Abstract
Background and purpose:
Pharmacological analysis of synergism or functional antagonism between different receptors commonly assumes that interacting receptors are located in the same cells. We have now investigated the distribution of α-adrenoceptors, β-adrenoceptors and cannabinoid-like (GPR55) receptors in the mouse arteries.
Experimental approach:
Fluorescence intensity from vascular tissue incubated with fluorescent ligands (α1-adrenoceptor ligand, BODIPY-FL-prazosin, QAPB; β-adrenoceptor ligand, TMR-CGP12177; fluorescent angiotensin II; a novel diarylpyrazole cannabinoid ligand (Tocrifluor 1117, T1117) was measured with confocal microscopy. Small mesenteric and tail arteries of wild-type and α1B/D-adrenoceptor-KO mice were used.
Key results:
T1117, a fluorescent form of the cannabinoid CB1 receptor antagonist AM251, was a ligand for GPR55, with low affinity for CB1 receptors. In mesenteric arterial smooth muscle cells, α1A-adrenoceptors were predominantly located in different cells from those with β-adrenoceptors, angiotensin receptors or cannabinoid-like (GPR55) receptors. Cells with β-adrenoceptors predominated at arterial branches. Endothelial cells expressed β-adrenoceptors, α-adrenoceptors and cannabinoid-like receptors. Only endothelial α-adrenoceptors appeared in clusters. Adventitia was a rich source of G protein-coupled receptors (GPCRs), particularly fibroblasts and nerve tracts, where Schwann cells bound α-adrenoceptor, β-adrenoceptor and CB-receptor ligands, with a mix of separate receptor locations and co-localization.
Conclusions and implications:
Within each cell type, each GPCR had a distinctive heterogeneous distribution with limited co-localization, providing a guide to the possibilities for functional synergism, and suggesting a new paradigm for synergism in which interactions may be either between cells or involve converging intracellular signalling processes.
This article is part of a themed section on Imaging in Pharmacology. To view the editorial for this themed section visit http://dx.doi.org/10.1111/j.1476-5381.2010.00685.x
Keywords: vascular smooth muscle, adrenoceptor, α1B/D-adrenoceptor knockout, fluorescent ligand binding, confocal microscopy, angiotensin, cannabinoid, adventitia, endothelium
Introduction
The concentration–response relationships of tissues to activation of G protein-coupled receptors (GPCRs) depend upon the number of receptors in those cells in the tissue that are activated, and the efficiency of signalling in these cells. For the classical expectations of this relationship to be fulfilled, it is generally assumed that these properties will be homogeneous throughout a tissue. However, little is known of how homogeneous or otherwise are the distributions of receptors within tissues, mainly because of the lack of spatial precision of the methods for visualizing receptors. In turn, this is due to the relatively low numbers of receptor molecules relative to other proteins and the unreliability of immunocytochemistry (Jensen et al., 2009; Pradidarcheep et al., 2009). This is compounded in tissues where different signals may be produced via receptors located on different cell types. In a first demonstration of heterogeneity among vascular smooth muscle cells (SMCs), we showed that the major α1-adrenoceptor-mediating contraction of small arteries, the α1A-adrenoceptor (receptor nomenclature follows Alexander et al., 2008), was present in only a minority of cells (Methven et al., 2009a). This contrasts with the relatively homogeneous distribution of the other vasoconstrictor α1-adrenoceptor, the α1D-adrenoceptor (Methven et al., 2009b).
We have taken advantage of the availability of fluorescent ligands for several different types of GPCR to examine the degree of heterogeneity of receptors in small arteries, both within each cell type and between cell types. Within each cell type, the distribution of different receptors should inform the possibilities for interaction between receptor types within the cell. The possibility of examining this in different cell types presents the additional possibility of determining whether any variability or similarity in distribution is a general or cell type-specific phenomenon.
In recent years, there has been a wealth of literature describing the GPCR cycle in single cells. The molecular mechanics of agonist-induced GPCR internalization, down-regulation and sequestration have been described for a large number of receptor families. In addition, the importance of dimerization of GPCRs has also been shown (Milligan, 2009). However, the existence of these receptor translocation and dimerization mechanisms has yet to be established in native tissues, and whether this varies between tissues and between cell types or whether it is homogeneous for a given cell type, is not known. At a more fundamental level, the general distribution of different receptor types within the vascular wall has not been given much attention. The technical difficulties associated with imaging live tissue coupled with the relative lack of specificity of some antibodies, most notably those for adrenoceptors and muscarinic receptors (Jensen et al., 2009; Pradidarcheep et al., 2009), have slowed progress in this area.
We have previously described the advantages and disadvantages of using fluorescent ligands for examining receptor distribution in segments of small artery (McGrath et al., 1996; Daly and McGrath, 2003; Methven et al., 2009a,b;), and believe that, on balance, there is significant gain in using fluorescent drugs, imaged at a known concentration, in equilibrium with their binding sites. The pharmacology of two such fluorescent ligands for α1-adrenoceptors (BODIPY FL-Prazosin; aka QAPB) and β-adrenoceptors (TMR-CGP12177) has been fully characterized (Daly et al., 1998; Baker et al., 2003; Briones et al., 2005). A recently developed fluorescent form of the cannabinoid CB1-receptor antagonist AM251 has just become commercially available. Presently, there are no published data on either its affinity at CB receptor subtypes or its fluorescence characteristics. Therefore, we report an initial assessment of the pharmacology and cellular labelling characteristics of this novel fluorescent ligand.
In this paper, we also describe the binding of four dissimilar fluorescent ligands (for α1-, β-, angiotensin II and cannabinoid receptors), and report the distribution of binding sites in various vascular cell types of mouse mesenteric artery (MMA) and rat tail artery. This is discussed in relation to the implications for the mechanisms underlying interactions between receptor types and the physiological roles of the receptors.
Methods
Animals
All animal experiments were performed at Glasgow University, and conformed to the UK Animals Act 1986 (scientific procedures). Mice were bred in-house at the University of Glasgow. The α1B/D-knockout mice were created by cross-breeding single knockout strains (α1B-KO and α1D-KO). Control [wild type (WT)] mice and knockout strains were maintained on a C57Bl6/J background. Male mice (4 months old) were used throughout. Mouse weight varied between 25 and 36 g for all strains with no apparent difference between strains. The mice were killed by CO2 inhalation. In one set of experiments, tail and mesenteric arteries from male Wistar rats (250 g) were used for comparison.
