Abstract
Background and purpose:
Activation of the proteinase-activated receptor-2 (PAR-2) induces scratching behaviour in mice. Here, we have investigated the role of kinin B1 and B2 receptors in the pruritogenic response elicited by activators of PAR-2.
Experimental approach:
Scratching was induced by an intradermal (i.d.) injection of trypsin or the selective PAR-2 activating peptide SLIGRL-NH2 at the back of the mouse neck. The animals were observed for 40 min and their scratching response was quantified.
Key results:
I.d. injection of trypsin or SLIGRL-NH2 evoked a scratching behaviour, dependent on PAR-2 activation. Mice genetically deficient in kinin B1 or B2 receptors exhibited reduced scratching behaviour after i.d. injection of trypsin or SLIGRL-NH2. Treatment (i.p.) with the non-peptide B1 or B2receptor antagonists SSR240612 and FR173657, respectively, prevented the scratching behaviour caused by trypsin or SLIGRL-NH2. Nonetheless, only treatment i.p. with the peptide B2receptor antagonist, Hoe 140, but not the B1receptor antagonist (DALBK), inhibited the pruritogenic response to trypsin. Hoe 140 was also effective against SLIGRL-NH2-induced scratching behaviour when injected by i.d. or intrathecal (i.t.) routes. Also, the response to SLIGRL-NH2 was inhibited by i.t. (but not by i.d.) treatment with DALBK. Conversely, neither Hoe 140 nor DALBK were able to inhibit SLIGRL-NH2-induced scratching behaviour when given intracerebroventricularly (i.c.v.).
Conclusions and implications:
The present results demonstrated that kinins acting on both B1 and B2 receptors played a crucial role in controlling the pruriceptive signalling triggered by PAR-2 activation in mice.
Keywords: PAR-2, scratching behaviour, kinins, kinin B2 and B1 receptors
Introduction
Kinins are endogenous peptides exerting a critical role in controlling physiological and pathological processes, particularly during nociceptive and inflammatory conditions. Once formed from their precursors by the action of kallikrein enzymes, kinins are released and exert their actions via activation of two subtypes of G-protein-coupled receptors, named kinin B1 and B2 receptors (nomenclature follows Alexander et al., 2007). B2receptors are usually expressed in a constitutive manner throughout the central and peripheral tissues, and mediate most of the physiological effects of kinins, presenting higher affinity for bradykinin (BK) and kallidin peptides. On the other hand, B1 receptors displays high affinity for the kinin metabolites des-Arg9-BK and Lys-des-Arg9-BK, and they are generally absent under physiological conditions, being rapidly expressed after tissue trauma and in certain inflammatory states (Calixto et al., 2000; 2001; 2004; Marceau and Regoli, 2004). Nevertheless, the constitutive expression of B1 receptors in the sensory neurons has been previously reported (Ma and Heavens, 2000; Ma, 2001; Wothersponn and Winter, 2000).
During the last two decades, it has become clear that both B1 and B2 receptors are involved in the onset and maintenance of several inflammatory and nociceptive conditions (Calixto et al., 2000; 2001; 2004;). There is some evidence that kinins and their receptors might play a role as effectors of pruriceptive signalling. For instance, intradermal application of BK into the injured skin of patients with atopic dermatitis evoked a very intense itch sensation (Hosogi et al., 2006). Interestingly, it has also been verified that itching behaviour induced by sodium deoxycholic acid in mice is mediated by the kallikrein-kinin system, essentially through the activation of B2 receptors (Hayashi and Majima, 1999). In a recent study, we have inferred the possible roles of such systems in the scratching behaviour in mice caused by trypsin, a non-selective agonist of proteinase-activated receptors (PAR)-2 (Costa et al., 2008). In line with these findings, earlier works have demonstrated that the activation of BK B2 receptors is a downstream event of PAR-2 activation in pain (Kawabata et al., 2006; Paszcuk et al., 2008).
