Abstract
Background and purpose:
Results from several studies point to voltage-gated Na+ channels as potential mediators of the immobility produced by inhaled anaesthetics. We hypothesized that the intrathecal administration of tetrodotoxin, a drug that blocks Na+ channels, should enhance anaesthetic potency, and that concurrent administration of veratridine, a drug that augments Na+ channel opening, should reverse the increase in potency.
Experimental approach:
We measured the change in isoflurane potency for reducing movement in response to a painful stimulus as defined by MAC (minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects) caused by intrathecal infusion of various concentrations of tetrodotoxin into the lumbothoracic subarachnoid space of rats, and the change in MAC caused by the administration of a fixed dose of tetrodotoxin plus various doses of intrathecal veratridine.
Key results:
Intrathecal infusion of tetrodotoxin (0.078–0.63 µM) produced a reversible dose-related decrease in MAC, of more than 50% at the highest concentration. Intrathecal co-administration of veratridine (1.6–6.4 µM) reversed this decrease in a dose-related manner, with nearly complete reversal at the highest veratridine dose tested.
Conclusions and implications:
Intrathecal administration of tetrodotoxin increases isoflurane potency (decreases isoflurane MAC), and intrathecal administration of veratridine counteracts this effect in vivo. These findings are consistent with a role for voltage-gated Na+ channel blockade in the immobility produced by inhaled anaesthetics.
Keywords: inhaled anaesthetics, isoflurane, MAC, mechanisms of anaesthetic action, sodium channels, tetrodotoxin, veratridine
Introduction
Considerable evidence supports the notion that inhaled anaesthetics act to produce immobility in the face of noxious stimulation by blocking excitatory neurotransmission in the spinal cord (Antognini and Schwartz, 1993; Rampil et al., 1993). Recent work suggests that inhaled anaesthetics produce immobility by depressing the locomotor network in the ventral spinal cord rather than by effects on the dorsal horn (Jinks et al., 2008), and might involve depression of glutamatergic mechanisms rather than potentiation of GABAergic mechanisms (Sonner et al., 2003; Kungys et al., 2009). Consistent with this notion, considerable neurochemical and electrophysiological evidence indicates that anaesthetizing concentrations of inhaled anaesthetics block neuronal voltage-gated Na+ channels and the consequent release of excitatory neurotransmitters such as glutamate in vitro (Hemmings, 2009).
Voltage-gated Na+ channels are critical to neuronal excitability, neurotransmitter release and action potential initiation and propagation. We reported previously several results consistent with a role for Na+ channels in the immobilizing effects of the inhaled anaesthetic isoflurane in rats in vivo. Increases in intrathecal, but not intracerebroventricular, Na+ concentration reduce anaesthetic potency as evidenced by an increase in MAC (the minimum alveolar concentration of anaesthetic that eliminates movement in response to noxious stimulation in 50% of subjects) (Laster et al., 2007). I.v. administration of lidocaine, a voltage- and use-dependent blocker of Na+ channels, decreased isoflurane MAC in a dose-related manner (Zhang et al., 2007). Conversely, intrathecal (but not intracerebroventricular) administration of veratridine, a lipid-soluble alkaloid neurotoxin derived from the Liliaceae plant family that enhances the activity of Na+ channels by binding preferentially to the open state and decreasing their rate of inactivation (Wang and Wang, 2003), increased MAC (Zhang et al., 2008).
The present study extended these studies by testing the predictions: (i) that intrathecal administration of tetrodotoxin, a highly specific heterocyclic guanidine puffer fish toxin that blocks the ion conducting pore of Na+ channels (Catterall et al., 2007), would decrease MAC in a dose-related manner; and (ii) that the concurrent administration of veratridine to increase net Na+ channel activation would reverse this effect of Na+ channel blockade.
Methods
Animal preparation
All animal care and experimental procedures were approved by the Committee on Animal Research of the University of California, San Francisco; we studied 52 male, 12–15-week-old, Long-Evans rats weighing 300–450 g obtained from Charles River Laboratories (Hollister, CA, USA). Each animal was caged with up to two additional rats before surgical preparation, and singly after preparation, with a 12 h light/dark cycle. All had continuous access to standard rat chow and tap water before study.
