Abstract
Background and purpose:
The endocannabinoid system and the cannabinoid CB1 receptor have been identified in human sperm, and it is well known that endocannabinoids have pronounced adverse effects on male and female reproduction. In order to elucidate further the pathophysiological role of the endocannabinoid system in male fertility, we investigated the activity of the CB1 receptor antagonist rimonabant (SR141716) on the fertilizing ability of human sperm.
Experimental approach:
We evaluated in vitro the effects of rimonabant on motility, survival, capacitation, acrosin activity and metabolism of human sperm. Particularly, capacitation was studied by using three different approaches: intracellular free Ca2+ content assay, cholesterol efflux assay and protein tyrosine phosphorylation analysis.
Key results:
Rimonabant significantly increased sperm motility and viability through the induction of pAkt and pBcl2, key proteins of cell survival and metabolism, and it induced acrosome reaction and capacitation as well. Rimonabant reduced the triglyceride content of sperm, while enhancing lipase and acyl-CoA dehydrogenase activities, implying an overall lipolytic action in these cells. Rimonabant also affected sperm glucose metabolism by decreasing phosphorylation of glycogen synthase kinase 3 and increasing glucose-6-phosphate dehydrogenase activity, suggesting a role in inducing sperm energy expenditure. Intriguingly, agonism at the CB1 receptor, with an anandamide analogue or a selective inhibitor of fatty acid amide hydrolase, produced opposing effects on human sperm functions.
Conclusions and implications:
Our data suggest that blockade of the CB1 receptor by rimonabant induces the acquisition of fertilizing ability and stimulates energy expenditure in human sperm.
Keywords: rimonabant, CB1 receptor, human sperm, male fertility, reproduction, endocannabinoids
Introduction
The endogenous cannabinoid (EC) system, comprising the most abundant cannabinoid type-1 (CB1) and the more restricted CB2 receptors, their endogenous ligands (endocannabinoids) and the enzymes catalyzing their biosynthesis and degradation, is an almost ubiquitous signalling system involved in the control of several physiological functions from energy homeostasis to movement, memory and pain (Elphick and Ergetova, 2001; Bifulco et al., 2007; Bellocchio et al., 2008). During the last years, evidence has accumulated for an important role of the EC system in both male and female fertility (Wang et al., 2006). The CB1 receptor is expressed in human testis (Gerard et al., 1991), and sea urchin and human sperm cells possess a functional EC system (Schuel et al., 1987; 1994; Wang et al., 2006). In human sperm, the CB1 receptor subtype is present in the membranes of the head and middle piece, and is also localized on the mitochondria (Rossato et al., 2005; Aquila et al., 2009a). Moreover, the CB1 agonist anandamide (AEA) inhibited sperm motility and viability (Rossato et al., 2005; Aquila et al., 2009a,b;). These observations together with the finding that agonism at CB1 receptors, either by the endogenous agonist AEA or by the natural CB1 agonist delta-9-tetrahydrocannabinol (THC) has negative effects on human reproduction, suggest that targeting the CB1 receptor or controlling the endogenous tone of endocannabinoids may represent a therapeutic tool in reproductive pathological situations involving an imbalance of the EC system (Maccarrone and Finazzi-Agrò, 2004; Bifulco et al., 2007; Maccarrone, 2008). So far, there are very few data on the effects of CB1 receptor blockade on male fertility, whereas there is a large body of the literature focused on the interaction and possible regulation of reproductive processes by endocannabinoids (Wang et al., 2006). The only available data concern the ability of the highly selective CB1 antagonist SR141716 (rimonabant) to counteract AEA-mediated inhibition of human sperm viability and motility, when administered prior to AEA (Rossato et al., 2005). Similar results were reported in the frog Rana esculenta by Cobellis et al. (2006) who found that micromolar concentrations of rimonabant alone were able to induce a slight, but significant, increase of viable and motile spermatozoa. Rimonabant also induced penile erection in male rats when injected into the paraventricular nucleus of male rat hypothalamus (Melis et al., 2006; Succu et al., 2006). This effect was associated with an increase of extracellular glutamic acid leading to the activation of NO synthase in oxytocinergic neurons mediating penile erection (Succu et al., 2006).
In order to increase our knowledge of the pathophysiological role of the EC system in male fertility, and to analyse the activity of rimonabant on human sperm functions, we investigated the effects of rimonabant on sperm viability and motility, and focused on different aspects of the capacitation process, acrosome reaction and metabolism. Human spermatozoa do not possess the ability to fertilize an oocyte immediately after ejaculation, but they acquire this ability after some time in contact with the female reproductive tract. This time-dependent acquisition of fertilizing ability is known as capacitation (Yanagimachi, 1994), and includes acquisition of hyperactivated motility (Suarez, 2008) increase in both intracellular Ca2+ concentration and protein phosphorylation (Visconti et al., 2002; Jha et al., 2003), and efflux of cholesterol from sperm (Travis and Kopf, 2002). Capacitation enables the sperm to bind to the zona pellucida (ZP) and undergo the acrosome reaction, a process by which powerful hydrolyzing enzymes present in the acrosome are released into its surroundings. Acrosome reaction serves at least two functions: first, to facilitate the penetration of the ZP by the and, subsequently, to aid in the oocyte–sperm fusion process (Yanagimachi, 1994). Hence, by analyzing the key biochemical changes of capacitation and acrosome reaction, we could assess the influence of rimonabant on male fertilizing potential. In addition, as there is a close link between energy balance and reproduction (Chehab, 2000; Altarejos et al., 2008), and we recently reported that sperm cells are able to modulate their own metabolism independently of systemic regulation by expressing and secreting both insulin and leptin (Andò and Aquila, 2005; Aquila et al., 2005; 2006;), we evaluated the action of rimonabant on lipid and glucose metabolism in human sperm.
Methods
Semen samples and spermatozoa preparations
Human semen was collected, according to the World Health Organization (WHO) recommendations, by masturbation from healthy volunteer donors of proven fertility undergoing semen analysis in our laboratory. Spermatozoa preparations were performed as previously described (Aquila et al., 2006). Briefly, sperm samples with normal parameters of semen volume, sperm count, motility, vitality and morphology, according to the WHO Laboratory Manual (World Health Organization, 1999), were included in this study. Each sperm sample was obtained by pooling the ejaculates of three different normozoospermic healthy donors. In our experience, this was necessary to obtain enough cells to perform all the tests (Aquila et al., 2005; 2009a,b;). In addition, each assay was performed at least three times using three different sperm samples. Washed pooled sperm samples were subjected to the indicated treatments, and incubated for 30 min at 37°C and 5% CO2. Then, the samples were centrifuged and the pellet containing sperm was lysed to perform Western blots, triglyceride assay, Ca2+ assay, acyl-CoA dehydrogenase assay, glucose-6-phosphate dehydrogenase (G6PDH) activity and lipase activity. Prior to the centrifugation, several aliquots were used to measure sperm motility and viability. The study was approved by the local medical ethical committees, and all participants gave their informed consent.
