Abstract
Background and purpose:
Elephantopus scaber L. (Asteraceae) is a traditional herbal medicine with anti-cancer effects. We evaluated the in vitro and in vivo efficacy of a major sesquiterpene lactone constituent of E. scaber, deoxyelephantopin (DET), against mammary adenocarcinoma and the underlying molecular mechanism.
Experimental approach:
A variety of cellular assays, immunoblotting and immunohistochemistry, as well as both orthotopic and metastatic TS/A tumour models in BALB/c mice, were used. Test mice were pretreated and post-treated with DET or paclitaxel and mammary tumour growth evaluated.
Key results:
DET (≤2 µg·mL−1) significantly inhibited colony formation, cell proliferation, migration and invasion of TS/A cells and induced G2/M arrest and apoptosis in TS/A cells. c-Jun N-terminal kinase-mediated p21Waf1/Cip1 expression and caspase activation cascades were up-regulated by DET, effects suppressed by N-acetyl-L-cysteine. Moreover, tumour necrosis factor α-induced matrix metalloproteinase-9 enzyme activity and expression and nuclear factor-kappa B activation were abolished by DET. Pretreatment with DET was more effective than paclitaxel, for profound suppression of orthotopic tumour growth (99% vs. 68% reduction in tumour size) and lung metastasis of TS/A cells (82% vs. 63% reduction in metastatic pulmonary foci) and prolonged median survival time (56 vs. 37 days, P < 0.01) in mice. The levels of cyclooxygenase-2 and vascular endothelial growth factor in metastatic lung tissues of TS/A-bearing mice were attenuated by DET.
Conclusions and implications:
Our data provide evidence for the suppression of mammary adenocarcinoma by DET with several mechanisms and suggest that DET has potential as a chemopreventive agent for breast cancer.
Keywords: Elephantopus scaber, deoxyelephantopin, chemoprevention, mammary adenocarcinoma, lung metastasis
Introduction
Natural products are important players in medical research and development and have been used for treatment or prevention of various human diseases throughout history (Newman and Cragg, 2007). Because of the high mortality rate of most cancers and the difficulty in treating the diseases by standard pharmacological means, nutrient and non-nutrient phytocompounds have recently been explored for their potential preventive effects against cancer (Surh, 2003; Singh et al., 2006). An essential approach in cancer control is chemoprevention – the use of natural products or synthetic agents to prevent, interrupt or reverse the carcinogenic process or to reduce disease recurrence (Sporn and Suh, 2002; Tsao et al., 2004). Phytocompounds with antioxidant, anti-inflammatory, cell cycle modulatory, apoptotic, anti-tumour immune-escape or antiangiogenic activities have therapeutic potential in cancer chemoprevention (Coussens and Werb, 2002; Sun et al., 2004; Albini et al., 2005). Currently, several phytocompounds with anti-inflammatory or antiangiogenic activities (e.g. curcumin and epigallocatechin gallate) are in or are close to clinical trials of their chemoprevention for various cancers (Fan et al., 2006; Thomasset et al., 2007). Chinese cabbage, one of the most popular crucifers consumed worldwide, has significant bioactivity against tumorigenesis and tumour growth via the action of constituent multifunctional brassinin derivatives (Banerjee et al., 2008).
Breast cancer is the most frequent tumour, with an alarming year-by-year increase in incidence, and is one of the main causes of death by cancer in women. Conventionally, breast cancer treatment regimens provide acceptable response rates, disease control and patient survival; however, both systemic adjuvant chemotherapy and hormone therapy still remain palliative treatments for patients with metastatic tumours and cause severe side effects (Longo et al., 2007). The genetic complexity of cancers suggests the use of novel multifunctional rather than monofunctional preventive or treatment agents (Liby et al., 2007). Therefore, the pressing need for such anti-cancer agents has spurred the search for phytocompounds from medicinal or edible plants with novel modes of action.
Elephantopus scaber L. (Asteraceae) is a popular perennial medicinal plant, anecdotally effective for diuresis, infection and hepatitis. The antiviral and hepatoprotective effects of E. scaber extracts have been investigated (Rajesh and Latha, 2001; Li et al., 2004). Recently, two germacranolide sesquiterpene lactones (SLs), major components of extracts of E. scaber have been shown to possess anti-cancer activity. Isodeoxyelephantopin potentiates tumour necrosis factor (TNF)-α-induced apoptosis in leukemia KBM-5 cells (Ichikawa et al., 2006), and its isomer deoxyelephantopin (DET) inhibited HeLa cell activity in vivo in xenografted mice (Xu et al., 2006; Zou et al., 2008). However, whether DET is efficacious for breast cancer is unknown.
In this study, we elucidate the novel in vitro and in vivo cancer chemopreventive properties and the underlying molecular mechanisms of DET against the proliferation and invasion of TS/A cells, a murine mammary adenocarcinoma cell line. DET suppressed TS/A tumour growth and lung metastasis, which led to increased overall survival in mice bearing mammary tumours and was more effective than the chemotherapeutic drug paclitaxel used to treat breast cancer.
Methods
Cell lines and culture conditions
TS/A (a gift from Dr Ning-Sun Yang of the Agriculture Biotechnology Research Center, Academia Sinica, Taiwan), a murine mammary adenocarcinoma cell line; MCF-7 (American Type Culture Collection; ATCC, Manassas, VA, USA), a human breast adenocarcinoma cell line; and CCD966SK (ATCC), a human breast skin fibroblast cell line, were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Grand Island, NY, USA)). MDA-MB-231 (ATCC), a metastatic human breast cancer cell line, was cultured in DMEM-F12 medium, and H184B5F5/M10 (ATCC), a non-cancerous human mammary epithelial cell line, was grown in minimum essential medium (MEM; Life Technologies). All cell lines were grown in specific media supplemented with 10% fetal bovine serum (FBS), 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin (Invitrogen, Carlsbad, CA, USA), in a humidified 5% CO2 incubator at 37°C.
Animals
All animal care and experimental procedures followed the institutional guidelines for the care and use of animals. Female BALB/c mice (National Laboratory Animal Center, Taipei, Taiwan) were given a standard laboratory diet and distilled water ad libitum and kept on a 12 h light/dark cycle at 22 ± 2°C.
Cell proliferation, colony-forming and cell cycle analyses, immunoblotting and electrophoretic mobility shift assay (EMSA)
In cell proliferation assay, TS/A, MCF-7, MDA-MB-231, M10 and CCD966SK cells were cultured in 96-well plates at 1 × 104 cells·well−1 and allowed to adhere overnight, then were treated for 48 h with vehicle (0.1% dimethyl sulphoxide; DMSO) or DET. Cell growth was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)-based colorimetric assays according to Chiang et al. (2005).
For colony-forming assay, TS/A cells (400 cells·well−1) were cultured in a six-well plate for 6 days, then treated with vehicle, DET or paclitaxel for 3 days. Cell colonies were fixed and stained in 0.1% crystal violet solution; then the deposited crystal violet was dissolved in 20% acetic acid before quantitation by absorbance at 595 nm.
Analysis of cell cycle was carried out as described by Shyur et al. (2004). TS/A cells (2 × 105 cells·mL−1) were synchronized by incubation in medium containing 1% FBS for 12 h. The low-serum (1% FBS) medium was replaced by medium containing 10% FBS, and the TS/A cells were treated with vehicle or DET for 48 h. Both adherent and floating cells were collected, washed with phosphate-buffered saline (PBS) and fixed with 1 mL of ice-cold 70% ethanol overnight at 4°C. Cells were stained with 0.2 mg·mL−1 propidium iodide in darkness for 30 min at room temperature and analysed on flow cytometry (Coulter Epics XL; Beckman Coulter, Miami, FL, USA).
Western blot analysis followed the procedures described by Chiang et al. (2005). Protein content was measured by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were resolved by 5–20% gradient SDS-PAGE and then were immunoblotted by use of enhanced chemiluminescence reagents (Amersham, Arlington Heights, IL, USA).
Electrophoretic mobility shift assay was conducted using a LightShift Chemiluminescent EMSA kit (Pierce Biotechnology, Rockford, IL, USA) following a published method (Hou et al., 2007).
Cancer cell metastasis assays
For wound healing assay, TS/A cells were cultured in a six-well plate until they formed a confluent monolayer, then a wound (width 2 mm) was created with a standard 2 mm wide strip. TS/A cells were treated with vehicle, DET or paclitaxel for 2 days. Images were recorded at 0 h (immediately after wounding) and 48 h after compound treatment, at 100× original magnification by inverted microscopy (Axiovert 200M; Zeiss, Jena, Germany). The mean wound area is expressed as percent of recovery (% R).
Cell migration and invasion assays involved use of a modified Boyden chamber (6.5 mm diameter, 8 µm pore size; Costar, Cambridge, MA, USA); the upper surface of the chamber was coated or not, with 50 µL of Matrigel (Chemicon, Temecula, CA, USA). The lower well was coated with a thin layer of Matrigel (250 µL) as a chemoattractant and filled with culture medium containing DET (2 µg·mL−1) or vehicle. TS/A cells were placed into the upper chamber (1 × 105 cells per insert) and incubated at 37°C for 48 h. Non-migrating or non-invading cells were scraped from the upper surface of the membrane using a cotton swab, and the remaining cells were fixed, permeabilized with 0.1% Triton X-100 and stained with dye (4,6-diamidino-2-phenylindole; DAPI). The migrating or invading cells remaining in the well were counted in six randomly chosen low-power fields at 100× original magnification by inverted fluorescence microscopy.
