Abstract
Biotherapies involve the utilization of antibodies, genetically modified viruses, bacteria or cells for therapeutic purposes. Molecular imaging has the potential to provide unique information that will guarantee their biosafety in humans and provide a rationale for the future development of new generations of reagents. In this context, non-invasive imaging of gene expression is an attractive prospect, allowing precise, spacio-temporal measurements of gene expression in longitudinal studies involving gene transfer vectors. With the emergence of cell therapies in regenerative medicine, it is also possible to track cells injected into subjects. In this context, the Na/I symporter (NIS) has been used in preclinical studies. Associated with a relevant radiotracer (123I-, 124I-, 99mTcO4-), NIS can be used to monitor gene transfer and the spread of selectively replicative viruses in tumours as well as in cells with a therapeutic potential. In addition to its imaging potential, NIS can be used as a therapeutic transgene through its ability to concentrate therapeutic doses of radionuclides in target cells. This dual property has applications in cancer treatment and could also be used to eradicate cells with therapeutic potential in the case of adverse events. Through experience acquired in preclinical studies, we can expect that non-invasive molecular imaging using NIS as a transgene will be pivotal for monitoring in vivo the exact distribution and pharmacodynamics of gene expression in a precise and quantitative way. This review highlights the applications of NIS in biotherapy, with a particular emphasis on image-guided radiotherapy, monitoring of gene and vector biodistribution and trafficking of stem cells.
This article is part of a themed section on Imaging in Pharmacology. To view the editorial for this themed section visit http://dx.doi.org/10.1111/j.1476-5381.2010.00685.x
Keywords: Na/I symporter, gene transfer, non-invasive molecular imaging, radiotherapy, cell trafficking, biotherapy
Introduction
The development of technologies allowing the imaging of biological processes in live subjects started 40 years ago and is now in an exponentially growing phase. By definition, molecular imaging is the visual representation and characterization of biological processes at both the cellular and subcellular level (Blasberg, 2003). Applications of molecular imaging technology in biomedicine are large. It can facilitate tumour detection at an early stage for diagnosis (Kelly et al., 2008), provide early information on the efficiency of a treatment (Geus-Oei and Oyen, 2008), offer opportunities to study fundamental biological processes in living animal models (Doubrovin et al., 2001) and provide alternative treatments (Barton et al., 2008).
One of the fields that could most benefit from this technology is biotherapies. Biotherapies use reagents of biological origin (antibodies, viruses, bacteria) for therapeutic effect. Considering the complexity of these reagents, molecular imaging has the potential to provide unique information that will guarantee their biosafety in humans and provide a rationale for the future development of new generations of reagents. In this context, non-invasive imaging of gene expression is an attractive prospect, allowing precise, spacio-temporal measurements of gene expression in longitudinal studies involving gene transfer vectors (Vassaux and Groot-Wassink, 2003; Raty et al., 2007). With the emergence of cell therapies in regenerative medicine, it is also possible to track cells injected into subjects. The monitoring of in vivo gene expression is critical for the evaluation of the success or failure of biotherapeutic approaches. Tissue sampling can provide useful information to monitor clinical response, but repeatedly performing biopsies on multiple tissues is inconvenient and impossible in patients. Hence, the assessment of samples is poorly reproducible and is limited by the inaccessibility of some internal organs. Thus, techniques for repeated, non-invasive imaging of therapeutic gene expression are highly desirable. To be applicable, the imaging technique should provide precise information about the location, magnitude and persistence of transgene expression over time.
Different imaging technologies to visualize gene expression have been developed or are under development. They can be classed into two groups, according to whether or not radioisotopes are used to obtain visual information (Table 1) (Blasberg, 2003; Serganova et al., 2007).
Table 1.
Overview of imaging systems
| Imaging modalitya | Resolution | Depth | Temporal resolution | Sensitivityb | Quantitative | Costc | Clinical use |
|---|---|---|---|---|---|---|---|
| MRI | 10-100 µM | No limit | Minutes to hours | 10−3 to 10−5 M | Yes | Most expensive | Yes |
| CT | 50 µM | No limit | Minutes | no probes | No | Expensive | Yes |
| PET | 1–2 mm | No limit | Minutes to hours | 10−11 to 10−12 M | Yes | Most expensive | Yes |
| SPECT | 1–2 mm | No limit | Minutes to hours | 10−10 to 10−12 M | Yes | Most expensive | Yes |
| Bioluminescence | 3–5 mm | cm | Minutes | 10−15 to 10−17 M | No | Cheap | No |
| Fluorescence | 2–3 mm | cm | Minutes to hour | 10−9 to 10−12 M | No | Cheap | No |
Table adapted from Blasberg, 2003 and Vassaux and Groot-Wassink, 2003.
Probe concentrations.
Cost is based on purchase price of the imaging system.
The non-nuclear medicine group includes magnetic resonance imaging, bioluminescence and fluorescence imaging (Briat and Vassaux, 2006). These technologies are currently mostly used in laboratory studies but, in their current state of development, cannot realistically be applied to humans. The most widely used technique is bioluminescence imaging, which relies on the detection of light emitted by oxidation of the luciferin substrate upon catalysis by the luciferase enzyme (Sweeney et al., 1999; Negrin and Contag, 2006). This reaction requires ATP, magnesium and oxygen. The bioluminescence emitted can be detected and amplified, revealing the sites of luciferase expression. In transgenic animals, this technology can be used to detect the activation of specific signalling pathways (Hamstra et al., 2006; Briat and Vassaux, 2008). Fluorescence imaging using GFP has many advantages as GFP is easily introduced into cells via transfection and can be fused to other proteins allowing dynamic monitoring of cellular processes (Hadjantonakis et al., 2003). However, both of these methodologies are poorly sensitive and have limited depth of penetration and resolution. The second group (e.g. nuclear medicine-based technologies) is based on the ability of scanners to detect and localize gamma ray emission from the decay of a radiotracer. PET and SPECT scanners and the gamma camera are the three main types of scanners used in nuclear medicine. These currently offer the greatest potential for translation into clinical applications (Vassaux and Groot-Wassink, 2003; Penuelas et al., 2005). They are highly sensitive and provide good spatial resolution, especially when they are coupled by macroscopic imaging systems such as computed tomography. These technologies, however, require more expensive cameras, and radioactivity. Their uses and imaging modalities will be discussed in this review.
To image gene expression, a reporter gene and specific radio-labelled probes are required (Table 2) (Blasberg, 2003). The general principle is that, upon expression of the reporter gene, the biodistribution of a tracer molecule is altered, leading to its local concentration at the site of reporter gene expression (Vassaux and Groot-Wassink, 2003). Three types of reporter gene families are currently being developed, based on enzymatic activities (Tjuvajev et al., 1996; 1998;), the presence of specific receptors (Rogers et al., 2005) or ionic transport mediated by membrane proteins (Groot-Wassink et al., 2002; 2004a,b;). As an example, HSV-1-tk is able to phosphorylate a freely diffusible, radio-labelled substrate which becomes trapped inside target cells when phosphorylated by the enzyme. This local accumulation of the radio-labelled tracer can be detected using nuclear imaging techniques, depending on the isotope used (Tjuvajev et al., 1996; 1998;) and has been developed for various applications (Koehne et al., 2003).
Table 2.
Examples of human reporter genes, radiolabeled probes and imaging modalities
| Class of reporter genesa | Gene | Reporter probes | Imaging modalities |
|---|---|---|---|
| Enzyme | HSV-1-tk | [123,124, 125,131I]FIAU; [18F]FEAU [18F]GCV; [18F]FPCV; [18F]FHBG | Scintigraphy, PET, SPECT |
| Luciferase | D-Luciferin | Bioluminescence | |
| TK2 | [124I]FIAU; [18F]FEAU | SPECT, PET | |
| GFP | Fluorochromes | Fluorescence camera | |
| Transporter | NIS | [123,124,131I]NaI; [99mTc]pertechnetate [186,188Re]perrhenate | Scintigraphy, PET, SPECT |
| NET | [123I]MIBG | Scintigraphy, SPECT | |
| Receptor | SSTR2 | [188Re]P829, [99mTc]P829 [111In]DTPA-D-Phe1-octreotide | Scintigraphy, PET |
| D2 receptor | [18F]FESP | PET |
Table adapted from Blasberg, 2003; Vassaux and Groot-Wassink, 2003; Briat and Vassaux, 2006 and Serganova et al., 2007.