The understanding of the pathophysiological basis of itch was greatly advanced by the discovery of PAR-2 involvement in pruritus (Steinhoff et al., 2003). PARs comprise a family of four G-protein-coupled receptors, named PAR-1 to -4, that are activated by proteolytic cleavage of their extracellular terminal sequence, which exposes a new NH2-terminus. This new portion acts as a tethered ligand, activating the cleaved receptor molecule (Ramachandran and Hollenberg, 2008; Vergnolle, 2009). PAR-2 is highly expressed in the skin (Steinhoff et al., 1999), and can be activated by both tryptase (from mast cell degranulation) and trypsin (from pancreatic and/or extra-pancreatic sources) (Corvera et al., 1997; Cottrell et al., 2004). Furthermore, this receptor is located throughout the sensory system and it has been indicated as a potent effector of nociceptive and pruriginous processes in both humans and animals (Steinhoff et al., 2003; Vergnolle et al., 2003; 2001; Shimada et al., 2006; Ui et al., 2006). Activation of PAR-2 evoked itching behaviour when intradermally injected at the back of the mouse neck (Shimada et al., 2006; Ui et al., 2006; Costa et al., 2008) and this has been used as a reproducible model to evaluate pruritus in rodents (Sun and Chen, 2007; Tsujii et al., 2008; 2009; Akiyama et al., 2009).
In the present study, in order to provide new evidence for the relevance of both kinin B1 and B2 receptors in itch, we sought to analyse, by the use of mice genetically deficient in kinin B1 (B1R−/−) or B2 receptors (B2R−/−), the contribution of these receptors to the scratching behaviour induced by different pruriginous agents. Particular attention was given to evaluate the anti-pruriceptive effects of selective kinin B1 or B2 receptor antagonists in the scratching behaviour elicited by PAR-2 activators.
Methods
Animals
All animal care and experimental procedures were carried out in accordance with the National Institutes of Health Animal Care Guidelines (NIH publications No. 80-23), and were approved by the Ethics Committee of the Universidade Federal de Santa Catarina (protocol number PP00032). Male adult Swiss mice (8–10 weeks) kept in controlled room temperature (22 ± 2°C) and humidity (around 60–80%) under a 12:12 h light–dark cycle (lights on 06:00 am) were used in this study. Food and water were provided ad libitum except during the experiments. In some experiments, C57BL/6 wild-type and kinin B1 or B2 receptor knockout (B1R−/− and B2R−/− respectively) mice were also used. Wild-type and knockout mice were originally obtained from the Centro de Desenvolvimento de Modelos Experimentais para Medicina e Biologia, from the Universidade Federal de São Paulo (São Paulo, Brazil). Deletion of the entire coding sequence for kinin B1 and B2 receptors was as described by Pesquero et al. (2000) and Rupniak et al. (1997) respectively.
Induction of scratching behaviour
The experiments were carried out as described by Hayashi and Majima (1999), with minor modifications (Costa et al., 2008). Two days before the experiments, the hair at the back of the mouse neck was shaved. On the day of the experiments, the animals were individually placed into glass cylinders 20 cm in diameter, for at least 30 min, in order to acclimatize them to the experimental environment. After this period, each mouse was briefly removed from the cylinder and given an intradermal (i.d.) injection of saline (50 µL) containing the non-selective PAR-2 receptor agonist trypsin (200 µg·site−1), the selective peptide PAR-2 receptor agonist SLIGRL-NH2 (25 to 200 µg·site−1), sodium deoxycholic acid (100 µg·site−1), chloroquine (200 µg·site−1) or compound 48/80 (10 µg·site−1). Immediately after pruritic stimulus administration, the animals were returned to their chambers. The animals were observed for 40 min, and their scratching behaviour was quantified by counting the number of scratches with fore- and hindpaws close to the injected site. Scratching behind the ears, but not on the face, was also counted. When a mouse scratched continuously for about 1 s without stopping, and repeated it more than once, this episode of scratching was counted as one. The results were expressed as the number of scratches in 40 min. Saline-treated animals (50 µL·site−1) were used as control.