Preparation of vascular tissue
Segments of tail artery and mesenteric artery were dissected free from connective tissue, and placed in physiological salt solution (see below for composition) previously gassed with 95% O2, 5% CO2, at room temperature, and divided into segments of 3–4 mm length. Artery segments were incubated in Eppendorf tubes containing 0.3–1 µM each of varying combinations of Syto 61 (nuclear stain), BODIPY FL-prazosin (QAPB, α1-adrenoceptor ligand), TMR-CGP12177 (β-adrenoceptor ligand), TMR-angiotensin II (AT1 and AT2 receptor ligand) or T1117 (cannabinoid ligand). Artery segments were incubated in dark conditions for 1 h before being mounted on glass slides, along with the incubation medium, including fluo-ligands, and sealed under a coverslip (no. 1.5).
Microscopic examination
Vessels were imaged using a Bio-Rad Radiance 2100 (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) (Nikon mounted, Nikon UK Ltd., Kingston Upon Thames, UK) confocal laser scanning microscope (CLSM) using either an oil immersion 20× (NA 0.75) or 40× objective (NA 1.0). The CLSM was fitted with a 50 mW argon ion, Green HeNe and red diode laser. Vessels were scanned using lambda strobing to reduce bleed-through on individual channels. Kalman frame averaging varied between 3 and 6. In any comparative tests, laser intensity and PMT settings were identical. Each fluorescent probe was imaged as close as possible to its excitation and emission maxima: QAPB (ex 488 nm, em 515 nm); TMR CGP12177, TMR angiotensin and T1117 (ex 543 nm, em 590 nm); Syto 61 (ex 637 nm, em 660 nm LP).
Single optical slices or z-series (1 µm in z) were collected for each fluorescent channel, taking care to avoid saturation and ensure that each channel contained data in the 8-bit (0–255) range. Multi-channel images are displayed as merged channels.
For comparison of fluorescent binding in image volumes, a histogram method was used as previously described (Miquel et al., 2005). Briefly, histograms describing fluorescence intensity within a collection of data volumes (image z-series) were collected and averaged. These data describe the number of voxels achieving a particular intensity value in the 8-bit range. The data are then normalized to 100% showing which intensity value has the maximum number of voxels. Thus, an image volume with mainly dark voxels (low intensity) will lie to the left and will move right on the graph as the intensity within the volume increases.
Equilibrium binding assays
Binding assays were performed with the CB1 receptor agonist, [3H]CP55940 (0.7 nM), 1 mg·mL−1 BSA and 50 mM Tris buffer containing 0.1 mM EDTA and 0.5 mM MgCl2 (pH 7.4), total assay volume 500 µL. Binding was initiated by the addition of WT mouse brain membranes (30 µg). Assays were carried out at 37°C for 60 min before termination by the addition of ice-cold wash buffer (50 mM Tris buffer, 1 mg·mL−1 BSA) and vacuum filtration using a 24-well sampling manifold (Brandel Cell Harvester, Biomedical Research & Development Laboratories Inc, Gaithersburg, MD, USA) and Whatman GF/B glass fibre filters that had been soaked in wash buffer at 4°C for 24 h. Each reaction tube was washed five times with a 4 mL aliquot of buffer. The filters were oven-dried for 60 min, and then placed in 5 mL of scintillation fluid (Ultima Gold XR, Packard, Perkin Elmer Life and Analytical Sciences, Seer Green, Beaconsfield Bucks, UK), and radioactivity quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 µM of the corresponding unlabelled ligand, and was 70–80% of the total binding.
GPR55 cell expression and Ca2+ imaging
HEK293 cells expressing GPR55 were generated and maintained by the methods outlined in Henstridge et al. (2009). GPR55-expressing cells were incubated in Fura-2-AM (Ca2+ indicator; 6 µM) for 40–60 min at 25°C in HEPES-buffered saline (see below). Fluorescence was measured from ratiometric images collected at 5 s intervals (28–30°C).
Materials
The physiological salt solution used for tissue incubation was of the following composition: 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4.H2O, 1.2 mM KH2PO4, 24.9 mM NaHCO3 and 11.1 mM glucose. The HEPES saline was of the following composition: 135 mM NaCl, 10 mM HEPES, 5 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2 and 25 mM glucose, pH 7.4.
Stock concentrations of fluorescent ligands were dissolved in dimethyl sulphoxide, and diluted in distilled water as required. Ligands were obtained from the following sources: BODIPY FL-Prazosin (QAPB), TMR-CGP12177 and Syto 61 from Invitrogen (Invitrogen Ltd., Paisley, UK) (previously Molecular Probes); TMR angiotensin II from Phoenix Pharmaceuticals (Karlsruhe, Germany); T1117, (N-(piperidin-1-yl)-5-(4-(4-(3-(5-carboxamidotetramethylrhodaminyl)propyl))phenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide); O1602 (5-methyl-4-[(1R,6R)-3-methyl-6-(1-cyclohexen-1-yl]-1,3-benzenediol) and CP55940 ((-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol) from Tocris (Bristol, UK); Fura-2 AM (Fura-2 pentakis(acetoxymethyl) ester) from Sigma (Sigma-Aldrich Co. Ltd., Gillingham, UK).
Results
α1-Adrenoceptors in mesenteric artery from WT and α1BD-KO (BDKO) mice
Figure 1 shows optical sections through the tunica media of MMA. The overall reduction in fluorescence after removal of α1B and α1D adrenoceptors (BDKO mice) is quantified in the histogram, where the graph for fluorescence from WT samples stretches beyond the BDKO to the right and into the high-intensity regions. In the BDKO tissue, several cells appear to express high concentrations of α1A-adrenoceptors, and these appear to be distributed evenly through the media. A segment of mesenteric artery taken from a triple (α1ABD) KO showed no QAPB binding (image not shown), and thus the histogram of the remaining autofluorescence lies furthest to the left.
