent subjects with significant right- or left-hand preferences (p < .05). Gray data points that lie between the two theoretical z-score values represent those individuals who fail to show a significant hand preference. As can be seen, the majority of individuals that fail to show a significant hand preference fall at the front of the funnel, indicating that these individuals had the fewest observations.
4. Discussion
This study provides new findings that support the view that the prerequisite of human left-lateralization for language may be found in the gestural system of nonhuman primates. First, to our knowledge, this is the first evidence in chimpanzees that intraspecific species-typical gestures involve a group-level right-hand bias and exhibit similar pattern of handedness when directed to humans. Second, hand preferences for species-typical gestures not only exhibit a similar degree of right-hand bias with human-directed food beg gestures (see Hopkins and Cantero, 2003; Hopkins and Leavens, 1998; Hopkins et al. 2005; Hopkins and Wesley, 2002) but also correlate significantly with the measures of hand preferences for food begs in the same individuals. Third, by contrast, the hand preferences for a non-communicative self-directed action (nose wipe) did not correlate with the hand preferences of any category of communicative gestures and did not reveal group-level manual asymmetries. This nonsignificant result is consistent with previous reports in the literature on lateralized face-touching behaviors in monkeys and apes (Aruguete et al., 1992; Dimond and Harries, 1984; Marchant and McGrew, 1996; but see Hopkins et al., 2006; Rogers and Kaplan, 1996). Fourth, species-typical gestures, as well as human-directed food begs (Hopkins et al., 2005), elicited stronger degree of population-level right-handedness that manipulative bimanual actions (i.e., TUBE task). Such a difference of lateralization between the manipulative actions and communicative gestures has been also reported in captive baboons (Meguerditchian and Vauclair, 2006; Vauclair et al., 2005) and in children raised by deaf parents (Bonvillian et al., 1997). Fifth, we demonstrated the robustness and the consistency of the pattern of right-handedness for human-directed food beg gestures with a strong and significant correlation in the same individuals between the measures of hand preferences of the first session and the measures of the second session collected 3 years later by an observer blind to the previous handedness data. Finally, there were no significant sex differences in handedness for all manual behaviors investigated; however, it is of note that greater degree of right-handedness for species-typical gestures in females compared to males approached conventional levels of statistical significance.
These collective findings indicate that evidence of group-level right-handedness previously reported for human-directed food beg gestures are neither attributable to solely this context nor to human influences, but generalize to species-typical gestures and intraspecific signaling behaviors as it has been previously reported in captive baboons (Meguerditchian and Vauclair, 2006). By contrast to non-communicative actions, different categories of communicative gestures show the same patterns of right-handedness and may thus share the same the lateralized cerebral system. This evidence raises the possibility that chimpanzees and perhaps baboons may possess a specific left-lateralized communicative cerebral system (different from the one involved in purely motor manipulative actions), which may be involved in the production of gestures. Consequently, gestural behaviors in nonhuman primates may constitute an ideal prerequisite from the common ancestor of baboons, chimpanzees and humans for the emergence of language and its typical left-lateralization (Meguerditchian and Vauclair, 2006, 2008).
This latter hypothesis is supported by recent evidence using brain imaging (magnetic resonance imaging, MRI) in chimpanzees. It has been shown that morphological left-asymmetries in the homologue of “Broca’s area” (inferior frontal gyrus) are related to right-handedness for food-beg gestures (Taglialatela et al., 2006) whereas handedness for non-communicative bimanual motor actions (TUBE task) are correlated with asymmetries of the primary motor cortex but not with asymmetries of any homologous language areas (Hopkins and Cantalupo, 2004). These findings support the view that the specific communicative system involved in the production of gestures in chimpanzees may be located in the homologous regions of human language areas. Moreover, it is likely that this communicative system may be not only gestural but rather bimodal (vocal + gestural). Indeed, it has been described that chimpanzees voluntarily produce two novel atypical sounds (an “extended grunt” involving the vocal tract and a “raspberry” involving the air of the mouth which exhaled through the lips) exclusively in the presence of both out-of-reach food and a human experimenter in order to request food (Hopkins et al., 2007). Interestingly, these auditory signals when produced simultaneously with food beg gestures, induced a more pronounced right-hand preference than when the gestures were produced alone (Hopkins and Cantero, 2003), indicating a greater activation of a common left-lateralized system in case of bimodal signaling to humans. More impressively, a recent study using functional brain imaging (PET) demonstrated that communicative signaling (either food beg gestures, atypical novel sounds, or both of them simultaneously) activated the inferior frontal gyrus, usually considered as an homologous of Broca’s area (Taglialatela et al., 2008). Regarding these global findings, we support the hypothesis than left-lateralization for language may result from an ancestral gestural communicative system in which vocalizations have been progressively inserted in the common ancestor of chimpanzees and humans at least 6 millions years ago (Corballis, 2002; Hopkins and Cantero, 2003).