D2, dopamine receptor 2; FEAU, 2′-fluoro-5-ethyl-1-β-D-arabinofuranosyluracil; FESP, Fluoroethylspiperone; FHBG, fluoro-3-(hydroxymethyl)butyl] guanine; FIAU, 2'deoxy-2′-fluoro-1-beta -d-arabinofuranosyl-5- iodo-uridine; FPCV, fluoropenciclovir; GCV, ganciclovir; MIBG, metaiodobenzylguanidine; NET, Norepinephrine transporter; SSTR2, Somatostatin subtype 2; TK2, Mitochondrial thymidine kinase2.
In this review, we will focus on imaging technology exploiting NIS as a reporter gene. The first part of the review will describe the biological function of NIS. In the second part, we will discuss the imaging potential of NIS to monitor gene expression in the live subject using nuclear imaging technology. Direct application of NIS as a gene reporter for biotherapy purposes will be developed in a final section. We will emphasize in particular its potential in (i) image-guided radiotherapy; (ii) monitoring of gene and vector biodistribution; and (iii) trafficking of stem cells.
The sodium-iodide symporter
Biological function
The sodium iodide symporter (NIS) is a member of the sodium/solute family of proteins composed of the sodium/glucose co-transporter (SLC5), the sodium/myo-inositol symporter (SMIT), the sodium/proline symporter (NPT), the sodium/multivitamin transporter (SMVT) and the high-affinity choline transporter (CHT). All of these proteins share the same biological function to transport molecules across a phospholipid membrane using an electrochemical gradient of sodium ions (Na+) as carrier and an ATP-driven pump (Jung, 2002).
NIS is present on the basolateral membrane of thyroid follicular cells and is responsible for the active transport of iodide into the thyroid gland. Its presence in thyrocytes is required for the synthesis of thyroid hormones, T3 and T4 (Kogai et al., 2006). NIS co-transports sodium and iodide ions into the cells in a 2:1 (Na+:I-) ratio. The energy required for the transport of iodide against the cellular electrochemical gradient is generated via the sodium/potassium (Na+/K+) ATPase. Therefore, in the thyroid, NIS is inhibited by factors that reduce the activity of the Na+/K+ ATPase, such as ouabain, and also by molecules capable of competing with iodide transport, such as thiocyanate (SCN-), perchlorate (ClO4-) and other anions. Within thyroid cells, iodide is transported into the thyroid colloid by passive transport across the apical plasma membrane, implicating two potential iodide transporters: pendrin and apical iodide transporter (AIT). In the colloid, iodide is oxidized by thyroid peroxidase enzyme (TPO) to form active iodine, which is incorporated into tyrosine residues of thyroglobulin molecules for the synthesis of the thyroid hormones, T3 and T4. Iodine is, therefore, trapped in the thyroid gland by organification (Kogai et al., 2006).
In addition to its expression in the thyroid, NIS is detectable and active in the salivary glands, gastric mucosa and lactating mammary glands (Tazebay et al., 2000; Kogai et al., 2006). However, the level of expression of NIS is lower in these tissues than in thyroid tissues. As in the thyroid, NIS is capable of mediating iodide uptake in these other tissues and this process can be blocked by thiocyanate and perchlorate anions. In contrast to thyroid tissues, there is no long-term retention of iodine and the expression of NIS is not regulated by TSH (Tazebay et al., 2000; Kogai et al., 2006). This indicates that iodide organification is a particular and unique characteristic of the thyroid gland, which can not be easily transposed to other tissues. The physiological function of NIS in extra-thyroidal tissues is still unclear.
NIS cloning, structure and expression in normal cells
The rat NIS gene was first cloned and characterized in 1996 (Dai et al., 1996), rapidly followed by the human NIS gene (hNIS) (Smanik et al., 1996). The knowledge of the coding sequence of NIS was crucial to understanding the regulation of NIS in the thyroid and its implication in thyroid disorders and treatments. The hNIS gene encodes a 3.9 kb mRNA transcript coding for a glycoprotein of 643 amino acids with a molecular mass of 70 to 90 kDa, depending on post-translational modifications. NIS is a 13 transmembrane domain protein with an extracellular amino- and intracellular carboxy-terminus that has strong amino acid sequence homology with the mouse, rat and pig NIS proteins (82.8%, 83% and 84.1% respectively).
NIS expression and functional activity are regulated at many different levels, from transcriptional activation to post translational modifications. As thyroidal NIS is only functional in the basolateral membrane of thyroid follicular cells, subcellular shuttling and plasma membrane retention signals also control NIS activity (Kogai et al., 2006).
Stimulation of NIS expression by TSH represents the main regulatory system of NIS expression in the thyroid. TSH is a 30 kDa glycoprotein that binds to its receptor, expressed on the basolateral membrane of thyroid follicular cells. Upon binding, the cAMP cell signalling pathway is activated, inducing downstream transcriptional activation of the NIS gene (Fenton et al., 2008) and iodide uptake in TSH stimulated-cells (Riedel et al., 2001). TSH was found also to control translocation and retention of NIS at the plasma membrane, providing an additional level of control of its activity (Riedel et al., 2001; Ferreira et al., 2005). NIS regulation by TSH-cAMP signalling is mediated to a large extent by an enhancer region (Fenton et al., 2008) located upstream of the proximal promoter of the human NIS gene (Fenton et al., 2008). These regulatory domains contain binding sites for thyroid-selective transcription factors such as Pax-8 and TTF-1 as well as a cAMP-responsive element binding protein (CREB) (Taki et al., 2002; Fenton et al., 2008). In addition to TSH, IGF-1, retinoic acid, IFN-γ and direct activation of the PI3-kinase and p38 MAPK pathways were found to be involved in the regulation of NIS expression in thyroid, whereas in lactating breast and in breast cancer cells, prolactin enhances the expression of NIS (Pekary and Hershman, 1998; Filetti et al., 1999; Garcia and Santisteban, 2002; Arturi et al., 2005; Kogai et al., 2008). Conversely, thyroglobulin suppresses the expression of NIS mRNA and of other thyroid-specific genes such as TPO and TRs, providing a sensitive control of NIS activity in the thyroid (Kogai et al., 2006; Suzuki and Kohn, 2006).
NIS expression in thyroidal and non-thyroid cancer cells
Advanced but well-differentiated thyroid cancer has been treated successfully for more than 50 years with radioiodine (131I) therapy (Siegel, 1999). This tumour type expresses high levels of NIS, although its expression is heterogeneous among the tumour, and expresses as well other thyroid-specific genes which are required for the organification of radioiodine (Saito et al., 1998; Wapnir et al., 2003; Lee et al., 2007). Consequently, upon injection of 131I, thyroid tumour cells accumulate and retain radiodide resulting in tumour radioablation by emission of β-particles and γ-radiation.
For other thyroidal cancers, the potency of radioiodide treatment is directly related to the effective radiation dose delivered to the tumour. NIS expression in poorly differentiated thyroid cancer tissues is variable and in some cases is down-regulated as compared with normal thyroid tissues. Consequently, iodide uptake is diminished and radiotherapy is largely ineffective. However, this limitation could be overcome using TSH stimulation of the thyroid prior to radioiodide administration (Jarzab et al., 2003) or by using pharmacological agents such as lithium to prolong the biological half-life of iodide in thyroid tissues (Elisei et al., 2006).