Figure 1.
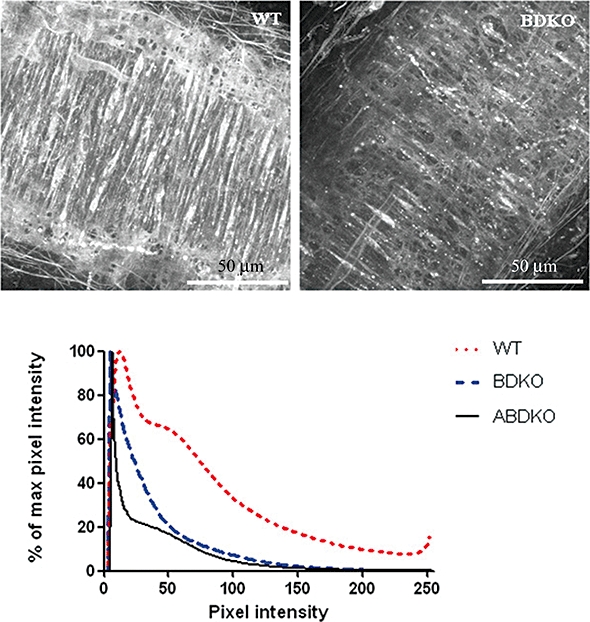
QAPB binding (1 µM) in MMA taken from WT, α1BD- and α1ABD-adrenoceptor knockout (KO) mice. Reduced binding was observed in arteries from both KO mice. Histogram analysis of image stacks from six samples from WT, five from BDKO and one from ABD KO shows an overall reduction in total fluorescence/binding in KO arteries throughout the vascular wall.
Assessment of the binding and fluorescence characteristics of the novel fluorescent cannabinoid ligand Tocrifluor 1117 (T1117)
T1117 was constructed by adding a tetramethylrhodamine group to the CB1-receptor antagonist AM251 (Figure 2A). In ligand binding studies, AM251 competed with CP55940-labelled membranes to give a Ki of 0.8 nM. The addition of the TMR group to AM251 significantly reduced the binding affinity, providing only 10% displacement of CP55940 at 1 µM T1117. However, at a lower concentration (0.3 µM), binding of T1117 to vascular smooth muscle of WT MMA was observed, using 543 nm excitation (590 nm emission). Such binding was inhibited (26%) in the presence of unlabelled AM251 (3 µM; Figure 2B–D).
Figure 2.
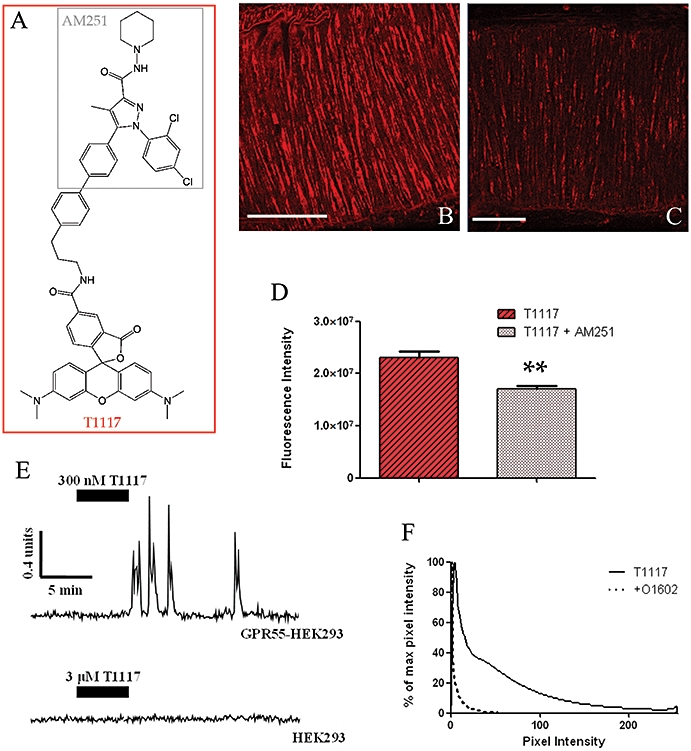
Characterization of the novel fluorescent cannabinoid ligand T1117. (A) The structure of T1117 is derived from the combination of tetramethylrhodamine and AM251. (B) Confocal scan of the media of a WT MMA segment after incubation with 0.3 µM T1117 (size bar 50 µm). (C) Following pre-incubation with non-fluorescent AM251 (3 µM), the T1117 binding-induced fluorescence is reduced (size bar 50 µm). (D) Quantitative fluorescence measurement of the T1117 binding in the presence and absence of AM251. Images shown in (B) and (C) are representative of those used for calculations in (D) (n= 3). (E) Representative tracing of the Ca2+ response induced by T1117 in GPR55-HEK293 and in control (HEK293) cells (tracing representative of n= 3). (F) Histogram analysis of z-series fluorescence (seven z-series from n= 3 each) shows a marked reduction in fluorescence induced by T1117 (0.3 µM), after exposure to the GPR55 agonist O1602 (10 µM).
At low concentrations (0.1–0.3 µM), AM251 activates GPR55 receptors (Henstridge et al., 2009) and, at these concentrations, T1117 evoked an oscillatory Ca2+ response in HEK293 cells, stably expressing GPR55 (Figure 2E). At a much higher concentration (3 µM), T1117 failed to activate control HEK293 cells, lacking GPR55 (Figure 2E). In WT MMA, the histogram of fluorescence induced by T1117 binding was shifted to the left following pre-incubation with the putative GPR55 agonist, O1602 (10 µM; Figure 2F), indicating a reduction in fluorescence (each data set measured from a group of seven z-series from n= 3 arteries).
Co-expression of different receptor types in vascular smooth muscle
Segments of MMA were incubated in a combination of QAPB (1 µM), and either TMR-CGP12177 (1 µM), TMR-angiotensin (0.3 µM) or T1117 (0.3 µM). Figure 3A,B shows examples of the binding pattern of QAPB and TMR-CGP12177 in the tunica media of MMA taken from a BDKO mouse. It is apparent that SMCs do not simply express α1- and β-adrenoceptors in equal proportions. A small proportion of SMCs (shown in yellow) have both α- and β-adrenoceptors in relatively equal numbers. However, the greater proportion of cells express mainly α1 (green) or β-adrenoceptors (red) (Figure 3A and also Supporting Information Video Clip S1). In particular, at the branch points, there appears to be a predominance of β-adrenoceptors (Figure 3B and Supporting Information Video Clip S2).
