Abstract
Background and purpose:
Despite decreased presynaptic 5-HT1A and altered 5-HT2A receptor function in genetically-deficient serotonin (5-HT) transporter (SERT) mice, the 5-HT1A receptor antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY 100635) still induced head twitches in these mice, a well-established 5-HT2A receptor-mediated response.
Experimental approach:
Interactions between 5-HT1A and 5-HT2A receptors were assessed using the head-twitch response following 5-HT1A and 5-HT2A receptor agonists and antagonists in SERT wild-type (+/+), heterozygous (+/−), and knockout (−/−) mice. The role of brain 5-HT availability in WAY 100635 induced head twitches was also examined.
Key results:
WAY 100635 induced head twitches in a SERT gene-dose dependent manner, inducing 5-fold more head twitches in SERT −/− versus SERT +/+ mice. In SERT −/− mice, inhibition of 5-HT synthesis with p-chlorophenylalanine (PCPA) markedly depleted tissue 5-HT in all five brain areas examined and abolished WAY 100635 induced head twitches. Further, the selective 5-HT reuptake inhibitor fluvoxamine increased WAY 100635 induced head twitches in SERT +/+ and +/− mice. Head twitches following the 5-HT2A receptor agonist (+/−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI) were robust in SERT +/+ and +/− mice but much reduced in SERT −/− mice. DOI-induced head twitches were decreased by the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in SERT +/+ and +/− mice. All drug-induced head twitches were blocked by the 5-HT2A receptor antagonist a-Phenyl-1-(2-phenylethyl)-4-piperidinemethanol (MDL 11,939).
Conclusions and implications:
These data show that indirect activation of 5-HT2A receptors via blockade of presynaptic 5-HT1A receptors potentiated head-twitch responses, suggesting functional interactions between these receptors, interactions affected by altered 5-HT availability. Our findings strongly support the correlation of WAY 100635 induced head twitches with increased 5-HT availability, induced genetically or pharmacologically.
Keywords: head twitch response; 5-HT transporter (SERT) knockout mice; 5-HT1A receptors; 5-HT2A receptors; 8-OH-DPAT; DOI; fluvoxamine; L745870; MDL 11,939; WAY 100635; serotonin (5-HT)
Introduction
In the central nervous system (CNS), 5-HT2A receptors (nomenclature follows Alexander et al., 2008) are a target for drugs effective in the treatment of schizophrenia and other psychotic disorders and mediate the hallucinogenic effects of hallucinogenic 5-hydroxytryptaminergic drugs such as lysergic acid diethylamide (LSD). The head-twitch response in mice is mediated by 5-HT2A receptors, providing a measure of 5-HT2A function and possibly providing a ‘behavioural proxy’ in mice to assess hallucinogenic effects in humans (Willins and Meltzer, 1997; Gonzalez-Maeso et al., 2003; 2007; Moya et al., 2007).
Recent studies show that altered 5-HT2A receptor-mediated responses are associated with alterations in serotonin 5-HT transporter (SERT) expression, the main mechanism for maintaining homeostatic levels of 5-HT. For example, SERT knockout (−/−) mice have marked decreases in head twitches induced by (+/−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and also by the recently described high-affinity 5-HT2A receptor agonist (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2) (McLean et al., 2006) compared with SERT wild-type (+/+) mice (Qu et al., 2005; Basselin et al., 2009; Fox et al., in press) and demonstrate a decrease in the ability to establish stimulus control to LSD within a drug discrimination paradigm (Krall et al., 2008). These functional alterations in SERT-deficient mice are associated with brain-area dependent alterations in 5-HT2A receptor density and diminished 5-HT2A receptor-mediated activation of the PLA2-arachidonic acid (AA) signalling pathway (Rioux et al., 1999; Li et al., 2003; Qu et al., 2005; Basselin et al., 2009).
Despite these reports of decreased 5-HT2A receptor function in SERT-deficient mice, we recently reported in preliminary results that the selective 5-HT1A receptor antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY 100635) induced an enhanced head-twitch response in SERT −/− mice (Fox et al., 2008), which has been demonstrated to be a response mediated by 5-HT2A receptors (Willins and Meltzer, 1997; Gonzalez-Maeso et al., 2003; Moya et al., 2007).
It has been suggested that selective 5-HT1A receptor antagonists such as S-(-)UH 301 and WAY 100635 might induce 5-HT2A receptor -mediated head twitches in an indirect manner (Darmani et al., 1990; Darmani and Reeves, 1996; Darmani, 1998). This effect might be dependent upon the presence of elevated 5-HT levels, as the effects of WAY 100635 were observed only when 5-HT levels were enhanced. For example, although WAY 100635 alone does not affect 5-HT levels, when a selective 5-HT reuptake inhibitor (SSRI), which increases 5-HT levels, was administered, WAY 100635 enhanced 5-HT levels and decreased firing rates in mice and rats (Hjorth, 1993; Gartside et al., 1995; Romero et al., 1996; Gundlah et al., 1997; Hjorth et al., 1997; Gobert et al., 2000). Further, WAY 100635 induced head twitches in mice during the light phase and not the dark phase, which correlates with altered 5-HT levels during these time periods (Darmani, 1998). These effects are likely to be due to inhibition of presynaptic 5-HT1A receptors, which when activated serve as a negative feedback loop for 5-HT synthesis and release (Hoyer et al., 1994; Sharp et al., 2007).