On day 1, rats were anaesthetized with isoflurane, and a vertical incision was made through the skin of the neck at the base of the skull down to the atlantooccipital membrane. A small incision through the atlantooccipital membrane allowed placement of a 32 gauge polyurethane catheter (Micor Inc., Allison Park, PA, USA) through the membrane as described by Yaksh and Rudy (1976). The distal end of the catheter was threaded caudally 6–8 cm towards the lumbar sac, the length depending on the size of the rat. The proximal end of the catheter then was tunnelled through to the external auditory meatus where it exited and could be accessed and sutured in place. The skin over the posterior wound was sutured closed, and the wound was infiltrated with 0.25% (w/v) bupivacaine. Rats recovered from anaesthesia and surgery for 24 h before study.
Study 1: effect of intrathecal tetrodotoxin on isoflurane MAC (28 rats)
The upper portion of Figure 1 shows the outline of the experimental plan. On day 2, groups of 4–8 rats were placed in individual clear plastic cylinders for determination of baseline MAC (MAC0). Each cylinder was connected to a flow of 1 L·min−1 oxygen containing 2% (v/v) isoflurane to induce anaesthesia, and an infusion of artificial cerebrospinal fluid (aCSF) was initiated at 4 µL·min−1 via the previously placed intrathecal catheter. We previously demonstrated that this inflow rate did not affect MAC, and that methylene blue added to the perfusate was primarily confined to the lumbar/lower thoracic area of the cord (Zhang et al., 2003b). The aCSF contained (mM): NaCl 127, NaHCO3 27.4, KCl 2.4, KH2PO4 0.50, Na2SO4 0.49, CaCl2 1.10, MgCl2 0.83 and glucose 5.5. The pH was adjusted to 7.4 by bubbling the mixture with carbon dioxide. A rectal temperature probe was inserted, and temperature maintained at 37.5 ± 1°C by a heating blanket. The isoflurane concentration was decreased to 1.0–1.2% and sustained at this concentration for 50 min, after which the tail was clamped and moved by rolling the clamp at 1–2 Hz for up to 1 min (less if the rat moved). After checking that movement had occurred, the isoflurane concentration was increased by 0.15–0.2%, and after a 30 min period of equilibration the tail clamp was again applied and movement or lack of movement determined. Isoflurane partial pressures were monitored using an infrared analyser (Datascope, Helsinki, Finland), and immediately after determination of the response to tail clamp, a sample of gas was obtained from one of the chambers and analysed for isoflurane by gas chromatography (see below). This process continued until all rats failed to move in response to application of the tail clamp. MAC was calculated as the average of the greatest concentration that permitted movement and the smallest concentration that suppressed movement. This value was designated MAC0. Anaesthetic administration then was discontinued, and the rats recovered.
Figure 1.
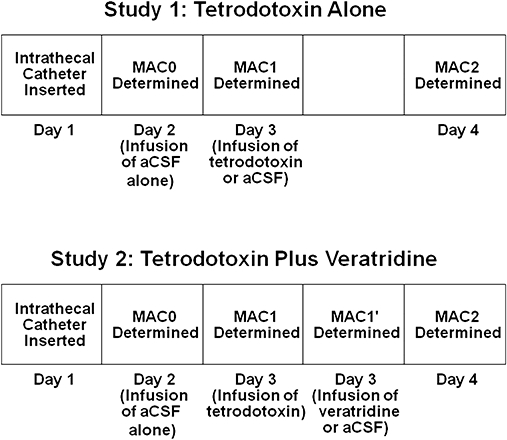
Schematic representations of the experimental design for the two studies: (i) effect on MAC of various concentrations of intrathecal tetrodotoxin infused for 30 min (tetrodotoxin alone); and (ii) capacity of various concentrations of intrathecal veratridine to antagonize the reduction in MAC produced by 0.47 µM tetrodotoxin infused intrathecally for 30 min before infusion of various concentrations of intrathecal veratridine (tetrodotoxin plus veratridine). aCSF, artificial cerebrospinal fluid; MAC, minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects.