Regarding non-thyroid cancer tissues, up to 83% of invasive human breast carcinomas expressed NIS, whereas normal breast tissues are negative for the expression of NIS (Wapnir et al., 2003). However, in two independent studies, only 20 to 25% of NIS-positive tumours showed functional iodide-radiotracer uptake. This could be explained by the fact that, in these malignant tissues, both plasma membrane-associated and intracellular cytoplasmic NIS have been reported (Wapnir et al., 2003). These studies emphasize that, as for non-differentiated thyroid cancer cells, NIS protein synthesis, modification and membrane targeting are important factors for high iodide uptake.
Molecular imaging of gene expression
Imaging detection methods to visualize NIS expression in vivo
Different modalities have been reported for imaging hNIS-expressing tissues. These include PET, scintigraphic imaging and SPECT. The main advantages of nuclear detection methods is that they offer increased sensitivity, allow temporal and spatial resolution and, importantly, are relevant in translation to use in human patients. Both PET and SPECT give quantitative and non-invasive information at the level of reporter gene expression.
Scintigraphic imaging
Planar scintigraphic images of radioisotope biodistribution are limited in their resolution and quantitative potential and, unlike SPECT and PET, do not permit a three-dimensional reconstruction of the image obtained (Blasberg, 2003; Serganova et al., 2007). This imaging modality is, however, straightforward and less expensive and has been used recently to visualize the activation of specific signalling pathways using NIS as reporter gene (Che et al., 2007; Kim et al., 2007a,b; Yeom et al., 2008).
PET imaging
The emission of positrons is an essential requirement for PET scanning. When the positrons emitted from the radioisotope collide with local neighbouring electrons, high-energy gamma (γ) rays are produced, which travel in opposite directions from the point of collision and can be detected by a γ camera surrounding the subject. Detectors form a ring around the subject (from which photons are emitted) and only process events that occur simultaneously. Reconstruction of three-dimensional images (probe distribution) from the raw data is performed by different mathematical methods. They differ in their resolution, resolution–noise ratio, contrast and required processing time. The most widely used positron-emitting isotopes are generally short lived. Isotopes with the shortest half-lives (e.g. 20 min for 11C, 109 min for 18F) have to be produced in cyclotrons near to the laboratory or hospital where tracer synthesis, transport and application take place, or have to be shipped by air. Small variations in timing have important consequences. However, isotopes with longer half-lives (e.g. 4.2 days for 124I) can be produced further away from the eventual site of use. Because it is completely absorbed in the tissue, the energy received from positron-emitting isotopes is higher than the energy received from isotopes used in SPECT (Briat and Vassaux, 2006).
SPECT imaging
For NIS-expression imaging, SPECT uses detectors to identify individual photons emitted by isotopes such as 123I- or 99mTcO4-. The direction of a photon emitted is determined by fitting lead collimators between the source of emission and the detectors. These collimators stop all photons not travelling in a certain direction relative to the detector panel. Therefore, the collimator rejects the photons not travelling at a right angle to the detectors or through a pinhole. As a result, the sensitivity of SPECT is much lower than for PET devices (Briat and Vassaux, 2006). Endogenous p53 transcriptional activity has been visualized using a γ-camera and the hNIS as a reporter gene placed under the control of a p53-responsive promoter (Doubrovin et al., 2001). SPECT/CT images were obtained in nude mice before and after treatment with adriamycin, a known activator of the p53 pathway, following an intravenous injection of [99mTc] pertechnetate. The images showed a clear increase in tracer uptake following treatment, which correlated with p53 activation and expression of its target genes. These results demonstrated the feasibility of monitoring the regulation of gene expression using SPECT. The SPECT imaging approach is readily adaptable to the clinic and has been widely used.
As for PET imaging, various manufacturers have developed small-animal SPECT imaging devices [microSPECT (Gamma Medica-Ideas), HiSPECT (Bioscan), and NanoSPECT (Bioscan)]. These systems, as it is the case for microSPECT (Carlson et al., 2006), now offer good resolution and the possibility of being coupled with computerized tomography for anatomical imaging. In contrast to human studies, small-animal imaging requires a high spatial resolution because of the small size of the organism to be studied. This is achieved using pinhole collimators, which can be adapted to the γ-camera. In addition, multiple pinholes can be used to increase both the spatial resolution and sensitivity.
Applications in biotherapies
Biodistribution of gene transfer in gene therapy
Soon after the rat NIS gene was cloned in 1996 (Dai et al., 1996), imaging of the transgene in non-thyroid cells was demonstrated by gamma camera (Shimura et al., 1997). Lately, intra-tumoural injection of 2 × 109 infectious adenovirus particles encoding NIS was shown to be able to image tumour sites in vivo and to redirect 11% ID/g of the injected radioiodine to the tumour (Boland et al., 2000). These first reports validated the use of NIS as a reporter gene for imaging gene expression in the live subject using non-invasive imaging technology. Since then, a large number of reports have been published (Boland et al., 2000; Spitzweg et al., 2000; Groot-Wassink et al., 2002; 2004a; Barton et al., 2003; Cho et al., 2007; Raty et al., 2007; Montiel-Equihua et al., 2008) confirming the feasibility of the approach for small-animal imaging. Recently, non-invasive imaging of NIS expression upon viral gene transfer has been demonstrated as feasible and safe in humans (Barton et al., 2008). In a phase I study, detection of gene transfer and persistence of gene expression in the whole body of patients with localized prostate cancer was imaged accurately using a SPECT/CT scanner and a replication-competent adenovirus armed with two suicide genes and NIS as the gene reporter. Data from nuclear imaging demonstrated in this study that intra-prostatic injection of 1012 viral particles led to the detection of NIS expression in the prostate in seven out of nine patients over a period of 7 days. Peak transgene expression, and therefore maximal viral spread, was detected 1 to 2 days after administration, covering on average 18% of the total volume of the prostate. Whole-body serial imaging of NIS expression in these patients indicated that there was no extra-prostatic dissemination of the virus, explaining in part the absence of serious adverse events observed. This study is first carried out in human patients and validates the use of NIS as a reporter gene for monitoring both gene and vector biodistribution in humans (Barton et al., 2008).
Imaging using NIS has several advantages. Iodide is the radiotracer; therefore, radio-labelling and radiochemistry are not required, reducing costs. The imaging of NIS-expressing tissues is particularly versatile as NIS can promote the cellular uptake of different radioisotopes 123I- (SPECT), 124I- (PET), 99mTcO4- (SPECT), 131I- (scintigraphic imaging) (Vassaux and Groot-Wassink, 2003). However, the lack of organification of iodide in extra-thyroidal tissues is a disadvantage using NIS as a reporter gene. There is a dynamic system of passive efflux of iodide and other NIS-specific radiotracers from cells transduced with NIS that produces difficulties in monitoring the process. The accumulation of iodide can be predicted to be a dynamic phenomenon, largely dependent on the clearance of the tracer, and for which the clearance effect will vary between organs. However, our laboratory (Groot-Wassink et al., 2002; 2004b;) and others (Boland et al., 2000; Smit et al., 2002; Haberkorn et al., 2004) have shown that the rate of efflux of iodide is slow: 1 h after iodide injection, 5 to 7% of the injected dose is still present in the plasma (Groot-Wassink et al., 2002; 2004b;). The residual iodide was enough to allow concentration in NIS-expressing organs and to monitor precisely the localization of gene transfer. In another study, we (Groot-Wassink et al., 2004b) took advantage of the high sensitivity and quantitative potential of PET scanning to assess whether gene transfer could provide quantitative information on gene expression in mice. Increasing doses of an adenovirus coding for NIS were injected intravenously into experimental mice before the administration of 124I. Post-mortem γ counting of liver biopsies was found to be directly correlated with the dose of adenovirus injected and with both NIS mRNA concentration and the number of NIS-expressing cells detected in these tissues. In order to assess whether PET scanning could also gather accurate and quantitative information on gene expression, iodide uptake measured by PET was compared to post-mortem γ counting values. The results revealed a good correlation (r2= 0.9581) between the uptake value of 124I detected in the liver by PET and the value detected by post-mortem γ counting (Groot-Wassink et al., 2004b). These data provided evidence that PET measurements of iodide uptake are quantitative and provide accurate information to evaluate precisely the expression level of a therapeutic transgene in gene therapy approaches.
