Abstract
Background and purpose:
Big endothelin-1 (ET-1) circulates in plasma but does not bind to ET receptors until converted to ET-1 by smooth muscle converting enzymes. We hypothesized that tissue-specific conversion of [18F]-big ET-1 to [18F]-ET-1 could be imaged dynamically in vivo within target organs as binding to ET receptors.
Methods:
[18F]-big ET-1 conversion imaged in vivo following infusion into rats using positron emission tomography (PET).
Key results:
[18F]-big ET-1 was rapidly cleared from the circulation (t1/2= 2.9 ± 0.1 min). Whole body microPET images showed highest uptake of radioactivity in three major organs. In lungs and liver, time activity curves peaked within 2.5 min, then plateaued reaching equilibrium after 10 min, with no further decrease after 120 min. Phosphoramidon did not alter half life of [18F]-big ET-1 but uptake was reduced in lung (42%) and liver (45%) after 120 min, consistent with inhibition of enzyme conversion and reduction of ET-1 receptor binding. The ETA antagonist, FR139317 did not alter half-life of [18F]-big ET-1 (t1/2= 2.5 min) but radioactivity was reduced in all tissues except for kidney consistent with reduction in binding to ETA receptors. In kidney, however, the peak in radioactivity was higher but time to maximum accumulation was slower (∼30 min), which was increased by phosphoramidon, reflecting renal excretion with low conversion and binding to ET receptors.
Conclusions and implications:
A major site for conversion was within the vasculature of the lung and liver, whereas uptake in kidney was more complex, reflecting excretion of [18F]-big ET-1 without conversion to ET-1.
This article is part of a themed section on Imaging in Pharmacology. To view the editorial for this themed section visit http://dx.doi.org/10.1111/j.1476-5381.2010.00685.x
Keywords: ET-1, big ET-1, endothelin converting enzyme, positron emission tomography, microPET, [18F]-ET, in vivo imaging, phosphoramidon, FR139317
Introduction
The potent vasoactive peptide endothelin-1 (ET-1) plays an important role in the maintenance of normal vascular tone. It is synthesized in human endothelial cells from its precursor peptide, big ET-1 by a unique cleavage of the Trp21-Val22 bond catalysed by ET converting enzymes (ECE) (Davenport and Maguire, 2006). ET-1 is continuously released from the endothelium, causing long-lasting vasoconstriction by stimulation of predominantly ETA receptors present on the underlying smooth muscle (Russell and Davenport, 1999). In contrast, ET-1 acting on ETB receptors expressed by the endothelium causes vasodilatation through release of nitric oxide and prostacyclin (de Nucci et al., 1988) counterbalancing the vasoconstriction (Haynes and Webb, 1998). Some big ET-1 escapes conversion by either the intracellular or cell-surface enzymes of the endothelium. Both big ET-1 and the mature peptide, ET-1 are secreted into the medium from human umbilical vein endothelial cells in a ratio of about 1:4 (Plumpton et al., 1994), consistent with the detection of both peptides in human plasma (Suzuki et al., 1990; Matsumoto et al., 1994)
Although Big ET-1 is present in human plasma (Suzuki et al., 1990; Matsumoto et al., 1994) it does not bind to ET receptors at the concentrations circulating in blood and must be converted to ET-1 for receptor activity (Kimura et al., 1989). Infused big ET-1 produces pronounced forearm vasoconstriction with a corresponding increase in plasma ET-1 and the biologically inactive C-terminal fragment. Phosphoramidon, an inhibitor that does not normally cross the plasma membrane, blocks this vasoconstriction, implying local conversion by an ectoenzyme at the site of action (Plumpton et al., 1995). This is most probably vascular smooth muscle ECE, as little or no conversion of big ET-1 to ET-1 has been detected in human blood in vitro (Watanabe et al., 1991) and constriction of human isolated blood vessels by big ET-1 persists after endothelial denudation (Mombouli et al., 1993; Maguire et al., 1997). Infusion of big ET-1 into rats (Gardiner et al., 1991; 1993; 1997; Mcmahon et al., 1991; Pollock and Opgenorth, 1991a) causes vasoconstriction, which could be blocked by the neutral endopeptidase/ECE inhibitor, phosphoramidon but not thiorphan, distinguishing this ECE activity from neutral endopeptidase (Pollock and Opgenorth, 1991b). Big ET-1 and ECE are up-regulated in disease (Minamino et al., 1997; Grantham et al., 1998; Maguire and Davenport, 1998). For example, tissue levels of ET-1 and big ET-1 are significantly increased in human vessels with atherosclerotic plaques compared with normal tissue (Bacon et al., 1996). ECE activity is up-regulated in atherosclerotic human coronary arteries resulting in an increased response to big ET-1 (Maguire and Davenport, 1998). These results suggest that significant tissue-specific conversion of big ET-1 may occur in the vasculature and hence add to the detrimental effects caused by increased levels of ET-1 in disease.
Positron emission tomography (PET) is used to image classical transmitter systems in vivo although peptides have been less widely studied owing to a lack of suitable ligands. Dedicated tomographs such as microPET have recently been introduced for laboratory animals with spatial resolution to allow the delineation of discrete organs and their larger substructures in rodents (Chatziioannou, 2002; Lewis et al., 2002). We have previously demonstrated using the microPET that binding of [18F]-ET-1 to ET receptors in the rat can be imaged dynamically and that this binding could be blocked when the rats were pretreated with an ETB selective antagonist (Johnström et al., 2005a). Importantly, binding of [18F]-ET-1 can be imaged using ‘tracer’ amounts of the peptide, which allows visualization of the receptors without causing vasoconstriction and altering haemodynamics.