Figure 3.
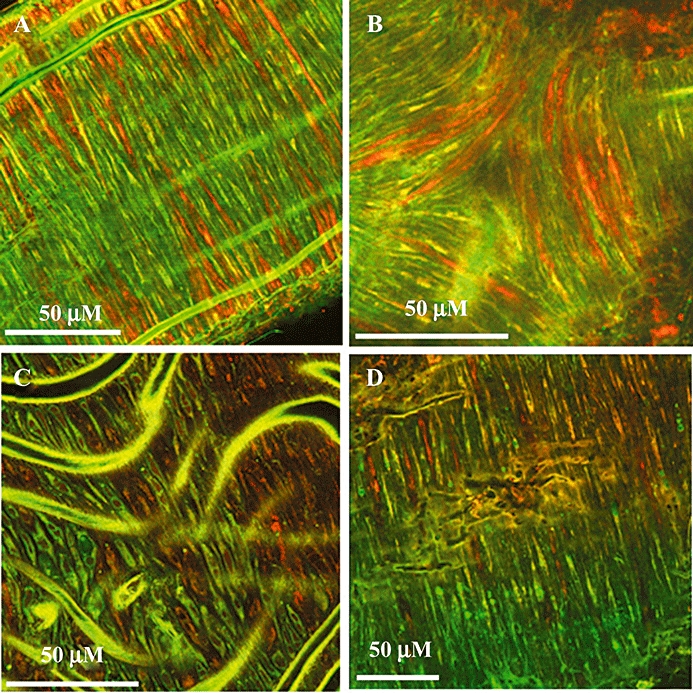
Fluorescent ligand co-localization in mouse vascular smooth muscle. (A and B) Binding of both QAPB and BODIPY-TMR-CGP12177 [both (1 µM) in MMA from BDKO mice] shows a combination of cells expressing mainly α1-adrenoceptors (green), mainly β-adrenoceptors (red) or both in relatively equal amounts (yellow). WT mesenteric arteries showed a similar pattern (see Supporting Information Video Clip S1) (B) A predominance of β-adrenoceptors was observed around the mesenteric branch points (see also Supporting Information Video Clip S2). (C) A similar heterogeneity was observed when combining QAPB (1 µM) with TMR-angiotensin II (0.3 µM) in WT carotid artery, or (D) combining QAPB (1 µM) with the fluorescent cannabinoid ligand T1117 (0.3 µM) in WT MMA. In all cases, images are representative of at least n= 3.
The apparently low expression of α1-adrenoceptors in certain SMCs is also reflected in the studies with both TMR-angiotensin II (Figure 3C) and the novel fluorescent cannabinoid ligand T1117 (Figure 3D). In both cases, SMCs expressing high levels of angiotensin or cannabinoid receptors were observed. Surprisingly, little co-localization was observed with α1-adrenoceptors and angiotensin AT receptors (Figure 3C). However, the yellow fluorescence in many SMCs shown in Figure 3D indicates a degree of co-localization of QAPB and T1117.
Co-expression of different receptor types in vascular adventitia and endothelium
Focusing on the outermost adventitial surface of the MMA permitted visualization of nerve tracts. No co-localization was observed between α1- and β-adrenoceptors in these tracts (Figure 4A and Supporting Information Video Clip S3) with both ligands producing distinct binding patterns. β-Adrenoceptors appear to be expressed strongly around the nerves. Co-localization of QAPB and T1117 was more apparent with the binding sites for both being more evenly distributed (Figure 4B). A similar binding pattern for nerves located at branch points was seen in rat mesenteric artery (Supporting Information Video Clip S4). Interestingly, examination of other adventitial cell types revealed the presence of cells rich in either QAPB binding sites or T1117 binding sites (Figure 4B inset).
Figure 4.
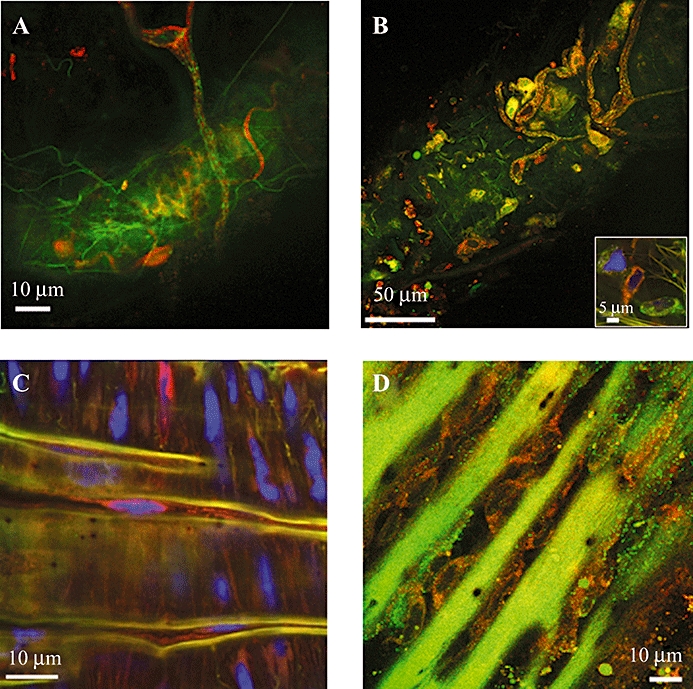
Fluorescent ligand binding in vascular adventitial and endothelial cells. In all images, QAPB (1 µM) is present and shown in green. (A) α1A- and β-adrenoceptors (TMR-CGP12177, 1 µM) are present, but not co-localized, on nerve cells of MMA from a BDKO mouse (see Supporting Information Video Clip S3). (B) Nerve cells also appear to express binding sites for T1117 (0.3 µM) which do co-localize, in certain areas, with α1-adrenoceptors. Insert image shows a group of adventitial cells mainly expressing either α1-adrenoceptors (green) or cannabinoid-like receptors (red). (C) β-Adrenoceptors are present on MMA (ABD-KO) endothelial cells (see Supporting Information Video Clip S5). (D) α1-Adrenoceptors (QAPB, green) are expressed on endothelial cells of rat tail artery which also express binding sites for T1117. In all cases, images are representative of at least n= 3.