In addition to monitoring gene transfer mediated by viral vectors, nuclear imaging of NIS expression could be developed for use with new synthetic vector formulations. A synthetic vector could be defined as a nanoscale-size molecule with a chemical formulation allowing to stably entrap DNA to transfect efficiently targeted cells for DNA transgene expression. The majority of synthetic vectors are cationic, lipid-derived formulations and have been used successfully for in vitro transfection. This is in sharp contrast to the in vivo situation. Experimental data (Pack et al., 2005; Cho et al., 2007), obtained at autopsy of animals, have provided evidence that the diffusion of synthetic vectors in tumour masses is largely limited by non-specific absorption in the liver, lungs and kidneys and by macrophages of the reticuloendothelial system (Pack et al., 2005; Cho et al., 2007). This non-specific accumulation in healthy organs creates important toxicity issues and has largely hampered the widespread application of this type of gene delivery vehicle in the clinic. Recently, with advances in the field of nanotechnology, PEG- or HPMA-modified polymeric nanoparticles have been shown to transform the size, charge and surface characteristics of the particles, resulting in longer circulation times in the blood, reduced uptake by the reticuloendothelial system and altered biodistribution in normal tissues (Pack et al., 2005). Classically, biodistribution in mice is assessed by radioisotope-labelled nanoparticles followed by post-mortem radioactive counting and/or scintigraphic imaging. Therefore, to obtain final conclusive data for the identification of the best chemical formulation allowing high tumour targeting with limited diffusion in healthy organs, important radiochemistry and autopsy analyses are required. In this context, coupling a synthetic vector with an expression plasmid encoding hNIS could greatly reduce the cost and time of procedures and might also provide qualitative and quantitative information about the efficiency of gene transfer. In a recent study (Chisholm et al., 2009), we demonstrated that a nanoparticle formulation composed of third generation polypropylenimine dendrimers, when complexed to hNIS plasmid, was capable of tumour transfection upon systemic administration to tumour-bearing mice. Whole-body nuclear imaging using a small-animal nano-SPECT/CT scanner and the human Na/I symporter (hNIS) as the reporter gene showed a unique and highly specific tumour targeting with no detection of gene transfer in any other tissues of tumour-bearing mice. Tumour-selective transgene expression was confirmed by quantitative RT-PCR at autopsy of scanned animals, while genomic PCR demonstrated that the tumour sites were the predominant sites of nanoparticle accumulation. Using this approach, we were able to demonstrate that the kinetic of NIS expression in tumours was transient. Peak of transgene expression was indeed detected 24 h after administration. Altogether, these data validated the use of the NIS as a reporter gene to monitor the exact biodistribution of gene transfer mediated by a synthetic vector, allowing the anticipation of potential toxicity issues.
Image-guided radiotherapy and dosimetry
In addition to its potential as an imaging transgene, NIS has therapeutic potential. These properties offer the unique possibility to combine the potential of nuclear imaging technology to provide precise, qualitative and quantitative information about gene expression with the potential of radiotherapy to kill cells expressing NIS by ionizing irradiation, as well as neighbouring cells through the physical crossfire effect. The utilization of NIS in cancer gene therapy could lead to individualized therapy, as NIS expression upon gene transfer can be assessed in a living subject to determine the exact dose of radiation needed to efficiently irradiate tumour cells (Figure 1).
Figure 1.
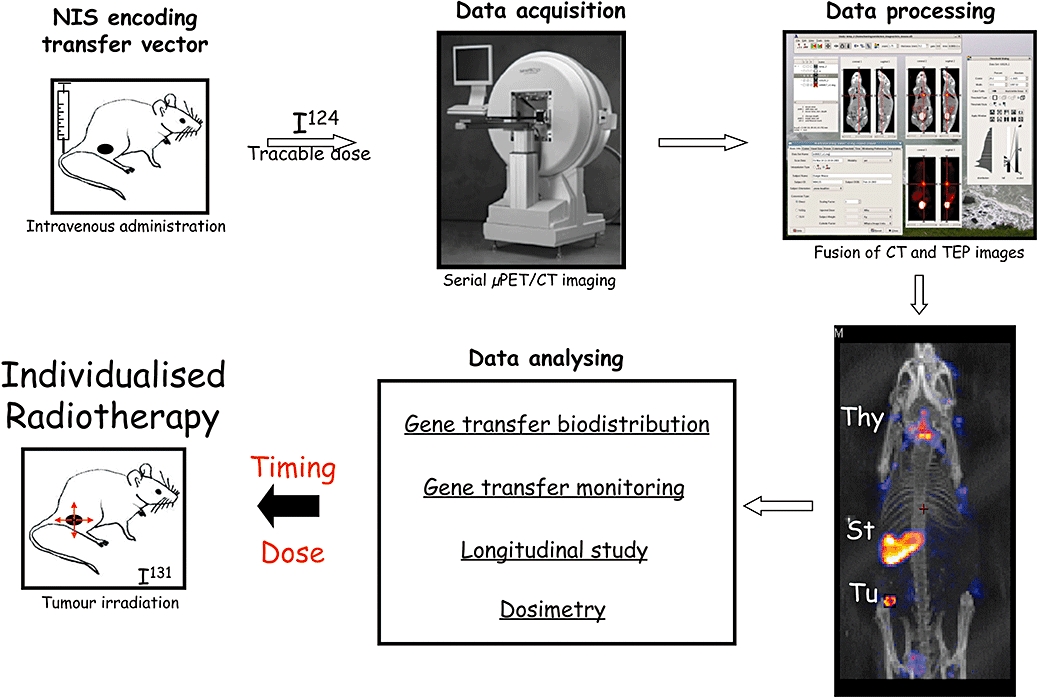
Schematic representation of the principle of image-guided radiotherapy mediated by NIS gene transfer in the living animal. Viral or synthetic gene transfer vectors encoding hNIS are injected intravenously into tumour-bearing mice. For kinetic studies, mice are anaesthetized, injected intravenously with radioiodide [124I] and positioned in a µPET/CT scanner. PET images are obtained from various acquisition times depending on the specificity of radioactivity levels in mouse. A CT scan is taken at the same time. The reconstruction of images and fusion between PET and CT images are processed with adequate software such MEDISO and PMOD (Medical Imaging Systems). Final images are shown in coronal, axial or sagittal view in the plane of the tumour or, alternatively, as seen in this figure in three-dimensions (3D). Coloured dot points reveal radiotracer uptake in the whole body of mice while CT images allow the precise determination of the localization of the radiotracer emission source. In this example, radiotracer uptakes is detectable in the thyroid (Thy) and the stomach (St) as the result of endogenous expression of mNIS, and in the tumour (Tu) as the result of hNIS gene transfer. The kinetics of hNIS expression at the tumour site could be determined by quantification of the radiotracer activity in tumour tissues at different times in a longitudinal study carried out on the same animal. When expression of hNIS in the tumour is found to be maximal, a dosimetry study is then performed. Dosimetric calculation determines accurately the minimal therapeutic dose to administrate for selectively irradiated the tumour volume with minimal side effects. Such protocol fully validates the principle of individualized image-guided radiotherapy.