On day 3, the rats again were anaesthetized with isoflurane and MAC determined (MAC1). However, on this day, one or two of the rats (control group) received an intrathecal infusion of aCSF at 4 µL·min−1. The other rats (experimental groups) received infusions of 0.078, 0.16, 0.31, 0.47 or 0.63 µM tetrodotoxin in aCSF at 4 µL·min−1 for 30 min (only one dose for a given experiment). We then determined MAC, anaesthetic administration was discontinued, and the rats recovered. The investigator making the determination of MAC was unaware of the contents of the infusions.
On day 4, the rats again were anaesthetized with isoflurane and the process of MAC determination repeated (MAC2). On this day, rats did not receive an intrathecal infusion. The rats again were allowed to recover and were examined for hind limb weakness assessed by visual inspection of the rats stimulated by gentle prodding.
These measurements supplied two control assessments. The change in MAC with treatment (MAC1) could be compared with the MAC in the same rat when given aCSF (MAC0). And the change in MAC with treatment could be compared with the MAC in a comparable group of rats given aCSF. Injury from treatment could be assessed by testing motor function after the third MAC determination, and whether MAC2 differed from MAC0 in experimental groups more than in the control group.
Study 2: effect of intrathecal veratridine on isoflurane MAC following intrathecal tetrodotoxin (24 rats)
The lower portion of Figure 1 shows the outline of the experimental plan for this series of experiments designed to determine whether a Na+ channel agonist can antagonize the effect of a Na+ channel antagonist on MAC. On day 1, rats were prepared as in the preceding study. On day 2, MAC0 was determined as in the preceding study.
On day 3, isoflurane MAC was again determined (MAC1) after the rats received an intrathecal infusion of 0.47 µM tetrodotoxin in aCSF at 4 µL·min−1 for 30 min. The anaesthetized rats then were separated into subgroups and given aCSF plus 4% (v/v) dimethyl sulphoxide (DMSO) (control group) or intrathecal infusions of 1.6, 3.2 or 6.4 µM veratridine in aCSF plus 4% DMSO (experimental groups) at 4 µL·min−1 for 50 min and MAC (MAC1′) redetermined. We previously demonstrated that infusion of 4% DMSO does not alter MAC (Zhang et al., 2003a). The investigator making the determination of MAC1′ was unaware of the contents of the infusions.
On day 4, the rats again were anaesthetized with isoflurane and the MAC determination repeated with no infusions (MAC2). The rats were allowed to recover and were examined for hind limb function using visual inspection of rats stimulated by gentle prodding. These measurements supplied two control assessments. The change in MAC with treatment (MAC1) could be compared with the MAC in the same rat when given aCSF (MAC0). MAC without or with veratridine after tetrodotoxin administration (MAC1′) could be compared between groups. Injury from treatment could be assessed by testing motor function after the third MAC determination, and whether MAC2 differed from MAC0 in the experimental groups more than in the control group.
Analysis of inhaled isoflurane
We used a Gow-Mac gas chromatograph (Gow-Mac Instrument Corp., Bridgewater, NJ, USA) equipped with a flame ionization detector to measure concentrations of inspired isoflurane. The 4.6 m long, 0.22 cm (ID) column was packed with SF-96. The column temperature was 100°C. The detector was maintained at temperatures approximately 50°C warmer than the column. The carrier gas flow was nitrogen at a flow of 15–20 mL·min−1. The detector received 35–38 mL·min−1 hydrogen and 240–320 mL·min−1 air. Primary standards were prepared, and the linearity of the response of the chromatograph was determined. We also used secondary (cylinder) standards referenced to primary standards.
Statistical analysis
For each concentration of tetrodotoxin infused we determined the ratios MAC1/MAC0 and MAC2/MAC0. A one-way analysis of variance (anova) with Fisher's protected least significant difference (PLSD) was done to determine whether infusion of tetrodotoxin significantly affected MAC and whether one or more groups differed significantly. Data were fitted using Prism v. 5.01 (GraphPad Software, San Diego, CA, USA). Similarly, we used a one-way anova with PLSD to determine whether infusion of any of the three doses of veratridine influenced the decrease in MAC produced by the infusion of 0.47 µM tetrodotoxin. We accepted a value of P < 0.05 as significant.