We hypothesized that tissue-specific conversion of [18F]-big ET-1 to [18F]-ET-1 could be imaged dynamically in vivo within target organs as binding to ET receptors, to provide evidence that big ET-1 could act as a long range signalling hormone. To test our hypothesis, big ET-1 was labelled for the first time with 18F and imaged in vivo following infusion into rats. Our aim was to identify the major organs mediating enzymatic conversion of [18F]-big ET-1 to [18F]-ET-1 and whether this could be inhibited by phosphoramidon.
Methods
Animals
All experiments were conducted in accordance with the United Kingdom Animal Scientific Procedures Act, 1986 and complied with guidelines of the local animal ethics committee. Rats were housed with free access to standard rat food and water prior to the experimental procedure. PET experiments were performed in male Sprague-Dawley rats (392 ± 19 g).
Animal preparation
Rats were anaesthetized with 3% isofluorane (Baker Norton, Bristol, UK) vaporized in N2O/O2 (0.8/0.4 L per min) and maintained with 2% isofluorane. Body temperature was monitored and maintained in the normal range. A femoral vein was cannulated for administration of [18F]-big ET-1 and preinfusion of phosphoramidon, at a concentration chosen to inhibit the in vivo conversion of big ET-1 to ET-1 (Mcmahon et al., 1991; Plumpton et al., 1995) and FR139317 the selective ETA receptor antagonist (Davenport and Maguire, 2006). The contralateral femoral artery was cannulated for blood sampling and for monitoring blood pressure. As expected injection of [18F]-big ET-1 at these tracer levels did not alter blood pressure compared with baseline. During PET scanning, anaesthesia was reduced to 1.5–2% isofluorane in N2O/O2 (0.8/0.4 L per min).
MicroPET imaging
Study design
Dynamic in vivo imaging of ECE conversion of [18F]-big ET-1 to [18F]-ET-1 and subsequent binding to ET receptors was studied using microPET. For control experiments using [18F]-big ET-1 alone (n= 3 animals per group according to license PPL80/1439), dynamic scans were performed for up to 2 h. To test the effect of enzyme inhibition, three rats were pretreated with phosphoramidon (10 mg·kg−1) immediately prior to injection of [18F]-big ET-1. In one experiment, to confirm that uptake of [18F]-big ET-1 could be blocked by an ETA selective antagonist, FR139317 (10 mg·kg−1) was infused immediately prior to injection of [18F]-big ET-1. Blood samples were collected in Eppendorf tubes at timed intervals.
MicroPET system
Animals were imaged using a microPET P4 scanner (Tai et al., 2001) (Concorde Microsystems, Knoxville, TN, USA). Rats were placed prone on the scanning bed and located in a purpose-built plastic stereotaxic frame. The computer controlled scanning bed was positioned so that the axial field of view (7.8 cm) encompassed the organs of interest.
Acquisition protocol
[18F]-big ET-1 (10.1 ± 0.8 MBq) was administered to the rats as a bolus intravenous injection. A timing window of 10 ns was used in conjunction with an energy window of 250–750 keV to increase sensitivity. The data were acquired in list mode and were binned into the following time frames starting at the time of tracer administration: 6 × 5 s, 9 × 0.5 min, 5 × 1 min, 15 × 2 min and then in 5 min frames to the end of the experiment.
Image reconstruction and analysis
All images were reconstructed using the 3D filtered backprojection algorithm (Kinahan and Rogers, 1989) adapted in-house to work with data from the microPET P4 scanner. Corrections were applied to the data during reconstruction as outlined in Johnström et al. (2005b). Images were reconstructed into 0.5 × 0.5 × 0.5 mm voxels in an array of 200 × 200 × 151 and a Hanning window cut-off at 0.8 × Nyquist frequency was incorporated into the reconstruction filters.
Regions of interest were delineated for the organs of interest using Analyze (AnalyzeDirect Inc, Lenexa, KS, USA) to construct time-activity curves (Robb et al., 1989). Consistently sized regions were used for all studies and were of sufficient size (≥1.3 mL) that quantification error due to the partial volume effect should not be significant for the resolution of the microPET. Data were corrected for radioactive decay measured in MBq·mL−1 and normalized for the dose of injected activity (%ID·g−1 tissue or %ID·mL−1 blood).
Ex vivo tissue analysis
At the end of scanning, animals were killed by intravenous injection of pentobarbitone and organs dissected, weighed and analysed for amount of radioactivity using a gamma counter. These data were quantified by counting a set of 18F standards prepared from the radioligand stock solution. Additionally, cryostat cut sections (30 µm) of tissues were apposed, together with 18F standards prepared from the radioligand stock solution, to a storage phosphor imaging screen (Cyclone, PerkinElmer Life Sciences Ltd, Cambridge, UK). Tissue sections were subsequently stored at −70°C to allow for the decay of 18F and then stained with haematoxylin and eosin or antisera to α-actin as a marker of smooth muscle cells to facilitate histological identification using methods described previously (Davenport and Kuc, 2005). The concentration of radioactivity in weighed blood samples was determined using a well counter.
Statistical analysis
Data are expressed as mean ± SEM. There was no evidence of non-normality and data were analysed by analysis of variance and differences were considered significant at P < 0.05.