Experimentally, several groups have used gene therapy methodologies to transfer the NIS gene into non-thyroidal cancer cells to achieve a successful radioablation of the experimental tumour, as in differentiated thyroid cancer (Boland et al., 2000; Spitzweg et al., 2000; Dingli et al., 2004; Dwyer et al., 2005; 2006; Hasegawa et al., 2006; Goel et al., 2007; Montiel-Equihua et al., 2008). Overall, the success of this approach resides in the level of NIS expression achieved in tumour cells as well as in the ability of tumour tissues to retain radioiodide within the cells. Because of their cellular origin, non-thyroidal cancer cells do not express endogenous TPO and are not capable of organifying iodide, even in the presence of exogenous hNIS. To overcome this problem, several approaches have been developed, with conflicting results. Some reports have demonstrated that co-expression of NIS with TPO (Huang et al., 2001) or TIFF-1 (Furuya et al., 2004) results in higher radioiodide uptake and retention by tumour cells, while another study (Boland et al., 2002) reported that induced-iodide organification by TPO was not enough to increase the retention time of the radiotracer in target cells. Another promising approach consists of the identification of small molecules capable of influencing radioiodide uptake by NIS-expressing cells. A recent, high-throughput screening identified an imidazothiazole derivative component capable of increasing by 4.5-fold the retention of radioiodine in NIS-expressing cells (Lecat-Guillet et al., 2007). Although these results are promising, dose-dependent experiments performed in vitro demonstrated that this component was capable of retaining iodide in NIS-expressing cells only when sub-micromolar concentrations of iodide were used (Lecat-Guillet and Ambroise, 2008). Considering the concentration of iodide in blood (10−8 to 10−9 M), this component is, therefore, unlikely to be effective in vivo. Thus, low or absent iodide organification in tissues remains a limiting factor in the use of NIS as a transgene for radiotherapy purposes. Indeed, the accumulation of iodide in cells has been demonstrated to be a dynamic process. Rapid efflux of iodide occurs through the cells when the radiotracer is removed from the extracellular medium. In vivo, a similar efflux of iodide takes place, although we (Groot-Wassink et al., 2002; 2004b;) and others (Boland et al., 2000; Smit et al., 2002; Haberkorn et al., 2004) have demonstrated that iodide clearance is slow: There is still 5–7% ID/g of iodide in the plasma within 1 h after injection (Groot-Wassink et al., 2004b). This circulating dose of the radiotracer in animals has been sufficient to eradicate experimental thyroidal and non-thyroidal tumour cells following hNIS expression. Interesting data from Faivre et al. (2004) indicate that iodide accumulation and retention is high in the liver without organification. Using chemically induced hepatocarcinomas model in rats, and injection of adenovirus encoding rat NIS directly into the liver portal vein, they observed a marked (20 to 30% of the injected dose) and sustained (>11 days) iodine uptake and retention in the hepatic tumours (Faivre et al., 2004). Radioiodine therapy by injection of 650 MBq 131I after gene transfer achieved a strong inhibition of tumour growth, complete regression of small nodules and prolonged survival of hepatocarcinoma-bearing rats. The sustained iodide uptake in vivo was attributed to permanent recycling of the effluent radioiodide via the hepatic blood flow and not to an active retention mechanism (Faivre et al., 2004). Recently, Hingorani et al. demonstrated that radiation increased the magnitude and duration of NIS expression from replication-deficient adenoviruses (Hingorani et al., 2008b). This effect was observed when various promoters were used to drive NIS expression and has important implications for future clinical applications. In another study, the same group demonstrated that the enhancement of transgene expression induced by the radiation was mediated in part by the formation of dsDNA breaks and subsequent MAPK pathway signalling, both in vitro and in vivo (Hingorani et al., 2008a).
Various radioisotopes can be used in conjunction with NIS. Radioisotope species with greater energy deposition could also increase radiotherapy potency. NIS can promote cellular uptake of several isotopes such as Rhenium (Re) (Shen et al., 2004) and Astatine (At) (Willhauck et al., 2008). Rhenium (188Re) is a β-emitter with a half-life of 16.7 h and a tissue penetration radius of 23–32 mm, making it particularly suitable for the treatment of large tumours. Dosimetry calculations indicate that 188Re-perrhenate delivers up to a 4.5-fold higher dose of radioactivity than 131I (Shen et al., 2004). Astatine is a particularly attractive isotope for radiotherapy purposes. 211At is an α-emitting radiohalide with a half-life of 7.2 h, tissue penetration of 60 µm and very high energy deposition. However, because of this short half-life and safety concerns, Astatine is not currently suitable for use in the clinic.
The successful treatment of NIS-expressing tumours with 131I is likely to involve high radiotherapy doses which may be beyond to the limit of toxicity when scaled to use in human patients (Wenzel et al., 2003). In the clinic, the most common approach to radionuclide dosimetry has been the MIRD S factor- (mean absorbed dose per unit cumulative activity) based method (Zanzonico, 2000). This approach is based on an algorithmic mathematical model which predicts the minimal, therapeutic, injected dose of a radioisotope required to irradiate tumour cells while sparing normal, healthy organs. As an example, the treatment of differentiated thyroid cancer has been carried out using fixed standard activities of I131 based on MIRD S factor software (Zanzonico, 2000). Although effective, this approach does not take into account the exact biokinetic distribution of the radioisotope within the tumour, nor individual differences between tumour architecture, blood supply and tumour volume. As a result, the correlation between assessed doses and observed toxicities in targeted radiotherapy has not been completely satisfactory. Some patients have shown only partial tumour regression, while others have developed toxicity symptoms (Wiseman et al., 2003). Therefore, accurate calculation of the therapeutic isotope dose to administer is a prerequisite for successful clinical response and safety. More recently, with advances in nuclear medicine imaging technology and the development of biophysical software, new dosimetric calculations have been made possible (Lassmann and Hanscheid, 2007). For example, three-dimensional imaging-based dosimetry methods combining 124I PET images have allowed the acquisition of information on the spatial distribution of absorbed doses within a tumour, and the accurate determination of tumour volume and mean absorbed dose within a tumour. Data (Sgouros et al., 2004) from a pilot study of a total of 56 patients with thyroid cancer showed high variability of 124I uptake within thyroid tissues. The mean absorbed dose within individual patients was widely variable, ranging from 1.2 to 540 Gy, while the distribution of absorbed doses within individual tumours was also highly variable, ranging from a minimum of 0.3 Gy to a maximum of 4000 Gy. This study illustrated well the potential of NIS imaging to allow personalized therapy (Figure 1). Pre-therapy dosimetric calculations based on NIS imaging can allow very accurate estimations of the amount of radioisotope necessary to deliver a given dose of radiation to a particular tumour. Moreover, repeated radioisotope monitoring in tumours allows adjusting the 123I therapeutic dose to administrate to the treated patient according to the tumour response. Finally, NIS-mediated radiotherapy could be combined with more classical external beam radiotherapy (Harrington et al., 2008).
Image-guided radiovirotherapy
The development of molecular imaging strategies capable of monitoring the propagation of oncolytic viruses in tumours is crucial to obtain an optimal therapeutic effect. Clinical trials using oncolytic adenoviruses have emphasized that, although these genetically modified viruses are capable of replicating selectively in cancer cells, their therapeutic efficacy is limited (Waehler et al., 2007). One reason for this is that virus spread within the tumour is often restricted to the initial site of infection. Viral propagation and potency are known to be prevented by intra-tumoural physical barriers such as stromal tissue composition, hypoxic and necrotic areas (Waehler et al., 2007). A combination of the therapeutic potential of the oncolytic adenovirus with additional therapeutic intervention(s) is required to obtain greater tumour reduction. Combined use of oncolytic adenovirus with radiotherapy or conventional chemotherapy has been reported (Dilley et al., 2005; Homicsko et al., 2005; Cheong et al., 2008). However, the mistimed combination of additional therapy has been described in preclinical studies (Wildner and Morris, 2000), resulting in loss of efficacy and even in virostatic effects. Therefore, the capability for non-invasive monitoring of virus spread in a tumour would provide unique information to rationalize the timing of these treatment combinations. Using two different engineered adenoviruses encoding NIS, and a small nano-SPECT/CT camera, we recently demonstrated that the kinetics of intratumoural spread could be monitored effectively in vivo. The peak of radiotracer uptake was detected 48 h post-infection for the first virus and at 72 h for the second one (Merron et al., 2007). In addition, these data demonstrate that adenovirus replication in tumours is very transient and dependent on viral design. Of additional interest in the use of NIS for radiotherapy in combination with oncolytic viruses is the physical crossfire effect (Dingli et al., 2004; Mitrofanova et al., 2006). As the average path length of the β particle emitted by 131I is 0.4 mm, non-transduced tumour cells could be destroyed by radiation emitted from neighbouring cells transduced by the recombinant oncolytic viruses expressing NIS. This effect can be particularly powerful (Dingli et al., 2004; Mitrofanova et al., 2006). Using an oncolytic measles virus, results from myeloma xenografts showed complete regression of the tumour after 131I therapy even when only half of the tumour cells expressed NIS (Dingli et al., 2004; Mitrofanova et al., 2006).
