Abstract
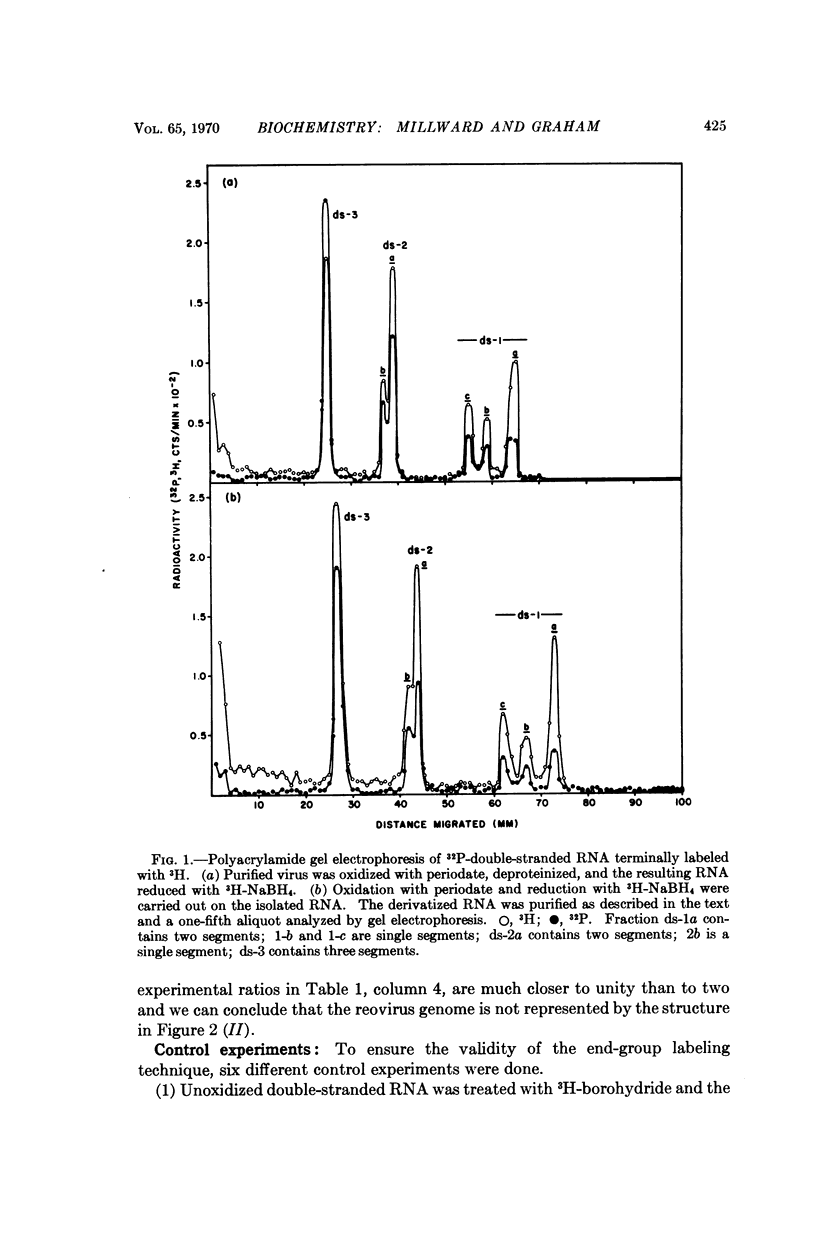
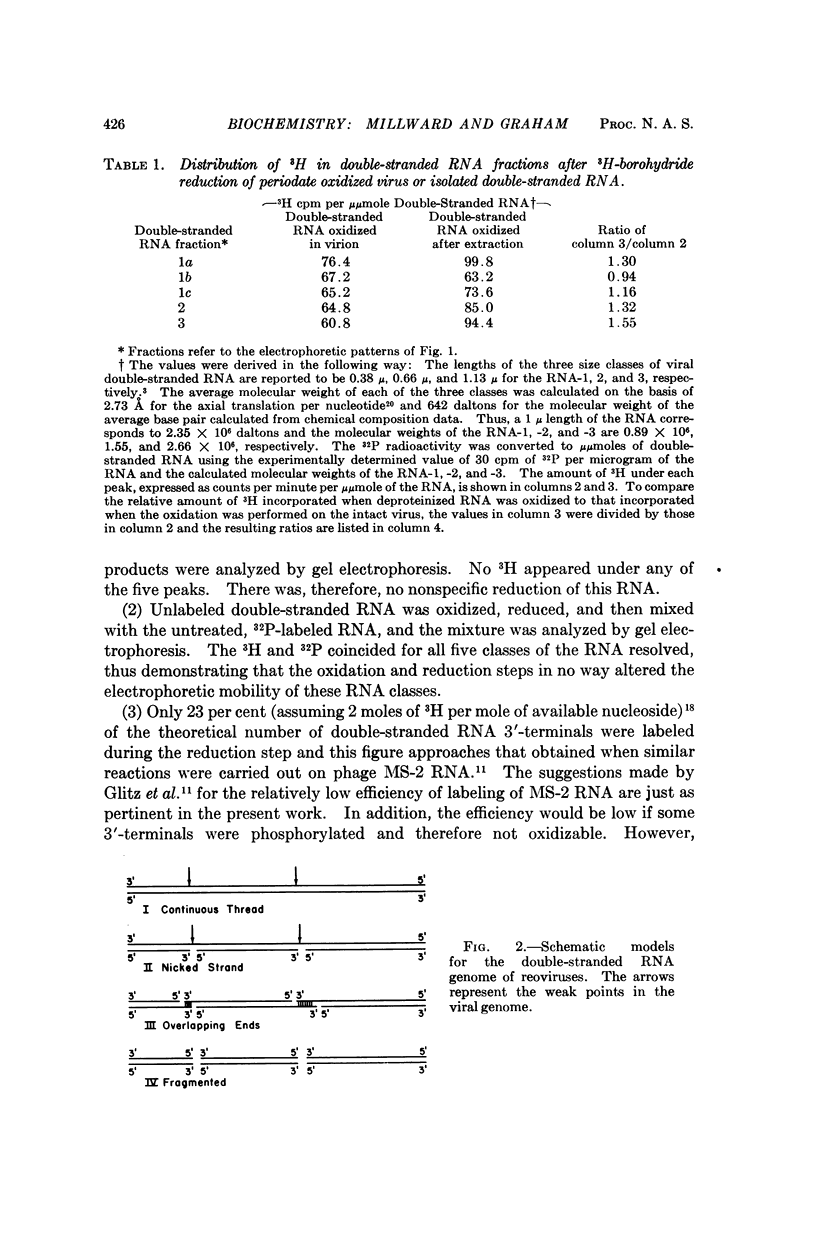
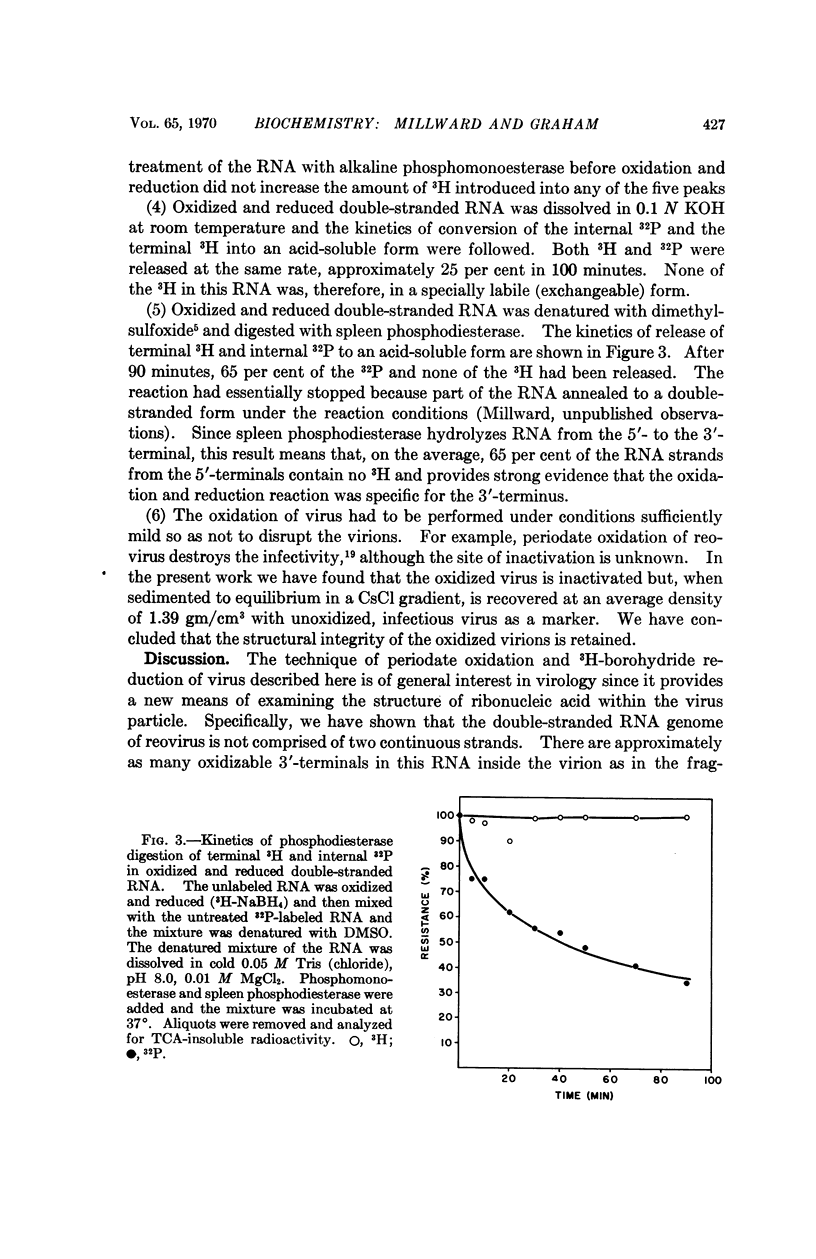
The double-stranded RNA genome of reovirus breaks reproducibly into ten segments upon extraction from purified virions. Reovirus-induced messenger RNA's formed in infected cells correspond in length to these ten genomic segments and some real structural and biological implication must be accorded to the ready manner in which the genome is fragmented on attempted isolation. In the present work we have posed the question whether the reovirus genome is a continuous, double-stranded molecule or whether there are discontinuities in the complementary strands of RNA that might constitute „weak points” in the structure. To answer this question, a method was developed to estimate the 3′-terminal nucleosides of RNA within intact virions. Approximately as many 3′-ends are free inside the virion as in RNA extracted from purified virus. Thus, the viral genome exists as a discontinuous structure inside the virus particle and both strands of the duplex are interrupted at intervals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Wilkins M. H., Fuller W., Langridge R. Molecular and crystal structures of double-helical RNA. 3. An 11-fold molecular model and comparison of the agreement between the observed and calculated three-dimensional diffraction data for 10- and 11-fold models. J Mol Biol. 1967 Aug 14;27(3):535–548. doi: 10.1016/0022-2836(67)90057-5. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Joklik W. K. Studies on reovirus RNA. II. Characterization of reovirus messenger RNA and of the genome RNA segments from which it is transcribed. J Mol Biol. 1967 Oct 14;29(1):19–26. doi: 10.1016/0022-2836(67)90178-7. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- De Wachter R., Fiers W. Studies on the bacteriophage MS2. IV. The 3'-OH terminal undecanucleotide sequence of the viral RNA chain. J Mol Biol. 1967 Dec 28;30(3):507–527. [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Glitz D. G., Bradley A., Fraenkel-Contrat H. Nucleotide sequences at the 5'-linked ends of viral ribonucleic acids. Biochim Biophys Acta. 1968 Jun 18;161(1):1–12. doi: 10.1016/0005-2787(68)90288-8. [DOI] [PubMed] [Google Scholar]
- HIRST G. K. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmakoff J., Lewandowski L. J., Black D. R. Comparison of the ribonucleic Acid subunits of reovirus, cytoplasmic polyhedrosis virus, and wound tumor virus. J Virol. 1969 Dec;4(6):851–856. doi: 10.1128/jvi.4.6.851-856.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R. Infectivity of bacteriophage R17 RNA after sequential removal of 3' terminal nucleotides. Nature. 1969 Jan 25;221(5178):321–325. doi: 10.1038/221321a0. [DOI] [PubMed] [Google Scholar]
- LERNER A. M., CHERRY J. D., FINLAND M. Hemagglutination with reoviruses. Virology. 1963 Jan;19:58–65. doi: 10.1016/0042-6822(63)90024-2. [DOI] [PubMed] [Google Scholar]