Analysis of gelatin zymography of matrix metalloproteinase (MMP)-2 and MMP-9 activities followed Lin et al. (2008a). The TS/A cells were seeded and allowed to grow to confluence for 24 h and then treated with DET (2 µg·mL−1) for 1 h followed by TNF-α (1 ng·mL−1) for an additional 24 h in serum-free medium. After the treatments, the conditioned medium were collected, concentrated 50-fold using a Nanosep 10K centrifugal device (Pall Corporation, Ann Arbor, MI, USA), then mixed with non-reducing sample buffer and subjected to SDS-PAGE with 10% polyacrylamide gels copolymerized with 1 mg·mL−1 gelatin. Gels were rinsed in washing buffer (50 mM Tris–HCl, pH 7.5, 2.5% Triton X-100) at room temperature for 1 h and incubated overnight at 37°C in zymogram development buffer (50 mM Tris–HCl, pH 7.5, 10 mM CaCl2 and 150 mM NaCl). Gels were fixed and stained with filtrated 0.1% Coomassie blue R250. After destaining, gelatinolytic signals were quantified by densitometry. Gelatinolytic activity was visualized as a clear band against a dark background of stained gelatin. MMP-2 and MMP-9 activity was detected by a clear band appearing at 72 and at 92 kDa respectively.
Immunofluorescent cell staining
TS/A cells were seeded on 12 mm glass slips in 24-well plates for 24 h, then co-treated with vehicle or DET with or without TNF-α (1 ng·mL−1) for 30 min. Cells were fixed, permeabilized and counterstained with DAPI and α-tubulin, or anti-p65, cleaved caspase-3, or phospho-c-Jun antibody, then visualized with goat anti-mouse FITC-labelled or goat anti-rabbit Cy3-labelled secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA). The nuclear translocation of nuclear factor-kappa B (NF-κB)(p65) was visualized at 400× original magnification by fluorescent microscopy (Nikon Eclipse E800), and image analysis followed the manufacturer's software.
Measurement of reactive oxygen species (ROS)
TS/A cells were treated with 2 µg·mL−1 DET for 2 h, then were incubated with 25 µM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFH-DA) for 30 min. After incubation, cells were collected, washed with PBS, resuspended in PBS and then analysed on flow cytometry.
Inhibition of mammary tumour growth in BALB/c mice
The mammary fat pad region of mice was injected with 1 × 106 TS/A cells on day 0. The tumour volume (V) was measured with calipers once a week for 4 weeks and calculated by the formula V= (L×W2)/2, where L is the length and W is the width of tumour. The time course of this experimental design is shown in Figure S1A. Mice were assigned to eight groups. Paclitaxel (5 mg·kg−1) and DET (1 and 10 mg·kg−1) dissolved in DMSO were injected i.p. in a volume of 1 mL·kg−1 every day. Sham control mice were given vehicle i.p. or p.o. throughout the experimental period. In the preventive groups, test mice were pretreated i.p. with paclitaxel (pre-paclitaxel) or DET (pre-DET) at the indicated doses every day, starting at 14 days before the injection of tumour cells. In the therapeutic groups, vehicle (tumour control), paclitaxel or DET were i.p. injected every day for 28 days beginning when the injected tumour cells grew to 55–105 mm3 (∼1 week). At the end of the study, mice were killed by cervical dislocation. Tumours were removed, fixed with 10% buffered formalin and examined visually and microscopically for growth of tumour cells.
The effects on mammary tumour growth of p.o. administered DET were examined. The time course of this experimental design is shown in Figure S1B. Tumour volumes were measured with calipers every 3 days beginning on day 7. Mice were assigned to 10 groups. Paclitaxel and DET were dissolved in 4% DMSO. Paclitaxel (5 mg·kg−1) and doses of DET (2, 10 and 50 mg·kg−1) were delivered p.o. in a volume of 25 mL·kg−1 every 2 days. All control mice were treated in parallel with vehicle. Test mice were pretreated with p.o. doses of paclitaxel or DET every 2 days, starting from day −14, for 2 weeks before tumour cell inoculation. In parallel, test mice were inoculated with tumour cells at day 0 and treated with vehicle, paclitaxel or DET 1 day later (day 1). At the end of the experiment (day 31), tumours were removed, fixed in 10% buffered formalin and then embedded in paraffin. Paraffin-embedded tumours were sectioned (4 µm) for haemotoxylin and eosin (H&E) staining and for immunofluorescent staining with DAPI, mouse proliferating cell nuclear antigen (PCNA) antibody or rabbit CD31 antibody visualized with goat anti-mouse or goat anti-rabbit Cy3-labelled secondary antibodies (Jackson ImmunoResearch) (Lin et al., 2008b). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay was used to detect in situ apoptotic cells according to the manufacturer's protocol (Chemicon). The mean proportions of CD31-positive areas and positive PCNA and TUNEL cells were measured by use of AxioVision software (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA).
Inhibition of lung metastasis of TS/A cells
The time course of this experimental design is shown in Figure S1C. Mice were assigned to 10 groups, including a sham control group, a tumour control group, a pre-paclitaxel group (pre-paclitaxel-5), pre-DET-2, DET-5 and DET-10 groups, a paclitaxel group (paclitaxel-5), and DET-2, DET-5 and DET-10 groups, after tumour cell inoculation through the tail vein. The lungs were removed and were processed for H&E staining or immunostaining with vascular endothelial growth factor (VEGF) or cyclooxygenase-2 (COX-2) antibody as described previously (Lin et al., 2008b). The number of tumour colonies in left lungs of test mice was counted.
Data analysis
All data are expressed as means ± SEM. For the survival data, the log-rank test was used to determine differences between drug- or compound-treated groups and the tumour control group. For other experiments, differences between treatments were determined by anova. A P < 0.05 was considered statistically significant.
Isolation and structure elucidation of DET
Approximately 5.0 kg of dried whole E. scaber L. (Asteraceae) (voucher specimen ES001 deposited in the Agricultural Biotechnology Research Center, Academia Sinica, Taiwan) was extracted with boiling water for 2 h, then the crude extract was partitioned with ethyl acetate (EtOAc). The EtOAc extract was chromatographed on a silica gel column with CHCl3/EtOAc eluting solvents. Subfraction-5 eluted with CHCl3/EtOAc (6:1, v/v) exhibiting potent bioactivity was then purified on an RP-C18 silica-gel column eluted with 50% methanol to obtain pure white crystals of deoxyelephantopin (designated DET, Figure 1A). The structure of DET was elucidated by electrospray ionization mass spectrometry (ThermoFinnigan LCQ, San Jose, CA, USA) and 1H and 13C NMR (Brüker ADVANCE 500 AV) spectrometry and confirmed by comparison of the spectral data with previously published results (But et al., 1997).
Figure 1.
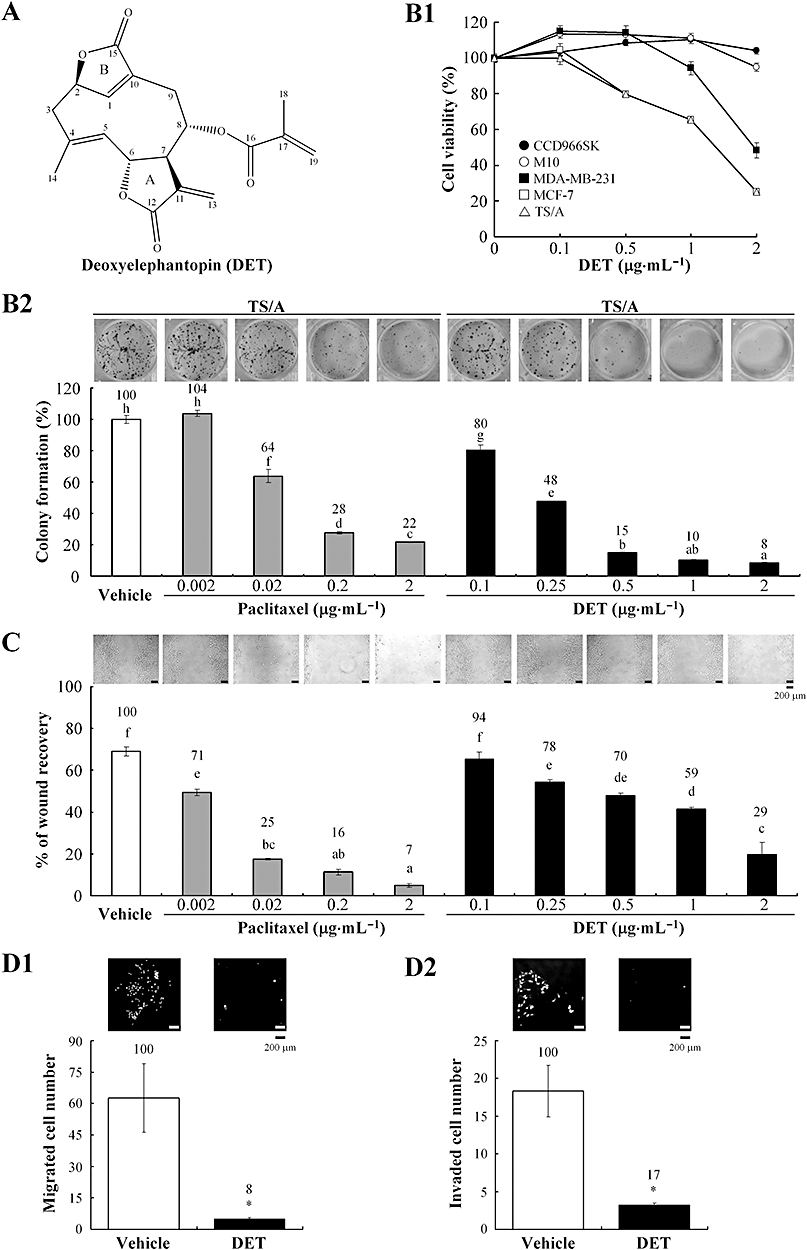
Effect of deoxyelephantopin (DET) on cell proliferation, colony formation, wound healing, migration and invasion of TS/A cells. (A) Chemical structure of DET. (B1) CCD966SK, M10, MDA-MB-231, MCF-7 and TS/A cells were treated with vehicle (0.1% dimethyl sulphoxide) or the indicated concentrations of DET at 37°C for 48 h, and cell viability (%) was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. (B2) Colony formation of TS/A cells was determined in a clonogenic assay. (C) Wound healing of TS/A cells treated with or without DET or paclitaxel at 37°C for 48 h; wound recovery was measured as a percentage of original wound size. (D1) and (D2) Migration and invasion analyses of TS/A cells. Data are mean ± SEM of six independent experiments. Different letters indicate significant differences (one-way anova). *P < 0.05.