Materials
Isoflurane was obtained from Baxter Healthcare Corp. (New Providence, NJ, USA). Tetrodotoxin was obtained from Tocris Bioscience (Ellisville, MO, USA). Veratridine was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Results
Control MAC values for isoflurane (MAC0) were 1.51 ± 0.12 vol% (mean ± SD) isoflurane, consistent with prior determinations in our laboratory (Zhang et al., 2001; Laster et al., 2007). Infusion of tetrodotoxin significantly (P < 0.0001 by anova) decreased MAC in a dose-related manner (Table 1; Figure 2). PLSD analysis indicated that infusions of 0.16 µM or greater significantly decreased MAC (decreased MAC1/MAC0) relative to lesser concentrations of tetrodotoxin. The lowest concentration of tetrodotoxin did not significantly decrease MAC from control, and the two greatest concentrations tested (0.47 and 0.63 µM) produced decreases of 58 ± 12% and 63 ± 10% (mean ± SD), values that did not differ significantly. The data were fitted to a sigmoidal dose–response curve with 50% maximal effective concentration (IC50) = 0.33 µM and a maximal effect (Imax) of 64% reduction of MAC. Recovery values for MAC (i.e. MAC2/MAC0) did not differ significantly (P= 0.16). However, two of eight rats given 0.63 µM tetrodotoxin died before MAC1 was determined. It is possible that these rats were more susceptible to the effects of tetrodotoxin, such that the MAC changes seen in the remaining (more resistant to tetrodotoxin toxicity) rats could underestimate the effect of tetrodotoxin.
Table 1.
Effect of intrathecal tetrodotoxin on isoflurane MAC
| Tetrodotoxin (µM) | n | MAC1/MAC0 (mean±SD) | MAC2/MAC0 (mean±SD) |
|---|---|---|---|
| 0 (Control) | 6 | 1.02 ± 0.03 | 1.01 ± 0.02 |
| 0.078 | 4 | 0.99 ± 0.01 | 1.03 ± 0.00 |
| 0.16 | 4 | 0.91 ± 0.02 | 0.98 ± 0.06 |
| 0.31 | 4 | 0.75 ± 0.15 | 0.96 ± 0.06 |
| 0.47 | 4 | 0.42 ± 0.12 | 1.00 ± 0.01 |
| 0.63 | 6* | 0.37 ± 0.10 | 0.97 ± 0.07 |
All rats received an intrathecal infusion of the indicated concentrations of tetrodotoxin in aCSF at 4 µL·min−1 for 30 min before determination of MAC1; MAC0 was determined the previous day during infusion of aCSF. MAC2/MAC0 was determined after 24 h of recovery.
n indicates the number of rats completing the indicated MAC studies. All MAC1/MAC0 values for tetrodotoxin concentrations of 0.16 µM or more differed significantly from the control value. No significant differences were found between the MAC2/MAC0 values.
Two rats out of eight died before completion of the determination of MAC1/MAC0. An additional rat died before completion of MAC2/MAC0.
aCSF, artificial cerebrospinal fluid; MAC, minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects.
Figure 2.
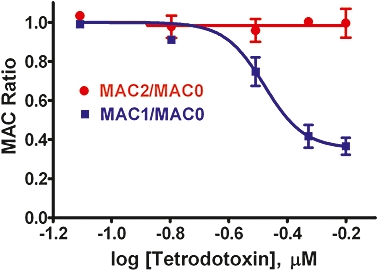
Intrathecal infusion of tetrodotoxin alone decreased isoflurane MAC (minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects). The effect of tetrodotoxin on MAC (MAC1/MAC0) was dose-related as indicated by non-linear curve fitting of the data with variable slope. The IC50 value for the infusion was 0.33 µM tetrodotoxin with a best fit plateau at a MAC ratio of 0.36 (Hill slope =−6.4). This effect was reversible with subsequent recovery of MAC to control values 24 h after cessation of tetrodotoxin infusion (MAC2/MAC0). Data are shown as mean ± SD (n= 4–6 rats for each concentration).
Intrathecal infusion of all veratridine concentrations tested reversed the decrease in MAC produced by 0.47 µM tetrodotoxin (P < 0.0001; Table 2; Figure 3). All three doses of veratridine increased MAC1′/MAC0 relative to control (P < 0.0001). The effect of the lowest concentration of veratridine (1.6 µM) differed significantly from that of the highest (6.4 µM; P= 0.025). No significant differences were found for MAC1/MAC0 (P= 0.055) or MAC2/MAC0 (P= 0.50).
Table 2.
