Abstract
Background and purpose:
T-cells may play a role in the evolution of ischaemic damage and repair, but the ability to image these cells in the living brain after a stroke has been limited. We aim to extend the technique of real-time in situ brain imaging of T-cells, previously shown in models of immunological diseases, to models of experimental stroke.
Experimental approach:
Male C57BL6 mice (6–8 weeks) (n= 3) received a total of 2–5 × 106 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled lymphocytes from donor C57BL6 mice via i.v. injection by adoptive transfer. Twenty-four hours later, recipient mice underwent permanent left distal middle cerebral artery occlusion (MCAO) by electrocoagulation or by sham surgery under isoflurane anaesthesia. Female hCD2-green fluorescent protein (GFP) transgenic mice that exhibit GFP-labelled T-cells underwent MCAO. At 24 or 48 h post-MCAO, a sagittal brain slice (1500 µm thick) containing cortical branches of the occluded middle cerebral artery (MCA) was dissected and used for multiphoton laser scanning microscopy (MPLSM).
Key results:
Our results provide direct observations for the first time of dynamic T-cell behaviour in living brain tissue in real time and herein proved the feasibility of MPLSM for ex vivo live imaging of immune response after experimental stroke.
Conclusions and Implications:
It is hoped that these advances in the imaging of immune cells will provide information that can be harnessed to a therapeutic advantage.
This article is part of a themed section on Imaging in Pharmacology. To view the editorial for this themed section visit http://dx.doi.org/10.1111/j.1476-5381.2010.00685.x
Keywords: inflammation, leukocytes, brain imaging, middle cerebral artery occlusion, stroke, multiphoton microscopy
Introduction
Leukocyte infiltration is a major mechanism contributing to the pathogenesis of cerebral ischaemic damage. However, the role of these immune cells in ischaemic damage still needs to be fully understood. Some intriguing proposals that lymphocytes may not be completely harmful for the brain or that manipulation of selected immune cells may result in a therapeutic advantage (Gee et al., 2007; Villoslada et al., 2008) need to be supported by a deep knowledge of the behaviour of these cells during the evolution of ischaemic damage and repair.
Direct observation of dynamic cellular behaviour in living tissues has been facilitated by recent advances in multiphoton laser scanning microscopy (MPLSM) (Zinselmeyer et al., 2005; Schneider et al., 2006; Maffia et al., 2007; Millington et al., 2007; Cahalan and Parker, 2008). MPLSM provides the ability to illuminate tissues at depth in the absence of significant phototoxicity, allowing analysis of physiological or pathological events over time, and has been exploited to study T-cells in the brain in experimental autoimmune encephalomyelitis (Flügel et al., 2007; Smorodchenko et al., 2007). We now report, for the first time, real-time in situ imaging of T-cell movement in brain tissue after a focal cerebral ischaemic insult.
Methods
All mice (6–8 weeks) were maintained on a 12/12 h light/dark cycle with free access to food and water at the Biological Procedures Unit, University of Strathclyde, in accordance with local ethical and UK Home Office regulations. Male C57BL6 mice (Harlan-Olac, Bicester, UK) were killed (n= 3) to obtain lymphocytes for adoptive transfer. A total of 2–5 × 106 CFSE (Invitrogen, Paisley, UK)-labelled lymphocytes from C57BL6 mice, prepared as previously described (Zinselmeyer et al., 2005; Schneider et al., 2006; Millington et al., 2007), were injected i.v. into age-matched C57BL6 recipients. Twenty-four hours later, the recipient animals were anaesthetized using 3% isoflurane anaesthesia in 100% O2 and were maintained using 1.5–2.0% isoflurane in 100% O2 as assessed by interdigital reflex. The mice were subjected to permanent left distal middle cerebral artery occlusion (MCAO) by electrocoagulation (Carswell et al., 2005) (males, n= 3) or by sham surgery (female, n= 1). Body temperature was maintained at 37°C throughout the procedure.
One female hCD2-GFP transgenic mouse that exhibited GFP-labelled T-cells (de Boer et al., 2003) with a very small proportion of GFP-labelled B-cells (kindly gifted by Dr. Dimitris Kioussis, NIMR, London, UK) also underwent MCAO.
At 24 or 48 h post-MCAO, a sagittal brain slice (1500 µm thick) containing cortical branches of the occluded MCA (Figure 1) was cut using a Vibratome (Intracel, Herts, UK), in oxygenated artificial cerebrospinal fluid (ACSF, containing the following in mM: NaCl, 124; KCl, 3; NaHCO3, 26; NaH2PO4, 2.5; MgSO4, 2; CaCl2, 2; and D-glucose, 10 with 95% O2/5% CO2, pH ∼ 7.3) (Bushell et al., 2002), glued (Vetbond, 3M, Loughborough, UK) onto a coverslip adhered to the bottom of the imaging chamber and continuously perfused with warmed (35–36°C), gassed (95% O2 and 5% CO2) ACSF before and throughout imaging. Imaging was performed using a Radiance 2000MP MPLSM (Bio-Rad Laboratories Ltd, Hemel Hempstead, UK) as described previously (Zinselmeyer et al., 2005; Schneider et al., 2006; Maffia et al., 2007; Millington et al., 2007). The scans were made with 500 lines per second (lps) and between 256 × 256 pixel boxes, and acquired at 20 frames per minute. Images were analysed using Volocity software (Improvision, Coventry, UK).