SERT-deficient mice have elevated extracellular 5-HT levels and thus enhanced 5-HT availability (Fabre et al., 2000; Mathews et al., 2004; Shen et al., 2004). However, life-long exposure to these enhanced levels of 5-HT have resulted in significant alterations in the density and function of several 5-HT receptor subtypes in SERT −/− mice (see Fox et al., 2007b). In addition to these changes in 5-HT2A receptors and receptor function, SERT-deficient mice have more marked decreases in presynaptic 5-HT1A receptor density and function, including decreased or absent temperature, neural firing, and hormonal responses following 5-HT1A receptor agonists (Li et al., 1999; 2000; Gobbi et al., 2001; Bouali et al., 2003; Holmes et al., 2003b; Fox et al., 2008).
The current studies were performed to examine functional pharmacological interactions between 5-HT2A and presynaptic 5-HT1A receptors in SERT +/+, heterozygous (+/−), and −/− mice. First, to replicate our preliminary findings for the present series of more detailed studies and to evaluate baseline head twitches, SERT +/+, +/−, and −/− mice were treated with vehicle or WAY 100635, and head twitches were recorded. On the basis of recent reports that suggest alterations in SERT expression and the consequent changes in availability of 5-HT affect DOI-induced head twitches (Jennings et al., 2008; Basselin et al., 2009), we next examined the role of 5-HT availability in this response in SERT −/− mice via pretreatment with p-chlorophenylalanine (PCPA), which depletes 5-HT via tryptophan hydroxylase inhibition (Cesana et al., 1993; O'Leary et al., 2007). Next, we determined the effects of pretreatment with the SSRI fluvoxamine, which blocks SERT and increases extracellular 5-HT levels in wild-type mice and in rats (Gobert et al., 2000; Kobayashi et al., 2008), on head twitches induced by WAY 100635 in SERT +/+, +/−, and −/− mice. To further examine pharmacological interactions between 5-HT1A and 5-HT2A receptors in SERT +/+, +/− and, −/− mice, we examined head twitches following administration of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), the 5-HT2A/2C receptor agonist DOI, and again the 5-HT1A receptor antagonist WAY 100635, either alone or in combination. As previous reports have shown that WAY 100635 might also have agonist properties at dopamine D4 receptors (Chemel et al., 2006; Marona-Lewicka and Nichols, 2009; but see Martel et al., 2007), we assessed the effects of pretreatment with the selective dopamine D4 receptor antagonist 3-(4-[4-Chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[2 ,3-b]pyridine trihydrochloride (L745870) or vehicle on head twitches induced by WAY 100635 in SERT −/− mice. Finally, to confirm 5-HT2A receptor mediation of the head-twitch responses described above, mice were treated with a-Phenyl-1-(2-phenylethyl)-4-piperidinemethanol (MDL 11,939), a selective 5-HT2A receptor antagonist (Pehek et al., 2006), or vehicle prior to the test drug(s). Despite earlier findings of altered 5-HT2A and presynaptic 5-HT1A receptor function in SERT-deficient mice, the current studies show a functional interaction between these receptors in these mice, an interaction that appears directly associated with 5-HT availability.
Methods
Animals
All animal care and experimental procedures adhered to the guidelines of the National Institutes of Health and were approved by the National Institute of Mental Health Animal Care and Use Committee. Mice were SERT +/+, +/− and −/− strains, originally produced by homologous recombination in ES cells (Bengel et al., 1998) and currently the product of 19–23 heterozygous backcrosses on a C57BL/6J genetic background. Mice were approximately 20–35 g in weight at the beginning of the experiments and were housed in groups of three to five animals per cage with food and water available ad libitum. Animals were maintained on a 12 h light : 12 h dark cycle (lights on at 0600 h).
Behavioural assessments
Mice were moved to the testing room in their home cage 1 h prior to testing. Following 15 min of habituation in a plexiglass container, mice were administered the test drug(s). Head twitches were recorded for five 1 min periods (once every 5 min starting 5 min after drug administration) over a 30 min period; the scores from the five 1-min periods were summed together. When 5-HT2A receptor mediation was assessed, the selective 5-HT2A receptor antagonist MDL 11,939 (5 mg·kg−1) was administered 30 min prior to administration of drug(s) (test drugs at the same doses as in initial experiments, administered at the same time). All experiments were conducted between 1000 h and 1300 h.
5-HT depletion
To deplete 5-HT levels, SERT −/− mice were given PCPA (300 mg·kg−1) or its vehicle twice daily for 3 days (Cesana et al., 1993; O'Leary et al., 2007; Basselin et al., 2009). On the fourth day, 18 h following the final PCPA treatment, half of the vehicle-treated and half of the PCPA-treated SERT −/− mice were administered WAY 100635 (1 mg·kg−1) and head twitches were counted as described above. The remaining mice were killed via cervical dislocation for examination of 5-HT and other monoamine levels (see below).
Analysis of brain region monoamines and metabolites
Following the regimen with vehicle or PCPA described above, brains were removed 18 h following the last PCPA treatment and were immediately dissected on a glass plate set in ice. After the removal of the hypothalamus and the frontal cortex, brains were bisected sagitally to expose and dissect the hippocampus and the striatum from each hemisphere, followed by isolation of the brainstem containing pons and medulla (Bengel et al., 1998). Brain samples were stored at −80°C before HPLC analysis using electrochemical detection, as previously described (Andrews and Murphy, 1993; Kim et al., 2005; Fox et al., 2008). Briefly, brain tissue samples were homogenized in 200–250 µL of 0.1 N HC1O4 using sonication and centrifuged at 7200×g for 10 min. For quantification of monoamines in the supernatant, an Axxichrom ODS C18 (5 µm, 25 cm × 0.46 cm) analytical column, an ESA Coulochem II detector with analytical cell (E1 = 100 mV, E2 = 300 mV; Model 5014), and a guard cell (100 mV; Model 5020) were set up with an ESA solvent pump (Model 582) and a Gibson autosampler (Model 231) fitted with a 50 µL sample loop. The mobile phase was composed of 8.6 mM heptane sulfonic acid, 0.3% phosphoric acid, 0.27% triethylamine, 0.34 mM EDTA and 12% acetonitrile delivered at a flow rate of 0.6 mL·min−1. Samples were prepared using 100 µL of the supernatant with the addition of an internal standard (50 µL of 1 µM N-methyl 5-HT for monoamines). A 55 µL aliquot of this mixture was injected onto the analytical column for HPLC-EC analysis.