Peptides and radiolabelling of big ET-1
Big ET-1 and phosphoramidon were obtained from Peptide Institute Inc. (Osaka, Japan). FR139317 was synthesized by Dr A. M. Doherty, Parke-Davis Pharmaceutical Research Division, Ann Arbor, Michigan USA. Phosphoramidon (10 mg·mL−1) and FR139317 (10 mg·mL−1) for injection were dissolved in saline.
Big ET-1 was labelled with 18F in the ε-amino group of Lys9 by conjugation with the Bolton-Hunter-type reagent N-succinimidyl 4-[18F]-fluorobenzoate using the method previously reported for ET-1 (Johnström et al., 2002). Briefly, N-succinimidyl 4-[18F]-fluorobenzoate (synthesized from [18F]-fluoride) in anhydrous acetonitrile (20 µL) was added to solution of big ET-1 (100 µL, 1.0 mg·mL−1 in sodium bicarbonate (0.04 M)) and left for 30 min at room temperature. The reaction was quenched with HCl (70 µL, 0.3 M) and the mixture was purified using reverse-phase HPLC (Spherisorb ODS2, 5 µ, 4.6 × 250 mm). [18F]-big ET-1 was eluted using a five-step gradient [50 mM NaH2PO4 with 0.1% trifluoroacetic acid (pH 4.0)] : [acetonitrile] 66:34 (v/v) for 5 min, 63:37 for 5 min, 60:40 for 5 min, 57:43 for 5 min and finally 54:46 for 5 min and a flow of 1 mL·min−1. The fraction corresponding to [18F]-big ET-1 was collected (retention time 21–23 min), phosphate buffer (8 mL, 10 mM, pH 7.4) was added and the resulting solution was loaded onto a SepPak C18 Light cartridge. The isolated [18F]-big ET-1 was eluted with ethanol/phosphate buffer (10 mM, pH 7.4) 80:20. Subsequent evaporation of the ethanol using a rotary evaporator and re-dissolving in saline yielded a solution of [18F]-big ET-1 suitable for injection.
Results
Radiolabelling of big ET-1
Big ET-1 was labelled in Lys9 by conjugation with N-succinimidyl 4-[18F]-fluorobenzoate. The radiochemical yield was 12–15% (corrected for decay) and the radiochemical purity of the isolated [18F]-big ET-1 was >95%. The specific activity at injection was 230 ± 21 GBq·µmol−1. The identity of [18F]-big ET-1 was confirmed using HPLC and co-elution with reference (4-fluorobenzoyl)-big ET-1 ([F]-big ET-1) synthesized using the method of Johnström et al. (2002). Identity of reference (4-fluorobenzoyl)-big ET-1 was confirmed by mass spectroscopy (MS (m/z) 2203.8 [M+2H]2+, 1469.2 [M+3H]3+, 1102.7 [M+4H]4+).
[18F]-big ET-1 biodistribution in control animals
In control animals, the blood curve constructed over a period of 120 min (Figure 1A), showed that [18F]-big ET-1 was initially rapidly cleared from the circulation with a t1/2= 2.9 ± 0.1 min followed by slower β-phase (t1/2= 104.6 ± 8.5 min).
Figure 1.
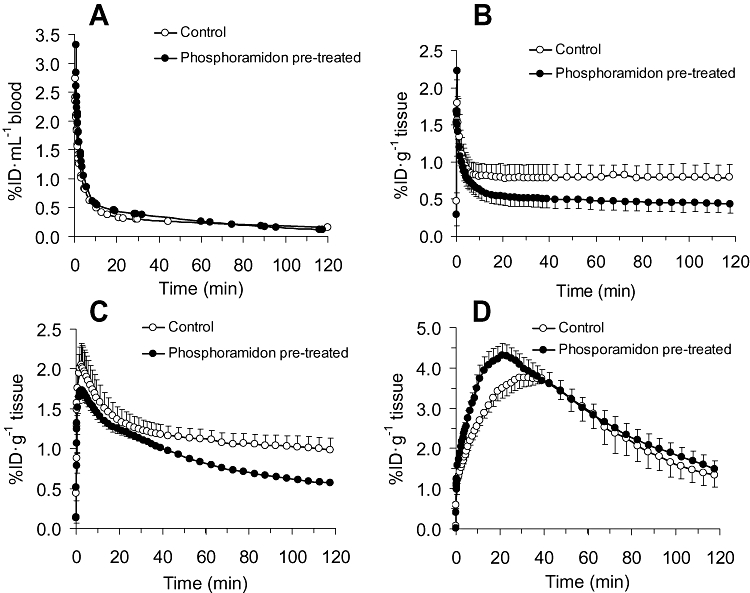
Blood and microPET time-activity curves after [18F]-big ET-1 administration in control, and phosphoramidon pretreated rats: (A) blood curve (B) lung (C) liver and (D) kidney. Activity is shown as % injected dose (%ID) per mL blood or g tissue. By analysis of variance there was a significant reduction in phosphoramidon treated animals in the liver and lung over the complete time-activity curve of 120 min (P < 0.05) and in the kidney a significant increase from 1-40 min (P < 0.05). ET-1, endothelin-1; PET, positron emission tomography.
Whole body microPET images of the rat showed the highest uptake of radioactivity in three major organs: kidney, liver and lung. These were selected for further detailed analysis by constructing time-activity curves over 120 min (Figure 1B–D). The decay-corrected concentration of radioactivity in the lungs peaked within 15 s, followed by an initial rapid decrease, then plateaued and reached equilibrium after 10 min, with no further decrease over the time studied (Figure 1B), consistent with conversion of [18F]-big ET-1 to [18F]-ET-1 and subsequent binding to ET receptors. In liver (Figure 1C) a similar pattern was observed with a slightly later peak of radioactivity after 2.5 min. In marked contrast, in the kidney (Figure 1D), the peak in radioactivity was higher but time to maximum accumulation was slower (about 30 min), followed by an exponential decline with a t1/2= 59.7 ± 2.8 min.