Endothelial cells were examined in MMA and rat tail artery. In a segment of MMA taken from an α1ABD-KO mouse (i.e. devoid of all α1-adrenoceptors) and incubated in both QAPB and TMR-CGP12177, only β-adrenoceptors were visible in both SMCs and endothelial cells (Figure 4C & Supporting Information Video Clip S5). In a segment of rat tail artery (cut open to facilitate endothelial cell imaging), both QAPB and T1117 bound to endothelial cells aligned within the folds (grooves) of the unpressurized internal elastic lamina (Figure 4D).
Discussion
The receptor cycle involving agonist activation, sequestration and internalization has been well characterized (Moore et al., 2007), in some cases using fluorescent ligands (Pediani et al., 2005). Homodimerization of the three α1-adrenoceptor subtypes has been reported, while heterodimerization appears to be more selective (Milligan et al., 2004). Heterodimerization studies between individual α1-adrenoceptor subtypes have suggested that in isolated transfected cells, one particular subtype (the α1B-adrenoceptor) facilitates cell surface expression of another (the α1D-adrenoceptor) (Hague et al., 2004). Further studies revealed that the β2-adrenoceptors can also, via dimerization, facilitate surface expression of the α1D-adrenoceptors (Uberti et al., 2005). Functional interactions between angiotensin and noradrenaline have long been recognized (Dunn et al., 1991), and more recently we have shown a functional interaction between anandamide (endogenous cannabinoid) and noradrenaline (Daly et al., 2008). Thus, the range of possible adrenoceptor heterodimers may extend beyond αα and αβ interactions. However, the occurrence of dimerization or the physiological importance of this in vascular cells in situ has not been widely studied. This is in part due to the technical difficulties of maintaining live tissue on a microscope stage coupled with the limits of resolution, depth of penetration, physical properties of fluorophores and the availability of suitable probes. Antibodies generally require fixed tissue and have been criticized for their lack of specificity (Jensen et al., 2009; Pradidarcheep et al., 2009). We have therefore chosen to exploit the advantages of using fluorescent drugs (McGrath et al., 1996), and now report the binding pattern of several ligands, in all three vascular layers (i.e. tunica adventitia, tunica media and tunica intima). While our study does not offer sufficient resolution to confirm the existence of receptor dimers, it does provide an indication of where co-localization, and thus dimerization, may or may not occur.
Validation of fluorescent ligands
We have previously shown that the fluorescent form of prazosin (QAPB) is a highly selective α1-adrenoceptor antagonist (McGrath and Daly, 1995; 2005; McGrath et al., 1996; Daly et al., 1998).
BODIPY-TMR-CGP12177 is a long acting partial agonist at β2-adrenoceptors which binds to cell surface receptors, of HEK293 cells, at concentrations of 30 nM and above (Baker et al., 2003). Quantitative analysis (from the same study) estimated an apparent KD for BODIPY-TMR-CGP12177 of 27 nM at recombinant β2-adrenoceptors, and also demonstrated cAMP activation with an EC50 of 28 nM. The same fluorescent ligand was also characterized functionally and in fluorescence studies in rat mesenteric artery by Briones et al. (2005) who showed that it acted as a β1-adrenoceptor antagonist. In their functional assay, the isoprenaline pEC50 (vs. phenylephrine pre-constriction in rat mesenteric artery; a preparation expressing mainly β1-adrenoceptors) was shifted from 7.75 to 6.9 in the presence of 10 nM BODIPY-TMR-CGP12177. Therefore, BODIPY-TMR-CGP12177 (like the parent compound) appears to have affinity for both β1- and β2-adrenoceptors. In support of this conclusion, the irreversible β-adrenoceptor antagonist BAAM (10 µM) significantly inhibited the binding of TMR-CGP 12177 (1 µM) in all three vascular layers of rat mesenteric artery (Briones et al., 2005).
Presently, there is only one commercially available fluorescent ligand for CB receptors (T1117), and it had not been tested prior to this study. Given the importance of the endocannabinoids in the cardiovascular system, and the possibility of an interaction with α1-adrenoceptors (Daly et al., 2008), we examined the binding and fluorescence characteristics of T1117. AM251 is a potent CB1 receptor antagonist; however, addition of the fluorescent tetramethylrhodamine (to produce T1117) appears to have removed virtually all of its activity at CB1 receptors. Interestingly, T1117 still bound to all three vascular layers, and binding was inhibited in the presence of an excess of unlabelled AM251 (Figure 2B–D). Recent studies suggest that AM251 can activate the cannabinoid-like receptor, GPR55 (Henstridge et al., 2009; Kapur et al., 2009). We now report that its fluorescent derivative T1117 can also activate GPR55, promoting a characteristic, oscillatory Ca2+ response in HEK293 cells, stably expressing recombinant GPR55. Because T1117 could bind to GPR55, and fluorescence was observed in the MMA, we decided to look at T1117 binding following maximal agonist binding, using the putative GPR55 ligand O1602. Our finding that pretreatment with O1602 reduced the fluorescence induced by T1117 binding (Figure 2F) suggests that, following agonist (O1602) stimulation, GPR55 receptors are internalized/down-regulated and are unavailable for binding. Overall, the data point to the likelihood that T1117 is a novel fluorescent GPR55 agonist. Once confirmed by other groups or in different tissues, this could prove to be an extremely useful tool for investigating non-CB1/CB2 receptor-mediated responses.