Statistical analysis
The number of head twitches was analysed using two-way (genotype × drug condition) anova or Student's t-tests when only two groups were compared, and 5-HT and other monoamines and metabolite concentrations were compared using t-tests. Post hoc analyses were performed using Tukey HSD tests or t-tests, as appropriate, to evaluate differences between the genotypes and between the drug conditions. Significance was based on P < 0.05.
Materials
WAY 100635, 8-OH-DPAT, DOI, PCPA and fluvoxamine were obtained from Sigma Chemical Company (St. Louis, MO, USA), and L745870 and MDL 11,939 were obtained from Tocris Bioscience (Ellisville, MO, USA). WAY 100635 (1 mg·kg−1), 8-OH-DPAT (1 mg·kg−1), DOI (2.5 mg·kg−1), fluvoxamine (10 mg·kg−1) and L745870 (1 or 3 mg·kg−1) were dissolved in saline; PCPA (300 mg·kg−1) was dissolved in distilled deionized water; MDL 11,939 (5 mg·kg−1) was dissolved in a few drops of acetic acid and diluted in distilled water and adjusted to a pH of 6.5 ± 0.1. Doses were selected based on previous work performed in our laboratory in addition to existing literature confirmed by pilot studies in our laboratory [WAY 100635 (Fox et al., 2007a; 2008; 2009;), 8-OH-DPAT (Fox et al., 2007a; 2008;), DOI (Basselin et al., 2009), PCPA (Cesana et al., 1993; O'Leary et al., 2007; Basselin et al., 2009), fluvoxamine (Daws et al., 2006), L745870 (Iasevoli et al., 2009; Milstein et al., 2010) and MDL 11,939 (Fox et al., 2007a)]. All drugs were administered by i.p. injection at a volume of 10 mL·kg−1.
Results
WAY 100635 induced head twitches
There were significant main effects for genotype (F2,45= 6.36, P= 0.004) and drug condition (F1,45= 32.21, P < 0.0001), and a significant genotype × drug condition interaction (F2,45= 6.24, P= 0.004). Mice of all three SERT genotypes given WAY 100635 (1 mg·kg−1) displayed significantly more head twitches than their respective counterparts receiving vehicle (P's < 0.05). Compared with SERT +/+ mice, this effect of WAY 100635 was increased ∼5-fold in SERT −/− mice (P= 0.007), with an intermediate, ∼3-fold increase in SERT +/− mice (Figure 1). We evaluated the effects of WAY 100635 over a range of doses (0, 0.1, 0.25, 0.5 and 1.0 mg·kg−1) in SERT −/− mice. There was a significant effect for dose (F4,26= 10.42, P < 0.0001); WAY 100635 induced head twitches in SERT −/− mice in a dose-dependent manner, with doses of 0.25, 0.5 and 1.0 mg·kg−1 inducing more head twitches than vehicle (P's < 0.001), with no difference between these three doses, and an intermediate response following 0.1 mg·kg−1 (data not shown).
Figure 1.
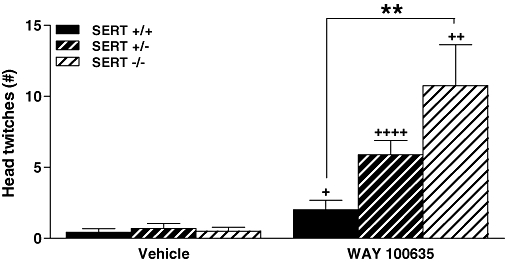
Head twitches in SERT +/+, +/− and −/− mice following vehicle or the selective 5-HT1A receptor antagonist WAY 100635 (1 mg·kg−1) (n= 8–10). Data represent the mean ± SEM. +P < 0.05, ++P < 0.01, ++++P < 0.0001 compared with vehicle-treated mice of the same genotype; **P < 0.01 compared with SERT +/+ mice in the same drug condition. SERT, 5-HT transporter; WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt.
Effects of PCPA pretreatment on WAY 100635 induced head twitches and brain region monoamines and metabolites in SERT −/− mice
PCPA pretreatment (300 mg·kg−1 twice daily for 3 days) completely abolished head twitches induced by WAY 100635 (1 mg·kg−1) in SERT −/− mice (P < 0.0001) (Figure 2) and decreased 5-HT levels by 94% in the frontal cortex, 93% in the hippocampus, 88% in the hypothalamus, 94% in the striatum and 67% in the brainstem compared with levels in vehicle-pretreated SERT −/− mice (all P's < 0.0001) (Table 1). Tissue levels of the major 5-HT metabolite, 5-hydroxyindole acetic acid (5-HIAA), were also decreased in all five brain areas examined in PCPA-pretreated mice compared with vehicle pretreated mice (all P's < 0.0003). 5-HT turnover (as measured by the 5-HIAA : 5-HT ratio) was not altered by PCPA pretreatment (NS; data not shown). Brain tissue levels of 5-HT, 5-HIAA, noradrenaline, dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC), a metabolite of dopamine, are presented in Table 1.
Figure 2.