At the end of the 120 min scanning period, organs were removed for ex vivo measurement of radioactivity using a gamma counter (Figure 2), confirming high levels of activity in lung, liver and kidney with lower but detectable levels of uptake in three further organs: heart, muscle and the highly vascular spleen. As expected from previous studies imaging ET receptors in vivo following infusion of [18F]-ET-1 in the periphery which did not cross the blood-brain barrier (Johnström et al., 2005a), there was no detectable radioactivity in the brain.
Figure 2.
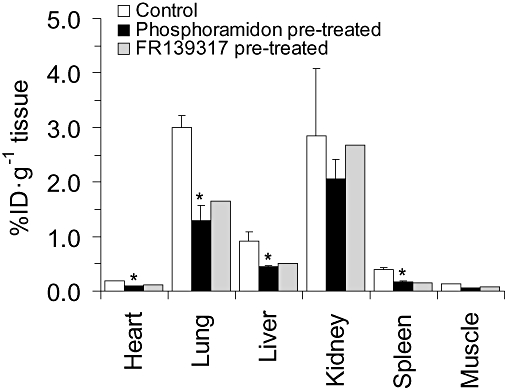
Concentration of radioactivity following excision of tissues at the end of experiment following [18F]-big ET-1 administration in control (n= 3) and after pretreatment with either phosphoramidon (n= 3) rats or FR139317 (n= 1). Phosphoramidon significantly reduced (P < 0.05) radioactivity in heart, lung, liver and spleen consistent with inhibition of conversion of [18F]-big ET-1 to [18F]-ET-1. In a single experiment, the ETA selective antagonist FR139317 reduced radioactivity in the same tissues, consistent with blocking [18F]-ET-1 binding to ETA receptors. ET-1, endothelin-1.
Effect of enzyme inhibition with phosphoramidon
In the blood activity curves (Figure 1), the half-life for [18F]-big ET-1 when the rats where pretreated with phosphoramidon (10 mg·kg−1, t1/2= 2.4 ± 0.1 min, Figure 1A) was similar to the controls. However, uptake of [18F]-big ET-1 in lung and liver (Figure 1B,C) was significantly reduced over the time period of 120 min measured by microPET imaging and in all tissues analysed ex vivo (Figure 2) at the end of the experiments, with the exception of kidney and muscle. Analysis of the images showed in the lung and liver that pretreatment resulted in a reduction in uptake by 42% and 45%, respectively, after 120 min. Furthermore the late kinetic data suggests a change from steady-state to a slow wash-out phase in lung (t1/2= 297.9 ± 32.1 min) and in the liver (t1/2= 131.9 ± 3.7 min).
In marked contrast in kidney, the amount of radioactivity increased significantly from 1 to 40 min in the presence of phosphoramidon but it did not change the shape of the subsequent time activity curve (Figure 1D) with a comparable exponential decline (t1/2= 62.3 ± 2.6 min). There was no significant change in the level of accumulated radioactivity at the end of the experiment compared with the control (Figures 1 and 2).
Effect of ETA receptor blockade with FR139317
In a single experiment, pretreatment with the ETA selective antagonist, FR139317 did not alter the half-life of [18F]-big ET-1 (t1/2= 2.5 min) in the blood whereas a reduction in uptake of radioactivity in the tissue comparable to that in the phosphoramidon pretreated rats was observed in all tissues except for kidney at the end of 120 min, consistent with reduction in binding to ETA receptors. In agreement with the control and the phosphoramidon results, there was no change in the kidney at end of 120 min consistent with the signal being dominated by excretion of radioactivity (Figure 2).
Comparison of microPET images and ex vivo autoradiography
The distribution of radioactivity was examined in more detail by comparing in vivo microPET images with ex vivo autoradiographical sections of the liver, lung and kidney. After 120 min the reconstructed microPET images in the liver and lung revealed a discrete pattern of high levels of radioactivity suggesting binding to the vasculature (Figure 3A,C). This distribution was observed in ex vivo tissue sections of liver and lung, confirming that the radioactivity was localized to the lung vasculature (Figure 3B,D) identified by comparison with the sections stained for the smooth muscle marker, α-actin or haematoxylin and eosin. High levels of radioactivity correlated with larger blood vessels although some radioactivity may be associated with capillary beds. A similar distribution pattern has previously been observed in animals treated with BQ788 to block ETB receptors in order to visualize binding of [18F]-ET-1 to the ETA sub-type using microPET imaging (Figure 3E) and autoradiography (Figure 3F) (Johnström et al., 2005a).
Figure 3.