Effect of intrathecal veratridine on the reduction in isoflurane MAC by intrathecal tetrodotoxin
| Tetrodotoxin (µM) | n | MAC1/MAC0 (mean±SD) | Veratridine (µM) | n | MAC1′/MAC0 (mean±SD) | n | MAC2/MAC0 (mean±SD) |
|---|---|---|---|---|---|---|---|
| 0.47 | 6 | 0.35 ± 0.04 | 0.0 | 4 | 0.38 ± 0.08 | 4 | 0.98 ± 0.07 |
| 0.47 | 6 | 0.48 ± 0.10 | 1.6 | 5 | 0.75 ± 0.11 | 5 | 0.98 ± 0.08 |
| 0.47 | 7 | 0.37 ± 0.12 | 3.2 | 6 | 0.83 ± 0.12 | 6 | 1.02 ± 0.03 |
| 0.47 | 4 | 0.34 ± 0.02 | 6.4 | 4 | 0.93 ± 0.08 | 4 | 1.03 ± 0.07 |
All rats received an intrathecal infusion of 0.47 µM tetrodotoxin in aCSF at 4 µL·min−1 for 30 min before the determination of MAC1; MAC0 was determined the previous day during infusion of aCSF alone. Veratradine at the indicated concentrations in aCSF plus 4% (v/v) DMSO at an intrathecal infusion rate of 4 µL·min−1 was begun after determination of MAC1 and continued throughout the determination of MAC1′. MAC2/MAC0 was determined after 24 h of recovery during infusion of aCSF. n indicates the number of rats completing the indicated MAC ratio studies. No significant differences were found for the MAC1/MAC0 or the MAC2/MAC0 values.
aCSF, artificial cerebrospinal fluid; DMSO, dimethyl sulphoxide; MAC, minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects.
Figure 3.
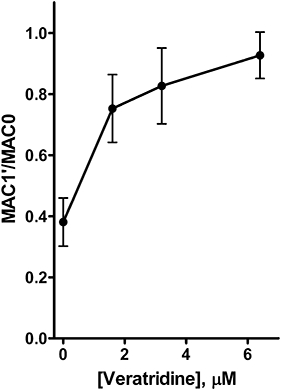
Intrathecal veratridine reversed the reduction in isoflurane MAC (minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects) produced by intrathecal tetrodotoxin. Intrathecal infusion of 0.47 µM tetrodotoxin decreased isoflurane MAC (MAC1/MAC0) to 35 ± 4% of control. Infusion of veratridine at all three concentrations reversed the effect of the tetrodotoxin (MAC1′/MAC0; P < 0.0001 by anova with Dunnett's multiple comparison test). Data shown are mean ± SD (n= 4–6 rats for each concentration).
Three deaths occurred in the first series of studies of intrathecal tetrodotoxin alone (Table 1), and six deaths occurred in rats given the combination of tetrodotoxin and veratridine (Table 2), all occurring with infusion of the highest tetrodotoxin doses of 0.47 or 0.63 µM. These deaths appeared to result from central respiratory depression, probably due to rostral spread of tetrodotoxin to medullary respiratory centres and respiratory arrest (Borison and McCarthy, 1977). MAC2/MAC0 values indicated complete recovery 24 h after tetrodotoxin alone or in combination with veratridine in all other animals (Tables 1 and 2; Figures 2 and 3). No rat displayed evidence of hind limb weakness on day 4.
Discussion and conclusions
Intrathecal infusion of the highly specific Na+ channel blocker tetrodotoxin increased the potency (decreased the MAC) of isoflurane in rats in a dose-related manner with an apparent plateau effect of ∼60% reduction in MAC. Furthermore, veratridine antagonized the reduction of MAC by tetrodotoxin, with nearly complete normalization of MAC at the greatest concentration of veratridine infused. Thus tetrodotoxin (0.47 µM) reduced MAC by >60%, while subsequent administration of veratradine (6.4 µM) increased MAC to 93% of control. This effect of veratradine on isoflurane MAC was more than twice the maximum increase (21%) produced by intrathecal infusion of veratridine alone in the absence of tetrodotoxin observed previously (Zhang et al., 2008). This suggests the possibility of a synergistic interaction between two allosteric Na+ channel antagonists (isoflurane and tetrodotoxin) at low levels of Na+ channel activation compared with the antagonistic interaction between an antagonist (isoflurane or tetrodotoxin) and allosteric agonist (veratridine). The mechanisms of these interactions at ion channel and/or neuronal circuit levels will require further investigation. These predicted drug interactions are consistent with the known pharmacological specificity of these ion channel modulators, and further support a role for Na+ channel actions in the immobilization produced by inhaled anaesthetics (Hemmings, 2009).