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DAPI and paclitaxel (Taxol®) were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and H2DCFH-DA was from Molecular Probes (Invitrogen). TNF-α (recombinant mouse type) was from R&D Systems (Minneapolis, MN, USA). Silica gel, RP-18 F254 TLC plates (Merck, Darmstadt, Germany) and RP-18 silica gel (Cosmosil, Kyoto, Japan) were used. All other chemicals and solvents were of reagent or HPLC grade.
Primary antibodies against α-tubulin (Oncogene Science, Cambridge, MA, USA), phospho-Cdk1 (BioSource International, Hopkinton, MA, USA), cyclin B1 and p27Kip1 (Medical & Biological Laboratories, Nagoya, Japan), COX-2 (Cayman, Ann Arbor, MI, USA), PCNA, caspase-7 and cytochrome c (BD Pharmingen, San Diego, CA, USA), poly(ADP-ribose) polymerase (PARP), cleaved caspase-3, phospho-c-Jun and MMP-9 (Cell Signaling Technology, Danvers, MA, USA) and CD31 (Abcam, Cambridge, MA, USA) were used. All other antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The nomenclature of drug and molecular targets in this article conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Results
DET inhibits TS/A cell proliferation, migration and invasion
Figure 1B1 shows that the viability of mammary cancer TS/A, MDA-MB-231 and MCF-7 cells was significantly suppressed, with IC50 1–2 µg·mL−1 (2.9–5.8 µM), whereas normal mammary M10 and CCD966SK control cells remained almost 100% viable after 48 h treatment with DET. Colony formation assay further revealed that 0.1–2 µg·mL−1 DET greatly suppressed TS/A cell growth, with 20–92% reduction as compared with vehicle control. Paclitaxel, a microtubule-stabilizing chemotherapeutic drug (0.02–2 µg·mL−1), also inhibited TS/A cell growth (Figure 1B2). In comparison, paclitaxel had a greater effect than did DET on inhibiting colony forming of TS/A cells at lower concentrations, 0.02–0.2 µg·mL−1, but the inhibition with DET at higher concentrations (1–2 µg·mL−1) was greater. This result shows that DET specifically inhibits mammary cancer cell proliferation, a key anti-neoplastic feature.
Important in metastasis is horizontal and vertical migration of cancer cells. DET at 0.25–2 µg·mL−1 and paclitaxel at 0.002–2 µg·mL−1 significantly inhibited migration and wound closure of TS/A cells concentration-dependently, after 48 h treatment (Figure 1C). On trans-well migration assay, treatment with DET (2 µg·mL−1) for 48 h almost abolished TS/A cell migration, compared with the vehicle control (Figure 1D1). Trans-well invasion assay (Figure 1D2) further revealed that DET inhibited invasion of TS/A cells through a thin Matrigel matrix, which suggests that DET reduced the ability of TS/A cells to invade basement membrane barriers.
DET regulates key biomarkers in the G2/M cycle transition and in apoptosis in TS/A cells
Flow cytometry revealed that 24 h treatment with 2 µg·mL−1 DET decreased the G0/G1 phase DNA content, from 38.8% to 19.6%, whereas the G2/M DNA content increased, from 26% to 48.4%, with a typical time-dependent G2/M arrest in TS/A cells. After longer treatment (36–48 h), apoptotic sub-G1 DNA content (32.8%) was increased (Figure 2A1). Vehicle-treated cells showed a typical flow cytometric profile of a dominant G0/G1 phase after 36–48 h treatment (Figure 2A2).
Figure 2.
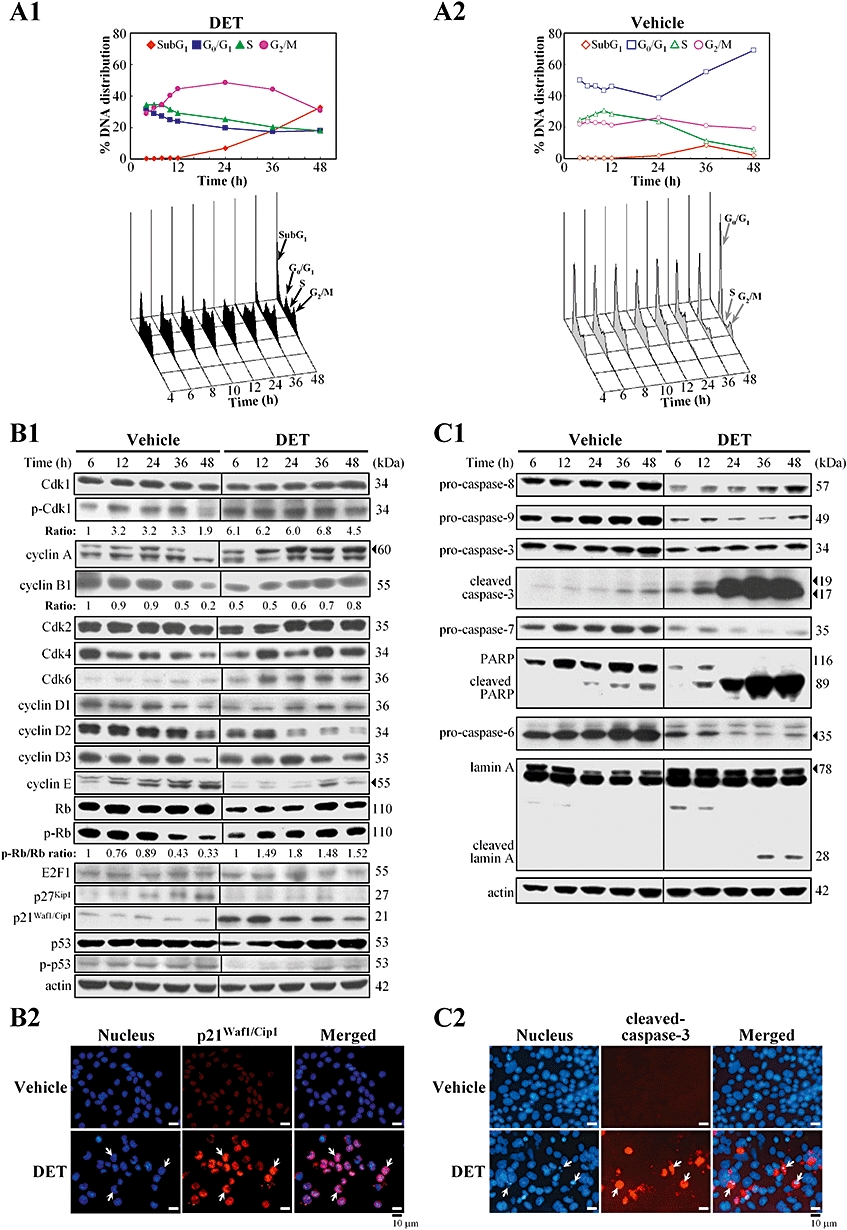
DET-induced cell cycle arrest and apoptosis in TS/A cells. In (A1) and (A2), flow cytometry analysis of DNA distribution in propidium iodide-stained cells. The percentage of subG1, G0/G1, S and G2/M cells was calculated. Data are representative of three independent experiments. (B1) Immunoblotting of key biomarkers involved in G2/M phase transition in TS/A cells treated with vehicle or DET (2 µg·mL−1 at times indicated). Protein levels were normalized to the level of actin. (B2) Immunostaining of p21Waf1/Cip1 (arrows) in the nuclei of TS/A cells treated with DET or vehicle for 12 h. Cells were stained with DAPI (nuclear marker; blue) and rabbit anti-p21Waf1/Cip1 (red). (C1) Immunoblotting of apoptotic mediators in the cell lysates of TS/A cells treated with vehicle or DET. (C2) Immunostaining of cleaved form of caspase-3 (red) in TS/A cells treated with DET or vehicle for 48 h. Typical apoptotic cells (arrows) with condensed nuclei in DET-treated cells. Cdk, cell cycle-dependent kinase; DAPI, 4,6-diamidino-2-phenylindole; DET, deoxyelephantopin; PARP, poly(ADP-ribose) polymerase.
Immunoblotting revealed the key biomarkers involved in cell cycle and mammalian cell apoptosis in vehicle- and DET-treated TS/A cells. Vehicle-treated cells showed a gradual and significant decrease in levels of the cyclins A, B1, D1, D2 and D3, and cell cycle-dependent kinase (Cdk)4 with increasing treatment time, which may be responsible for the decreased level of phosphorylated Rb protein at 36–48 h (Figure 2B1) and therefore revealed a dominant G0/G1 phase. In comparison, DET-treated cells showed levels of the cyclins A, D1 and E and Cdks 2 and 4 decreased with early treatment (6 or 12 h) and then gradually increased from 24 to 48 h (Figure 2B1). The ratio of phosphorylated Rb to Rb protein was up-regulated with DET during prolonged treatment for 12–48 h (1.48- to 1.8-fold increase); however, E2F1 level was changed little or not at all in vehicle- or DET-treated cells. Most importantly, the significant increase in inactive phospho(Tyr15)-Cdk1 level and decrease in cyclin B1 level from 6 to 24 h support the G2/M arrest induced by DET in TS/A cells from 10 to 24 h (Figure 2A1).