Trafficking of cells with therapeutic potential
Another application of NIS as a gene reporter for imaging gene expression involves adoptive cell-based therapies. In this field, in vitro expansion of lymphocyte T cells with anti-tumour activity, followed by the re-implantation of these cells in the same patient, has been used for two decades with some clinical benefit, particularly for the treatment of melanoma (Rosenberg et al., 2008). Although tumour T cell tropism was shown to be effective in patients, the exact biodistribution of these re-infused cells has not been characterized fully (Pittet et al., 2007). In addition to T cells, other cell types such as mesenchymal stem cells, also exhibit a marked tropism for damaged areas including cancer tissues (Fritz and Jorgensen, 2008). Recently, mesenchymal stem cells have been the object of important research activity in the fields of both gene and cellular therapies. Promising results have been reported in graft versus host disease treatment, heart regeneration, cartilage and bone repair, skin wound healing and neuronal regeneration after strokes (Uccelli et al., 2008). Mechanistically, inherent migration potential as well as their transdifferentiation property has been proposed as the cause of the plasticity of these cells. However, to date, there is insufficient information about the exact in vivo biodistribution, survival and biological comportment of these cells in targeted tissues (Uccelli et al., 2008). In this context, constitutive expression of the hNIS gene in cells transduced with a lentiviral vector would provide a unique opportunity to study trafficking, homing and tumour targeting following injection of a radiotracer dose of I124. Proof of principle of this approach has been demonstrated recently (Hwang et al., 2008). The behaviour of stem cells incorporated into biocompatible chitosan or poly L-lactic acid (PLLA) scaffolds and then grafted subcutaneously in nude mice was visualized successfully using NIS as a gene reporter and a gamma camera as an imaging modality. Serial quantitative analyses of regions of interest drawn manually from the scintigraphy images demonstrated that radiotracer uptake by NIS-expressing stem cells was higher in cells grafted onto PLLA scaffolds than in cells not seeded onto a scaffold. Assuming that NIS quantitative data are directly proportional to the number of viable cells, this imaging modality mediated by NIS allowed the identification in mice of the best scaffold polymeric structure capable of promoting efficient proliferation and survival of stem cells. This work provides a new rationale for the design of cell-scaffold complexes for tissue-regenerative and cell-replacement therapies (Hwang et al., 2008). However, transduction of human patients with integrative vectors has been shown to lead to adverse events, in particular leukaemia (Hacein-Bey-Abina et al., 2003). Therefore, besides imaging stem cell and other cell-type migration, the use of NIS as a gene reporter could also be exploited for the irradiation (I131) of transduced, NIS-positive cells in the case of adverse events.
Moving towards clinical applications
Non-invasive imaging technology will help in the design and improvement of gene therapy applications. The capability to visualize and monitor in vivo the regulation of gene expression (either of endogenous genes or genes that have been delivered to cells using a viral, synthetic or cellular vector) in small animals will lead to novel therapeutic approaches in clinical research, as well as advances in the design of safe and efficient vectors such as viruses to be used in clinical settings. Among the safety issues, specific targeting of diseased sites is an important safety consideration for all trials involving patients. As NIS imaging has been validated recently in humans (Barton et al., 2008), non-invasive molecular imaging will help clinicians to monitor in vivo the distribution and pharmacodynamics of gene expression in a precise and quantitative way.
Acknowledgments
The work in the authors' laboratory is supported by grants from INSERM, la Ligue Nationale Contre le Cancer, ARC, and by grant 0607-3D1615-66/AO INSERM from the French National Cancer Institute (INCa). The publication of this article was possible with thanks to financial support brought by Association Française pour l'Etude du Foie and BMS laboratories.
Glossary
Abbreviations:
- DAB-AM16
Diaminobutane based polypropylenimine dendrimers
- GFP
green fluorescent protein
- HPMA
N-2-hydroxypropylmethacrylamide
- HSV-1-tk
the herpes simplex virus thymidine kinase gene
- MRI
Magnetic resonance imaging
- NIS
Na/I symporter
- PEG
Poly(ethylene glycol)
- PET
Positron emission tomography
- SPECT
single photon emission computed tomography
- Tg
thyroglobulin
- TIFF-1
thyroid transcription factor-1
- %ID/g
percentage of injected dose per gram
- TRs
thyroid stimulating hormone receptor
- TSH
thyroid stimulating hormone
Note
The drug and molecular target nomenclatures used in this manuscript are in agreement with the BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Statement of conflict of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arturi F, Ferretti E, Presta I, Mattei T, Scipioni A, Scarpelli D, et al. Regulation of iodide uptake and sodium/iodide symporter expression in the MCF-7 human breast cancer cell line. J Clin Endocrinol Metab. 2005;90:2321–2326. doi: 10.1210/jc.2004-1562. [DOI] [PubMed] [Google Scholar]
- Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, Lu M, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;8:508–518. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KN, Tyson D, Stricker H, Lew YS, Heisey G, Koul S, et al. GENIS: gene expression of sodium iodide symporter for noninvasive imaging of gene therapy vectors and quantification of gene expression in vivo. Mol Ther. 2003;8:508–518. doi: 10.1016/s1525-0016(03)00153-9. [DOI] [PubMed] [Google Scholar]
- Blasberg RG. Molecular imaging and cancer. Mol Cancer Ther. 2003;2:335–343. [PubMed] [Google Scholar]
- Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–3492. [PubMed] [Google Scholar]
- Boland A, Magnon C, Filetti S, Bidart JM, Schlumberger M, Yeh P, et al. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfers. Thyroid. 2002;12:19–26. doi: 10.1089/105072502753451922. [DOI] [PubMed] [Google Scholar]
- Briat A, Vassaux G. Preclinical applications of imaging for cancer gene therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S1462399406000044. [DOI] [PubMed] [Google Scholar]
- Briat A, Vassaux G. A new transgenic mouse line to image chemically induced p53 activation in vivo. Cancer Sci. 2008;99:683–698. doi: 10.1111/j.1349-7006.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SK, Classic KL, Hadac EM, Bender CE, Kemp BJ, Lowe VJ, et al. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006;8:324–332. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
- Che J, Doubrovin M, Serganova I, Ageyeva L, Beresten T, Finn R, et al. HSP70-inducible hNIS-IRES-eGFP reporter imaging: response to heat shock. Mol Imaging. 2007;6:404–416. [PubMed] [Google Scholar]
- Cheong SC, Wang Y, Meng JH, Hill R, Sweeney K, Kirn D, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm EJ, Vassaux G, Martin-Duque P, Chevre R, Lambert O, Pitard B, et al. Cancer-specific transgene expression mediated by systemic injection of nanoparticles. Cancer Res. 2009;69:2655–2662. doi: 10.1158/0008-5472.CAN-08-2657. [DOI] [PubMed] [Google Scholar]