Trypsin-induced overt nociception
Initially, the animals were separately placed into glass cylinders 20 cm in diameter, for at least 30 min, in order to acclimatize them to the experimental environment. After the adaptation period, each mouse received an intraplantar (i.pl.) injection of saline (20 µL) containing trypsin (300 µg·paw−1) into the right hindpaw. Control animals received saline (20 µL) by i.pl. route. The mice were observed individually for 10 min following trypsin injection. The amount of time (in seconds) spent licking the injected paw, was recorded with a stopwatch and was considered as an index of overt nociception (Paszcuk et al., 2008).
Intrathecal and intracerebroventricular drug injections
Intrathecal (i.t.) drug injections were performed in accordance with the method described by Hylden and Wilcox (1980), with minor modifications (Ferreira et al., 2002). The animals were lightly anaesthetized with isoflurane and a needle connected to a microsyringe by a polyethylene tubing was introduced through the skin. Subsequently, a volume of 5 µL of saline solution (0.9% NaCl) alone (control) or containing the drugs was injected between the L5 and L6 vertebral spaces. For intracerebroventricular (i.c.v.) injections, the animals were lightly anesthetized with isoflurane and a volume of 5 µL of sterile saline containing the drugs was injected directly into the lateral ventricle (coordinates from bregma: 1 mm lateral; 1 mm rostral; 3 mm vertical), as described previously by Laursen and Belknap (1986). Control animals received the same volume of saline.
Pharmacological treatments
In order to confirm the involvement of PAR-2 in the scratching behaviour elicited by SLIGRL-NH2 (100 µg·site−1), mice were treated with the selective peptide PAR-2 receptor antagonist FSLLRY-NH2 (100 µg·site−1). The sensitivity of the pruriceptive effects produced by trypsin (200 µg·site−1) or SLIGRL-NH2 (100 µg·site−1) to clinically used anti-pruritic treatments was assessed by systemic pretreatment with the corticoid dexamethasone (0.5 mg·kg−1, s.c., 4h) or the selective histamine H1 receptor antagonist pyrilamine (10 mg·kg−1, s.c., 30 min).
To assess the involvement of kinin B1 receptors in the scratching behaviour induced by either trypsin or SLIGRL-NH2, animals were treated with the selective peptide or non-peptide B1 receptor antagonists, des-Arg9-Leu8-bradykinin (DALBK) and SSR240612, respectively, by different pathways of administration. Firstly, DALBK (150 nmol·kg−1) or SSR240612 (1 mg·kg−1) were given by i.p. injection, 30 min before the injection of PAR-2 activators. In other experimental groups, the local effect of DALBK (0.3 nmol·site−1) was tested by i.d. co-injection with the PAR-2 activator. In another set of experiments, the animals received DALBK (25 pmol·site−1) by i.t. or i.c.v. routes, 15 min before SLIGRL-NH2 administration.
The contribution of kinin B2 receptors to the scratching behaviour induced by either trypsin (200 µg·site−1) or SLIGRL-NH2 (100 µg·site−1) was analysed by the treatment with the selective peptide or non-peptide B2 receptor antagonists Hoe 140 and FR173657, respectively, by different pathways of administration. Initially, Hoe 140 (50 nmol·kg−1) or FR173657 (30 mg·kg−1) were administered i.p., 30 min before PAR-2 activators. The local effect of Hoe 140 (3 nmol·site−1) was tested by i.d. co-injection with PAR-2 activators. In another set of experiments, the animals received Hoe 140 (100 pmol·site−1) by i.t. or i.c.v. routes, 15 min before SLIGRL-NH2.
The protocols of all tested drugs (doses and time of injection) were chosen on the basis of pilot studies (data not shown), or in accordance with previous publications (Ferreira et al., 2002; 2004; 2008; Costa et al., 2006; 2008; Ui et al., 2006; Paszcuk et al., 2008; Quintão et al., 2008).
Data analysis
The results are presented as the mean ± SEM of six to 10 animals. Statistical comparison of the data was performed by one way analysis of variance (anova) followed by Dunnett's or Newman-Keuls tests, as appropriate. P values less than 0.05 were considered significant. The inhibition of scratching behaviour are given as the difference (in percentage) between the mean of the responses in drug-treated group (or B1R−/− or B2R−/− group) in relation to vehicle-treated group (or wild-type group).