Processing of ejaculated sperm
After liquefaction, the normal semen samples were pooled and subjected to centrifugation (800×g) on a discontinuous Percoll density gradient (80:40% v : v) (World Health Organization, 1999). The 80% Percoll fraction was examined using an optical microscope at 1000× magnification to ensure that a pure sample of sperm was obtained. An independent observer, who observed several fields for each slide, checked the cells. Percoll-purified sperm was washed with uncapacitating medium (Earle's balanced salt solution medium without supplementation with BSA, sodium bicarbonate or Ca2+), and incubated for 30 min at 37°C and 5% CO2, without (control, NC) or with the indicated concentrations of rimonabant. When combined treatments were performed, the cells were pretreated for 15 min with the CB1 receptor antagonist rimonabant (1 µM), and then 2-methylarachidonyl-2′-fluoro-ethylamide (MF-AEA) at the indicated concentrations or URB597 (0.1 µM) was added.
Evaluation of sperm motility and viability
Sperm motility was assessed by means of light microscopy examining aliquots of each sperm. An independent observer scored at least 200 cells. Sperm motility was expressed as percentage of total motile sperm.
Viability was assessed by red eosin exclusion test using eosin Y. Sperm vitality was assessed by means of light microscopy examining an aliquot of each sperm sample in the absence (NC) or in the presence of increasing concentrations of rimonabant, and then incubated for 30 min. An independent observer scored 200 cells for stain uptake (dead cells) or exclusion (live cells), and sperm viability was expressed as percentage of total live sperm.
Evaluation of Ca2+ in sperm
Intracellular Ca2+ concentration has been estimated spectrophotometrically with the indicator arsenazo III (Thomson and Wishart, 1989) using sperm lysates according to our previous study (Aquila et al., 2009b). At a neutral pH, the Ca2+ forms with arsenazo III a complex, the colour intensity of which is directly proportional to the concentration of Ca2+ in the sample. Normal sperm samples were treated as mentioned earlier. Ca2+ content was measured at 600 nm. The Ca2+ standard used was 2.5 mM (100 mg·L–1). Inter- and intra-assay variations were 0.24 and 0.37% respectively. Ca2+ results are presented as µM per 107 spermatozoa.
Measurement of cholesterol in the sperm culture medium
Cholesterol was measured in duplicate by a cholesterol oxidase–peroxidase (CHOD–POD) enzymatic colorimetric method according to the manufacturer's instructions in the incubation medium from human spermatozoa. Each sperm sample, washed twice with uncapacitating medium, was incubated in the same medium (control) in the presence or in the absence of testing compounds for 30 min at 37°C and 5% CO2. At the end of the sperm incubation, culture media were recovered by centrifugation, lyophilized and subsequently dissolved in 1 mL of reaction buffer. The samples were incubated for 10 min at room temperature, then the cholesterol content was measured spectrophotometrically at 505 nm. The cholesterol standard used was 2 g·L–1. The limit of sensitivity for the assay was 0.005 mg·L–1. Inter- and intra-assay variations were 0.04 and 0.03% respectively. Cholesterol results are shown as mg per 107 spermatozoa.
Acrosin activity assay
Acrosin activity was assessed by the method of Kennedy et al. (1989) and as previously described (Aquila et al., 2003). Sperm cells were washed in Earle's medium and centrifuged at 800×g for 20 min, and then were resuspended in different tubes (final concentration of 107 spermatozoa mL–1) in the presence and absence of treatments. One millilitre of substrate–detergent mixture (23 mM BAPNA in DMSO and 0.01% Triton X-100 in 0.055 M NaCl, 0.055 M HEPES at pH 8.0, respectively) was added for 3 h at room temperature. Aliquots (20 µL) were removed at 0 and 3 h, and the percentage of viable cells was determined for each treatment. After incubation, benzamidine 0.5 M final concentration was added to each tube, and then centrifuged at 1000×g for 30 min. Supernatants were collected, and the acrosin activity was measured by using a spectrophotometer at 410 nm. In this assay, the total acrosin activity was defined as the amount of the active (non-zymogen) acrosin associated with sperm plus the amount of active acrosin that is obtained by pro-acrosin activable. The acrosin activity was expressed as µIU/106 spermatozoa. Quantification of acrosin activity was performed as previously described (Aquila et al., 2003).
Western blot analysis of sperm proteins
Each sperm sample, washed twice with an uncapacitating medium, was incubated and treated as mentioned earlier and then centrifuged for 5 min at 5000×g. The pellet was resuspended in lysis buffer as previously described (Aquila et al., 2002). An equal amount of protein (80 µg) was boiled for 5 min, separated on a 10% polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and probed with an appropriate dilution of the indicated primary antibody. The binding of the secondary antibody was revealed with the ECL Plus Western blotting detection system, according to the manufacturer's instructions. As internal control, all membranes were subsequently stripped (glycine 0.2 M, pH 2.6 for 30 min at room temperature) and reprobed with anti-β-actin antibody or with antibody to total Akt, Bcl2 or glycogen synthase kinase 3 (GSK3). The protein bands were quantified by scanning densitometry (Imaging Densitometer GS-700, Bio-Rad, Hercules, CA, USA). Western blot analysis was performed in at least three independent experiments, and more representative results are shown.
Triglyceride assay
Triglycerides were measured in duplicate by a glycerol-3-phosphate oxidase–POD enzymatic colorimetric method according to the manufacturer's instructions and as previously described (Aquila et al., 2006). Sperm samples, washed twice with an uncapacitating medium, were incubated in the same medium (control) for 30 min at 37°C and 5% CO2 in the presence or in the absence of the testing compounds. At the end of the incubation, 10 µL of sperm lysate was added to 1 mL of reaction buffer, and incubated for 10 min at room temperature. Triglyceride content was measured at 505 nm by using a spectrophotometer. Data are presented as µg/106 spermatozoa.
Lipase activity assay
Lipase activity was evaluated by the method of Panteghini et al. (2001) based on the use of 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester (DGGR) as substrate (Aquila et al., 2009b). Then, 50 µg of sperm extracts, treated as described earlier, was loaded into individual cuvettes containing buffer for spectrophotometric determination. DGGR was cleaved by lipase, resulting in an unstable dicarbonic acid ester which was spontaneously hydrolysed to yield glutaric acid and methylresorufin, a bluish purple chromophore with peak absorption at 580 nm. The absorbance of the samples was read every 20 s for 1.5 min. The rate of methylresorufin formation is directly proportional to the lipase activity in the sample. Analysis of total imprecision gave a coefficient of variation of between 0.01 and 0.03%. The estimated reference interval was 6–38 U·L–1 (µmol·min–1·mg–1 protein). The enzymatic activity was determined with three control media: one without the substrate, one without the coenzyme (colipase) and the third without either substrate or coenzyme (data not shown).