Effect of DET on Cdk inhibitors p21Waf1/Cip1 and p27Kip1
The changes of p21Waf1/Cip1 and p27Kip1 in DET-treated TS/A cells were examined. The protein level of p27Kip1 increased from 24 to 48 h in the vehicle control but was inhibited in DET-treated cells (Figure 2B1). The p21Waf1/Cip1 protein maintained a constant level in the vehicle group but was increased after DET treatment (6–12 h) then decreased with longer treatment time (24–48 h). Immunofluorescent analysis further confirmed the significant induction of p21Waf1/Cip1 protein in the nuclei of cells after 12 h treatment with DET (Figure 2B2). Both p53 and phospho-p53 were constantly expressed in the vehicle group but were attenuated in cells on early treatment with DET (Figure 2B1), which implies that the p21Waf1/Cip1 up-regulation by DET was p53 independent.
DET induces apoptosis in TS/A cells
In light of the increase of sub-G1 populations by DET, we further analysed some key apoptotic hallmarks in TS/A cells. The levels of initiator procaspases-8 and 9 and executioner procaspases-3, 7 and 6 were significantly lower in DET-treated cells than in vehicle-treated cells (Figure 2C1). As well, proteolytic cleavage of caspase-3 and its substrate PARP was time-dependently increased with DET treatment. The increase of the active form of caspase-3 is believed to activate the caspase-6-mediated specific cleavage of lamin A in DET-treated cells with 36–48 h treatment (Figure 2C1). Fluorescent microscopic analysis revealed some TS/A cells with characteristic condensed nuclei after 48 h treatment, concomitant with increased level of activated caspase-3, which indicates an increase in apoptotic cell number (Figure 2C2). The mitochondrial membrane potential (Dψm) and the protein levels of some Bcl-2 family members (Bax, Bid and Bcl-2) and cytochrome c showed little or no change in DET-treated cells (data not shown).
Role of ROS and c-Jun N-terminal kinase (JNK)-mediated signalling pathway in DET-treated cells
The JNK signalling pathway is involved in regulating various cellular processes, including apoptosis. Oxidative stress can activate JNK, which subsequently induces c-Jun expression and phosphorylation, thus resulting in apoptosis. We demonstrated that 2 µg·mL−1 DET treatment for 0.5–2 h increased ROS level on flow cytometry (Figure 3A). To further understand whether ROS production can trigger JNK activation under DET treatment, cells were treated with the ROS scavenger N-acetyl-L-cysteine (NAC) and DET alone or NAC plus DET for 6–24 h, then subjected to immunoblotting. Pretreatment with NAC significantly inhibited DET-mediated phosphorylation of JNK, c-Jun expression and phosphorylation and p21Waf1/Cip1 expression, but the expression of JNK was not affected (Figure 3B). Co-treating TS/A cells with DET and JNK inhibitor SP600125 significantly decreased the phospho-c-Jun protein level in most DET-treated cells on immunofluorescent staining (Figure 3C). Activation of p21Waf1/Cip1, caspase-3 and PARP were also associated with up-regulation of the JNK pathway by DET and was significantly inhibited by the JNK inhibitor (Figure 3D). These results demonstrate that induction of oxidative stress and activation of JNK are required for apoptotic cell death in TS/A cells with DET treatment.
Figure 3.
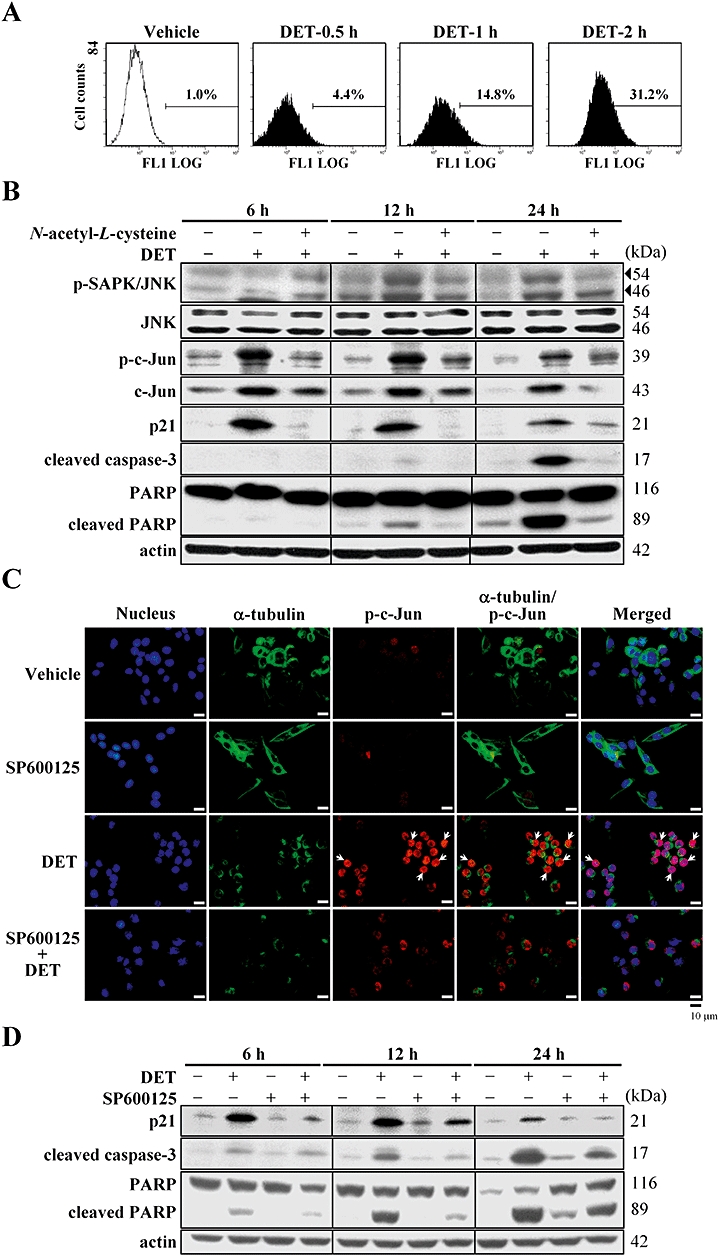
Deoxyelephantopin (DET) induces oxidative stress and c-Jun N-terminal (JNK)-mediated p21Waf1/Cip1 protein expression and apoptosis in TS/A cells. (A) Reactive oxygen species generation in DET-treated TS/A cells was quantified by flow cytometry. (B) Immunoblotting of JNK, phospho-JNK, c-Jun, phospho-c-Jun, p21Waf1/Cip1, cleaved caspase-3 and poly(ADP-ribose) polymerase (PARP) protein expression in TS/A cells with or without pretreatment with N-acetyl-L-cysteine (5 mM) for 1 h followed by DET (2 µg·mL−1) treatment at the indicated times. (C) Expression of phospho-c-Jun in DET-treated TS/A cells (6 h) examined on immunostaining and fluorescent microscopy at 400× original magnification. DET-up-regulated expression of phospho-c-Jun was blocked by JNK inhibitor SP600125 (10 µM). (D) Immunoblotting of DET-induced p21Waf1/Cip1 expression, activation of caspase-3 and PARP cleavage in TS/A cells blocked by 10 µM SP600125.
DET suppresses TNF-α-induced MMP-9 and NF-κB activation
Because MMP-9 activation has been shown in the metastasis of breast carcinoma cells, we examined the effect of DET on TNF-α-induced MMP-9 enzyme activity and protein expression. DET significantly suppressed MMP-9 and MMP-2 enzymatic activities with or without TNF-α stimulation (Figure 4A1). Immunoblotting further demonstrated MMP-9 protein level down-regulated by DET (Figure 4A2).
Figure 4.