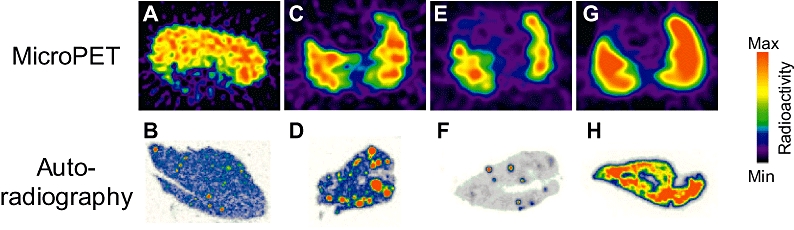
MicroPET images showing localized distribution of radioactivity in liver (A) and lung (C) after [18F]-big ET-1 administration in control rats. This was corroborated by autoradiography of ex vivo tissue sections of liver (B) and lung (D) confirming that the radioactivity was localized to the vasculature. For comparison, data from Johnström et al. (2005a) are shown where a comparable localized distribution in the lung was observed when the ETB receptor had been blocked using the ETB antagonist BQ788 prior to infusion of [18F]-ET-1: (E) Micropet image of the lung and (F) corresponding autoradiography of ex vivo tissue section. The Micropet image of [18F]-ET-1 binding in the control rat lung is shown in (G) together with the corresponding autoradiography of ex vivo tissue section (H). ET-1, endothelin-1; PET, positron emission tomography.
In the kidney, microPET images reveal initial high accumulation of radioactivity in the cortex after 15 min, corresponding to the peak of radioactivity measured in the time activity curve with subsequent redistribution with time to the papilla, a pattern consistent with excretion of radioactivity (Figure 4A–D). The autoradiographical images of kidney sections removed at the end of the experiment showed a similar distribution with most of the radioactivity redistributed to the renal papilla (Figure 4E), with some low levels of radioactivity localized to the vasculature of the cortex after 120 min.
Figure 4.
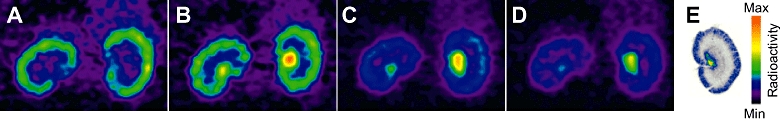
MicroPET images showing the distribution of radioactivity in the kidney as a function of time: (A–D) 15, 40, 65 and 75 min post big-ET-1 injection. The pattern over time is dominated by that expected for excretion rather than binding of the radioligand to receptors. Ex vivo autoradiography of kidney sections at end of the imaging experiment shows that most of the radioactivity is in the renal papilla with low levels of radioactivity localized to the vasculature of the cortex (E). ET-1, endothelin-1; PET, positron emission tomography.
A comparable localization of radioactivity to blood vessels was observed in autoradiography of ex vivo tissue sections of heart and spleen although levels were too low to allow visualization with microPET (Figure 5A,B).
Figure 5.
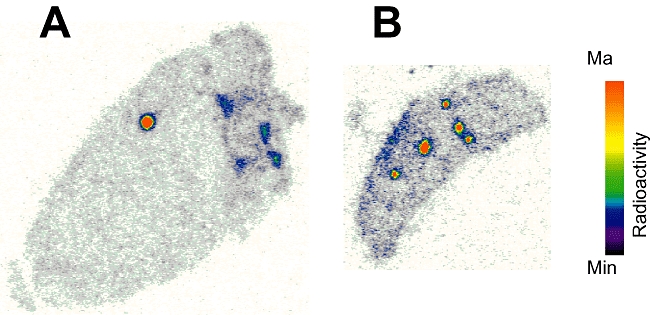
Ex vivo autoradiography following PET imaging showing localization of radioactivity to the vasculature in tissue sections of heart (A) and spleen (B). PET, positron emission tomography.
Discussion
Our results showed that following infusion of [18F]-big ET-1 into anaesthetized rats, the peptide is rapidly cleared from the circulation. Major organs for accumulation of radioactivity were identified as the lung, liver and kidney, visualized by dynamic PET imaging, with lower but detectable levels in the heart, spleen and muscle measured by gamma counting of excised organs 2 h after [18F]-big ET-1 administration. Dynamic PET data in the lungs revealed an initial rapid clearance of [18F]-big ET-1, which then levelled out and reached a steady state after 10 min, similar to that previously observed with [18F]-ET-1 infused into rats, consistent with binding to ET receptors (Johnström et al., 2005a). For liver, the steady state was reached later at ∼30–40 min which may reflect differences in ECE activity in these organs.
Big ET-1 has no affinity for the ET receptor at the tracer concentrations used in this study and the cleavage of this peptide to ET-1 is essential for receptor binding (Kimura et al., 1989). We have previously shown that the peptide labelling technique used in this study results in conjugation of a [18F]-fluorobenzoyl group with the ε-amino group of Lys9 of ET-1 (Johnström et al., 2002). [18F]-ET-1 labelled in this position retains subnanomolar affinity for ET receptors and has pharmacokinetic and pharmacodynamic properties allowing dynamic imaging of ET receptors in vivo using PET (Johnström et al., 2005a). It has been shown that the His27-Gly34 sequence in big ET-1 is important for enzyme recognition and conversion to ET-1 (Okada et al., 1991; 1993; Brooks and Ergul, 1998). Our strategy avoids labelling within this His27-Gly34 region, permitting both enzymic cleavage and formation of [18F]-ET-1.