Assay of acyl-CoA dehydrogenase activity
Assay of acyl-CoA dehydrogenase was performed on sperms, using a modification of the method described by Lehman et al. (1990) (Aquila et al., 2006). In brief, after protein lysis, 70 µg of sperm proteins was added to the buffer containing 20 mM Mops, 0.5 mM EDTA and 100 µM FAD+ at pH 7.2. Reduction of FAD+ to FADH was read at 340 nm upon the addition of octanoyl-CoA (100 µM) every 20 s for 1.5 min. Data are expressed as nmol·min–1·mg–1 protein. The enzymatic activity was determined with three control media: one without octanoyl-CoA as substrate, one without the coenzyme (FAD+) and the third without either substrate or coenzyme (data not shown).
G6PDH activity
The G6PDH activity was performed as previously described (Aquila et al., 2005). Briefly, sperm samples, washed twice with an uncapacitating medium, were treated as mentioned earlier and incubated in the same medium for 30 min at 37°C and 5% CO2. After incubation, 50 µL of sperm extracts was loaded into individual cuvettes containing buffer (100 mM triethanolamine, 100 mM MgCl2, 10 mg·mL–1 glucose-6-phosphate, 10 mg·mL–1 NADP+, pH 7.6) for spectrophotometric determination. The conversion of NADP+ to NADPH, catalysed by G6PDH, was measured by the increase of absorbance at 340 nm every 20 s for 1.5 min. Data are expressed in nmol·min–1/106 spermatozoa. The enzymatic activity was determined with three control media: one without glucose-6-phosphate as substrate, one without the coenzyme (NADP+) and the third without either substrate or coenzyme (data not shown).
Statistical analysis
The data obtained from Western blots, Ca2+ assay, cholesterol efflux assay, triglyceride assay, G6PDH activity, acyl-CoA dehydrogenase activity, lipase activity, acrosin activity, viability and motility (six replicate experiments using duplicate determinations) are presented as the mean ± SEM. The differences in mean values were calculated using anova with Newman–Keuls post hoc test. Values of P < 0.05 were taken to show a significant difference between means.
Materials
BSA protein standard, Laemmli sample buffer, pre-stained molecular weight markers, Percoll (colloidal PVP-coated silica for cell separation), sodium bicarbonate, sodium lactate, sodium pyruvate, Earle's balanced salt solution (uncapacitating medium) and all other chemicals were purchased from Sigma Chemical (Milan, Italy). Acrylamide bisacrylamide was from Labtek Eurobio (Milan, Italy). Triton X-100 and eosin Y were from Farmitalia Carlo Erba (Milan, Italy). ECL Plus Western blotting detection system, Hybond ECL and HEPES sodium salt were purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). Triglyceride assay kit, cholesterol assay kit, lipase activity kit, calcium (Ca2+) assay kit, G6PDH activity assay and CHOD–POD enzymatic colorimetric kit were from Inter-Medical (Biogemina Sas, Catania, Italy). Goat polyclonal β-actin antibody (Ab); polyclonal rabbit anti-phosphoAkt (S473); and total Akt Abs, POD-coupled anti-mouse, anti-rabbit and anti-goat IgG secondary Abs were from Santa Cruz Biotechnology (Heidelberg, Germany). Polyclonal anti-phosphoBcl2 (S70), anti-total Bcl2 Abs, anti-phosphoGSK3-beta (S9) and total GSK3 were from Cell Signaling (Milan, Italy). The CB1 receptor antagonist rimonabant was kindly provided by Sanofi-Aventis (Montpellier, France). The MF-AEA was purchased from Sigma Chemical, and the fatty acid amide hydrolase (FAAH) inhibitor, 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate (URB597) was from Alexis Biochemicals (Firenze, Italy).
The nomenclature of cannabinoid receptors, their agonists and antagonists and endocannabinoid-metabolizing enzymes follows Alexander et al. (2008).
Results
CB1 receptor blockade by rimonabant influences sperm motility and viability
Flagellar sperm motility was determined in increasing concentrations of rimonabant from 1 nM to 1 µM. Our data indicated that sperm motility was increased by rimonabant at 1 and 10 nM (Figure 1A), but unchanged at higher concentrations (100 nM or 1 µM). Similar results were obtained when we analysed the effects of the same concentrations of rimonabant on sperm viability, even though 10 nM appears to be more efficacious compared with 1 nM of rimonabant (Figure 1B). Notably, the CB1 receptor agonist, MF-AEA, at 0.1 µM caused opposing effects either on sperm motility or viability. URB597 (URB, 0.1 µM), which increases the level of endogenous AEA by inhibiting the activity of its degrading enzyme, FAAH (Piomelli et al., 2006), also reduced the motility and viability of sperm. In particular, MF-AEA plus rimonabant or URB plus rimonabant resulted in the strengthening of the rimonabant-induced effects.
Figure 1.
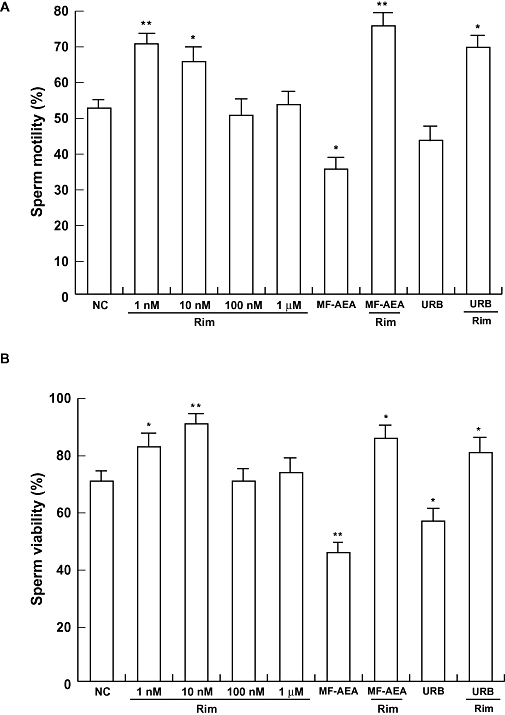
Rimonabant positively affects sperm motility and viability. Spermatozoa were incubated in unsupplemented Earle's medium for 30 min at 37°C and 5% CO2, in the absence (NC) or in the presence of increasing concentrations of rimonabant, MF-AEA (0.1 µM) and URB (0.1 µM) alone or in combination with 1 µM rimonabant. Sperm motility (A) and viability (B) were assessed as reported in Methods. Histograms represent mean ± SEM of three independent experiments, each in duplicate. *P < 0.05 and **P < 0.02 versus control.