The precise role of the cannabinoid system in the control of blood pressure is still not entirely clear. What has become apparent in recent years is that the endocannabinoid system is involved in a range of cardiovascular functions and pathologies (see Pacher and Steffens, 2009). The blood pressure response to administration of anandamide is triphasic with both pressor and depressor components (Varga et al., 1996). The response of isolated blood vessels to cannabinoids is varied and seems to depend largely on experimental conditions and the particular vascular bed being studied (see Mendizábal and Adler-Graschinsky, 2007). In addition to the reported location of CB1 receptors on vascular smooth muscle and endothelium (Högestätt and Zygmunt, 2002), a non-CB1/CB2 receptor on endothelium has been reported (Waldeck-Weiermair et al., 2008). Therefore, it is of interest to investigate the distribution of cannabinoid receptors on all three vascular layers in a variety of blood vessels. We now present evidence that T1117 is a fluorescent ligand for the GPR55 receptor with little or no affinity at the CB1 receptor. Although not tested, we have no reason to suspect that this fluorescent form of AM251 (a selective CB1 receptor antagonist) should have any affinity for CB2 receptors. For the purposes of this discussion (and in line with Alexander et al., 2008), we regard GPR55 as ‘cannabinoid-like’ and therefore a member of the CB receptor family.
Tunica media (smooth muscle)
In their study of antibody specificity, Pradidarcheep et al. (2009) suggested that knockout animal models are the most reliable method for testing specificity. In support of this, our experiments (Figure 1, using KO animals) demonstrate the ability of a fluorescent ligand to report (in a semi-quantitative fashion) receptor number as a function of fluorescence.
Previous work has shown that removal of two α1-adrenoceptor subtypes (B and D) leaves a subpopulation of cells that strongly express the remaining α1A-adrenoceptors (Methven et al., 2009a; also figure 1 of this paper). This suggests that individual cells may have a preferred subtype expression profile, and that vascular SMCs do not express receptors in equal quantities. Histogram analysis of full image volumes (z-series) shows that a full range of fluorescence intensity exists within the QAPB-labelled WT tissues. When both α1B- and α1D-adrenoceptors were removed from the arteries, the histogram shifted to the left, demonstrating that the fluorescence (and thus binding) within the artery was reduced compared with WT arteries. When all three α1-ARs are removed, all that remains is the autofluorescence. Limited availability of the triple α1-AR KO mice provided only one artery segment for analysis. This demonstrates a way in which large amounts of image data, describing fluorescent ligand binding, can be reduced to a more quantitative form. This technique has previously been used to examine α1-adrenoceptors in the tunica media of mouse aorta (Miquel et al., 2005).
In the present study, TMR-CGP12177 bound to specific SMCs and only co-localized with QAPB in a subpopulation of cells (those shown in yellow). The pattern of binding of both ligands suggests that cells could be classed as expressing an abundance of either α-adrenoceptors (α-rich) or of β-adrenoceptors (β-rich). The significance of this is unknown at present, but is of particular interest when the mesenteric branch points are examined (Figure 3B and Supporting Information Video Clip S2). Here, we see a predominance of β-rich cells along with a degree of co-localization with the remaining α1A-adrenoceptors. The mesenteric branch points have a rich innervation (see Supporting Information Video Clip S4), which could be expected to liberate concentrations of noradrenaline high enough to activate β-adrenoceptors. As the images in Figure 3A,B are from BDKO mice, the suggestion is that β-adrenoceptors are co-localizing (in some but not all cells) with α1A-adrenoceptors. Heterodimerization between β2- and α1D-adrenoceptors has been reported (Uberti et al., 2005). The present light microscopy-based study does not offer sufficient resolution to identify dimerization between α1A- and β-adrenoceptors. However, co-localization is a pre-requisite for dimerization, and the data predict that mesenteric branch points would be an interesting place to look for functional heterodimers.
The presence of ‘subtype-rich’ cells was also observed following co-incubation with either TMR-angiotensin II or T1117 (Figure 3C,D). In both cases, cells could be classified as either α-rich, AT-rich or CB-rich. This result helps to explain why, in WT arteries using a fluorescent non-selective α1-receptor antagonist, we have never observed uniform fluorescence in all SMCs (McGrath and Daly, 1995; 2005; McGrath et al., 1996; Figure 1A). The notion of subdividing vascular SMCs is not new. It is generally accepted that there are at least two phenotypes of smooth muscle in normal healthy arteries: contractile and non-contractile (proliferative) (see Hao et al., 2003). Furthermore, one of the vessels we have examined in this study, the MMA is subject to α1B-adrenoceptor-driven remodelling under hypertensive conditions (Vecchione et al., 2002), and so could be expected to harbour a population of, normally quiescent, proliferative cells. However, with respect to the previous discussion of receptor dimers, the presence of receptor subtype ‘rich’ cells may contradict the importance of heterodimers, and highlights the need for further study in native tissue.
Adventitia
The observation that removal of the tunica adventitia can modulate agonist responses (Somoza et al., 2005) has demonstrated the importance of this vessel layer. Structurally, the adventitia is known to comprise nerves, fibroblasts, macrophages, mast cells and adipocytes. Removal of the fat around the adventitia of rat mesenteric arteries has been shown to enhance contractile responses (Verlohren et al., 2004). The presence of these cell types coupled with the nerves and structural elements of elastin makes the adventitia a highly complex, dynamic and yet poorly understood component of normal vascular function. There is, however, no doubt it plays an essential modulatory role.
In the present study, we have observed, on the outermost vascular surface, nerve tracts most likely to be Schwann cells. These are easily distinguished by their peripheral location, nerve-like appearance, the presence of a nucleus and general size and shape (Jessen and Mirsky, 1984). The presence of binding sites for TMR-CGP12177 and T1117 around the nucleus of a Schwann cell (Figure 3A,B; Supporting Information Video Clip S3) indicates a possible role for β-adrenoceptors and cannabinoids at these sites. It is interesting that QAPB does not appear to co-localize with TMR-CGP 12177, indicating different locations for the α1- and β-adrenoceptors on Schwann cells. However, co-localization of QAPB and T1117 indicates a similar location for α1-adrenoceptors and GPR55 on these cells. In contrast, other adventitial cell types appear to show a subtype preference. Figure 3B (inset) shows three adventitial cells where one expresses mainly GPR55 (red), and one expresses mainly α1-adrenoceptors (green).