Materials
The following drugs were used: trypsin (from porcine pancreas), compound 48/80, dexamethasone, sodium deoxycholic acid, chloroquine, pyrilamine and des-Arg9-[Leu8]-bradykinin (DALBK) all from Sigma Chemical Company (St. Louis, MO, USA). Hoe 140, FR173657 and SSR240612 were kindly donated by Sanofi-Aventis (Bridgewater, NJ, USA), Fujisawa Pharmaceutical Co. (Osaka, Japan) and Sanofi Recherché (Moontpellier, France) respectively. The peptide fragments FSLLRY-NH2 (Phe-Ser-Leu-Leu-Arg-Tyr-NH2), SLIGRL-NH2 (Ser-Leu-Ile-Gly-Arg-Leu-NH2) and LRGILS-NH2 (Leu-Arg-Gly-Ile-Leu-Ser-NH2) were synthesized by Dr Luis Juliano (UNIFESP, São Paulo, Brazil).
Results
Scratching behaviour induced by PAR-2 agonists in mice
As described earlier (Costa et al., 2008), trypsin evoked a scratching behaviour when injected at the back of the mouse neck in different mouse strains (Swiss and C57BL/6) compared with saline-treated group (Figures 1, 2 and 4). This effect is dependent on the trypsin serine-proteolytic activity, and is mediated by PAR-2 receptor activation (Costa et al., 2008). As shown in the Figure 1A, the i.d. injection of the selective PAR-2 activating peptide SLIGRL-NH2 (25 to 200 µg·site−1) into the back of the mouse neck also displayed a marked and dose-related scratching behaviour response. The dose of 100 µg·site−1 was chosen for the following experiments, as this was the dose capable of inducing reproducible effects with less variability. Moreover, this dose was equivalent to the effects induced by the positive control compound 48/80 (10 µg·site−1) (Figure 1A). Unlike the PAR-2 agonist, the i.d. injection of the reverse sequence LRGILS-NH2 (100 µg·site−1) did not cause any significant scratching response in comparison with the saline-treated group (Figure 1A). As expected, co-treatment with the selective peptide PAR-2 receptor antagonist FSLLRY-NH2 (100 µg·site−1) markedly inhibited SLIGRL-NH2-induced scratching behaviour (88 ± 10% of inhibition) (Figure 1B).