In order to provide further insights on the molecular action of rimonabant, we also evaluated the phosphorylation levels of key proteins controlling cell survival such as Akt and Bcl2. Our findings indicate (Figure 2) that rimonabant alone induced the phosphorylation of both Akt and Bcl2, whereas MF-AEA and URB reduced this effect.
Figure 2.
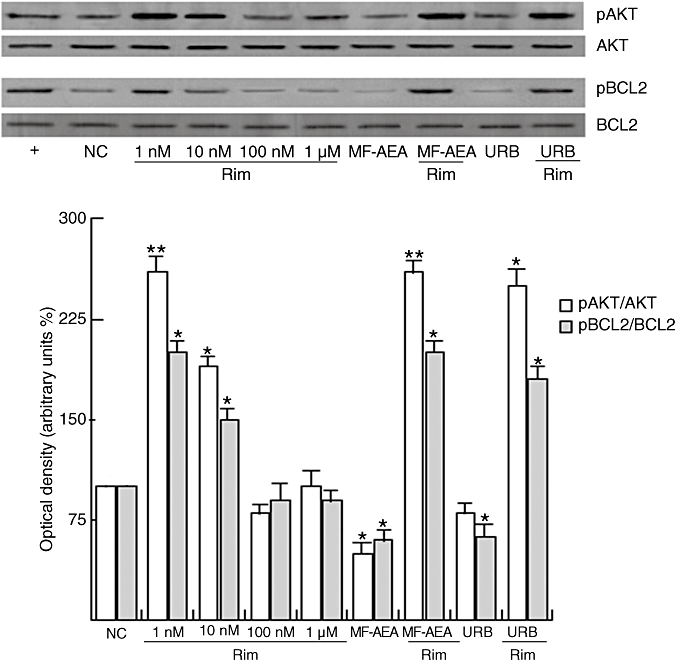
Rimonabant induces Akt and Bcl2 phosphorylation in sperm cells. Washed spermatozoa were incubated in uncapacitating medium for 30 min at 37°C and 5% CO2, in the absence (NC) or in the presence of increasing concentrations of rimonabant, MF-AEA (0.1 µM) and URB (0.1 µM) alone or in combination with 1 µM rimonabant. Capacitating medium (+) was used as a positive control. Representative Western blots of phosphoAkt (pAKT) and phosphoBcl2 (pBCL2) are shown. Densitometric analysis (mean ± SEM) of four independent experiments are reported below as pAkt/Akt and pBcl2/Bcl2 relative intensity. *P < 0.05 and **P < 0.02 versus control.
Effects of rimonabant on human sperm capacitation
To investigate the possible action of rimonabant on sperm capacitation, we studied the variation of intracellular free Ca2+ content, cholesterol efflux and tyrosine protein phosphorylation after treatment with increasing concentrations of rimonabant in the presence and absence of MF-AEA and URB. Results showed that rimonabant, up to 100 nM, produced a concentration-dependent increase in the intracellular free Ca2+ (Figure 3A). Increased cholesterol efflux (Figure 3B) was also observed upon treatment with rimonabant, even though the effect was not concentration dependent, and 10 nM was the most effective concentration. In addition, we observed an enhancement in protein tyrosine phosphorylation at 1 and 10 nM rimonabant (Figure 3C and D). All the effects induced by rimonabant were opposite to those of MF-AEA and URB (both used at concentrations of 0.1 µM) and 1 µM rimonabant fully reversed the agonist-mediated decrease of cholesterol efflux and protein tyrosine phosphorylation. Rimonabant also reversed the effect of the agonist on free Ca2+ content.
Figure 3.
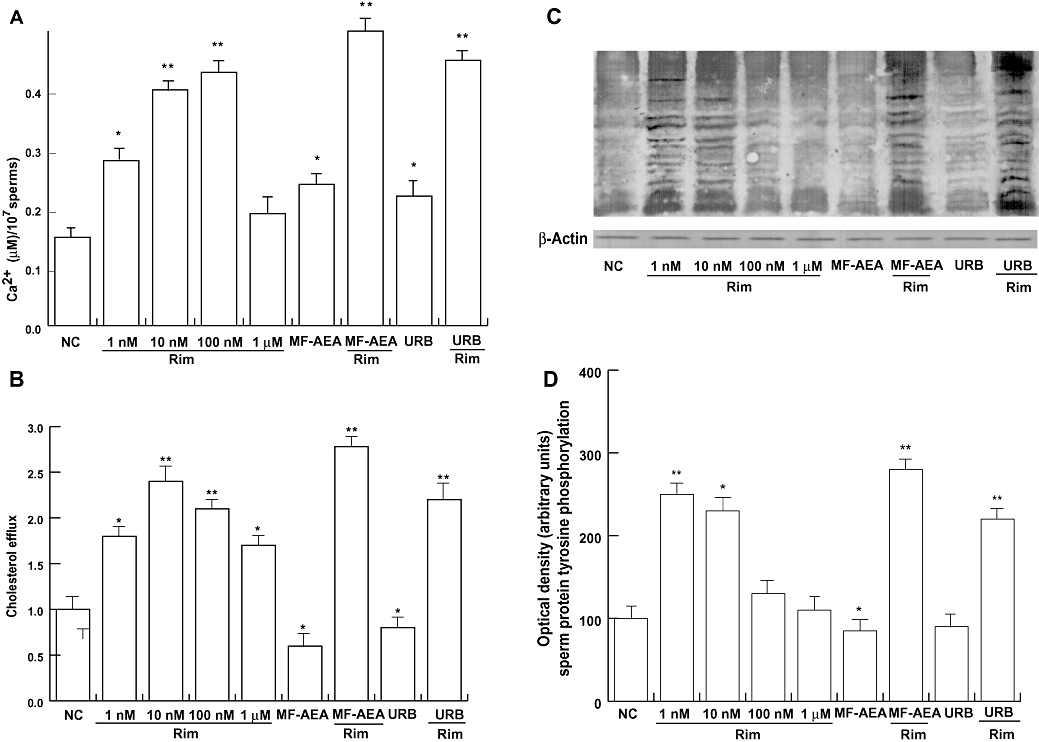
Rimonabant increases free intracellular Ca2+ cholesterol efflux and protein tyrosine phoshorylation. Sperm cells were incubated in unsupplemented Earle's medium (uncapacitating medium) for 30 min at 37°C and 5% CO2, in the absence (NC) or in the presence of increasing concentrations of rimonabant, MF-AEA and URB (0.1 µM, respectively) alone or in combination with 1 µM rimonabant. Free intracellular Ca2+ (A) and cholesterol efflux (B) were measured, and values shown represent mean ± SEM. *P < 0.05 and **P < 0.02 versus control. (C) Sperm lysates were used for Western blot analysis performed to determine protein tyrosine phosphorylation. Actin was used as a loading control. (D) Quantitative representation after densitometric evaluation of the 95 kDa band/actin. Autoradiograph presented is a representative example of independent experiments performed four times. *P < 0.05 versus control, **P < 0.02 versus control.