Figure 1.
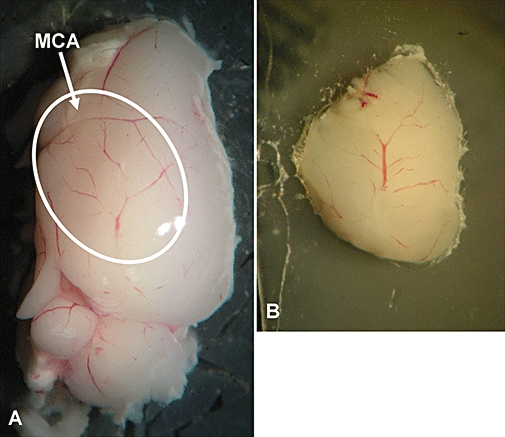
Representative images of the area of multiphoton laser scanning microscopy (MPLSM) imaging (white oval) containing the cortical branches of the occluded MCA (arrow) (A) and of a 1500 µm sagittal slice glued onto a coverslip for MPLSM imaging (B).
Results
Initially, brain slices were cut in ice-cold (<4°C) sucrose solution as described previously (Bushell et al., 2002) and were imaged at 35–36°C; however, lymphocytes observed were stationary. This concurs with our own unpublished observations in lymph nodes. Hence, all slices included in the present study were prepared in room-temperature ACSF.
Recruitment of labelled lymphocytes in the cerebrovasculature and in the cortex, downstream of occluded MCA in adoptive transfer mice, was observed at 24 and 48 h post-MCAO (Figure 2A,B respectively). No cells were detectable in the sham-operated mouse (data not shown). Furthermore, since CFSE will label all lymphocyte populations and not just T-cells, using an hCD2-GFP transgenic mouse, we imaged, selectively, endogenous T-cell movement in the brain at 48 h post-MCAO (Figure 2C and the accompanying online movie). The average velocity of T-cells was 9.4 µm min−1, although during the imaging period peak velocities approached 32 µm min−1 compared to average velocities in the region of 12–15 µm min−1 in lymph nodes previously reported (Miller et al., 2002; Zinselmeyer et al., 2005).
Figure 2.
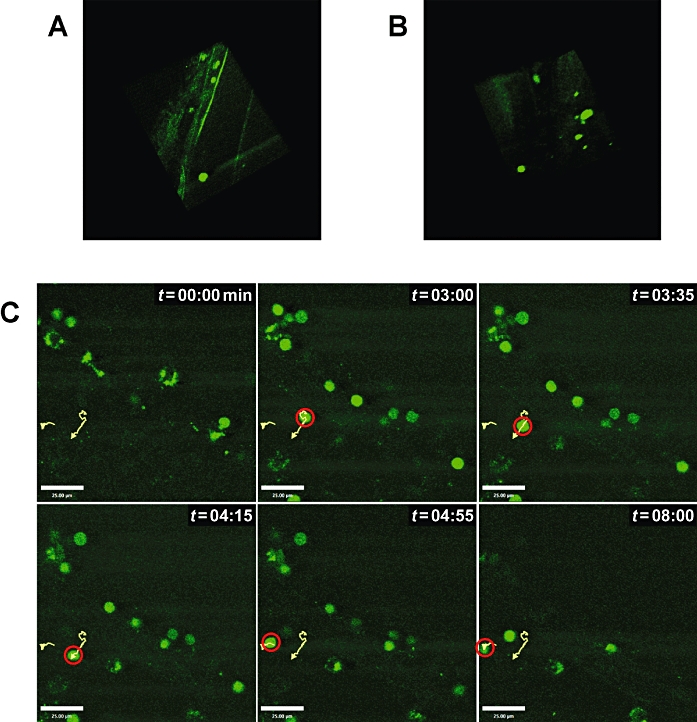
(A,B) Three-dimensional reconstruction of lymphocyte infiltration in the cortex post-middle cerebral artery occlusion (MCAO). At 24 h post-MCAO, fluorescently CFSE-labelled lymphocytes (green) were detectable in a cortical artery, and only few cells were infiltrating the parenchyma (A). On the contrary, lymphocyte infiltration in the parenchyma is clearly detectable at 48 h post-MCAO (B). Each imaged volume consisted of 13–21 planes, 2.5 µm apart. (C) Tracking of T-cells in the cortex post-MCAO. At 48 h post-MCAO, endogenous hCD2-GFP T-cells were clearly detectable in the brain with tracking (yellow lines, red circle) shown in six consecutive snapshots representative of 9 min of imaging of the same field of view. Bar = 25 µm. The scans were acquired with 500 lps and between 256 × 256 pixel boxes. Images were analysed using Volocity software (Improvision). The movie of T-cell movement in the ischaemic brain tissue is also available as online supplemental data (movie 1; view using Quick Time or Real Player).