The vascular adventitia is an ideal tissue type for confocal microscopy studies due to its peripheral location, relatively low cell density and heterogeneity of cell type. Studies with fluorescent ligands enjoy the benefit of reduced diffusion distances (if any) and the absence of local concentrations or pooling of ligand. Thus, experiments can be performed at true equilibrium. The peripheral location of the autonomic nerves within the adventitia enables easy visualization. Supporting Information Video Clip S4 shows binding of TMR-CGP12177 and QAPB to nerves and smooth muscle throughout a branched segment of rat mesenteric artery.
Endothelium
As above, fluorescent ligand binding on vascular endothelium could be studied at true equilibrium if a perfusion system was used. However, depending on the thickness of the vessel wall, it may not be possible to clearly resolve the endothelial cells, when viewed from the adventitial side. Fortunately, the MMA has a thin-enough wall to permit penetration of the confocal beam to enable imaging of single cells (Figure 4C; Supporting Information Video Clip S5). In a segment of MMA devoid of any α1-adrenoceptors, there was an absence of QAPB binding, compared with a significant amount of CGP 12177 binding, to smooth muscle and endothelial cells. The presence of endothelial β-adrenoceptors has been assumed from various functional studies. In the present study, we showed the expression of β-adrenoceptors to be almost equal in endothelium and smooth muscle. It is possible that β-adrenoceptors had been up-regulated in response to the absence of any α1-adrenoceptors.
In a segment of rat tail artery, where the vessel had been cut open to facilitate visualization, studies of endothelium showed binding sites for both T1117 and QAPB in different cellular locations (Figure 4D). QAPB binding was punctuate, whereas binding sites for T1117 appeared to be more diffuse and could represent the presence of an endothelial ‘cannabinoid-like’ receptor as described by Waldeck-Weiermair et al. (2008). Endothelial cannabinoid receptors have been identified. However, these appear to be distinct from either CB1 or CB2 receptors in that they are activated by abnormal cannabidiol (even in CB receptor KO mice) and are blocked by O-1918 (Offertáler et al., 2003).
The presence of QAPB and T1117 co-localization in adventitia and smooth muscle, but not in endothelial cells, could be due to the different distribution of α1-adrenoceptor subtypes throughout the vascular wall. All three α1-subtypes would be expected in the adventitia (Faber et al., 2001) with α1A- or α1D-adrenoceptors predominating in the media of small and large vessels, respectively (Daly et al., 2002), and α1D-adrenoceptors predominating in the endothelium (de Andrade et al., 2006). A possible role for GPR55 in modulating blood pressure (and influencing the adrenergic system) is supported by the observation that mRNA expression of GPR55 is higher in mouse adrenals than in any other tissue (Ryberg et al., 2007).
Conclusions
Fluorescent ligands offer significant advantages for the study of receptors in living cells and tissues (Daly and McGrath, 2003). Carefully constructed fluorescent ligands can be stable, non-toxic selective probes for receptors and ion channels of interest. In this report, we have used four fluorescent ligands to demonstrate the heterogeneity of receptor distribution in all three vascular layers. Our initial assumption was that vascular cells would express all receptor types and that the relative levels of each would vary only slightly. We did not expect to find cells that were particularly rich in one receptor type or another. However, the observed co-localization between different combinations of ligands suggests that in such cells, the ability of receptors to dimerize is possible, whereas in cells where they are not present together it is not possible. Future higher resolution studies with fluorescent ligands, in combination with techniques, such as fluorescence resonance transfer, fluorescence recovery after photobleaching, fluorescence correlation spectroscopy and bimolecular fluorescence complementation on multi-layered tissues, hold great promise.
Acknowledgments
We are grateful to Mrs Joyce Macmillan for genotyping and co-ordinating the breeding of the mice. This work was supported by the British Heart Foundation (PG/05/140/20094 and FS/04/034). We are also grateful to Karl Swift and Duncan Crawford (Tocris Bioscience) for the supply of T1117.
Glossary
Abbreviations:
- α1BD-KO
α1B/D-adrenoceptor knockout mouse
- BiFC
bimolecular fluorescence complementation
- CLSM
confocal laser scanning microscopy
- FCS
fluorescence correlation spectroscopy
- FRAP
fluorescence recovery after photobleaching
- FRET
fluorescence resonance transfer
- GPCR
G protein-coupled receptor
- QAPB
BODIPY FL-Prazosin
- SMC
smooth muscle cell
- T1117
Tocrifluor fluo-AM251
- TMR CGP12177
tertramethylthodamine CGP12177
- WT
wild type
Conflict of interest
The authors have no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Video Clip S1 WT MMA QAPB CGP full scan: The file shows a scan through a full segment of MMA (α1BD-knockout). Starting on the surface, the movie scans through nerves on the outer adventitial surface and then into the vascular media where smooth muscle cells show either green (α1-adrenoceptor rich) or red (β-adrenoceptor rich). Within the folds of the internal elastic lamina (at the lowest part of the scan), endothelial cells can be seen.
Video Clip S2 BDKO QAPB CGP branch: The file shows a scan through a full segment of MMA (α1BD-knockout) branch point. The movie scans through nerves on the outer adventitial surface and then into the vascular media where smooth muscle cells show either green (α1-adrenoceptor rich) or red (β-adrenoceptor rich). β-Adrenoceptor rich cells are apparent at the branch point.
Video Clip S3 QAPB CGP nerve scan: A nerve tract (presumably a non-mylenating Schwann cell) is shown to wrap around the vessel. α1-Adrenoceptors (green) and β-adrenoceptors (red) are shown to have differing distributions with the β-adrenoceptors (red staining) prominent around the cell nucleus.
Video Clip S4 Rat MA QABP CGP nerve and branch: A rat mesenteric artery branch point is shown for comparison and to illustrate the complexity of innervation at the branch. In this scan, β-adrenoceptors (red) appear to be in abundance on the nerves. α-Adrenoceptors (green) are present mainly (but not exclusively) in the smooth muscle.