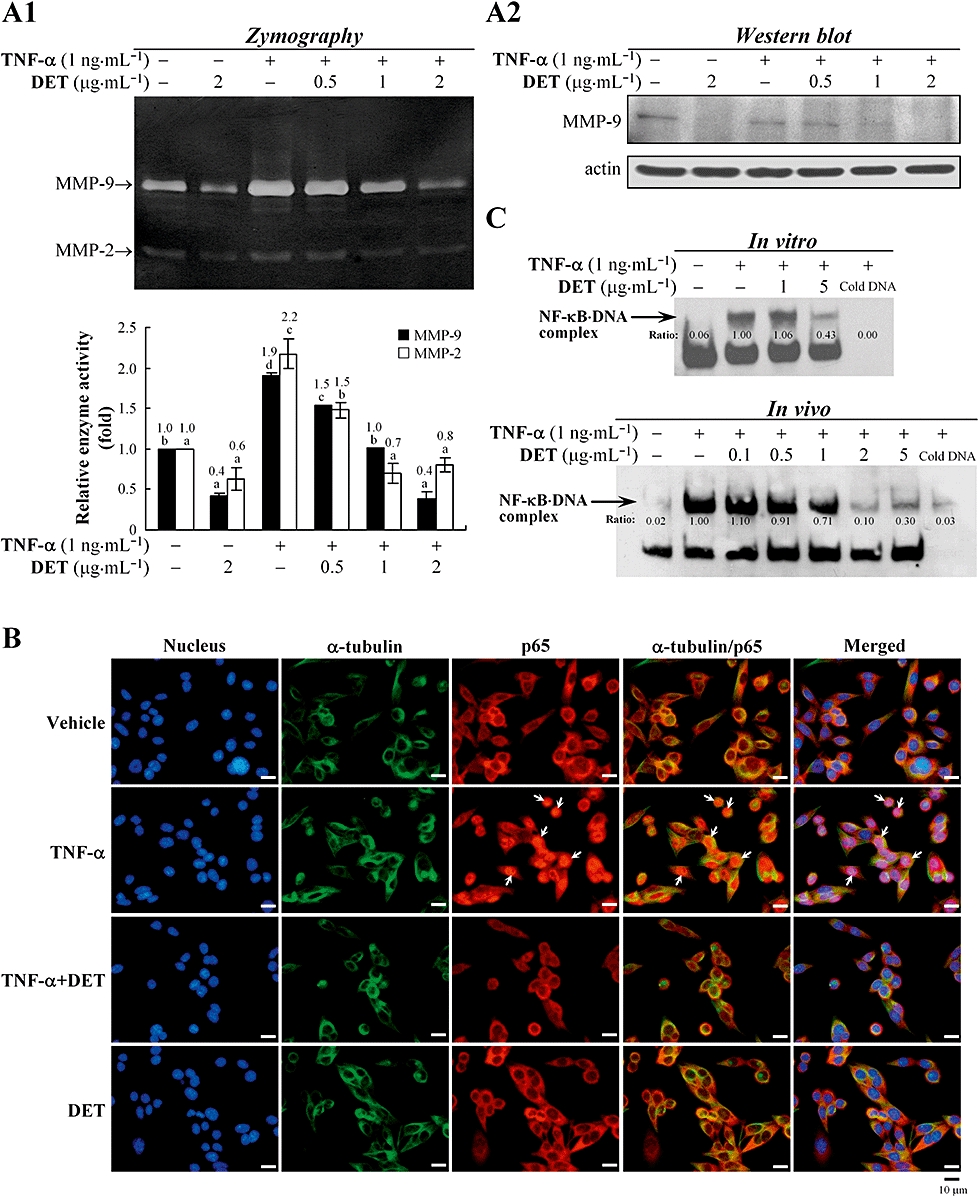
Inhibitory effects of deoxyelephantopin (DET) on tumour necrosis factor α (TNF-α)-induced activation of matrix metalloproteinase (MMP)-9, MMP-2 and nuclear factor-kappa B (NF-κB), and p65 translocation in TS/A cells. TS/A cells were treated with DET, then TNF-α for 24 h. In (A1) and (A2), MMP-9 and MMP-2 enzyme activity was analysed by gelatin zymography and protein expression by immunoblotting. Data are presented as mean ± SEM of three independent experiments. Different letters between treatments within the same assay indicate significant differences (one-way anova). (B) Immunofluorescent cell staining and microscopy. TS/A cells were treated with vehicle or DET for 1 h, then stimulated with TNF-α (1 ng·mL−1) for 30 min. Cells were stained with 4,6-diamidino-2-phenylindole (blue), FITC-labelled anti-α-tubulin antibody (green) and rabbit anti-p65 antibody (red). (C) Electrophoretic mobility shift assay (EMSA) analysis. Nuclear extracts (8 µg) from the TS/A cells with or without stimulation with TNF-α were treated with DET for 30 min at 37°C in vitro or the cells with the same treatments in (B) in vivo were subjected to EMSA with a biotin-labelled DNA probe containing the NF-κB binding site. Arrow, NF-κB-bound DNA complex. Data are representative of three independent experiments.
Tumour necrosis factor α has been reported to act as a potent inducer of NF-κB activation in tumour cells (Ichikawa et al., 2006). The effect of DET on NF-κB(p65) activity and its nuclear translocation was investigated. In vehicle-treated cells, p65 protein mainly appeared in the cytoplasm of test cells, but TNF-α stimulation resulted in significant translocation of p65 from the cytosol to the nucleus, which was greatly inhibited by DET (Figure 4B). We used an in vitro binding assay to examine the effect of DET on perturbing the ability of activated NF-κB to bind to a biotin-labelled oligonucleotide containing κB DNA elements on EMSA. Nuclear extracts from TNF-α-stimulated TS/A cells were incubated with DET for 30 min at 37°C. A more significant effect was observed with higher DET concentrations (5 µg·mL−1 > 1 µg·mL−1) (Figure 4C). Moreover, EMSA also revealed that TNF-α stimulation triggered consensus κB DNA element binding to NF-κB in TS/A cells, which was prevented by adding excess cold DNA. In the in vivo experiments, TNF-α-stimulated cells treated with DET (0.5–5 µg·mL−1) for 30 min showed a dose-dependent inhibition of the formation of a NF-κB/DNA complex, with only 0.91–0.3 times as much binding as with TNF-α alone (Figure 4C).
DET induces apoptosis accompanied by reduced tumour growth and tumour blood vessel density in orthotopic mammary tumours in BALB/c mice
We compared DET and paclitaxel in terms of cancer prevention or therapeutic efficacy in syngeneic BALB/c mice. The experimental design is presented in Figure S1A. Mice were pretreated i.p. with 5 mg·kg−1·day−1 paclitaxel (pre-paclitaxel-5) or 1 or 10 mg·kg−1·day−1 DET (pre-DET-1 and pre-DET-10, respectively) for 14 days before tumour cell inoculation (day 0) and then for another 28 days; the mean tumour volumes were significantly reduced, by 68%, 64% and 99%, respectively, as compared with in tumour control mice (Figure 5A1). On treatment with paclitaxel or DET after full establishment of TS/A tumours in BALB/c mice (therapeutic group), only DET at 10 mg·kg−1·day−1 (DET-10) was able to significantly suppress tumour growth, by 61% (Figure 5A2). Paclitaxel-5 or DET-1 treatment produced no statistically significant effect on tumour growth. Staining for proliferative tumour cells and blood vessel marker in orthotopic mammary tumour sites with PCNA and CD31 antibody (Figure 5B,C), respectively, revealed that pre-paclitaxel-5, pre-DET-1 or DET-10 for 28 days decreased the number of positive-stained PCNA cells and CD31 cells and resulted in increased number of apoptotic tumour cells, as seen on positive TUNEL staining (Figure 5D).
Figure 5.
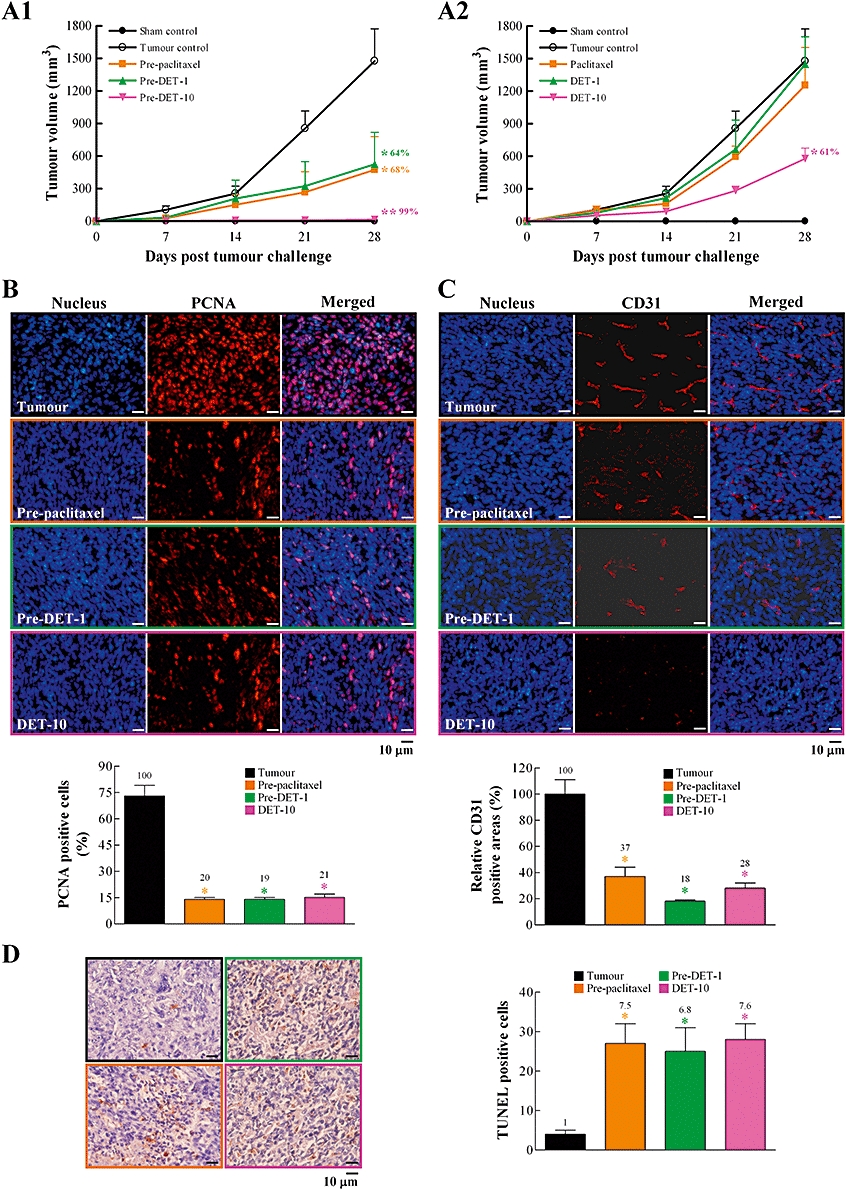
Intraperitoneal administration of deoxyelephantopin (DET) suppresses orthotopic TS/A mammary tumour growth in female BALB/c mice. Experimental design and details are in Figure S1A. In (A1) and (A2), tumour growth was measured once a week (days 7–28) in the eight experimental groups. At the end of the study, tumours were excised and processed for immunohistochemical staining for tumour growth with proliferating cell nuclear antigen (PCNA) in (B), tumour blood vessel growth with CD31 in (C) and apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay in (D). A representative image of each treatment group is shown. PCNA-positive cells (%) calculated by [(number of positive (red) cells/total number of cells counted) ×100]. Relative CD31-positive areas (%) calculated by [(areas of positive (red) CD31 in treatment group/areas of positive CD31 in tumour group) ×100]. TUNEL-postive cells in (D) calculated by number of positive (reddish brown) cells. Data are mean ± SEM of six independent tumour samples from each group of mice (*P < 0.05; **P < 0.005).