Discussion and conclusion
The presence of T lymphocytes has been previously described by immunohistochemistry at 1–7 days post-MCAO (Li et al., 2005), but their role and behaviour in the parenchyma remains elusive. Our aim was not to quantify T-cell recruitment after injury, but rather to provide a feasible protocol for cell detection in the living inflamed brain for future pharmacological targeting. We have proved the feasibility of MPLSM for in situ real-time imaging of immune response after focal cerebral ischaemia. To the best of our knowledge this is the first demonstration of T-cell movement in the brain after cerebral ischaemia and the first application of hCD2-GFP transgenic mice in cerebral ischaemia. Our results have three important implications. Firstly, our visualization of T-cells in the brain implies a dynamic role of T-cells, possibly involving direct, physical contact with other cells. Secondly, the use of hCD2-GFP transgenic mice combined with MPLSM represents an important tool for investigating the temporal profile, role and behaviour of endogenous T-cells during the evolution of ischaemic damage and repair. Finally, given the growing availability of transgenic mice expressing fluorescently labelled cell types (e.g. microglia), our use of adoptive lymphocyte transfer permits future investigations into cellular interactions, thereby clarifying lymphocyte activity.
One limitation of the current study is that immune cell mobility and activation could be drastically altered by the slice preparation; therefore, the ultimate goal would be in vivo imaging. In vivo imaging of immune response in the cortex with multiphoton microscopy has been possible so far only in autoimmune-type disease models, while the dynamic analysis of immune cell behaviour in murine stroke models in vivo until now has not been possible due to technical limitations. A major obstacle is the paucity of immune cells infiltrating the ischaemic area compared to autoimmune disease, and another hurdle is the difficulty to implant a cranial window enabling the imaging of the compromised area of interest. On the other hand, ex vivo real-time imaging bypasses this issue, allowing the imaging of the whole cortex, and therefore an easy detection of immune infiltrate. Furthermore, real-time imaging of sagittal brain slices could easily allow the visualization of some other brain regions of interest (i.e. striatum, hippocampus) which could be affected by post-ischaemic inflammatory response (Wiart et al., 2007), and which cannot be visualized using conventional in vivo multiphoton imaging, due to depth limitation.
For these reasons, we strongly believe that our in situ real-time imaging could represent the first important step towards the in vivo dynamic imaging of immune response after experimental stroke. Our advances in imaging techniques will help define and analyse the cellular interactions, role and behaviour of these immune cells in situ after a stroke and will help us to understand how intervening in these cellular interactions will affect stroke pathology for future therapeutic exploitations.
Acknowledgments
GD was funded by a British Heart Foundation project grant (PG/06/083/21198) awarded to PG; PM was funded by a Capacity Building Award in Integrative Mammalian Biology funded by the Biotechnology and Biological Sciences Research Council (BBSRC), British Pharmacological Society (BPS), Medical Research Council (MRC), Knowledge Transfer Network (KTN) and Scottish Funding Council (SFC); OM was supported through a Research Council UK (RCUK) fellowship; GR was supported by an Italian Society of Pharmacology travel grant.
Glossary
Abbreviations:
- ACSF
artificial cerebrospinal fluid
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- GFP
green fluorescent protein
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- MPLSM
multiphoton laser scanning microscopy
References
- de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- Bushell T, Clarke C, Mathie A, Robertson B. Pharmacological characterization of a non-inactivating outward current observed in mouse cerebellar Purkinje neurones. Br J Pharmacol. 2002;135:705–712. doi: 10.1038/sj.bjp.0704518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Flügel A, Odoardi F, Nosov M, Kawakami N. Autoaggressive effector T cells in the course of experimental autoimmune encephalomyelitis visualized in the light of two-photon microscopy. J Neuroimmunol. 2007;191:86–97. doi: 10.1016/j.jneuroim.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- Li GZ, Zhong D, Yang LM, Sun B, Zhong ZH, Yin YH, et al. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol. 2005;62:481–486. doi: 10.1111/j.1365-3083.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, et al. Images in cardiovascular medicine. Multiphoton microscopy for 3-dimensional imaging of lymphocyte recruitment into apolipoprotein-E-deficient mouse carotid artery. Circulation. 2007;115:e326–e328. doi: 10.1161/CIRCULATIONAHA.106.658492. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Millington OR, Gibson VB, Rush CM, Zinselmeyer BH, Phillips RS, Garside P, et al. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog. 2007;3:1380–1387. doi: 10.1371/journal.ppat.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- Smorodchenko A, Wuerfel J, Pohl EE, Vogt J, Tysiak E, Glumm R, et al. CNS-irrelevant T-cells enter the brain, cause blood-brain barrier disruption but no glial pathology. Eur J Neurosci. 2007;26:1387–1398. doi: 10.1111/j.1460-9568.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Moreno B, Melero I, Pablos JL, Martino G, Uccelli A, et al. Immunotherapy for neurological diseases. Clin Immunol. 2008;128:294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Wiart M, Davoust N, Pialat JB, Desestret V, Moucharrafie S, Cho TH, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, et al. In situ characterization of CD4+ T cell behaviour in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–1823. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]