Rimonabant induces acrosin activity
A sperm was treated with increasing concentrations of rimonabant (1, 10 and 100 nM, and 1 µM), and incubated under uncapacitating conditions (see Methods for details) to evaluate the activity of acrosin, the major enzyme contained in the acrosome. A significant increase was produced by rimonabant, and 10 nM was the most effective concentration (Figure 4). Intriguingly, in this assay, the effect of the treatment with the CB1 receptor agonist was not different to that of rimonabant alone and rimonabant 1 µM plus 10 nM MF-AEA (i.e. the concentration at which we have already observed an effect of MF-AEA on the acrosome reaction) (Aquila et al., 2009a). Similarly, URB (0.1 µM) alone or plus rimonabant (1 µM) all increase acrosin activity.
Figure 4.
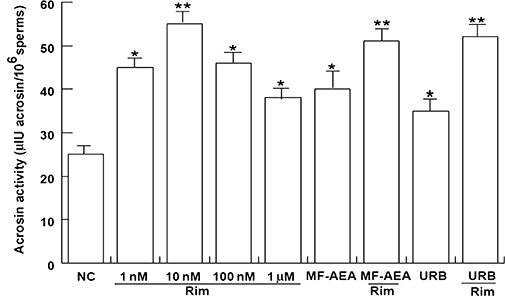
Rimonabant increases acrosin activity in human sperm. Washed spermatozoa were incubated in unsupplemented Earle's medium for 30 min at 37°C and 5% CO2, in the absence (NC) or in the presence of rimonabant (from 1 nM to 1 µM), MF-AEA (10 nM) and URB (0.1 µM) alone or in combination with 1 µM rimonabant. Acrosin activity was determined, and data shown represent mean ± SEM of three independent experiments each in duplicate. *P < 0.05 and **P < 0.02 versus control.
Rimonabant-induced effects on sperm lipid metabolism
In order to evaluate whether rimonabant had the ability to modulate sperm lipid metabolism, we first investigated its action on the intracellular content of triglycerides. Our data indicated that rimonabant decreased the triglyceride content in a concentration-independent manner (Figure 5A). Moreover, treatment with rimonabant induced an increase of lipase activity (Figure 5B) and the β-oxidation of fatty acids, as assayed by the octanoyl-CoA dehydrogenase activity (Figure 5C), within the same concentration range. MF-AEA and URB attenuated the effect of rimonabant alone (Figure 5) exerting per se a lipogenic action on human sperm cells.
Figure 5.
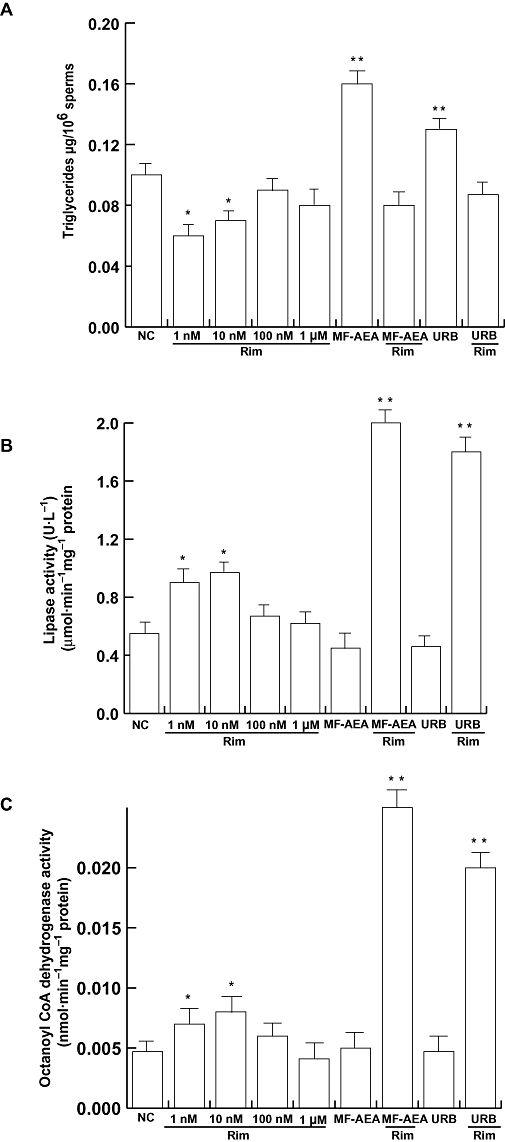
Rimonabant increases lipid metabolism in human sperm. Washed spermatozoa were incubated in uncapacitating medium for 30 min at 37°C and 5% CO2, in the absence (NC) or in the presence of rimonabant (from 1 nM to 1 µM), MF-AEA (0.1 µM) and URB (0.1 µM) alone or in combination with 1 µM rimonabant. Triglyceride content (A), lipase activity (B) and octanoyl-CoA dehydrogenase activity (C) were determined, and data shown represent mean ± SEM of four independent experiments each in duplicate. *P < 0.05 and **P < 0.02 versus control.
Rimonabant-induced effects on sperm glucose metabolism
To further investigate the potential effects of rimonabant on human sperm metabolism, we chose to evaluate expression of phosphorylated GSK3 and G6PDH activity. Rimonabant treatment was able to significantly induce G6PDH activity at 1 and 10 nM (Figure 6A). Enhancement of endogenous AEA by URB or treatment with the agonist MF-AEA at the same concentrations as used to study lipid metabolism induced no change in G6PDH activity. These effects were reversed by pretreatment with rimonabant. In terms of GSK3 (Figure 6B), at 1 nM rimonabant reduced GSK3 phosphorylation, whereas MF-AEA and URB induced the opposite effect. Also in this case, the treatment with both rimonabant and CB1 receptor agonist attenuated the effect of the agonist alone. Altogether, these data suggest that rimonabant increased glucose expenditure in human sperm.
Figure 6.