Metabolic degradation of big ET-1 to longer or shorter amino acid sequences than the optimal ET-1 (1–21) will result in peptides with little or no affinity for ET receptors (Kimura et al., 1988; 1989;) suggesting that our data reflects conversion of [18F]-big ET-1 to [18F]-ET-1 and subsequent binding to ET receptors. This is supported by the seven-fold increase in the initial half-life for [18F]-big ET-1 in the blood, compared with the half-life of [18F]-ET-1 (0.43 min) previously measured in the rat (Johnström et al., 2005a). Furthermore, pretreatment with phosphoramidon resulted in a reduction in the level of uptake and a more rapid clearance of radioactivity from lung over time in agreement with inhibition of enzyme conversion and reduction of ET-1 binding to ET receptors. These results are in concordance with previous studies where phosphoramidon treatment blocked in vivo vasoconstrictor actions of infused big ET-1 in the rat (Gardiner et al., 1991; Mcmahon et al., 1991; Pollock and Opgenorth, 1991a) and human (Plumpton et al., 1995). Importantly, Pollock and Opgenorth (1991a) demonstrated that phophoramidon had no effect on the vasoconstrictor actions of ET-1 infused in vivo, thus excluding the possibility that phosphoramidon blocked binding of ET-1 to its receptor rather than inhibiting conversion of big ET-1. The concentration of phosphoramidon used in this study was chosen as this dose has previously been shown to inhibit in vivo constrictor actions of big ET-1 in rats by about 60% (Mcmahon et al., 1991) In agreement, the magnitude of inhibition by phosphoramidon ranging from 51% for liver to 63% for muscle was similar for all tissues studied with the exception of kidney.
Several lines of evidence suggest the site of conversion that we measure is most likely to be ECE present on the smooth muscle, followed by [18F]-ET-1 binding immediately to receptors, particularly the ETA sub-type that predominates on the smooth muscle. Little or no conversion of big-ET-1 to the mature peptide is thought to occur in the blood (Watanabe et al., 1991) and phosphoramidon had no effect on the blood curve in this study. We have previously shown that most of the endothelial cell ECE activity (∼85%) is within the cytoplasm and not the cell surface (Davenport et al., 1998) and as phosphoramidon does not cross the plasma membrane, significant conversion of [18F]-big ET-1 is unlikely to be effected by the endothelium. Furthermore, isolated vessels denuded of endothelium rapidly convert big ET-1 to cause vasoconstriction, corresponding to formation of the mature peptide in the organ bath and this activity is significantly increased in vessels with atherosclerotic lesions (Maguire and Davenport, 1998). Our results with the ETA receptor selective antagonist FR139317 support the formation of [18F]-ET-1 and binding to the ETA receptor subtype (nomenclature follows Alexander et al., 2009). MicroPET images and the higher resolution afforded by autoradiography visualized high levels of binding to the vasculature in all tissues examined.
Our results for the accumulation of [18F]-big ET-1 in the kidney are intriguing in that the peak in radioactivity after 20 min in the cortex is the highest for all organs imaged. The magnitude of the peak is further increased in the presence of phosphoramidon, consistent with the reduction in tissue-specific conversion of [18F]-big ET-1 to [18F]-ET-1 that we measured in other organs and a subsequent increase in excretion of [18F]-big ET-1. After 20 min, radioactivity is redistributed to the medulla which is unchanged by phosphoramidon. Although autoradiography revealed very low levels of binding to the renal vasculature this pattern is consistent with most of the radioactivity being excreted. This may be either unconverted [18F]-big ET-1 or much shorter peptide fragments with little receptor binding action.
Previously we have shown that infusion of [18F]-ET-1 into the rat results in rapid binding to ET receptors in the kidney which can be blocked by antagonists (Johnström et al., 2005a) at densities comparable to lungs and liver. Thus if a similar high level of local conversion of [18F]-big ET-1 as that seen in lungs and liver had occurred within the kidney, we would have expected to see greater evidence for this in the kinetic data and ex vivo autoradiography at the end of the experiment. Big ET-1 has been identified and found to be more abundant than ET-1 in human urine by specific ELISA HPLC (Matsumoto et al., 1994) and Naruse et al. (1991) showed by HPLC that urinary levels are increased in patients with cardiovascular disease supporting the hypothesis that the kidney may have a previously unsuspected function to remove a significant component of big ET-1 from the circulation. Some binding was visualized to the renal vasculature which is consistent with previous in vivo studies in rats where low doses of big ET-1 (0.1 nmol·kg−1) had no effect on renal haemodynamics whereas vasoconstriction was detected in other vascular beds measured (hindquarters and mesentery) (Gardiner et al., 1991).
Our results show that the hepatic and the pulmonary vasculatures are a major site for [18F]-big ET-1 conversion and binding to ET receptors in the rat. It is not yet clear whether the results have clinical significance. However, in pulmonary arterial hypertension which is associated with increases in pulmonary vascular resistance, the ET system is up-regulated and the mixed ETA/ETB receptor antagonist, bosentan is used to treat this condition. The source of increased ET-1 is not established although enhanced production or reduced ETB clearance have been proposed (Dupuis et al., 1996; Dupuis et al., 1998). Our results suggest a third possibility, of increased conversion of big ET-1 within the target vasculature. Similarly, ET levels are higher in patients with cirrhosis of the liver (Martinet et al., 1996) and conversion of big ET-1 by the hepatic circulation may contribute to portal hypertension (Martinet et al., 1996). Lower but detectable conversion in the heart could also have pathophysiological actions particularly under conditions where smooth muscle ECE activity is increased, for example in atherosclerosis (Minamino et al., 1997; Grantham et al., 1998; Maguire and Davenport, 1998), that might promote vasospasm. The renal vasculature is very sensitive to the constrictor actions of ET-1 but surprisingly our results suggest most of the injected radioactivity is being excreted without conversion to ET-1 within the vasculature. Finally, specific inhibitors of ECE are being developed (Jeng et al., 2002; Jeng, 2003) to attenuate the proteolytic synthesis of ET-1. Our results suggest under conditions of ECE inhibition, renal excretion may be an important route for removal of big ET-1.