We examined the efficacy of DET administered p.o. The experimental design is presented in Figure S1B. Pretreatment every 2 days for 2 weeks and then post-administration of pre-paclitaxel-5 or DET (10 and 50 mg·kg−1, pre-DET-10 and pre-DET-50, respectively) for 31 days after tumour cell inoculation significantly decreased the mean tumour volumes, by 43%, 50% and 53%, respectively, as compared with the tumour control (Figure 6A1). In animals post-treated with paclitaxel or DET from day 1, only DET-50 significantly suppressed tumour growth, by 36% (Figure 6A2). Less effect was observed in animals treated with DET-2 and DET-10 (17% and 29%, respectively). Interestingly, mice treated p.o. with paclitaxel-5 showed an important reduction in tumour volume, although not statistically significant, as compared with control animals (1012 ± 283 vs. 1394 ± 243 mm3, 27% reduction). Typical skin tissue architecture of epithelial and endothelial cell linings was seen in sham controls, and a full growth of tumour cells was seen in tumour control groups, with a very thin stratum corneum, epidermis and dermis (see Figure S1C1). Far less defective skin tissue and fewer tumour cells were found with high-dose DET or paclitaxel treatment (see Figure S1C2). Immunohistochemistry revealed that the number of positive-stained PCNA and CD31 cells significantly decreased and TUNEL-positive (apoptotic) tumour cells significantly increased in the pre-paclitaxel, pre-DET-10 or 50 and DET-50 groups (Figure 6B–D). Thus, DET given i.p. or p.o. showed potent anti-tumour effects in vivo through anti-cell proliferative, anti-metastatic and pro-apoptotic activity, which agreed with the in vitro effects of DET (Figures 1–4).
Figure 6.
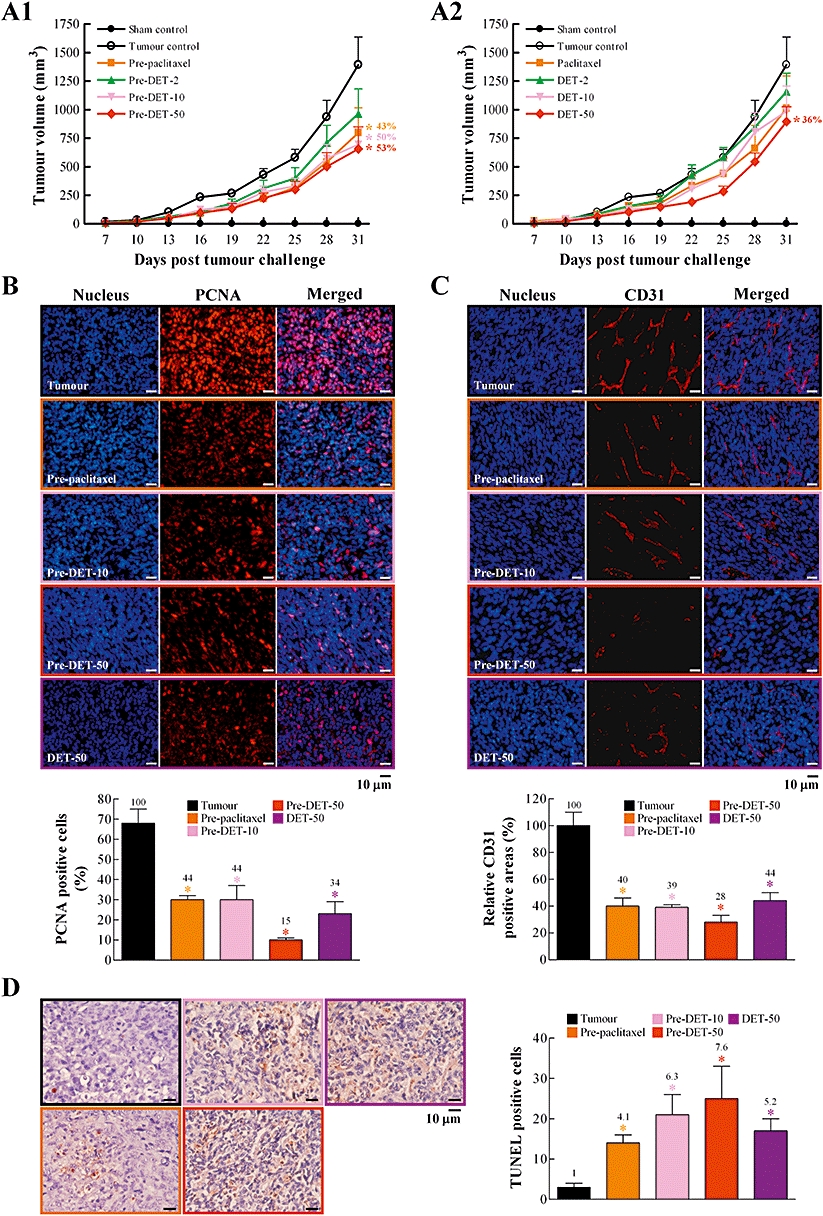
Oral administration of deoxyelephantopin (DET) suppresses orthotopic TS/A mammary tumour growth in female BALB/c mice. Experimental design and details are in Figure S1B. Tumour growth was measured once every 3 days (days 7–31) in the eight experimental groups in (A1) and (A2). At the end of the study, tumours were excised and processed for immunohistochemical staining for cell proliferation with proliferating cell nuclear antigen (PCNA) in (B), tumour blood vessel with CD31 in (C) and apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay in (D). A representative image of each treatment group is shown. PCNA-positive cells (%) calculated by [(number of positive (red) cells/total number of cells counted) ×100]. Relative CD31-positive areas (%) calculated by [(areas of positive (red) CD31 in treatment group/areas of positive CD31 in tumour group) ×100]. TUNEL-positive cells in (D) calculated by number of positive (reddish brown) cells. Data are mean ± SEM of six independent tumour samples from each group of mice. Each treatment group significantly different from tumour control is marked (*P < 0.05).
DET potently suppressed TS/A metastasis and prolonged survival time of test animals
We investigated the effect of DET on the lung metastasis of TS/A cells and overall survival in test mice using the experimental strategy described in Figure S1D. Test mice received i.v. injection of TS/A cells in the tail vein and i.p. administration of paclitaxel or DET. The survival time was significantly prolonged in pre-paclitaxel and pre-DET groups (P < 0.005, Figure 7A1). The median survival times were 27 ± 2, 37 ± 4, 41 ± 5, 42 ± 3 and 56 ± 6 days for tumour control, pre-paclitaxel-5, pre-DET-2, pre-DET-5 and pre-DET-10 groups respectively. Notably, the pre-DET-10 group showed the longest survival time, up to 90 days. However, of mice treated with DET or paclitaxel at the time of tumour cell inoculation, only the DET-10 group showed longer survival time than the tumour control group (31 ± 2 vs. 27 ± 2 days; P= 0.094, Figure 7A2). Consistent with this finding, the pre-paclitaxel-5 and pre-DET-2, 5 and 10 groups showed significantly suppressed number of metastatic pulmonary foci than did the tumour control group (Figure 7B1), whereas DET-5 and DET-10 groups showed a lower reduction of metastasis (Figure 7B2). Vehicle-treated mice showed a typical tissue architecture of lung alveoli on H&E staining, the tumour control group showed a fully grown tumour mass, and the DET- or pre-DET groups showed fewer alveolar defects (Figure 7C1,C2).
Figure 7.