The interpretation of such pharmacological studies depends heavily on the specificity of the drugs used. All isoforms of voltage-gated Na channels (Nav) can be blocked with high potency and specificity by the puffer fish toxin tetrodotoxin, but three isoforms, known as tetrodotoxin-resistant isoforms (Nav1.5, Nav1.8 and Nav1.9; nomenclature follows Alexander et al., 2008), are 200- to 10 000-fold less sensitive compared with other isoforms (Goldin, 2001). This suggests that pharmacological differences between isoforms could also exist for other drugs. Veratridine has a much greater effect on deactivation of tetrodotoxin-sensitive isoforms consistent with the relatively fast inactivation of these channels compared with the slowly inactivating tetrodotoxin-resistant isoforms (Farrag et al., 2008). We recently found that sensitivity to inhaled anaesthetics including isoflurane is conserved across diverse Nav family members including both tetrodotoxin-sensitive and resistant isoforms (Herold et al., 2009). Taken together, these observations suggest that the effects of intrathecal infusions of tetrodotoxin and veratridine reflect primarily actions on the tetrodotoxin-sensitive Nav isoforms, in particular at lower doses. While it is difficult to extrapolate from the concentrations of drugs injected intrathecally to their concentrations at the site of action, this would suggest in turn that the MAC-modulating effects of tetrodotoxin and veratridine are mediated by as yet unidentified tetrodotoxin-sensitive voltage-gated Na+ channel isoforms in the spinal cord. Although recent studies suggest that isoflurane produces immobility primarily by actions in the ventral spinal cord with relatively minor effects on the dorsal horn (Jinks et al., 2008; Kungys et al., 2009), it is likely that tetrodotoxin and veratridine affect both nociceptive and motor components of the limb withdrawal reflex. The observation that hind limb motor function is preserved following intrathecal tetrodotoxin or veratridine at the doses used indicates that the withdrawal reflex remains functional and suggests that these drugs interact with isoflurane at a common site to modulate MAC. The use of intrathecal drug injections does not allow us to distinguish between various sites in the spinal cord, but the prominent ventral actions of isoflurane strongly implicate drug interactions in the ventral horn (Jinks et al., 2008; Kungys et al., 2009).
It is critical to distinguish pharmacological actions of veratridine and tetrodotoxin mediated by Na+ channel interactions from toxic actions involving Na+ channel and possibly other non-specific targets. By facilitating excessive Na+ and Ca2+ entry, veratridine can induce neuronal necrosis and apoptosis that could result in irreversible neuronal damage in the spinal cord (Banasiak et al., 2004). Our previous finding that veratridine increases isoflurane MAC indicated that veratridine (6.4 µM) might cause residual injury, as might be evident in a failure of the ratio MAC2/MAC0 to recover to unity (Zhang et al., 2008). In the present study, however, recovery was complete, suggesting that the presence of tetrodotoxin can ameliorate the irreversible toxic effects of veratridine. Infusion of tetrodotoxin alone did not preclude observation of movement and thereby prevent MAC determination. Withdrawal responses at isoflurane concentrations below MAC were maintained in all animals. No animal exhibited hind limb weakness the day following the intrathecal infusions. However, several animals died at the higher doses of tetrodotoxin tested probably due to cephalad spread and respiratory depression (Chang et al., 1990). The reversible alterations in MAC observed (e.g. Tables 1 and 2; Figure 2) are consistent with pharmacological, rather than toxic, actions of veratridine and tetrodotoxin at the intrathecal doses used.