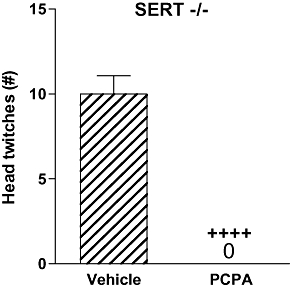
Effects of PCPA pretreatment (300 mg·kg−1 twice daily for 3 days) on head twitches induced by WAY 100635 (1 mg·kg−1) in SERT −/− mice (n= 4–5). Data represent the mean ± SEM. ++++P < 0.0001 compared with vehicle-pretreated mice. SERT, 5-HT transporter; WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; PCPA, p-chlorophenylalanine.
Table 1.
Tissue levels of 5-HT, 5-HIAA, noradrenaline, dopamine and DOPAC in SERT −/− mice treated with vehicle or PCPA (300 mg·kg−1 twice daily for 3 days)
| Measurement (pg·mg−1tissue) |
Drug condition |
|
|---|---|---|
| Brain area | Vehicle | PCPA |
| 5-HT | ||
| Frontal cortex | 233 ± 61 | 14 ± 4++++ |
| Brain stem | 193 ± 29 | 63 ± 14++++ |
| Hippocampus | 152 ± 28 | 10 ± 4++++ |
| Hypothalamus | 469 ± 99 | 59 ± 49++++ |
| Striatum | 216 ± 57 | 13 ± 6++++ |
| 5-HIAA | ||
| Frontal cortex | 128 ± 49 | 8 ± 5++ |
| Brain stem | 510 ± 131 | 151 ± 58++ |
| Hippocampus | 260 ± 90 | 13 ± 4++++ |
| Hypothalamus | 423 ± 124 | 37 ± 13++++ |
| Striatum | 252 ± 15 | 15 ± 9++++ |
| Noradrenaline | ||
| Frontal cortex | 366 ± 78 | 290 ± 12 |
| Brain stem | 398 ± 35 | 364 ± 42 |
| Hippocampus | 297 ± 18 | 236 ± 49 |
| Hypothalamus | 1627 ± 166 | 1228 ± 266+ |
| Dopamine | ||
| Striatum | 11 422 ± 639 | 10 660 ± 689 |
| DOPAC | ||
| Striatum | 516 ± 61 | 428 ± 89 |
Data represent the mean ± SEM; n= 4–5 per group.
P < 0.05,
P < 0.01,
P < 0.0001 compared with SERT −/− mice administered vehicle (t-test).
SERT, 5-HT transporter; PCPA, p-chlorophenylalanine; 5-HIAA, 5-hydroxyindole acetic acid; DOPAC, 3,4-dihydroxyphenylacetic acid.
Effects of pretreatment with the SSRI fluvoxamine on WAY 100635 induced head twitches
Administered alone, fluvoxamine (10 mg·kg−1) did not induce head twitches (data not shown). There were significant main effects for genotype (F2,29= 6.61, P= 0.004) and drug condition (F1,29= 12.04, P= 0.002) and a significant genotype × drug condition interaction (F2,29= 10.77, P < 0.0001). In mice pretreated with vehicle, WAY 100635 (1 mg·kg−1) again induced an enhanced head-twitch response in SERT −/− mice compared with SERT +/+ mice (P < 0.0001) (Figure 3). Pretreatment with fluvoxamine (10 mg·kg−1) 30 min earlier increased WAY 100635 induced head twitches in SERT +/+ and +/− mice compared with their respective vehicle-pretreated counterparts (P's < 0.004). In mice pretreated with fluvoxamine and then given WAY 100635, there were no differences between the three SERT genotypes. In fact, following pretreatment with fluvoxamine, WAY 100635 induced a similar number of head twitches in SERT +/+ and +/− mice compared with those observed in SERT −/− mice pretreated with vehicle and given WAY 100635.
Figure 3.
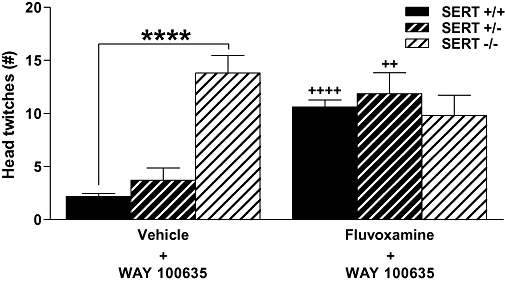
Effects of pretreatment with vehicle or the SSRI fluvoxamine (10 mg·kg−1) 30 min earlier on head twitches induced by WAY 100635 (1 mg·kg−1) in SERT +/+, +/− and −/− mice (n= 5–7). Data represent the mean ± SEM. ++P < 0.01, ++++P < 0.0001 compared with vehicle-pretreated mice of the same genotype; ****P < 0.0001 compared with SERT +/+ mice in the same drug condition. SERT, 5-HT transporter; WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; SSRI, selective 5-HT reuptake inhibitor.
Effects of 8-OH-DPAT and WAY 100635 on DOI-induced head twitches
There were significant main effects for genotype (F2,61= 7.70, P= 0.001) and drug condition (F4,61= 67.50, P < 0.0001), in addition to a significant genotype × drug condition interaction (F8,61= 6.84, P < 0.0001). DOI (2.5 mg·kg−1) induced ∼85% fewer head twitches in SERT −/− mice compared with SERT +/+ mice (P= 0.001) (Figure 4). Administration of WAY 100635 (1 mg·kg−1) alone again induced more head twitches in SERT −/− mice compared with SERT +/+ mice (P < 0.0001), and of note in SERT −/− mice, WAY 100635 induced more head twitches than did DOI (P= 0.014). The combination of WAY 100635 plus DOI induced more head twitches compared with DOI alone in SERT +/− mice (P= 0.004), with additive or super-additive effects in SERT −/− mice (the combination of WAY 100635 plus DOI compared with either drug alone, P's < 0.0001). Further, 8-OH-DPAT (1 mg·kg−1) decreased DOI-induced head twitches in SERT +/+ and +/− mice (P's < 0.001), with no effect when administered alone and no effects in SERT −/− mice.