Video Clip S5 ABDKO QAPB CGP full scan. The file shows a scan through a full segment of MMA (α1ABD-knockout). This artery segment is devoid of any α1-adrenoceptor subtypes. Therefore, no α1-adrenoceptor ligand binding (QAPB, green fluorescence) is observed. In contrast, the fluorescent β-adrenoceptor ligand (TMR-CGP12177, red) shows strong binding in all vascular tunics.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade CR, Fukada SY, Olivon VC, de Godoy MA, Haddad R, Eberlin MN, et al. Alpha1D-adrenoceptor-induced relaxation on rat carotid artery is impaired during the endothelial dysfunction evoked in the early stages of hyperhomocysteinemia. Eur J Pharmacol. 2006;543:83–91. doi: 10.1016/j.ejphar.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Baker GJ, Hall IP, Hill SJ. Pharmacology and direct visualisation of BODIPY-TMR-CGP: a long-acting fluorescent β2-adrenoceptor agonist. Br J Pharmacol. 2003;139:232–242. doi: 10.1038/sj.bjp.0705287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Daly CJ, Jimenez-Altayo F, Martinez-Revelles S, Gonzalez JM, McGrath JC, et al. Direct demonstration of β1- and evidence against β2- and β3-adrenoceptors, in smooth muscle cells of rat small mesenteric arteries. Br J Pharmacol. 2005;146:679–691. doi: 10.1038/sj.bjp.0706369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC. Fluorescent ligands, antibodies & proteins for the study of receptors. Pharmacol Ther. 2003;100:101–118. doi: 10.1016/j.pharmthera.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Daly CJ, Milligan CM, Milligan G, Mackenzie JF, McGrath JC. Cellular localisation and pharmacological characterisation of functioning a1-adrenoceptors by fluorescent ligand binding and image analysis reveals identical binding properties of clustered and diffuse populations of receptors. J Pharmacol Exp Ther. 1998;286:984–990. [PubMed] [Google Scholar]
- Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- Daly CJ, Wallace G, White K, Henstridge C, Irving A, McGrath JC. Visualisation of vascular cannabinoid receptors and their potential interaction with α1-adrenergic receptors. Themed Meeting of The Physiological Society. Vascular & Smooth Muscle Physiology. 2008 Dec 2008, PC4, p. 55. [Google Scholar]
- Dunn WR, Daly CJ, McGrath JC, Wilson VG. A comparison of the effects of angiotensin II and Bay K 8644 on responses to noradrenaline mediated via postjunctional α1- and α2-adrenoceptors in rabbit isolated blood vessels. Br J Pharmacol. 1991;103:1475–1483. doi: 10.1111/j.1476-5381.1991.tb09814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JE, Yang N, Xin X. Expression of alpha-adrenoceptor subtypes by smooth muscle cells and adventitial fibroblasts in rat aorta and in cell culture. J Pharmacol Exp Ther. 2001;298:441–452. [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J Biol Chem. 2004;279:15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand l-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Högestätt ED, Zygmunt PM. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot Essent Fatty Acids. 2002;66:343–351. doi: 10.1054/plef.2001.0346. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Nonmyelin-forming Schwann cells coexpress surface proteins and intermediate filaments not found in myelin-forming cells: a study of Ran-2, A5E3 antigen and glial fibrillary acidic protein. J Neurocytol. 1984;13:923–934. doi: 10.1007/BF01148594. [DOI] [PubMed] [Google Scholar]
- Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, et al. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Daly CJ. Viewing adrenoceptors; past, present, and future; commentary and a new technique. Pharmacol Commun. 1995;6:269–279. [Google Scholar]
- McGrath JC, Daly CJ. The use of fluorescent ligands and proteins to visualise adrenergic receptors. In: Perez DM, Neve K, editors. The Adrenergic Receptors in the 21st Century: The Receptor Series. Totowa, NJ: Humana Press, Inc; 2005. pp. 151–172. [Google Scholar]
- McGrath JC, Arribas SM, Daly CJ. Fluorescent ligands for the study of receptors. TiPS. 1996;17:393–399. doi: 10.1016/s0165-6147(96)40004-9. [DOI] [PubMed] [Google Scholar]
- Mendizábal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methven L, McBride M, Wallace GA, McGrath JC. The alpha(1B/D)-adrenoceptor knockout mouse permits isolation of the vascular alpha(1A)-adrenoceptor and elucidates its relationship to the other subtypes. Br J Pharmacol. 2009a;158:209–224. doi: 10.1111/j.1476-5381.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methven L, Simpson PC, McGrath JC. 1A/B-Knockout mice explain the native α1D-adrenoceptor's role in vasoconstriction and show that its location is independent of the other α1-subtypes. Br J Pharmacol. 2009b;158:1663–1675. doi: 10.1111/j.1476-5381.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Pediani J, Fidock M, López-Giménez JF. Dimerization of alpha1-adrenoceptors. Biochem Soc Trans. 2004;32(5):847–850. doi: 10.1042/BST0320847. Pt. [DOI] [PubMed] [Google Scholar]
- Miquel MR, Ali Z, Segura V, D'Ocon MP, McGrath JC, Daly CJ. 3D image analysis of fluorescent drug binding. Mol Imaging. 2005;4:1–13. doi: 10.1162/15353500200504172. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:469–470. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009;31:63–77. doi: 10.1007/s00281-009-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediani JD, Colston JF, Caldwell D, Milligan G, Daly CJ, McGrath JC. Beta-arrestin dependent spontaneous alpha1a-adrenoceptor endocytosis causes intracellular transportation of alpha-blockers via recycling compartments. Molec Pharmacol. 2005;67:992–1004. doi: 10.1124/mol.104.008417. [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W, Stallen J, Labruyère WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoza B, González MC, González JM, Abderrahim F, Arribas SM, Fernández-Alfonso MS. Modulatory role of the adventitia on noradrenaline and angiotensin II responses role of endothelium and AT2 receptors. Cardiovasc Res. 2005;65:478–486. doi: 10.1016/j.cardiores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-adrenergic receptors. J Pharmacol Exp Ther. 2005;313:16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Fratta L, Rizzoni D, Notte A, Poulet R, Porteri E, et al. Cardiovascular influences of α1b-adrenergic receptor defect in mice. Circulation. 2002;105:1700–1707. doi: 10.1161/01.cir.0000012750.08480.55. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, et al. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121(10):1704–1717. doi: 10.1242/jcs.020958. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