Acknowledgments
We are grateful to Jamie L. Russell and Jennifer A. Schaeffer for their helpful assistance with the chimpanzees as well as Jared P. Taglialatela for his comments. William D. Hopkins is the author of the picture (Fig. 1.). This work, as part of the European Science Foundation EUROCORES Programme OMLL, was supported by funds from the CNRS (OHLL Programme) and the EC Sixth Framework Programme under Contract no. ERAS-CT-2003-980409. This research was further supported by NIH grants RR-00165, NS-36605, NS-42867, HD-56232 and HD-38051.
Fig. 1.
Intraspecies extend arm in chimpanzees. An adult male extends his right arm toward an adult female in order to greet her.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adrien Meguerditchian, Email: adrien.meguerditchian@univ-provence.fr.
Jacques Vauclair, Email: jacques.vauclair@univ-provence.fr.
William D. Hopkins, Email: whopkin@emory.edu, whopkins@agnesscott.edu.
References
- Annett M. Left, right, hand and brain: The right shift theory. Hillsdale, NJ: Erlbaum; 1985. [Google Scholar]
- Aruguete MS, Ely EA, King JE. Laterality in spontaneous motor activity of chimpanzees and squirrel monkeys. American Journal of Primatology. 1992;27:177–178. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Aureli F, de Waal FBM. Inhibition of social behavior in chimpanzees under high-density conditions. American Journal of Primatology. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Blake J, O’Rourke P, Borzellino G. Form and function in the development of pointing and reaching gestures. Infant Behavior and Development. 1994;17:195–203. [Google Scholar]
- Bonvillian JD, Richards HC, Dooley TT. Early sign language acquisition and the development of hand preferences in young children. Brain and Language. 1997;58:1–22. doi: 10.1006/brln.1997.1754. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. The gestural communication of monkeys and apes. Oxford: Psychology Press; 2007. [Google Scholar]
- Corballis MC. The Origins of Language. Princeton, NJ: Princeton University Press; 2002. From Hand to Mouth. [Google Scholar]
- Corina DP, San Jose-Robertson L, Guillemin A, High J, Braun AR. Language lateralization in a bimanual language. Journal of Cognitive Neurosciences. 2003;15:718–730. doi: 10.1162/089892903322307438. [DOI] [PubMed] [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Laterality: Asymmetries of Body, Brain and Cognition. 2004;9:233–242. [Google Scholar]
- Dimond S, Harries R. Face touching in monkeys, apes and Man: Evolutionary origins and cerebral asymmetry. Neuropsychologia. 1984;22:227–233. doi: 10.1016/0028-3932(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Emmorey K, Mehta S, Grabowski TJ. The neural correlates of sign versus word production. Neuroimage. 2007;36:202–208. doi: 10.1016/j.neuroimage.2007.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Carriba S, Loeches A, Morcillo A, Hopkins WD. Asymmetry in facial expression of emotions by chimpanzees. Neuropsychologia. 2002;40:1523–1533. doi: 10.1016/s0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Goodall J. Glossary of chimpanzee behaviors. Tucson: Jane Goodall Institute; 1989. [Google Scholar]
- Grossi G, Semenza C, Corazza S, Volterra V. Hemispheric specialization for sign language. Neuropsychologia. 1996;34:737–740. doi: 10.1016/0028-3932(96)00008-5. [DOI] [PubMed] [Google Scholar]
- Hauser MC. Right hemisphere dominance in the production of facial expression in monkeys. Science. 1993;261:475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hauser MC. The evolution of a lopsided brain: Asymmetries underlying facial and vocal expressions in primates. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. Cambridge, MA: MIT/Bradford; 1999. pp. 597–628. [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36:1265–1273. doi: 10.1016/s0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, editor. Evolution of hemispheric specialization in primates. Oxford: Academic Press; 2007. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor cortex but not with homologous language areas. Behavioural Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantero M. From hand to mouth in the evolution on language: the influence of vocal behavior on lateralized hand use in manual gestures by chimpanzees (Pan troglodytes) Developmental Science. 2003;6:55–61. [Google Scholar]
- Hopkins WD, de Waal F. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 1995;16:261–276. [Google Scholar]
- Hopkins WD, Fernandez-Carriba S. Laterality of communicative behaviors in nonhuman primates: A critical analysis. In: Rogers LJ, Andrew RJ, editors. Comparative Vertebrate Lateralization. Cambridge: Cambridge University Press; 2002. pp. 445–479. [Google Scholar]