Figure 4.
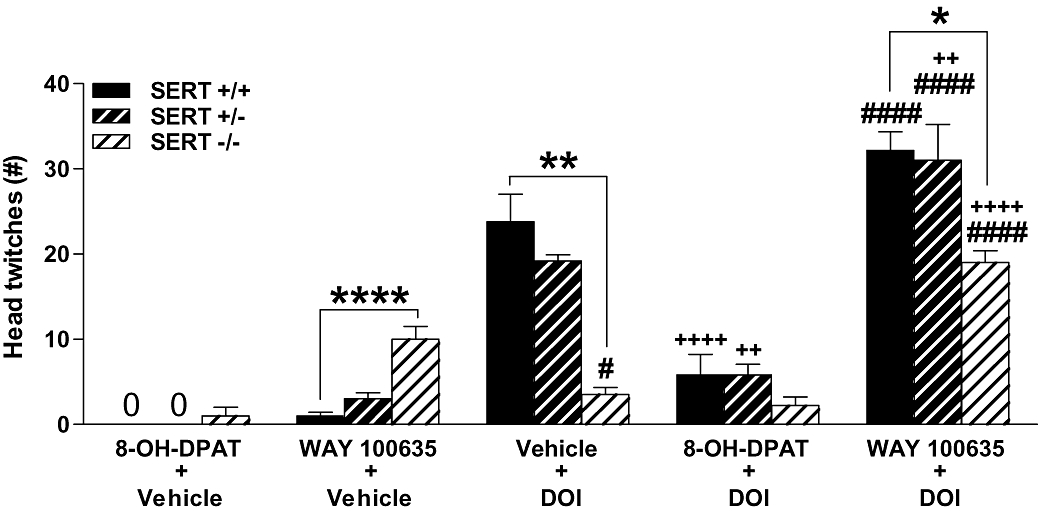
Head twitches in SERT +/+, +/−, and −/− mice following 8-OH-DPAT (1 mg·kg−1), WAY 100635 (1 mg·kg−1) and DOI (2.5 mg·kg−1) administered alone or in combination (administered at the same time) (n= 4–9). Data represent the mean ± SEM. #P < 0.05, ####P < 0.0001 compared with WAY 100635 alone; ++P < 0.01, ++++P < 0.0001 compared with DOI alone; *P < 0.05, **P < 0.01, ****P < 0.0001 compared with SERT +/+ mice in the same drug condition. SERT, 5-HT transporter; WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)tetralin; DOI, (+/−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane.
Effects of pretreatment with the selective dopamine D4 receptor antagonist L745870 on WAY 100635 induced head twitches in SERT −/− mice
Administered alone, the selective dopamnine D4 receptor antagonist L745870 (1 or 3 mg·kg−1) did not alter head twitches compared with vehicle (data not shown). Further, pretreatment with L745870 did not affect head twitches induced by WAY 100635 (1 mg·kg−1) in SERT −/− mice (F2,15= 0.86, NS) (mean ± SEM; vehicle + WAY 100635, 8.33 ± 2.58; 1 mg·kg−1 L745870 + WAY 100635, 9.67 ± 2.07; 3 mg·kg−1 L745870 + WAY 100635, 9.67 ± 1.21; n= 6 per group), thus ruling out a role for dopamine D4 receptors in this response.
Assessments of 5-HT2A receptor mediation of head twitches
MDL 11,939 administered alone did not affect head twitches (data not shown). In SERT −/− mice, pretreatment with MDL 11,939 completely blocked WAY 100635 induced head twitches (P < 0.0001) (Figure 5A). Similarly, MDL 11,939 completely blocked head twitches induced by the combination of WAY 100635 plus DOI in mice of all three SERT genotypes (P's < 0.05) (Figure 5B) [main effect of genotype (F2,20= 7.05, P= 0.005); main effect of drug condition (F1,20= 160.89, P < 0.0001); genotype × drug condition interaction (F2,20= 7.05, P= 0.005)]. Additionally, regardless of genotype, MDL 11,939 pretreatment completely blocked the head twitches induced by fluvoxamine plus WAY 100635 in SERT +/+ and +/− mice (P < 0.0001) (Figure 5C) [main effect of genotype (F1,14= 0.67, NS); main effect of drug condition (F1,14= 45.08, P < 0.0001); genotype × drug condition interaction (F1,14= 0.67, NS)].
Figure 5.