Previous studies have shown that several inhaled anaesthetics including isoflurane, but not the non-immobilizer 1,2-dichloro-hexafluorocyclobutane, block endogenous neuronal Na+ channels (Ratnakumari and Hemmings, 1998; Ratnakumari et al., 2000; Ouyang et al., 2003; Herold et al., 2009) as well as various Navα-subunit isoforms heterologously expressed in mammalian cell lines (Rehberg et al., 1996; Ouyang and Hemmings, 2007; Ouyang et al., 2009) or amphibian oocytes (Shiraishi and Harris, 2004), in a voltage- and use-dependent manner. Inhibition of presynaptic Na+ channels contributes to depression of neurotransmitter release by volatile anaesthetics (Schlame and Hemmings, 1995; Westphalen and Hemmings, 2003; Wu et al., 2004; Hemmings et al., 2005), although the specific presynaptic Na+ channel isoforms involved in the release of various neurotransmitters have not been identified. A role for Na+ channel blockade in the immobilizing action of isoflurane is also supported by several recent observations in vivo. Intrathecal, but not intracerebroventricular, increases in Na+ concentration increase isoflurane MAC (Laster et al., 2007), and i.v. administration of lidocaine decreases isoflurane MAC in a dose-related manner (Zhang et al., 2007). Although this study did not report a plateau for the effect of lidocaine on isoflurane MAC in rats, a prior study found a plateau at about 40% reduction in cyclopropane MAC in rats, a decrease comparable to the effect of tetrodotoxin (DiFazio et al., 1976). Conversely, intrathecal (but not intracerebroventricular) administration of veratridine increased MAC (Zhang et al., 2008). These results plus the present findings point to inhibition of Na+ channels as a plausible mechanism for the mediation of the immobility produced by inhaled anaesthetics (Eger et al., 2008; Hemmings, 2009).
Previous studies have shown that isoflurane and other inhaled anaesthetics inhibit Na+ channel-dependent release of glutamate, the principal excitatory neurotransmitter, from isolated cerebrocortical nerve terminals at concentrations near the MAC (Miao et al., 1995; Schlame and Hemmings, 1995; Westphalen and Hemmings, 2003; Shiraishi and Harris, 2004; Wu et al., 2004). Recent studies have shown that isoflurane and halothane also inhibit glutamate release from isolated nerve terminals from other brain regions including spinal cord (H.C. Hemmings and R.I. Westphalen, unpubl. experiments), which suggests that a critical molecular target for the immobilizing actions of inhaled anaesthetics could be inhibition of voltage-gated Na+ channels in the ventral spinal cord, thereby leading to reduced excitatory transmission due to inhibition of the release of glutamate, and possibly other transmitters (Kim et al., 2007; Jinks et al., 2008; Hemmings, 2009; Kungys et al., 2009). In conclusion, our observations that intrathecal administration of tetrodotoxin increases isoflurane potency (decreases isoflurane MAC), while intrathecal administration of veratridine counteracts this effect in vivo, provide further support for a role for voltage-gated Na+ channel blockade in the immobility produced by inhaled anaesthetics (Hemmings, 2009).
Acknowledgments
This work was supported in part by NIH grants 1P01GM47818 (EIE and RAH) and GM58055 (HCH).
Glossary
Abbreviations:
- aCSF
artificial cerebrospinal fluid
- MAC
minimum alveolar concentration of anaesthetic required to abolish movement in 50% of subjects
Conflicts of interest
Dr Eger is a paid consultant to Baxter Healthcare Corporation (Deerfield, IL, USA), which donated the isoflurane used in these studies.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- Borison HL, McCarthy LE. Respiratory and circulatory effects of saxitoxin in the cerebrospinal fluid. Br J Pharmacol. 1977;61:679–689. doi: 10.1111/j.1476-5381.1977.tb07561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Cestèle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Chang FC, Benton BJ, Salyer JL, Foster RE, Franz DR. Respiratory and cardiovascular effects of tetrodotoxin in urethane-anesthetized guinea pigs. Brain Res. 1990;528:259–268. doi: 10.1016/0006-8993(90)91666-5. [DOI] [PubMed] [Google Scholar]
- DiFazio CA, Neiderlehner JR, Burney RG. The anesthetic potency of lidocaine in the rat. Anesth Analg. 1976;55:818–821. doi: 10.1213/00000539-197611000-00016. [DOI] [PubMed] [Google Scholar]