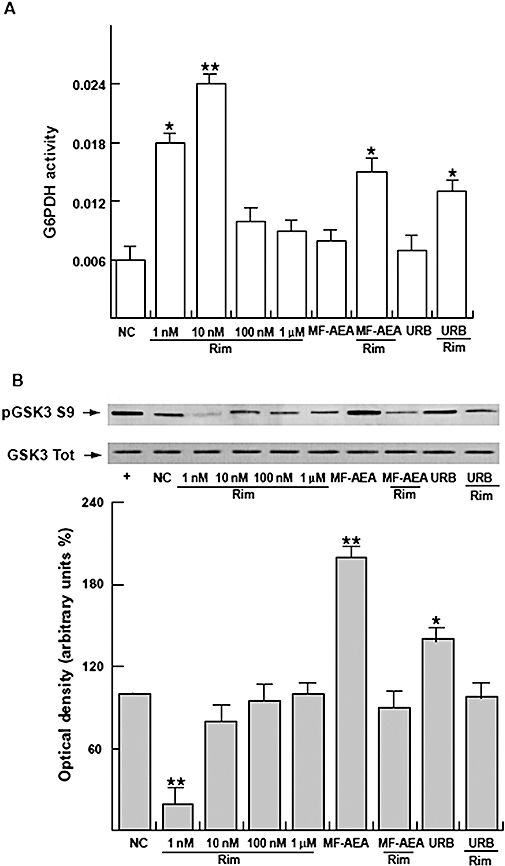
Rimonabant increases glucose metabolism in human sperm. Sperm samples, washed twice with an uncapacitating medium, were incubated in the same medium (NC) for 30 min at 37°C and 5% CO2, and treated with or without rimonabant in the presence or absence of MF-AEA and URB (0.1 µM respectively). (A) The conversion of NADP+ to NADPH catalysed by G6PDH was measured by the increase of absorbance at 340 nm every 20 s for 1.5 min. Data are expressed in nmol·min–1/106 spermatozoa, and represent mean ± SEM. *P < 0.05 and **P < 0.02 versus control. (B) Western blot and quantitative representation after densitometric evaluation of the ratio of phosphorylated GSK3/total GSK3. *P < 0.05 versus control, **P < 0.02 versus control. Autoradiograph presented is representative of three independent experiments. +, MCF7 tumour breast cancer cells used as positive control.
Discussion
The EC system is involved in mammalian reproduction, but the significance of (endo)cannabinoid signalling and the pharmacological implications of CB receptor modulation in spermatogenesis, fertilization and embryonic implantation and growth remain still largely unknown (Wang et al., 2006). An autonomous EC system has been found in sperm (Schuel et al., 1994; Rossato et al., 2005). Several reports have demonstrated CB1 receptor-mediated inhibitory effects of endocannabinoids on mammalian sperm functions (Maccarrone et al., 2005; Rossato et al., 2005; Aquila et al., 2009a,b;), supporting the idea that the physiological role of the EC system in sperm is to maintain a quiescent, uncapacitated condition before interacting with the egg (Rossato, 2008). In the present study, we clearly showed that rimonabant was able to increase human sperm motility and viability, and, at molecular level, it appears that Akt and Bcl2 proteins are involved. These enzymes control key pro-survival pathways, and the phosphatidylinositol 3′-kinase/Akt signalling pathway has also been shown to be a positive regulator of cell metabolism (Maddika et al., 2007). On the contrary, AEA reduced sperm motility and viability, and rimonabant completely reversed this action. We obtained similar effects by using the highly selective FAAH inhibitor URB597, suggesting that the blockade of CB1 receptor is involved in the observed effects. The present findings, together with our already reported evidence that the CB2 antagonist SR144528 and the vanilloid receptor antagonist capsazepine were unable to counteract AEA-mediated action on sperm survival and Akt phosphorylation (Aquila et al., 2009a), also support a CB1 receptor-mediated action as the underlying mechanism. Our results are also in agreement with previous data showing that rimonabant induced Akt activity and glucose uptake in skeletal muscle cells in a CB1-dependent manner (Esposito et al., 2008). Moreover, this evidence is corroborated by Ricci et al. (2007) who reported that sperm from CB1 receptor knockout mice showed a strong increase of motility in the head of epididymus, compared to wild-type mice.
During life, sperm exists in two different physiological conditions: a quiescent state in the seminal plasma and a capacitated state upon ejaculation and movement through the female reproductive tract. Capacitation involves numerous physiological changes including destabilization of the plasma membrane, cholesterol efflux, alterations of intracellular ion concentrations and protein phosphorylation (Yanagimachi, 1994). In order to gain further insight into the effects of rimonabant on sperm fertilization, we tested rimonabant's effects on Ca2+ concentration, cholesterol efflux and protein tyrosine phosphorylation, which are hallmarks of the capacitation status (Visconti et al., 2002; Jha et al., 2003; Suarez, 2008). Our results demonstrated a concentration-dependent increase of Ca2+ content, whereas cholesterol efflux was significantly induced until 1 µM in a concentration-independent manner. Protein tyrosine phosphorylation was also induced. These findings indicate a stimulation of the adenylate cyclase/cAMP/PKA signalling, which plays an important role not only in capacitation (Travis and Kopf, 2002; Jha et al., 2003), but also in the acrosome reaction (Salicioni et al., 2007). Moreover, our data demonstrating an increase in acrosin activity after treatment of sperm with rimonabant are in line with this finding and with previous observations showing that this pathway is inhibited by endocannabinoids in a CB1 receptor-dependent manner (Maccarrone et al., 2005; Aquila et al., 2009a). Interestingly, AEA did not decrease acrosin activity, and in accord with our previous findings, we found that acrosin was stimulated by the addition of 10 nM MF-AEA, which corresponds to the physiological concentration in mid-cycle oviductal fluid (Schuel et al., 2002). Furthermore, treatment with both MF-AEA and rimonabant potentiated, rather than reversed, the effect of MF-AEA alone. This finding is not surprising because synergic effects of MF-AEA and rimonabant have already been reported (Malfitano et al., 2008). On the other hand, we also demonstrated that treatment with MF-AEA plus rimonabant increased free Ca2+ content in human sperm, even though MF-AEA alone induced similar effects (Aquila et al., 2009b).
The interaction between energy balance and reproduction is subject of intensive investigations, and ATP generation is an important process in human sperm especially after the initiation of capacitation (Yanagimachi, 1994; Miki, 2007). Capacitated sperm displays an increased metabolic rate and overall energy expenditure, presumably to effect the changes in sperm signalling and function during capacitation. It appears that each event characterizing male fertilization requires different substrates and activates different metabolic pathways. For example, the acrosome reaction requires lactate or pyruvate for ATP production by oxidative phosphorylation, while successful gamete fusion requires glucose to produce NADPH through the pentose phosphate pathway (PPP) (Urner and Sakkas, 1999a; Miki, 2007). From this point of view, we investigated whether the capacitated status and increased motility induced by rimonabant would also involve specific changes in lipid and glucose metabolism. We found that the compound induced human sperm energy expenditure by stimulating lipase and octanoyl-CoA dehydrogenase activities concomitantly with reducing the level of triglycerides. As for glucose metabolism, we observed an enhanced G6PDH activity and a reduced phosphorylation of GSK3 at 1 and 10 nM rimonabant concentrations, also addressing to an induction of energy consumption. GSK3 is known to be implicated in the storage of glucose to form glycogen in mammalian sperm (Ballester et al., 2000; Andò and Aquila, 2005; Aquila et al., 2005). Particularly, in uncapacitated sperm, GSK3 is tightly blocked and phosphorylated, whereas during capacitation there is a de-phosphorylation and then an activation of the enzyme. G6PDH, the rate-limiting enzyme in the PPP that catalyses the oxidation of glucose 6-phosphate and regulates the production of NADPH by controlling glucose metabolism (Urner and Sakkas, 1999b) is crucial for sperm-fertilizing ability (Urner and Sakkas, 1999a; Travis et al., 2001; Urner et al., 2001). Metabolic fluxes between glycolysis and the PPP are also particularly relevant for maintaining the mitochondrial transmembrane potential, and balancing cellular requirements for energy and the production of reactive oxygen intermediates (Perl, 2007), and it has been reported that cannabinoid receptor agonists inhibit mitochondrial membrane potential and ATP production in rat testicular tissue (see Rossato, 2008). Our findings demonstrating that nanomolar concentrations of rimonabant favour G6PDH activity are in line with the observation that AEA, unlike rimonabant, is able to affect human sperm metabolism by exerting a lipogenic effect and favouring the accumulation of energy substrates (Aquila et al., 2009b). Moreover, the effect of the enhancement of endogenous AEA by URB-mediated action and the treatment with MF-AEA at the concentration used to study lipid metabolism, supported the hypothesis that rimonabant, through action at CB1 receptors, induced glucose metabolism in sperm.