Acknowledgments
We thank Mrs Rhoda Kuc, Miss Morgan Alexander, Dr Olivier Barret and Mr Paul Burke for technical support. This work was supported by grants from the British Heart Foundation, the Medical Research Council and for the microPET a JREI grant from HEFCE and Merck Sharpe & Dohme, Ltd.
Glossary
Abbreviations:
- ET-1
endothelin-1
- FR139317
(R)-2-[(R)-2-[(S)-2-[[1-(hexahydro-1H-azepinyl)]carbonyl]amino-4-methylpentanoyl]amino-3-[3-(1-methyl-1H-indoyl)]propionyl] amino-3-(2-pyridyl)propionic acid
- PET
positron emission tomography
- phosphoramidon
(2S)-2-[[(2S)-2-[[hydroxy-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl] oxyphosphoryl] amino]-4-methylpentanoyl]amino]-3-(1H-indol-3-yl) propanoic acid
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon CR, Cary NRB, Davenport AP. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ Res. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- Brooks C, Ergul A. Identification of amino acid residues in the C-terminal tail of big endothelin-1 involved in processing to endothelin-1. J Mol Endocrinol. 1998;21:307–315. doi: 10.1677/jme.0.0210307. [DOI] [PubMed] [Google Scholar]
- Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur J Nucl Med Mol Imaging. 2002;29:98–114. doi: 10.1007/s00259-001-0683-3. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Immunocytochemical localization of receptors using light and confocal microscopy with application to the phenotypic characterization of knock-out mice. Methods Mol Biol. 2005;306:155–172. doi: 10.1385/1-59259-927-3:155. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE, Mockridge JW. Endothelin-converting enzyme in the human vasculature: evidence for differential conversion of big endothelin-3 by endothelial and smooth-muscle cells. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S1–S3. doi: 10.1097/00005344-199800001-00002. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Maguire JJ. Endothelin. Handb Exp Pharmacol. 2006;176:295–329. doi: 10.1007/3-540-32967-6_9. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Cernacek P, Tardif JC, Stewart DJ, Gosselin G, Dyrda I, et al. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am Heart J. 1998;135:614–620. doi: 10.1016/s0002-8703(98)70276-5. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;94:1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Kemp PA, Bennett T. The effects of phosphoramidon on the regional haemodynamic responses to human proendothelin [1-38] in conscious rats. Br J Pharmacol. 1991;103:2009–2015. doi: 10.1111/j.1476-5381.1991.tb12368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Bennett T. Regional haemodynamic responses to intravenous and intraarterial endothelin-1 and big endothelin-1 in conscious rats. Br J Pharmacol. 1993;110:1532–1536. doi: 10.1111/j.1476-5381.1993.tb13997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Brunner-Ferber F, Bennett T. Effects of the dual metallopeptidase inhibitor, MDL 100,240, on regional haemodynamic responses to vasoactive peptides in conscious rats. Br J Pharmacol. 1997;122:1687–1693. doi: 10.1038/sj.bjp.0701550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham JA, Grantham JA, Schirger JA, Williamson EE, Heublein DM, Wennberg PW, et al. Enhanced endothelin-converting enzyme immunoreactivity in early atherosclerosis. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S22–S26. doi: 10.1097/00005344-199800001-00009. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–1098. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- Jeng AY. Utility of endothelin-converting enzyme inhibitors for the treatment of cardiovascular diseases. Curr Opin Investig Drugs. 2003;4:1076–1081. [PubMed] [Google Scholar]
- Jeng AY, Mulder P, Kwan AL, Battistini B. Nonpeptidic endothelin-converting enzyme inhibitors and their potential therapeutic applications. Can J Physiol Pharmacol. 2002;80:440–449. doi: 10.1139/y02-025. [DOI] [PubMed] [Google Scholar]
- Johnström P, Fryer TD, Richards HK, Barret O, Davenport AP. Dynamic in vivo imaging of receptors in small animals using positron emission tomography. Methods Mol Biol. 2005b;306:217–232. doi: 10.1385/1-59259-927-3:217. [DOI] [PubMed] [Google Scholar]
- Johnström P, Fryer TD, Richards HK, Harris NG, Barret O, Clark JC, et al. Positron emission tomography using 18F-labelled endothelin-1 reveals prevention of binding to cardiac receptors owing to tissue-specific clearance by ETB receptors in vivo. Br J Pharmacol. 2005a;144:115–122. doi: 10.1038/sj.bjp.0706064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnström P, Harris NG, Fryer TD, Barret O, Clark JC, Pickard JD, et al. 18F-Endothelin-1, a positron emission tomography (PET) radioligand for the endothelin receptor system: radiosynthesis and in vivo imaging using microPET. Clin Sci (Lond) 2002;103(Suppl. 48):4S–8S. doi: 10.1042/CS103S004S. [DOI] [PubMed] [Google Scholar]
- Kimura S, Kasuya Y, Sawamura T, Shinimi O, Sugita Y, Yanagisawa M, et al. Conversion of big endothelin-1-21-residue endothelin-1 is essential for expression of full vasoconstrictor activity: structure-activity relationships of big endothelin-1. J Cardiovasc Pharmacol. 1989;13(Suppl. 5):S5–S7. doi: 10.1097/00005344-198900135-00003. [DOI] [PubMed] [Google Scholar]