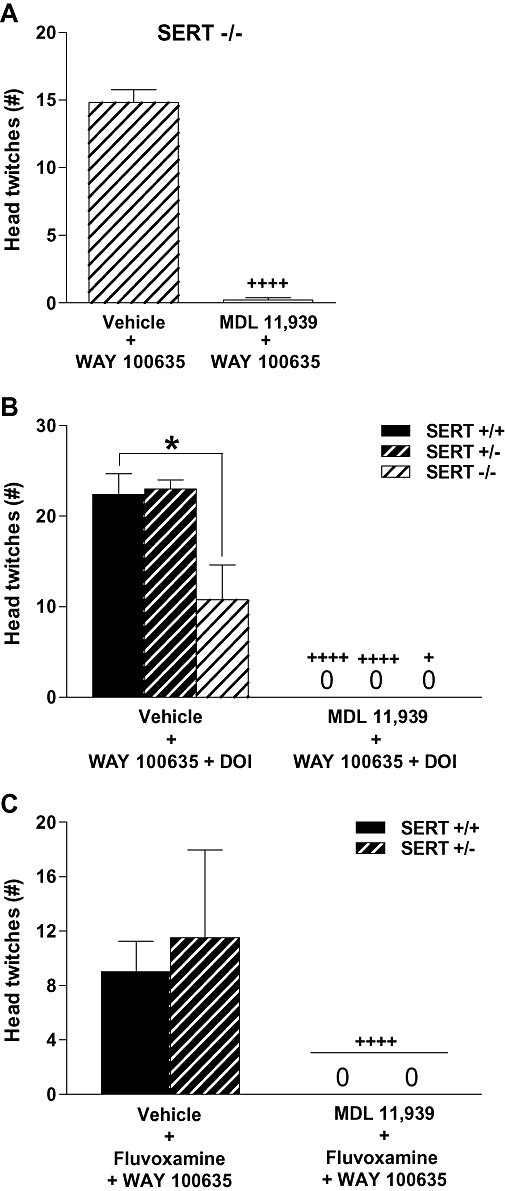
Effects of pretreatment 30 min earlier with vehicle or the selective 5-HT2A receptor antagonist MDL 11,939 (5 mg·kg−1) on head twitches induced (A) by WAY 100635 (1 mg·kg−1) in SERT −/− mice (n= 5), (B) by WAY 100635 (1 mg·kg−1) plus DOI (2.5 mg·kg−1) in SERT +/+, +/− and −/− mice (n= 4–5) or (C) by fluvoxamine (10 mg·kg−1) plus WAY 100635 (1 mg·kg−1) in SERT +/+ and +/− mice (n= 4–5). Data represent the mean ± SEM. +P < 0.05, ++++P < 0.0001 compared with vehicle-pretreated mice [regardless of genotype in panel (C)]; *P < 0.05 compared with SERT +/+ mice in the same drug condition. SERT, 5-HT transporter; WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; DOI, (+/−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane; MDL 11,939, a-Phenyl-1-(2-phenylethyl)-4-piperidinemethanol.
Discussion
In the current studies, the selective 5-HT1A receptor antagonist WAY 100635 induced an enhanced head-twitch response in SERT −/− mice. It is well established that the head-twitch response is mediated by 5-HT2A receptors (Darmani and Reeves, 1996; Willins and Meltzer, 1997; Darmani, 1998; Gonzalez-Maeso et al., 2003; Moya et al., 2007). For example, studies have established that direct injection of 5-HT2A receptor agonists such as DOI into the medial prefrontal cortex induce head twitches that are blocked by 5-HT2A receptor antagonists MDL 100907 and ketanserin, but not by the 5-HT2C/2B receptor antagonist SDZ SER 082 (Willins and Meltzer, 1997). Consistent with this, the selective 5-HT2A receptor antagonist MDL 11,939 blocked all head-twitch responses assessed in the current studies.
This enhanced 5-HT2A receptor-mediated response following the 5-HT1A receptor antagonist WAY 100635 in SERT −/− mice is of note as SERT-deficient mice have brain area-dependent alterations in 5-HT2A receptor density and function, in addition to markedly reduced post-receptor signalling by 5-HT2A receptors along the PLA2-AA pathway in multiple brain regions (Rioux et al., 1999; Li et al., 2003; Qu et al., 2005; Basselin et al., 2009; Fox et al., in press). Further, SERT-deficient mice have markedly reduced density and function of presynaptic 5-HT1A autoreceptors, including decreased temperature, neural firing, and hormonal responses following 5-HT1A receptor agonists (Li et al., 1999; 2000; Gobbi et al., 2001; Bouali et al., 2003; Holmes et al., 2003a; Fox et al., 2008).
SERT +/− and −/− mice have ∼3-fold and ∼6-fold increases in baseline extracellular 5-HT respectively (Fabre et al., 2000; Mathews et al., 2004; Shen et al., 2004). In SERT −/− mice, this elevated availability of 5-HT appears to play a role in WAY 100635 induced head twitches.
In the current studies in SERT −/− mice, pretreatment with the 5-HT synthesis inhibitor PCPA, using a regimen previously shown to decrease 5-HT levels by ∼80% in wild-type mice (Cesana et al., 1993; O'Leary et al., 2007), decreased tissue 5-HT levels by ∼67–94% compared with vehicle in all five brain areas examined, thus decreasing 5-HT availability. Further, this pretreatment with PCPA completely abolished WAY 100635 induced head twitches in SERT −/− mice, strongly suggesting a role for elevated 5-HT availability in this response.
We also report that pretreatment with the SSRI fluvoxamine, which blocks SERT and increases extracellular 5-HT levels (Gobert et al., 2000; Kobayashi et al., 2008), increased the number of head twitches induced by WAY 100635 in SERT +/+ and +/− mice, findings consistent with a previous report of treatment with fluoxetine plus WAY 100635 in rats (Gobert et al., 2000). As expected, pretreatment with fluvoxamine was without effect in SERT −/− mice. We had hypothesized that fluvoxamine might have an exaggerated effect in SERT +/− mice, as a previous report showed that fluvoxamine prolonged 5-HT clearance in an exaggerated manner in SERT +/− compared with SERT +/+ mice (Montanez et al., 2003); however, the effects of fluvoxamine were similar in SERT +/+ and +/− mice.