It is well known that the EC system contributes to the physiological regulation of energy balance, and rimonabant reduces food intake inducing favourable changes in lipid and glucose metabolism in obese patients, as well as in animal models (see Bifulco et al., 2007; 2009;). Moreover, endocannabinoids are regulated negatively by leptin and ob/ob and db/db mice, characterized by an impairment of leptin signalling are obese, infertile and fail to undergo normal sexual maturation (Chehab, 2000; Di Marzo et al., 2001). In ob/ob mice, exogenous leptin restored fertility (Chehab et al., 1996), and it has been demonstrated that leptin controls AEA degradation, but not its binding to the CB1 receptor. Rimonabant induced a significant decrease in food intake and body weight loss in ob/ob mice, but failed to improve female fertility when administered alone (Maccarrone et al., 2004). These results, together with ours, suggest that CB1 receptor modulation might be important in human fertility, and rimonabant might be used to develop special media to improve in vitro fertilization.
In addition, rimonabant, by acting as a CB1 antagonist, was able, at 0.1 µM, to reverse the negative effects of AEA on boar spermatozoa (Maccarrone et al., 2005), a finding in agreement with those we have obtained in human spermatozoa. In the majority of the tests we performed, rimonabant was active at concentrations in the range of the highest affinity for the CB1 receptor (1–20 nM) (Rinaldi-Carmona et al., 1994; Pertwee, 2005), and had opposite effects compared to those produced by the exogenous and endogenous AEA. Besides, the tested compound attenuated the effect of the agonist even though an enhancement of the antagonist-induced effect can be observed in the presence of both CB1 receptor agonist and antagonist. Moreover, above 100 nM, we did not observe any significant effects of rimonabant, suggesting that starting from this concentration rimonabant could display neutral antagonism, while, in combination with MF-AEA, it behaves as an inverse agonist. Rimonabant has been shown to act as a neutral antagonist, competitive antagonist and inverse agonist (Hurst et al., 2005; Pertwee, 2005). As an inverse agonist, rimonabant produces effects in some CB1 receptor containing bioassay systems that are opposite in direction from those produced by agonists for these receptors. It was proposed that inverse agonism at the CB1 receptor may be explained in terms of a three-state model in which the receptor can switch between two conformational states, a ground or inactive R state and an active R* state, which are in equilibrium with each other (Leff, 1995). An agonist has higher affinity for R*, and agonist binding is thought to shift the equilibrium towards R*, resulting in G protein activation with an increase in GDP/GTP exchange. An inverse agonist has higher affinity for R and its binding shifts the equilibrium towards R, resulting in a decrease in the activation of the signalling pathway. The binding of a neutral/null antagonist is thought not to alter the equilibrium between R and R*, because the neutral antagonist has equal affinity for both states. It is likely that the efficacy for the production of inverse cannabimimetic effects will be governed by the degree of endocannabinoid release at CB1 receptors (Aquila et al., 2009b).
Finally, we have to point out that in our experimental conditions, rimonabant is able to antagonize endocannabinoid-mediated inhibition of human sperm functions, in agreement with those obtained earlier in boar sperm by Maccarrone et al. (2005), in frog sperm by Cobellis et al. (2006) and human sperm by Rossato et al. (2005).
In conclusion, in the present study, we demonstrate for the first time that rimonabant is able to improve fertilization by the human male gamete, and stimulates energy expenditure in sperm.
Acknowledgments
This work was supported by Sanofi-Aventis (M.B.), MURST ex 60% (2007), and the Associazione Educazione e Ricerca Medica Salernitana. Our special thanks to Dr Vincenzo Cunsolo (Biogemina Italia Srl, Catania, Italy) for technical and scientific assistance. We also thank Serena and Maria Clelia Gervasi for the English language review of the manuscript.