- Hopkins WD, Leavens DA. Hand use and gestural communication in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;6:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Freeman H, Reynolds EAM, Griffis C, Leavens DA. Lateralized scratching in chimpanzees: Evidence of a functional asymmetry during arousal. Emotion. 2006;6:553–559. doi: 10.1037/1528-3542.6.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Leavens DA. Chimpanzees differentially produce novels vocalizations to capture the attention of a human. Animal Behaviour. 2007;73:281–286. doi: 10.1016/j.anbehav.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ. Gestural communication in chimpanzees (Pan troglodytes): The effect of situational factors on gesture type and hand use. Laterality. 2002;7:19–30. doi: 10.1080/13576500143000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees (Pan troglodytes) are predominantly right-handed: Replication in three populations of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Manual activity during speaking. I. Right-handers. Neuropsychologia. 1973a;11:45–50. doi: 10.1016/0028-3932(73)90063-8. [DOI] [PubMed] [Google Scholar]
- Kimura D. Manual activity during speaking. II. Left-handers. Neuropsychologia. 1973b;11:45–50. doi: 10.1016/0028-3932(73)90063-8. [DOI] [PubMed] [Google Scholar]
- Kimura D. Neuromotor mechanisms in human Communication. Oxford: Oxford University Press; 1993. [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD. Behavioral evidence for the cutaneous expression of emotion in a chimpanzee (Pan Troglodytes) Behaviour. 2004;141:979–997. [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Understanding the point of chimpanzee pointing: epigenesis and ecological validity. Current Directions in Psychological Science. 2005;14:185–189. doi: 10.1111/j.0963-7214.2005.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losin E, Russell JL, Freeman H, Meguerditchian A, Hopkins WD. Left hemisphere specialization for oro-facial movements of learned vocal signals by captive chimpanzees. PlosOne. 2008;3:e2529. doi: 10.1371/journal.pone.0002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous behaviors. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioural laterality of hand function in non human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- Meguerditchian A, Vauclair J. Vocal and gestural communication in nonhuman primates and the question of the origin of language. In: Roska-Hardy LS, Neumann-Held EM, editors. Learning from animals? Examining the Nature of Human Uniqueness. London: Psychology Press; 2008. pp. 61–85. [Google Scholar]
- Meguerditchian A, Vauclair J. Baboons communicate with their right hand. Behavioural Brain Research. 2006;171:170–174. doi: 10.1016/j.bbr.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kano T, Goodall J, McGrew WC, Nakamura M. Ethogram and ethnography of Mahale chimpanzees. Anthropological Science. 1999;107:141–188. [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: evaluating the evidence with funnel plots. American Journal of Physical Anthropology. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences, particularly for reaching. Journal of Comparative Psychology. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Pika S, Liebal K, Call J, Tomasello M. The gestural communication of apes. Gesture. 2005a;5:41–56. [Google Scholar]
- Pika S, Liebal K, Tomasello M. Gestural communication in subadult bonobos (Pan paniscus): repertoire and use. American Journal of Primatology. 2005b;65:39–61. doi: 10.1002/ajp.20096. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew JR, editors. Comparative vertebrate lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orang-utans, Pongo pygmaeus pygmaeus. Animal Behaviour. 1996;51:13–25. [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New-York: Springer-Verlag; 1993. pp. 267–283. [Google Scholar]
- Taglialatela JP. Functional and structural asymmetries for auditory perception and vocal production in nonhuman primates. In: Hopkins WD, editor. Evolution of hemispheric specialization in primates. Oxford: Academic Press; 2007. pp. 117–142. [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela JP, Russell JL, Schaeffer JA, Hopkins WD. Communicative signaling activates “Broca’s” homologue in chimpanzees. Current Biology. 2008;18:343–348. doi: 10.1016/j.cub.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid J, Bellugi U, Poizner H. Hand dominance for signing: Clues to brain lateralization. Neuropsychologia. 1989;27:949–960. doi: 10.1016/0028-3932(89)90070-5. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral Brain Sciences. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vauclair J, Meguerditchian A. The gestural origin of language and its lateralization: theory and data from studies in nonhuman primates. In: Kern S, Gayraud F, Marsico E, editors. Emergence of Linguistic Abilities: From Gestures to Grammar. Cambridge: Cambridge Scholars Publishing; 2008. pp. 43–59. [Google Scholar]
- Vauclair J, Meguerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cognitive Brain Research. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Williams NA, Close JP, Giouzeli M, Crow TJ. Accelerated evolution of Protocadherin 11X/Y: A candidate gene-pair for cerebral asymmetry and language. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:623–633. doi: 10.1002/ajmg.b.30357. [DOI] [PubMed] [Google Scholar]