- Loh P. C., Shatkin A. J. Structural proteins of reoviruses. J Virol. 1968 Nov;2(11):1353–1359. doi: 10.1128/jvi.2.11.1353-1359.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Prevec L., Watanabe Y., Gauntt C. J., Graham A. F. Transcription of the genomes of type 1 and type 3 reoviruses. J Virol. 1968 Apr;2(4):289–297. doi: 10.1128/jvi.2.4.289-297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L. Studies on polynucleotides. LXXVII. The labeling of end groups in polynucleotide chains: the selective modification of diol end groups in ribonucleic acids. J Biol Chem. 1968 Feb 10;243(3):556–564. [PubMed] [Google Scholar]
- Sato T., Kyogoku Y., Higuchi S., Mitsui Y., Iitaka Y., Tsuboi M., Miura K. I. A preliminay investigation on the molecular structure of rice dwarf virus ribonucleic acid. J Mol Biol. 1966 Mar;16(1):180–190. doi: 10.1016/s0022-2836(66)80271-1. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider A., Fraenkel-Conrat H. Studies of nucleotide sequences in tobacco mosaic virus ribonucleic acid. 3. Periodate oxidation and semicarbazone formation. Biochemistry. 1966 Aug;5(8):2729–2734. doi: 10.1021/bi00872a033. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C., Kleinschmidt A. K. Electron microscopy of RNA strands released from individual Reovirus particles. J Mol Biol. 1968 May 28;34(1):137–147. doi: 10.1016/0022-2836(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Verwoerd D. W. Purification and characterization of bluetongue virus. Virology. 1969 Jun;38(2):203–212. doi: 10.1016/0042-6822(69)90361-4. [DOI] [PubMed] [Google Scholar]
- WHITFELD P. R. A method for the determination of nucleotide sequence in polyribonucleotides. Biochem J. 1954 Nov;58(3):390–396. doi: 10.1042/bj0580390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]