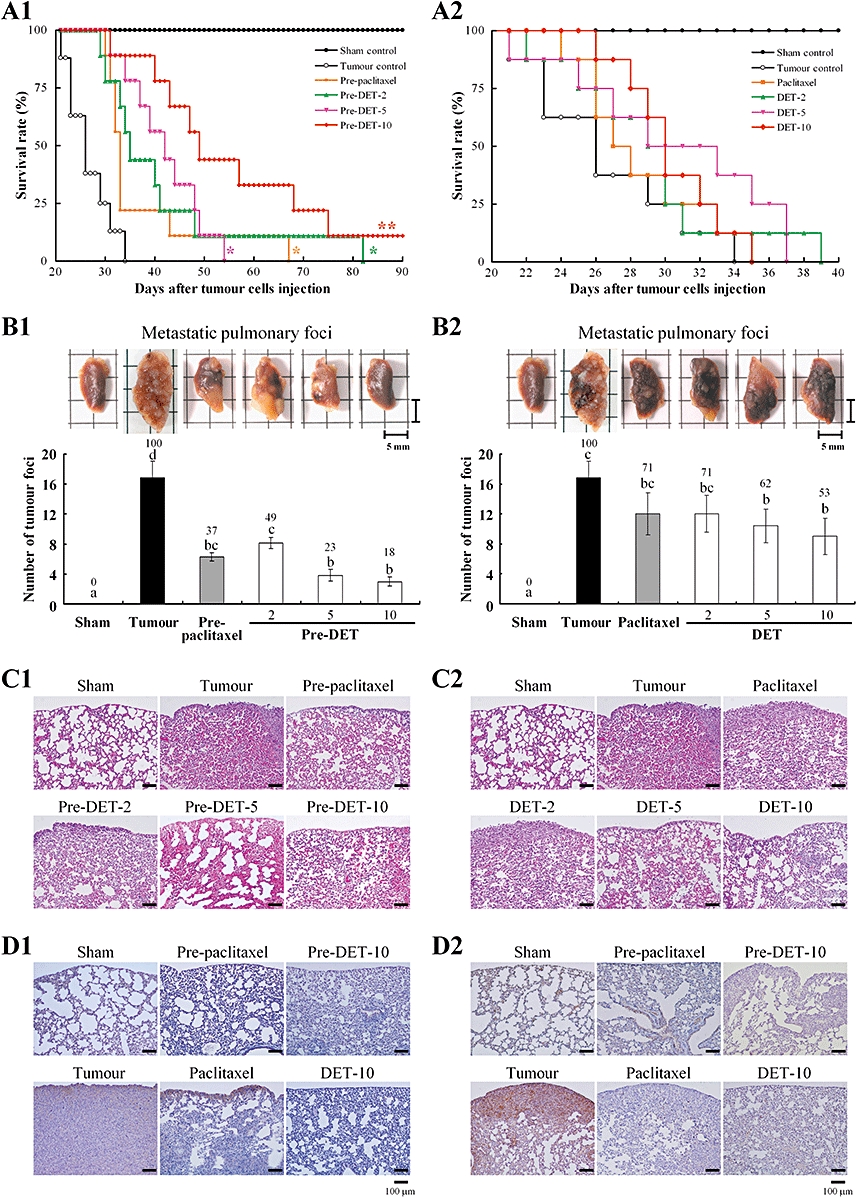
Effect of deoxyelephantopin (DET) on the survival rate and pulmonary foci formation in lung metastasis of TS/A mammary tumour cells in female BALB/c mice. Experimental design and details are in Figure S1C. The survival rate curve of test mice in the 10 experimental groups was determined at end points for lung tumour burden (*P < 0.005 and **P < 0.0001) in (A1) and (A2). The excised left lungs of test mice in the 10 experimental groups (top panels). In (B1) and (B2), the number of metastatic pulmonary foci (mean ± SEM) in the left lung was calculated (bottom panels). Values with different letter symbols are significantly different from each other (P < 0.05; one-way anova). (C1) and (C2), representative lung sections of mice from each group stained with haemotoxylin and eosin (100× original magnification). The typical tissue architecture of alveoli can be seen in sham control mice and a fully grown tumour mass in the tumour control group. Immunohistochemical analysis of the level of vascular endothelial growth factor in (D1) and cyclooxygenase-2 in (D2) protein (100× original magnification) in lung tissues of sham control, tumour control and DET- or paclitaxel-treated mice.
The overexpression of VEGF may be involved in the early stage of metastasis. In lung samples taken at 21 days of tumour induction, the overexpression of VEGF was detected by immunostaining in the tumour control group and was significantly suppressed in the DET or pre-DET groups (Figure 7D1). Expression of the important pro-inflammatory enzyme COX-2 was also significantly induced in metastatic lung tissue and was greatly attenuated in DET- and paclitaxel-treated mice (Figure 7D2).
Discussion
Herbal medicines, in addition to their traditional values, hold great public and medical interest. They also serve as sources of bioactive compounds and many are being used as drug discovery leads (Shyur and Yang, 2008). SLs have recently attracted a great deal of interest for their anti-cancer activities and molecular mechanisms of action and their potential chemopreventive and chemotherapeutic applications (Zhang et al., 2005). For instance, the germacranolide SL parthenolide has shown significant anti-cancer activities in vitro and in vivo in an MDA-MB-231 xenograft model (Sweeney et al., 2005). In this study, we isolated the major germacranolide SL DET from E. scaber. Compared with parthenolide, DET contains an extra α,β-unsaturated ketone and α-methylene-γ-lactone without an epoxy group in its structure. DET was first isolated from a different Elephantopus species in the 1970s (Lee et al., 1975), and recently its anti-tumour activity against HeLa cells and its function as a partial agonist against peroxisome proliferator-activated receptor γ were reported (Xu et al., 2006; Zou et al., 2008). In the present study, we demonstrated that DET exhibited novel activities against the mammary adenocarcinoma TS/A in cell culture-based experiments and in syngeneic mice. DET was effective and superior to the breast cancer drug paclitaxel against TS/A mammary tumour growth and lung metastasis, for increased overall survival of mice with mammary tumours.
Recently, an increasing number of chemopreventive agents have been shown to stimulate apoptosis or to function as cell cycle modulators (Singh et al., 2002; Sun et al., 2004). Cell cycle control is a highly regulated process that involves a complex cascade of events. Modulation of the expression and function of the cell cycle-regulatory proteins such as cyclins, Cdks, CdkIs, p53 and Rb provides an important means for the inhibition of cancer cell activity (Weng et al., 2005). We found that DET can cause cell cycle arrest in TS/A cells mainly through deregulation of the G2/M transition of checkpoint cyclin B1 and phospho-Cdk1 (Figure 2B1). Mechanistic studies demonstrated that with prolonged treatment, DET specifically up-regulated the caspase signalling cascades (such as caspase-8, 9, 3, 7 and 6) and down-regulated CdkI p27kip1, which resulted in the activation of downstream cellular death substrates, lamin A and PARP, leading to apoptosis (Figure 2C1). Down-regulating p27kip1 by accompanying PARP cleavage is a common occurrence in lymphoid and leukemic cells undergoing apoptosis (Frost and Sinclair, 2000; Quiney et al., 2006). Furthermore, we suggest that these apoptotic events triggered by DET might be in part through TNF/TNFR-mediated signalling because the TNF-R1 protein level was increased by DET treatment, whereas Fas and FasL were not affected (data not shown). Increased ROS production or oxidative stress is also likely to act as a signalling intermediate in the apoptotic events of mammalian cells. Our data show that DET can up-regulate JNK-mediated apoptosis in TS/A cancer cells, perhaps in part through induction of upstream oxidative stress and downstream CdkI p21Waf1/Cip1 expression (Figure 3). Curcumin, the major pigment of the dietary spice turmeric, has been reported to induce JNK-dependent apoptosis in human colon cancer cells (Collett and Campbell, 2004). Oxidative stress induced by curcumin can also up-regulate mitochondrion-initiated apoptosis in cancer cells (Sandur et al., 2007), but in our study, the DET-induced ROS formation did not trigger mitochondrion-mediated signalling in TS/A cells (data not shown).
Nuclear factor-kappa B is a hallmark of tumour angiogenesis and metastasis in mammals, and its activation by TNF-α apparently plays a key role in sustaining tumours (Aggarwal, 2004). NF-κB can regulate multiple angiogenic genes, including MMP-9, which is suggested to be important in tumour angiogenesis and metastasis of various cancers (Egeblad and Werb, 2002; Overall and Kleifeld, 2006; Wang et al., 2007). In the present study, DET significantly suppressed MMP-9 protein expression and activation, which might occur via blockade of TNF-α-stimulated NF-κB activation and consequent suppression of TS/A cell migration and invasion (Figure 4). A recent study demonstrated that isodeoxyelephantopin, an isomer of DET, suppresses NF-κB signalling by inhibiting TNF-α-induced phosphorylation and degradation of IκB and upstream activation of IKKα/β and IKK kinase activities (Ichikawa et al., 2006). Here, we demonstrated that DET blocked the nuclear translocation of NF-κB(p65) and its binding to consensus DNA elements in TNF-α-stimulated TS/A cells in vitro and in vivo (Figure 4B,C). Moreover, we observed that the carbonyl group at position 16 of DET can form hydrogen bonds with residue Lys122 of NF-κB(p65), which also forms hydrogen bonds with cis-acting DNA element of p65 on the basis of a molecular docking analysis with the computer programme Discovery Studio Modeling Client v2.5 (Accelrys, Inc., San Diego, CA, USA). This explains why DET blocked the NF-κB(p65)/DNA binding on EMSA. Helanalin, another SL, prevented the binding of NF-κB to DNA by directly targeting cysteine residues of p65 subunit of NF-κB (Lyss et al., 1998), a result in good agreement with our observations with DET. Thus, DET is an effective blocker of TNF-α–NF-κB axis signalling, which implies that DET may have potential in preventing NF-κB-mediated tumorigenesis and metastasis.
Investigation of anti-cancer agents commonly involves various experimental mouse models, including human tumour xenografts and syngeneic mouse tumours, grown as subcutaneous nodules or in orthotopic sites, or transgenic tumour models. TS/A cells, a spontaneous BALB/c tumour cell, which show similar progression in vivo to human breast cancer as aggressive, poorly immunogenic mammary adenocarcinoma, is a popular model system for studies of tumour heterogeneity, metastasis and immunological gene therapy (Nanni et al., 1983; Dobrzanski et al., 2006). In this study, DET had a more profound effect than paclitaxel on suppression of orthotopic tumour growth and lung metastasis of TS/A cells in BALB/c mice. A preventive approach by continuous administration of DET or paclitaxel for 14 days before implantation of TS/A tumour in BALB/c mice was more effective than the therapeutic approach with the administration of the compound or drug after tumour cell inoculation (Figures 5–7). Pretreatment with DET had a greater effect than paclitaxel on the prolongation of median survival time (56 vs. 37 days) in mice. DET may be more effective in the early stage of carcinogenesis by inhibiting TS/A cell proliferation and growth and cell cycle machinery in cells than when tumours are fully established. Furthermore, the i.p. administration of DET showed a greater effect than p.o. administration in the long-term efficacy experiments that might be due to incomplete absorption of DET or because DET underwent metabolism when passing through the GI tract by p.o. administration. Bioavailability and pharmacokinetic studies of DET are warranted in future studies.