Glossary
Abbreviations:
- AEA
anandamide, N-arachidonoylethanolamine
- CB receptor
cannabinoid receptor
- DGGR
1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester
- EC
endocannabinoid
- FAAH
fatty acid amide hydrolase
- G6PDH
glucose-6-phosphate dehydrogenase
- GSK3
glycogen synthase kinase 3
- MF-AEA
2-methylarachidonyl-2′-fluoro-ethylamide
- PPP
pentose phosphate pathway
- SR141716
rimonabant (N-(piperidino-1-yl)-5-(4-chlorophenyl)-1-(2,4dichlorophenyl)-4-methyl-pyrazole-3-carboxamide)
- THC
delta-9-tetrahydrocannabinol
- URB
URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate)
- ZP
zona pellucida
Conflict of interest
Authors declare that there is no conflict of interest to disclose.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Goebel N, Conkright MD, Inoue H, Xie J, Arias CM, et al. The Creb1 coactivator Crtc1 is required foe energy balance and fertility. Nat Med. 2008;14:1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andò S, Aquila S. Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Mol Cell Endocrinol. 2005;245:1–6. doi: 10.1016/j.mce.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87:3385–3390. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, et al. Towards a physiological role for cytocrome P450 aromatase in ejaculated human sperm. Hum Reprod. 2003;18:1650–1659. doi: 10.1093/humrep/deg340. [DOI] [PubMed] [Google Scholar]
- Aquila S, Gentile M, Middea E, Catalano S, Andò S. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology. 2005;146:552–557. doi: 10.1210/en.2004-1252. [DOI] [PubMed] [Google Scholar]
- Aquila S, Bonofiglio D, Gentile M, Middea E, Gabriele S, Belmonte M, et al. Peroxisome proliferator-activated receptor (PPAR) gamma is expressed by human spermatozoa: its potential role on the sperm physiology. J Cell Physiol. 2006;209:977–986. doi: 10.1002/jcp.20807. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Perrotta I, Santoro A, Laezza C, Bifulco M, et al. Human sperm anatomy: ultrastructural localization of the cannabinoid1 receptor (CB1-R) and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec. 2009a doi: 10.1002/ar.21042. in press. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Laezza C, Santoro A, Pezzi V, Panza S, et al. A new role of anandamide in human sperm: focus on metabolism. J Cell Physiol. 2009b;221:147–153. doi: 10.1002/jcp.21837. [DOI] [PubMed] [Google Scholar]
- Ballester J, Fernandez-Novell JM, Rutllant J, Garcia-Rocha M, Jesus Palomo M, Mogas T, et al. Evidence for a functional glycogen metabolism in mature mammalian spermatozoa. Mol Reprod Dev. 2000;56:207–219. doi: 10.1002/(SICI)1098-2795(200006)56:2<207::AID-MRD12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008;20:850–857. doi: 10.1111/j.1365-2826.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Grimaldi C, Gazzerro P, Pisanti S, Santoro A. Rimonabant: just an antiobesity drug? Current evidence on its pleiotropic effects. Mol Pharmacol. 2007;71:1445–1456. doi: 10.1124/mol.106.033118. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Santoro A, Laezza C, Malfitano AM. Cannabinoid receptor CB1 antagonists state of the art and challenges. Vitam Horm. 2009;81:159–189. doi: 10.1016/S0083-6729(09)81007-8. [DOI] [PubMed] [Google Scholar]
- Chehab FF. Leptin as a regulator of adipose mass and reproduction. Trends Pharmacol Sci. 2000;21:309–313. doi: 10.1016/s0165-6147(00)01514-5. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Cobellis G, Cacciola G, Scarpa D, Meccariello R, Chianese R, Franzoni MF, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol Reprod. 2006;75:82–89. doi: 10.1095/biolreprod.106.051730. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Ergetova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito I, Proto MC, Gazzerro P, Laezza C, Miele C, Alberobello AT, et al. The cannabinoid CB1 receptor antagonist rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol Pharmacol. 2008;74:1678–1686. doi: 10.1124/mol.108.049205. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollerau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, et al. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol. 2005;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- Jha KN, Kameshwari DB, Shivaji S. Role of signaling pathways in regulating the capacitation of mammalian spermatozoa. Cell Mol Biol. 2003;49:329–340. [PubMed] [Google Scholar]
- Kennedy WP, Kamisky JM, Van der Ven HH, Jeyendran RS, Reid DS, Blackwell J, et al. A simple, classical assay to evaluate the acrosin activity of human spermatozoa. J Androl. 1989;10:221–231. doi: 10.1002/j.1939-4640.1989.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Lehman TC, Hale DE, Bhala A, Thorpe C. An acyl-coenzyme A dehydrogenase assay utilizing the ferricenium ion. Annal Biochem. 1990;186:280–284. doi: 10.1016/0003-2697(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Maccarrone M. CB2 receptor in reproduction. Br J Pharmacol. 2008;153:189–198. doi: 10.1038/sj.bjp.0707444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agrò A. Anandamide hydrolase: a guardian angel of human reproduction? Trends Pharmacol Sci. 2004;25:353–357. doi: 10.1016/j.tips.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Fride E, Bisogno T, Bari M, Cascio MG, Battista N, et al. Up-regulation of the endocannabinoid system in the uterus of leptin knockout (ob/ob) mice and implications for fertility. Mol Hum Reprod. 2004;11:21–28. doi: 10.1093/molehr/gah130. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Barboni B, Bernabò N, Gasperi V, Pistilli MG, Fezza F, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci. 2005;118:4393–4404. doi: 10.1242/jcs.02536. [DOI] [PubMed] [Google Scholar]
- Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Malfitano AM, Laezza C, Pisanti S, Gazzerro P, Bifulco M. Rimonabant (SR141716) exerts anti-proliferative and immunomodulatory effects in human peripheral blood mononuclear cells. Br J Pharmacol. 2008;153:1003–1010. doi: 10.1038/sj.bjp.0707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mascia MS, Sanna F, Melis T, Castelli MP, et al. The cannabinoid receptor antagonist SR-141716A induces penile erection in male rats: involvement of paraventricular glutamic acid and nitric oxide. Neuropharmacology. 2006;50:219–228. doi: 10.1016/j.neuropharm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Miki K. Energy metabolism and sperm functions. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- Panteghini M, Bonora R, Pagani F. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem. 2001;38:365–370. doi: 10.1258/0004563011900876. [DOI] [PubMed] [Google Scholar]
- Perl A. The pathogenesis of transaldolase deficiency. IUBMB Life. 2007;59:365–373. doi: 10.1080/15216540701387188. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G, Cacciola G, Altucci L, Meccariello R, Pierantoni R, Fasano S, et al. Endocannabinoid control of sperm motility: the role of epididymus. Gen Comp Endocrinol. 2007;153:320–322. doi: 10.1016/j.ygcen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Healume M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rossato M. Endocannabinoids, sperm functions and energy metabolism. Mol Cell Endocrinol. 2008;286S:S31–S35. doi: 10.1016/j.mce.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor CB1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab. 2005;90:984–991. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, et al. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil. 2007;(Suppl 65):245–259. [PubMed] [Google Scholar]
- Schuel H, Schuel R, Zimmerman AM, Zimmerman S. Cannabinoids reduce fertility of sea urchin sperm. Biochem Cell Biol. 1987;65:130–136. doi: 10.1139/o87-018. [DOI] [PubMed] [Google Scholar]
- Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci USA. 1994;91:7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, et al. Evidence that Anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev. 2002;63:376–387. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- Succu S, Mascia MS, Sanna F, Melis T, Argiolas A, Melis MR. The cannabinoid CB1 receptor antagonist SR141716 induces penile erection by increasing extra-cellular glutamic acid in the paraventricular nucleus of male rats. Behav Brain Res. 2006;169:274–281. doi: 10.1016/j.bbr.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Thomson MF, Wishart GJ. Elucidation of the mechanism responsible for the temperature-dependent reversible inactivation of the motility of fowl spermatozoa. Br Poult Sci. 1989;30:687–692. doi: 10.1080/00071668908417191. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110:731–736. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, et al. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276:7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. A possible role for the pentose phosphate pathway of spermatozoa in gamete fusion in the mouse. Biol Reprod. 1999a;60:733–739. doi: 10.1095/biolreprod60.3.733. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. Characterization of glycolysis and pentose phosphate pathway during sperm entry into the mouse oocyte. Biol Reprod. 1999b;60:973–978. doi: 10.1095/biolreprod60.4.973. [DOI] [PubMed] [Google Scholar]
- Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod. 2001;64:1350–1357. doi: 10.1095/biolreprod64.5.1350. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53:133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK, Maccarrone M. Jekill and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev. 2006;27:427–448. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interactions. 4th edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press Ltd; 1994. pp. 189–317. [Google Scholar]