- Cho YW, Park SA, Han TH, Son DH, Park JS, Oh SJ, et al. In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials. 2007;28:1236–1247. doi: 10.1016/j.biomaterials.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Doubrovin M, Ponomarev V, Beresten T, Balatoni J, Bornmann W, Finn R, et al. Imaging transcriptional regulation of p53-dependent genes with positron emission tomography in vivo. Proc Natl Acad Sci USA. 2001;98:9300–93005. doi: 10.1073/pnas.161091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005;11:1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006;17:661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- Elisei R, Vivaldi A, Ciampi R, Faviana P, Basolo F, Santini F, et al. Treatment with drugs able to reduce iodine efflux significantly increases the intracellular retention time in thyroid cancer cells stably transfected with sodium iodide symporter complementary deoxyribonucleic acid. J Clin Endocrinol Metab. 2006;91:2389–2395. doi: 10.1210/jc.2005-2480. [DOI] [PubMed] [Google Scholar]
- Faivre J, Clerc J, Gerolami R, Herve J, Longuet M, Liu B, et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in Wistar rats. Cancer Res. 2004;64:8045–8051. doi: 10.1158/0008-5472.CAN-04-0893. [DOI] [PubMed] [Google Scholar]
- Fenton MS, Marion KM, Hershman JM. Identification of cyclic adenosine 3′,5′-monophosphate response element modulator as an activator of the human sodium/iodide symporter upstream enhancer. Endocrinology. 2008;149:2592–2606. doi: 10.1210/en.2007-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AC, Lima LP, Araujo RL, Muller G, Rocha RP, Rosenthal D, et al. Rapid regulation of thyroid sodium-iodide symporter activity by thyrotrophin and iodine. J Endocrinol. 2005;184:69–76. doi: 10.1677/joe.1.05643. [DOI] [PubMed] [Google Scholar]
- Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141:443–457. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- Fritz V, Jorgensen C. Mesenchymal stem cells: an emerging tool for cancer targeting and therapy. Curr Stem Cell Res Ther. 2008;3:32–42. doi: 10.2174/157488808783489462. [DOI] [PubMed] [Google Scholar]
- Furuya F, Shimura H, Miyazaki A, Taki K, Ohta K, Haraguchi K, et al. Adenovirus-mediated transfer of thyroid transcription factor-1 induces radioiodide organification and retention in thyroid cancer cells. Endocrinology. 2004;145:5397–5405. doi: 10.1210/en.2004-0631. [DOI] [PubMed] [Google Scholar]
- Garcia B, Santisteban P. PI3K is involved in the IGF-I inhibition of TSH-induced sodium/iodide symporter gene expression. Mol Endocrinol. 2002;16:342–352. doi: 10.1210/mend.16.2.0774. [DOI] [PubMed] [Google Scholar]
- Geus-Oei LF, Oyen WJ. Predictive and prognostic value of FDG-PET. Cancer Imaging. 2008;8:70–80. doi: 10.1102/1470-7330.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Carlson SK, Classic KL, Greiner S, Naik S, Power AT, et al. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood. 2007;110:2342–2350. doi: 10.1182/blood-2007-01-065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye EO, Glaser M, Lemoine NR, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13:1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Keith WN, Vassaux G. Noninvasive imaging of the transcriptional activities of human telomerase promoter fragments in mice. Cancer Res. 2004a;64:4906–4911. doi: 10.1158/0008-5472.CAN-04-0426. [DOI] [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Reader AJ, Vassaux G. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther. 2004b;9:436–442. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Haberkorn U, Beuter P, Kubler W, Eskerski H, Eisenhut M, Kinscherf R, et al. Iodide kinetics and dosimetry in vivo after transfer of the human sodium iodide symporter gene in rat thyroid carcinoma cells. J Nucl Med. 2004;45:827–833. [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Dickinson ME, Fraser SE, Papaioannou VE. Technicolour transgenics: imaging tools for functional genomics in the mouse. Nat Rev Genet. 2003;4:613–625. doi: 10.1038/nrg1126. [DOI] [PubMed] [Google Scholar]
- Hamstra DA, Bhojani MS, Griffin LB, Laxman B, Ross BD, Rehemtulla A. Real-time evaluation of p53 oscillatory behavior in vivo using bioluminescent imaging. Cancer Res. 2006;66:7482–7489. doi: 10.1158/0008-5472.CAN-06-1405. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Melcher A, Vassaux G, Pandha HS, Vile RG. Exploiting synergies between radiation and oncolytic viruses. Curr Opin Mol Ther. 2008;10:362–370. [PubMed] [Google Scholar]
- Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- Hingorani M, White CL, Merron A, Peerlinck I, Gore ME, Slade A, et al. Inhibition of repair of radiation-induced DNA damage enhances gene expression from replication-defective adenoviral vectors. Cancer Res. 2008a;68:9771–9778. doi: 10.1158/0008-5472.CAN-08-1911. [DOI] [PubMed] [Google Scholar]
- Hingorani M, White CL, Zaidi S, Merron A, Peerlinck I, Gore ME, et al. Radiation-mediated up-regulation of gene expression from replication-defective adenoviral vectors: implications for sodium iodide symporter gene therapy. Clin Cancer Res. 2008b;14:4915–4924. doi: 10.1158/1078-0432.CCR-07-4049. [DOI] [PubMed] [Google Scholar]
- Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- Huang M, Batra RK, Kogai T, Lin YQ, Hershman JM, Lichtenstein A, et al. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non-small cell lung cancer. Cancer Gene Ther. 2001;8:612–618. doi: 10.1038/sj.sgt.7700354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DW, Jang SJ, Kim YH, Kim HJ, Shim IK, Jeong JM, et al. Real-time in vivo monitoring of viable stem cells implanted on biocompatible scaffolds. Eur J Nucl Med Mol Imaging. 2008;35:1887–1898. doi: 10.1007/s00259-008-0751-z. [DOI] [PubMed] [Google Scholar]
- Jarzab B, Handkiewicz-Junak D, Roskosz J, Puch Z, Wygoda Z, Kukulska A, et al. Recombinant human TSH-aided radioiodine treatment of advanced differentiated thyroid carcinoma: a single-centre study of 54 patients. Eur J Nucl Med Mol Imaging. 2003;30:1077–1086. doi: 10.1007/s00259-003-1190-5. [DOI] [PubMed] [Google Scholar]
- Jung H. The sodium/substrate symporter family: structural and functional features. FEBS Lett. 2002;529:73–77. doi: 10.1016/s0014-5793(02)03184-8. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:0657–0668. doi: 10.1371/journal.pmed.0050085. e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Jeon YH, Kang JH, Lee YJ, Kim KI, Chung HK, et al. In vivo long-term imaging and radioiodine therapy by sodium-iodide symporter gene expression using a lentiviral system containing ubiquitin C promoter. Cancer Biol Ther. 2007a;6:1130–1135. doi: 10.4161/cbt.6.7.4342. [DOI] [PubMed] [Google Scholar]
- Kim KI, Kang JH, Chung JK, Lee YJ, Jeong JM, Lee DS, et al. Doxorubicin enhances the expression of transgene under control of the CMV promoter in anaplastic thyroid carcinoma cells. J Nucl Med. 2007b;48:1553–1561. doi: 10.2967/jnumed.106.038612. [DOI] [PubMed] [Google Scholar]
- Koehne G, Doubrovin M, Doubrovina E, Zanzonico P, Gallardo HF, Ivanova A, et al. Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat Biotechnol. 2003;21:405–413. doi: 10.1038/nbt805. [DOI] [PubMed] [Google Scholar]
- Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006;13:797–826. doi: 10.1677/erc.1.01143. [DOI] [PubMed] [Google Scholar]
- Kogai T, Sajid-Crockett S, Newmarch L, Liu YY, Brent G. Phosphoinositide-3-kinase (PI3K) inhibition induces sodium/iodide symporter expression (NIS) in rat thyroid cells and human papillary thyroid cancer Cells. J Endocrinol. 2008;199:243–252. doi: 10.1677/JOE-08-0333. [DOI] [PubMed] [Google Scholar]