- Kimura S, Kasuya Y, Sawamura T, Shinmi O, Sugita Y, et al. Structure-activity relationships of endothelin: importance of the C-terminal moiety. Biochem Biophys Res Commun. 1988;156:1182–1186. doi: 10.1016/s0006-291x(88)80757-5. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. Analytic 3D image-reconstruction using all detected events. IEEE Trans Nucl Sci. 1989;36:964–968. [Google Scholar]
- Lewis JS, Achilefu S, Garbow JR, Laforest R, Welch MJ. Small animal imaging. current technology and perspectives for oncological imaging. Eur J Cancer. 2002;38:2173–2188. doi: 10.1016/s0959-8049(02)00394-5. [DOI] [PubMed] [Google Scholar]
- McMahon EG, Palomo MA, Moore WM, McDonald JF, Stern MK. Phosphoramidon blocks the pressor activity of porcine big endothelin-1-(1-39) in vivo and conversion of big endothelin-1-(1-39) to endothelin-1-(1-21) in vitro. Proc Natl Acad Sci USA. 1991;88:703–707. doi: 10.1073/pnas.88.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. Increased response to big endothelin-1 in atherosclerotic human coronary artery: functional evidence for up-regulation of endothelin-converting enzyme activity in disease. Br J Pharmacol. 1998;125:238–240. doi: 10.1038/sj.bjp.0702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Johnson CM, Mockridge JW, Davenport AP. Endothelin converting enzyme (ECE) activity in human vascular smooth muscle. Br J Pharmacol. 1997;122:1647–1654. doi: 10.1038/sj.bjp.0701564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet JP, Legault L, Cernacek P, Roy L, Dufresne MP, Spahr L, et al. Changes in plasma endothelin-1 and Big endothelin-1 induced by transjugular intrahepatic portosystemic shunts in patients with cirrhosis and refractory ascites. J Hepatol. 1996;25:700–706. doi: 10.1016/s0168-8278(96)80241-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Suzuki N, Kitada C, Fujino M. Endothelin family peptides in human plasma and urine: their molecular forms and concentrations. Peptides. 1994;15:505–510. doi: 10.1016/0196-9781(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Minamino T, Kurihara H, Takahashi M, Shimada K, Maemura K, Oda H, et al. Endothelin-converting enzyme expression in the rat vascular injury model and human coronary atherosclerosis. Circulation. 1997;95:221–230. doi: 10.1161/01.cir.95.1.221. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Le SQ, Wasserstrum N, Vanhoutte PM. Endothelins 1 and 3 and big endothelin-1 contract isolated human placental veins. J Cardiovasc Pharmacol. 1993;22(Suppl. 8):S278–S281. doi: 10.1097/00005344-199322008-00073. [DOI] [PubMed] [Google Scholar]
- Naruse K, Naruse M, Watanabe Y, Yoshihara I, Ohsumi K, Horiuchi J, et al. Molecular form of immunoreactive endothelin in plasma and urine of normal subjects and patients with various disease states. J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S506–S508. doi: 10.1097/00005344-199100177-00144. [DOI] [PubMed] [Google Scholar]
- de Nucci G, Thomas R, D'Orleans-Juste P, Antunes E, Walder C, Warner TD, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;185:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Arai Y, Hata M, Matsuyama K, Yano M. Big endothelin-1 structure important for specific processing by endothelin-converting enzyme of bovine endothelial cells. Eur J Biochem. 1993;218:493–498. doi: 10.1111/j.1432-1033.1993.tb18401.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Takada J, Arai Y, Matsuyama K, Yano M. Importance of the C-terminal region of big endothelin-1 for specific conversion by phosphoramidon-sensitive endothelin converting enzyme. Biochem Biophys Res Commun. 1991;180:1019–1023. doi: 10.1016/s0006-291x(05)81167-2. [DOI] [PubMed] [Google Scholar]
- Plumpton C, Haynes WG, Webb DJ, Davenport AP. Phosphoramidon inhibition of the in vivo conversion of big endothelin-1 to endothelin-1 in the human forearm. Br J Pharmacol. 1995;116:1821–1828. doi: 10.1111/j.1476-5381.1995.tb16669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumpton C, Kalinka S, Martin RC, Horton JK, Davenport AP. Effects of phosphoramidon and pepstatin A on the secretion of endothelin-1 and big endothelin-1 by human umbilical vein endothelial cells: measurement by two-site enzyme-linked immunosorbent assays. Clin Sci (Lond) 1994;87:245–251. doi: 10.1042/cs0870245. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Opgenorth TJ. Comparison of the hemodynamic effects of endothelin-1 and big endothelin-1 in the rat. Biochem Biophys Res Commun. 1991a;179:1122–1126. doi: 10.1016/0006-291x(91)91936-7. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Opgenorth TJ. Evidence for metalloprotease involvement in the in vivo effects of big endothelin 1. Am J Physiol. 1991b;261:R257–R263. doi: 10.1152/ajpregu.1991.261.1.R257. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126:391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Matsumoto H, Kitada C, Kimura S, Miyauchi T, Fujino M. A sandwich-type enzyme immunoassay to detect immunoreactive big-endothelin-1 in plasma. J Immunol Methods. 1990;127:165–170. doi: 10.1016/0022-1759(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Tai YC, Ruangma A, Rowland D, Siegel S, Newport DF, Chow PL, et al. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys Med Biol. 2001;46:1845–1862. doi: 10.1088/0031-9155/46/7/308. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Naruse M, Monzen C, Naruse K, Ohsumi K, Horiuchi J, et al. Is big endothelin converted to endothelin-1 in circulating blood? J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S503–S505. doi: 10.1097/00005344-199100177-00143. [DOI] [PubMed] [Google Scholar]