- Eger EI, II, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–848. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag KJ, Bhattacharjee A, Docherty RJ. A comparison of the effects of veratridine on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in isolated rat dorsal root ganglion neurons. Pflugers Arch. 2008;455:929–938. doi: 10.1007/s00424-007-0365-5. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Hemmings HC., Jr Sodium channels and the synaptic mechanisms of anaesthesia. Br J Anaesth. 2009;103:61–69. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005;67:1591–1599. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- Herold KF, Nau C, Ouyang W, Hemmings HC., Jr Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009;111:591–599. doi: 10.1097/ALN.0b013e3181af64d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Bravo M, Hayes SG. Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology. 2008;108:1016–1024. doi: 10.1097/ALN.0b013e3181730297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yao A, Atherley R, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg. 2007;105:1020–1026. doi: 10.1213/01.ane.0000280483.17854.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108:1531–1537. doi: 10.1213/ane.0b013e31819d9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster MJ, Zhang Y, Eger EI, 2nd, Shnayderman D, Sonner JM. Alterations in spinal, but not cerebral, cerebrospinal fluid Na+ concentrations affect the isoflurane minimum alveolar concentration in rats. Anesth Analg. 2007;105:661–665. doi: 10.1213/01.ane.0000278090.88402.26. [DOI] [PubMed] [Google Scholar]
- Miao N, Frazer MJ, Lynch C., 3rd Volatile anesthetics depress Ca2+ transients and glutamate release in isolated cerebral synaptosomes. Anesthesiology. 1995;83:593–603. doi: 10.1097/00000542-199509000-00019. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Hemmings HC., Jr Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology. 2007;107:91–98. doi: 10.1097/01.anes.0000268390.28362.4a. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wang G, Hemmings HC., Jr Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol. 2003;64:373–381. doi: 10.1124/mol.64.2.373. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Herold KF, Hemmings HC., Jr Comparative effects of inhaled anesthetics on rat Nav1.4 function. Anesthesiology. 2009;110:582–590. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Hemmings HC., Jr Inhibition of presynaptic sodium channels by halothane. Anesthesiology. 1998;88:1043–1054. doi: 10.1097/00000542-199804000-00025. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Vysotskaya TN, Duch DS, Hemmings HC., Jr Differential effects of anesthetic and nonanesthetic cyclobutanes on neuronal voltage-gated sodium channels. Anesthesiology. 2000;92:529–541. doi: 10.1097/00000542-200002000-00037. [DOI] [PubMed] [Google Scholar]
- Rehberg B, Xiao YH, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84:1223–1233. doi: 10.1097/00000542-199605000-00025. [DOI] [PubMed] [Google Scholar]
- Schlame M, Hemmings HC., Jr Inhibition by volatile anesthetics of endogenous glutamate release from synaptosomes by a presynaptic mechanism. Anesthesiology. 1995;82:1406–1416. doi: 10.1097/00000542-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Harris RA. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J Pharmacol Exp Ther. 2004;309:987–994. doi: 10.1124/jpet.103.064063. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, et al. Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–740. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Laster MJ, Eger EI, II, Stabernack CR, Sonner JM. Blockade of 5-HT2A receptors may mediate or modulate part of the immobility produced by inhaled anesthetics. Anesth Analg. 2003a;97:475–479. doi: 10.1213/01.ANE.0000070229.94485.17. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Laster MJ, Hara K, Harris RA, Eger EI, II, Stabernack CR, et al. Glycine receptors may mediate part of the immobility produced by inhaled anesthetics. Anesth Analg. 2003b;96:97–101. doi: 10.1097/00000539-200301000-00021. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Laster MJ, Eger EI, II, Sharma M, Sonner JM. Lidocaine, MK-801, and MAC. Anesth Analg. 2007;104:1098–1102. doi: 10.1213/01.ane.0000260318.60504.a9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sharma M, Eger EI, II, Laster MJ, Hemmings HC, Jr, Harris RA. Intrathecal veratridine administration increases MAC in rats. Anesth Analg. 2008;107:875–878. doi: 10.1213/ane.0b013e3181815fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Stabernack C, Sonner JM, Dutton R, Eger EI., II Both cerebral GABAA receptors and spinal GABAA receptors modulate the capacity of isoflurane to produce immobility. Anesth Analg. 2001;92:1585–1589. doi: 10.1097/00000539-200106000-00047. [DOI] [PubMed] [Google Scholar]