The inhibition by DET of TS/A cell migration and invasion, MMP-9 activity and blood vessel density in tumour areas suggest that some of the in vivo effects of DET arise as a result of reducing tumour cell invasion through the basement membrane into the surrounding stroma, thus leading to the inhibition of lung metastasis. VEGF is the most critical molecule involved in angiogenesis, an important process for tumour growth and metastasis. We found significantly less VEGF immunoreactivity in metastatic lung tissues in DET-treated mice, suggesting that DET might also be a deregulator of angiogenesis and thus inhibit lung metastasis of TS/A cells by another mechanism. COX-2, an enzyme induced by inflammatory and mitogenic stimuli, is pro-tumorigenic in several human cancers. COX-2 also appears to be a biomarker for stratified breast cancer risk among women with atypia and is a novel target for breast cancer chemoprevention (Longo et al., 2007; Visscher et al., 2008). COX-2 overexpression in the lungs of mice with metastatic TS/A tumours was significantly suppressed by DET or paclitaxel treatment. We are currently undertaking a comparative proteomics study to decipher in depth the common or differential protein profiling and molecular mechanism(s) modulated by DET and paclitaxel in TS/A cells, to address in part why DET was more effective than paclitaxel in inhibiting mammary tumours.
The anti-mammary tumour activities of DET, from its targeting of multiple key components in inflammation, angiogenesis and cell apoptosis, suggest that DET has great potential for further development as an anti-breast cancer agent. Although overburden of ROS can lead to reversible or irreversible tissue injury, cytotoxic ROS can serve as a tumour terminator, by oxidation therapy, if it is produced selectively in tumours (Fang et al., 2009). How DET can appropriately, for future clinical application, increase susceptibility of cancer cells to ROS through modulating signal transduction cascades warrants further investigation.
Acknowledgments
This study was supported by an institutional grant from Academia Sinica, Taiwan. We thank Dr Harry Wilson (Academia Sinica) for his careful reading of the manuscript and Miss Ya-Wen Cheng, Dr Chih-An Hsu, Miss Meng-Chen Hsieh and Dr Meng-Shih Weng for their technical assistance.
Glossary
Abbreviations:
- Cdk
cell cycle-dependent kinase
- COX-2
cyclooxygenase-2
- DAPI
4,6-diamidino-2-phenylindole
- DET
deoxyelephantopin
- EMSA
electrophoretic mobility shift assay
- H2DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- JNK
c-Jun N-terminal kinase; MMP-9, matrix metalloproteinase-9
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NAC
N-acetyl-L-cysteine
- NF-κB
nuclear factor-kappa B
- PARP
poly(ADP-ribose) polymerase
- PCNA
proliferating cell nuclear antigen
- ROS
reactive oxygen species
- TNF-α
tumour necrosis factor α
- VEGF
vascular endothelial growth factor
Statement of conflicts of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 (A) Experimental design for study of preventive and therapeutic effects of deoxyelephantopin (DET) on orthotopic mammary tumour growth by i.p. administration. In total, 44 mice were assigned to eight groups: sham control (n = 6), tumour control (n = 5), pre-paclitaxel-5 (n = 6), pre-DET-1 (n = 6), pre-DET-10 (n = 6), paclitaxel-5 (n = 5), DET-1 (n = 5) and DET-10 (n = 5). (B) Experimental design for the study of preventive and therapeutic effects of DET against orthotopic mammary tumour growth by p.o. administration. Eighty-four mice were assigned to 10 groups: sham control (n = 8), tumour control (n = 8), pre-paclitaxel-5 (n = 9), pre-DET-2 (n = 9), pre-DET-10 (n = 9), pre-DET-50 (n = 9), paclitaxel-5 (n = 8), DET-2 (n = 8), DET-10 (n = 8) and DET-50 (n = 8). In (C1) and (C2), mammary tumour sections from p.o. administrated DET were stained with haemotoxylin and eosin (100× original magnification). The typical tissue architecture of epithelium and endothelium lining can be seen in sham control mice. Sections from mice in tumour control group show a very thin stratum corneum, epidermis and dermis. (D) Experimental design for the study of preventive and therapeutic effects of DET against experimental lung metastasis of TS/A cells by i.p. administration. In total, 84 mice were assigned to 10 groups: sham control (n = 8), tumour control (n = 8), pre-paclitaxel (n = 9), pre-DET-2 (n = 9), pre-DET-10 (n = 9), pre-DET-50 (n = 9), paclitaxel (n = 8), DET-2 (n = 8); DET-10 (n = 8) and DET-50 (n = 8).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Albini A, Tosetti F, Benelli R, Noonan DM. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee T, Duhadaway JB, Gaspari P, Sutanto-Ward E, Munn DH, Mellor AL, et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene. 2008;27:2851–2857. doi: 10.1038/sj.onc.1210939. [DOI] [PubMed] [Google Scholar]
- But PP, Hon PM, Cao H, Chan TWD, Wu BM, Mak TCW, et al. Sesquiterpene lactones from Elephantopus scaber. Phytochemistry. 1997;44:113–116. [Google Scholar]
- Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, et al. Ethyl caffeate suppresses NF-κB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2in vitro or in mouse skin. Br J Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA. CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer. J Immunol. 2006;177:8191–8201. doi: 10.4049/jimmunol.177.11.8191. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Frost V, Sinclair AJ. p27KIP1 is down-regulated by two different mechanisms in human lymphoid cells undergoing apoptosis. Oncogene. 2000;19:3115–3120. doi: 10.1038/sj.onc.1203657. [DOI] [PubMed] [Google Scholar]
- Hou CC, Chen YP, Wu JH, Huang CC, Wang SY, Yang NS, et al. A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Res. 2007;67:6907–6915. doi: 10.1158/0008-5472.CAN-07-0158. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Nair MS, Takada Y, Sheeja DB, Kumar MA, Oommen OV, et al. Isodeoxyelephantopin, a novel sesquiterpene lactone, potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Clin Cancer Res. 2006;12:5910–5918. doi: 10.1158/1078-0432.CCR-06-0916. [DOI] [PubMed] [Google Scholar]
- Lee KH, Cowherd CM, Wolo MT. Antitumor agents. XV: deoxyelephantopin, an antitumor principle from Elephantopus carolinianus Willd. J Pharm Sci. 1975;64:1572–1573. doi: 10.1002/jps.2600640938. [DOI] [PubMed] [Google Scholar]
- Li Y, Ooi LS, Wang H, But PP, Ooi VE. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother Res. 2004;18:718–722. doi: 10.1002/ptr.1518. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, et al. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008a;29:1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- Lin FM, Tsai CH, Yang YC, Tu WC, Chen LR, Liang YS, et al. A novel diterpene suppresses CWR22Rv1 tumor growth in vivo through antiproliferation and proapoptosis. Cancer Res. 2008b;68:6634–6642. doi: 10.1158/0008-5472.CAN-08-0635. [DOI] [PubMed] [Google Scholar]
- Longo R, Torino F, Gasparini G. Targeted therapy of breast cancer. Curr Pharm Des. 2007;13:497–517. doi: 10.2174/138161207780162890. [DOI] [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Nanni P, de Giovanni C, Lollini PL, Nicoletti G, Prodi G. TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin Exp Metastasis. 1983;1:373–380. doi: 10.1007/BF00121199. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment-opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Quiney C, Billard C, Faussat AM, Salanoubat C, Ensaf A, Naït-Si Y, et al. Pro-apoptotic properties of hyperforin in leukemic cells from patients with B-cell chronic lymphocytic leukemia. Leukemia. 2006;20:491–497. doi: 10.1038/sj.leu.2404098. [DOI] [PubMed] [Google Scholar]
- Rajesh MG, Latha MS. Hepatoprotection by Elephantopus scaber Linn. in CCl4-induced liver injury. Indian J Physiol Pharmacol. 2001;45:481–486. [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyur LF, Yang NS. Metabolomics for phytomedicine research and drug development. Curr Opin Chem Biol. 2008;12:66–71. doi: 10.1016/j.cbpa.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Shyur LF, Chen CH, Lo CP, Wang SY, Kang PL, Sun SJ, et al. Induction of apoptosis in MCF-7 human breast cancer cells by phytochemicals from Anoectochilus formosanus. J Biomed Sci. 2004;11:928–939. doi: 10.1159/000081840. [DOI] [PubMed] [Google Scholar]
- Singh RP, Dhanalakshmi S, Agarwal R. Phytochemicals as cell cycle modulators – a less toxic approach in halting human cancers. Cell Cycle. 2002;1:156–161. [PubMed] [Google Scholar]
- Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–543. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- Sun SY, Hail N, Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals – promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- Visscher DW, Pankratz VS, Santisteban M, Reynolds C, Ristimaki A, Vierkant RA, et al. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J Natl Cancer Inst. 2008;100:421–427. doi: 10.1093/jnci/djn036. [DOI] [PubMed] [Google Scholar]
- Wang J, An H, Mayo MW, Baldwin AS, Yarbrough WG. LZAP, a putative tumor suppressor, selectively inhibits NF-κB. Cancer Cell. 2007;12:239–251. doi: 10.1016/j.ccr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Weng MS, Ho YS, Lin JK. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: involvement of p38 mitogen-activated protein kinase. Biochem Pharmacol. 2005;69:1815–1827. doi: 10.1016/j.bcp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Xu G, Liang Q, Gong Z, Yu W, He S, Xi L. Antitumor activities of the four sesquiterpene lactones from Elephantopus scaber L. Exp Oncol. 2006;28:106–109. [PubMed] [Google Scholar]
- Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- Zou G, Gao Z, Wang J, Zhang Y, Ding H, Huang J, et al. Deoxyelephantopin inhibits cancer cell proliferation and functions as a selective partial agonist against PPARγ. Biochem Pharmacol. 2008;75:1381–1392. doi: 10.1016/j.bcp.2007.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