Together, these findings from the genetically-deficient SERT mice and the pharmacological inhibition of SERT suggest that in the presence of the consequently elevated 5-HT availability, such as in SERT −/− mice at baseline and in SERT +/+ and +/− mice pretreated with an SSRI, WAY 100635 further enhances 5-HT levels, most likely by blocking presynaptic 5-HT1A receptors, which when activated serve as a negative feedback loop for 5-HT synthesis and release, as shown earlier (Hoyer et al., 1994; Sharp et al., 2007). Activation of presynaptic 5-HT1A autoreceptors by 5-HT or other 5-HT1A agonists decreases neural activity and inhibits 5-HT synthesis, thus decreasing 5-HT levels (Hoyer et al., 1994). As mentioned, administration of a 5-HT1A receptor antagonist blocks this inhibition, in this case the inhibition induced by 5-HT, thus increasing neural activity. As such, administration of a 5-HT1A antagonist increases 5-HT availability by blocking 5-HT1A autoreceptors, and this extra 5-HT is then available to activate downstream postsynaptic 5-HT receptors including, in this case, 5-HT2A receptors.
5-HT availability as examined in SERT-deficient and SERT over-expressing mice also affects DOI-induced head twitches (Jennings et al., 2008; Basselin et al., 2009), and appears to do so in a manner opposite to the effect on WAY 100635 induced head twitches. In SERT over-expressing mice, which have decreased 5-HT levels, and in wild-type mice with decreased 5-HT levels (via PCPA administration), DOI-induced head twitches are increased (Jennings et al., 2008). As mentioned, in SERT −/− mice which have increased extracellular 5-HT, DOI- and TCB-2-induced head twitches are decreased (this study) (Qu et al., 2005; Basselin et al., 2009; Fox et al., in press). Further, we recently reported that PCPA pretreatment in SERT −/− mice essentially restored the DOI-induced head-twitch response to levels observed in SERT +/+ mice (Basselin et al., 2009). Thus, it appears that 5-HT might compete with DOI for occupancy at 5-HT2A receptors, resulting in fewer DOI-induced head twitches in the presence of elevated levels of 5-HT in the synapse.
Recent research shows that 5-HT and DOI induce head twitches by different post-receptor signalling pathways. Specifically, 5-HT induces head twitches via a β-arrestin-2-dependent pathway, whereas DOI-induced head twitches occur independent of β-arrestin-2 (Schmid et al., 2008). Evidence for this comes from studies in β-arrestin-2-knockout mice, which display an intact head-twitch response to DOI but no head-twitch response following 5-hydroxytryptophan (5-HTP), which increases 5-HT levels (Schmid et al., 2008). If WAY 100635 is inducing head twitches in SERT −/− mice by further increasing 5-HT availability, it is likely that these head twitches are being induced via a β-arrestin-2-dependent pathway, although confirmation of this is required by further studies, for example in β-arrestin-2-knockout mice. Additionally, it is possible that there are differences in post-receptor signalling in SERT −/− mice, which might underlie the current report of increased head twitches following WAY 100635 in the presence of elevated 5-HT availability despite DOI-induced head twitches in SERT −/− mice being reduced.
It has been hypothesized that head twitches induced via a β-arrestin-2-independent pathway, for example by 5-HT2A receptor agonists such as DOI, are associated with hallucinogenic activity in humans, whereas head twitches induced by 5-HT (and 5-HT-enhancing agents such as 5-HTP) via a β-arrestin-2-dependent pathway are not associated with hallucinogenic activity (Abbas and Roth, 2008). As such, the current findings might suggest that if WAY 100635 does indeed induce head twitches in a β-arrestin-2-dependent manner, that WAY 100635 and other structurally related 5-HT1A receptor agents might not have hallucinogenic effects, even in the presence of increased 5-HT availability.
Consistent with previous findings (Qu et al., 2005; Basselin et al., 2009; Fox et al., in press), the number of head twitches induced by the 5-HT2A/2C receptor agonist DOI in the current studies was decreased by ∼85% in SERT −/− mice compared with SERT +/+ mice, with intermediate changes in SERT +/− mice compared with SERT +/+ mice. Further, 8-OH-DPAT decreased DOI-induced head twitches in SERT +/+ and +/− mice in the present study, consistent with previous reports (Darmani et al., 1990; Bjork et al., 1991; Willins and Meltzer, 1997), and we show that 8-OH-DPAT was without effect in SERT −/− mice. Interestingly, the combination of WAY 100635 and DOI induced more head twitches than either drug alone in SERT +/− and −/− mice. In SERT −/− mice, despite decreased responses following DOI, this effect following the combination of WAY 100635 and DOI was super-additive, confirming an important functional change in this genetic mouse model. We recently reported another super-additive receptor–receptor interaction in SERT −/− mice involving presynaptic 5-HT1A receptors (Fox et al., 2008). Although in SERT +/+ and +/− mice, WAY 100635 blocked hypothermia following the 5-HT precursor 5-HTP, WAY 100635 had no effect on the exaggerated 5-HTP-induced hypothermic response in SERT −/− mice. Pretreatment with the selective 5-HT7 receptor antagonist SB 269970 (Hagan et al., 2000) did decrease 5-HTP-induced hypothermia in SERT −/− mice, and when administered in combination, WAY 100635 and SB 269970 had super-additive effects in these mice despite no effect of WAY 100635 administered alone. Together, these studies show that although presynaptic 5-HT1A receptors are downregulated in SERT −/− mice, interesting functional interactions exist between this 5-HT1A receptor and other 5-HT receptor subtypes.
The current studies used this unique animal model to further explore interactions between 5-HT1A and 5-HT2A receptors. We show that despite numerous reports of decreased or absent 5-HT2A and presynaptic 5-HT1A receptor function in SERT-deficient mice, indirect activation of 5-HT2A receptors via inhibition of presynaptic 5-HT1A receptors results in an exaggerated response, showing that these receptors have functional interactive consequences that are strongly correlated with both genetically and pharmacologically induced alterations in the availability of 5-HT.