- Lassmann M, Hanscheid H. Spatial dose mapping for individualizing radioiodine treatment. J Nucl Med. 2007;48:2–4. [PubMed] [Google Scholar]
- Lecat-Guillet N, Ambroise Y. Enhanced iodide sequestration by 3-biphenyl-5,6-dihydroimidazo[2,1-b]thiazole in sodium/iodide symporter (NIS)-expressing cells. ChemMedChem. 2008;3:1211–1216. doi: 10.1002/cmdc.200800052. [DOI] [PubMed] [Google Scholar]
- Lecat-Guillet N, Merer G, Lopez R, Pourcher T, Rousseau B, Ambroise Y. A 96-well automated radioiodide uptake assay for sodium/iodide symporter inhibitors. Assay Drug Dev Technol. 2007;5:535–5340. doi: 10.1089/adt.2007.068. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Choi KC, Han JP, Park YE, Choi MG. Relationship of sodium/iodide symporter expression with I131 whole body scan uptake between primary and metastatic lymph node papillary thyroid carcinomas. J Endocrinol Invest. 2007;30:28–34. doi: 10.1007/BF03347392. [DOI] [PubMed] [Google Scholar]
- Merron A, Peerlinck I, Martin-Duque P, Burnet J, Quintanilla M, Mather S, et al. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14:1731–1738. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
- Mitrofanova E, Unfer R, Vahanian N, Link C. Rat sodium iodide symporter allows using lower dose of 131I for cancer therapy. Gene Ther. 2006;13:1052–1056. doi: 10.1038/sj.gt.3302758. [DOI] [PubMed] [Google Scholar]
- Montiel-Equihua CA, Martin-Duque P, de la Vieja A, Quintanilla M, Burnet J, Vassaux G, Lemoine NR. Targeting sodium/iodide symporter gene expression for estrogen-regulated imaging and therapy in breast cancer. Cancer Gene Ther. 2008;15:465–473. doi: 10.1038/cgt.2008.6. [DOI] [PubMed] [Google Scholar]
- Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- Pekary AE, Hershman JM. Tumor necrosis factor, ceramide, transforming growth factor-beta1, and aging reduce Na+/I- symporter messenger ribonucleic acid levels in FRTL-5 cells. Endocrinology. 1998;139:703–712. doi: 10.1210/endo.139.2.5760. [DOI] [PubMed] [Google Scholar]
- Penuelas I, Haberkorn U, Yaghoubi S, Gambhir SS. Gene therapy imaging in patients for oncological applications. Eur J Nucl Med Mol Imaging. 2005;32(Suppl. 2):S384–S403. doi: 10.1007/s00259-005-1928-3. [DOI] [PubMed] [Google Scholar]
- Pittet MJ, Grimm J, Berger CR, Tamura T, Wojtkiewicz G, Nahrendorf M, et al. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc Natl Acad Sci USA. 2007;104:12457–12461. doi: 10.1073/pnas.0704460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty JK, Liimatainen T, Unelma Kaikkonen M, Grohn O, Airenne KJ, Yla-Herttuala S. Non-invasive imaging in gene therapy. Mol Ther. 2007;15:1579–1586. doi: 10.1038/sj.mt.6300233. [DOI] [PubMed] [Google Scholar]
- Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- Rogers BE, Parry JJ, Andrews R, Cordopatis P, Nock BA, Maina T. MicroPET imaging of gene transfer with a somatostatin receptor-based reporter gene and (94m)Tc-Demotate 1. J Nucl Med. 2005;46:1889–1897. [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Endo T, Kawaguchi A, Ikeda M, Katoh R, Kawaoi A, et al. Increased expression of the sodium/iodide symporter in papillary thyroid carcinomas. J Clin Invest. 1998;101:1296–1300. doi: 10.1172/JCI1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganova I, Ponomarev V, Blasberg R. Human reporter genes: potential use in clinical studies. Nucl Med Biol. 2007;34:791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Sgouros G, Kolbert KS, Sheikh A, Pentlow KS, Mun EF, Barth A, et al. Patient-specific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med. 2004;45:1366–1372. [PubMed] [Google Scholar]
- Shen DH, Marsee DK, Schaap J, Yang W, Cho JY, Hinkle G, et al. Effects of dose, intervention time, and radionuclide on sodium iodide symporter (NIS)-targeted radionuclide therapy. Gene Ther. 2004;11:161–169. doi: 10.1038/sj.gt.3302147. [DOI] [PubMed] [Google Scholar]
- Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I- symporter gene. Endocrinology. 1997;138:4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- Siegel E. The beginnings of radioiodine therapy of metastatic thyroid carcinoma: a memoir of Samuel M. Seidlin, M.D. (1895–1955) and his celebrated patient. Cancer Biother Radiopharm. 1999;14:71–79. doi: 10.1089/cbr.1999.14.71. [DOI] [PubMed] [Google Scholar]
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, et al. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun. 1996;226:339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- Smit JW, Schroder-van der Elst JP, Karperien M, Que I, Stokkel M, van der Heide D, et al. Iodide kinetics and experimental (131)I therapy in a xenotransplanted human sodium-iodide symporter-transfected human follicular thyroid carcinoma cell line. J Clin Endocrinol Metab. 2002;87:1247–1253. doi: 10.1210/jcem.87.3.8307. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, O'Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- Suzuki K, Kohn LD. Differential regulation of apical and basal iodide transporters in the thyroid by thyroglobulin. J Endocrinol. 2006;189:247–255. doi: 10.1677/joe.1.06677. [DOI] [PubMed] [Google Scholar]
- Sweeney TJ, Mailander V, Tucker AA, Olomu AB, Zhang W, Cao Y, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki K, Kogai T, Kanamoto Y, Hershman JM, Brent GA. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3′,5′-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol Endocrinol. 2002;16:2266–2282. doi: 10.1210/me.2002-0109. [DOI] [PubMed] [Google Scholar]
- Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6:871–888. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- Tjuvajev JG, Finn R, Watanabe K, Joshi R, Oku T, Kennedy J, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56:4087–4095. [PubMed] [Google Scholar]
- Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Vassaux G, Groot-Wassink T. In vivo noninvasive imaging for gene therapy. J Biomed Biotechnol. 2003;2003:92–101. doi: 10.1155/S1110724303209050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, et al. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003;88:1880–1888. doi: 10.1210/jc.2002-021544. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Upadhyay G, Schmitt TL, Loos U. Iodination of proteins in TPO transfected thyroid cancer cells is independent of NIS. Mol Cell Endocrinol. 2003;213:99–108. doi: 10.1016/j.mce.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Wildner O, Morris JC. The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity. Cancer Res. 2000;60:4167–4174. [PubMed] [Google Scholar]
- Willhauck MJ, Samani BR, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, Meyer GJ, et al. The potential of (211)Astatine for NIS-mediated radionuclide therapy in prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:1272–1281. doi: 10.1007/s00259-008-0775-4. [DOI] [PubMed] [Google Scholar]
- Wiseman GA, Kornmehl E, Leigh B, Erwin WD, Podoloff DA, Spies S, et al. Radiation dosimetry results and safety correlations from 90Y-ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory non-Hodgkin's lymphoma: combined data from 4 clinical trials. J Nucl Med. 2003;44:465–474. [PubMed] [Google Scholar]
- Yeom CJ, Chung JK, Kang JH, Jeon YH, Kim KI, Jin YN, et al. Visualization of hypoxia-inducible factor-1 transcriptional activation in C6 glioma using luciferase and sodium iodide symporter genes. J Nucl Med. 2008;49:1489–1497. doi: 10.2967/jnumed.107.044461. [DOI] [PubMed] [Google Scholar]
- Zanzonico PB. Internal radionuclide radiation dosimetry: a review of basic concepts and recent developments. J Nucl Med. 2000;41:297–308. [PubMed] [Google Scholar]