Acknowledgments
The current research was supported by the NIMH Intramural Research programme. The authors thank Drs Pablo R. Moya and Anne M. Andrews for valuable suggestions during the course of these studies, and Su-Jan Huang and Teresa J. Tolliver for their continued assistance with animal care and genotyping. All experiments adhered to the guidelines of the National Institutes of Health and were approved by the National Institute of Mental Health Animal Care and Use Committee.
Glossary
Abbreviations:
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- DOI
(+/−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane
- L745870
3-(4-[4-Chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[2,3-b]pyridine trihydrochloride
- MDL 11,939
a-Phenyl-1-(2-phenylethyl)-4-piperidinemethanol
- PCPA
p-chlorophenylalanine
- SSRI
selective 5-HT reuptake inhibitor
- TCB-2
(4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide
- WAY 100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt
Conflict of interest
None.
References
- Abbas A, Roth BL. Arresting serotonin. Proc Natl Acad Sci U S A. 2008;105:831–832. doi: 10.1073/pnas.0711335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews AM, Murphy DL. Sustained depletion of cortical and hippocampal serotonin and norepinephrine but not striatal dopamine by 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP): a comparative study with 2′-CH3-MPTP and MPTP. J Neurochem. 1993;60:1167–1170. doi: 10.1111/j.1471-4159.1993.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Basselin M, Fox MA, Chang L, Bell JM, Greenstein D, Chen M, et al. Imaging elevated brain arachidonic acid signaling in unanesthetized serotonin transporter (5-HTT)-deficient mice. Neuropsychopharmacology. 2009;34:1695–1709. doi: 10.1038/npp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (‘Ecstasy’) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bjork L, Cornfield LJ, Nelson DL, Hillver SE, Anden NE, Lewander T, et al. Pharmacology of the novel 5-hydroxytryptamine1A receptor antagonist (S)-5-fluoro-8-hydroxy-2-(dipropylamino)tetralin: inhibition of (R)-8-hydroxy-2-(dipropylamino)tetralin-induced effects. J Pharmacol Exp Ther. 1991;258:58–65. [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Cesana R, Ceci A, Ciprandi C, Borsini F. Mesulergine antagonism towards the fluoxetine anti-immobility effect in the forced swimming test in mice. J Pharm Pharmacol. 1993;45:473–475. doi: 10.1111/j.2042-7158.1993.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. Short communication. J Neural Transm. 1998;105:635–643. doi: 10.1007/s007020050085. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Reeves SL. The mechanism by which the selective 5-HT1A receptor antagonist S-(-) UH 301 produces head-twitches in mice. Pharmacol Biochem Behav. 1996;55:1–10. doi: 10.1016/0091-3057(96)00072-x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, et al. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, et al. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen C, Gallagher P, Murphy D. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007a;53:643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007b;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT2A receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) doi: 10.1007/s00213-009-1694-1 in press. [DOI] [PubMed]
- Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, et al. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology (Berl) 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, Murphy DL. Tramadol and another atypical opioid meperidine have exaggerated serotonin syndrome behavioural effects, but decreased analgesic effects, in genetically deficient serotonin transporter (SERT) mice. Int J Neuropsychopharmacol. 2009;12:1055–1065. doi: 10.1017/S146114570900011X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Gobert A, Dekeyne A, Millan MJ. The ability of WAY100,635 to potentiate the neurochemical and functional actions of fluoxetine is enhanced by co-administration of SB224,289, but not BRL15572. Neuropharmacology. 2000;39:1608–1616. doi: 10.1016/s0028-3908(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlah C, Hjorth S, Auerbach SB. Autoreceptor antagonists enhance the effect of the reuptake inhibitor citalopram on extracellular 5-HT: this effect persists after repeated citalopram treatment. Neuropharmacology. 1997;36:475–482. doi: 10.1016/s0028-3908(97)00052-x. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, et al. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S. Serotonin 5-HT1A autoreceptor blockade potentiates the ability of the 5-HT reuptake inhibitor citalopram to increase nerve terminal output of 5-HT in vivo: a microdialysis study. J Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Westlin D, Bengtsson HJ. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology. 1997;36:461–465. doi: 10.1016/s0028-3908(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003b;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Iasevoli F, Tomasetti C, Ambesi-Impiombato A, Muscettola G, de Bartolomeis A. Dopamine receptor subtypes contribution to Homer1a induction: insights into antipsychotic molecular action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:813–821. doi: 10.1016/j.pnpbp.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, et al. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi E, Shimamura M, Kinoshita M, Murphy NP. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology (Berl) 2008;197:567–580. doi: 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- Krall CM, Richards JB, Rabin RA, Winter JC. Marked decrease of LSD-induced stimulus control in serotonin transporter knockout mice. Pharmacol Biochem Behav. 2008;88:349–357. doi: 10.1016/j.pbb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem. 2003;84:1256–1265. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. WAY 100635 produces discriminative stimulus effects in rats mediated by dopamine D(4) receptor activation. Behav Pharmacol. 2009;20:114–118. doi: 10.1097/FBP.0b013e3283242f1a. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormiere AM, Faucillon V, Danty N, Culie C, et al. WAY-100635 has high selectivity for serotonin 5-HT(1A) versus dopamine D(4) receptors. Eur J Pharmacol. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- Milstein J, Dalley J, Robbins T. Methylphenidate-induced impulsivity: pharmacological antagonism by {beta}-adrenoreceptor blockade. J Psychopharmacol. 2010 doi: 10.1177/0269881108098146. in press. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, et al. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, et al. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Romero L, Hervas I, Artigas F. The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci Lett. 1996;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]