Figure 1.
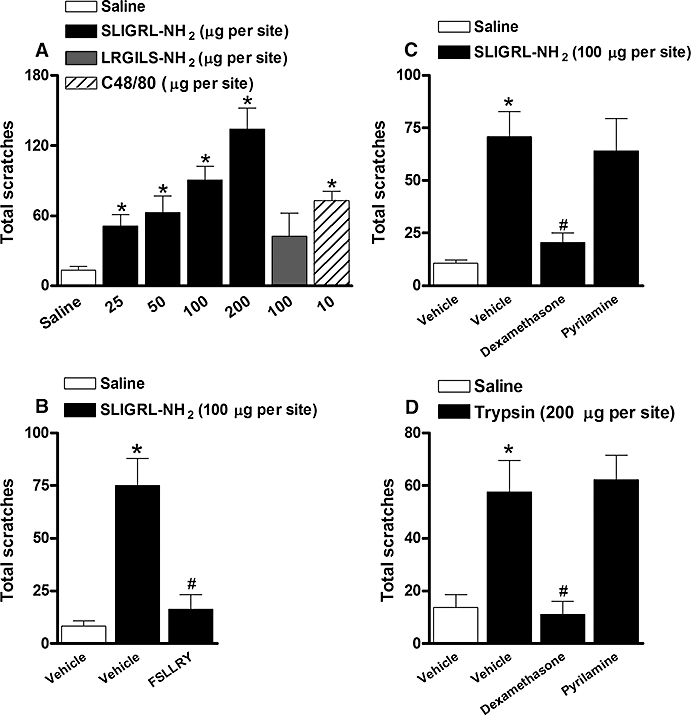
(A) Scratching behaviour induced by the selective PAR-2 activating peptide SLIGRL-NH2 (25 to 200 µg·site−1, i.d.), the inactive control peptide LRGILS-NH2 (100 µg·site−1, i.d.) or compound 48/80 (C48/80; 10 µg·site−1, i.d.) in Swiss mice. (B) Effect of the treatment with the selective peptide PAR-2 antagonist FSLLRY-NH2 (100 µg·site−1, co-injection) on the SLIGRL-NH2 (100 µg·site−1)-induced scratching behaviour in Swiss mice. (C, D) Effect of the treatment with the corticoid dexamethasone (0.5 mg·kg−1, s.c., 4h) or the selective histamine H1 receptor antagonist pyrilamine (10 mg·kg−1, s.c., 30 min) on the (C) SLIGRL-NH2 (100 µg·site−1)- or (D) trypsin (200 µg·site−1)-induced scratching behaviour in Swiss mice. Each column represents the mean of six to 10 animals and the vertical bars represent the SEM. Significantly different when compared with saline group (*P < 0.05) and SLIGRL-NH2- or trypsin-treated group (#P < 0.05). PAR-2, proteinase-activated receptor-2.
Figure 2.
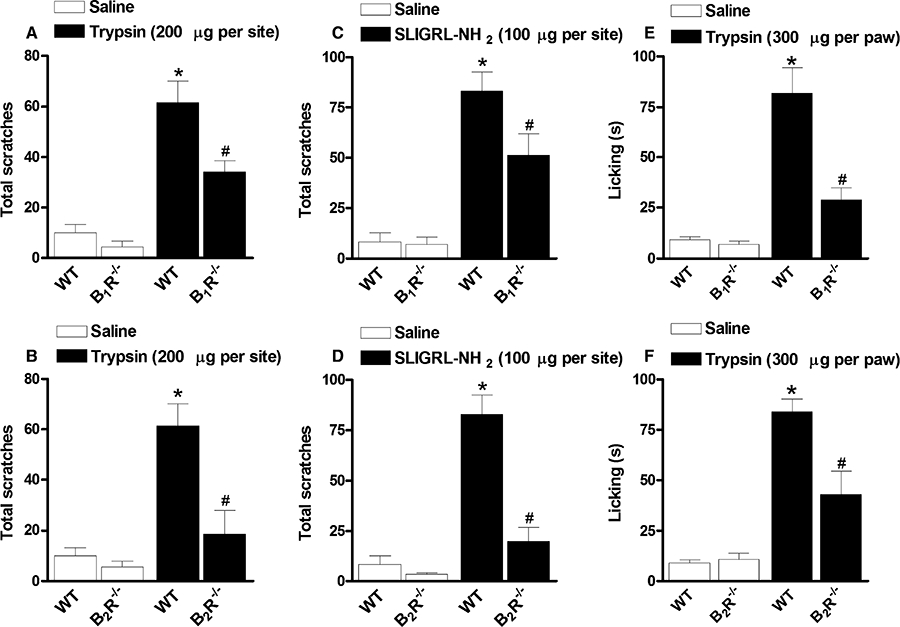
(A, B) Scratching behaviour induced by trypsin (200 µg·site−1, i.d.) in kinin (A) B1 (B1R−/−) or (B) B2 (B2R−/−) receptor deficient mice. (C, D) Scratching behaviour induced by SLIGRL-NH2 (100 µg·site−1, i.d.) in (C) B1R−/− or (D) B2R−/− mice. (E, F) Overt nociception (licking) induced by trypsin (300 µg·site−1, i.pl.) in (E) B1R−/− or (F) B2R−/− mice. Each column represents the mean of six to 10 animals and the vertical bars represent the SEM. Significantly different when compared with wild-type (WT) saline group (*P < 0.05) and WT SLIGRL-NH2- or trypsin-treated group (#P < 0.05).
Figure 4.
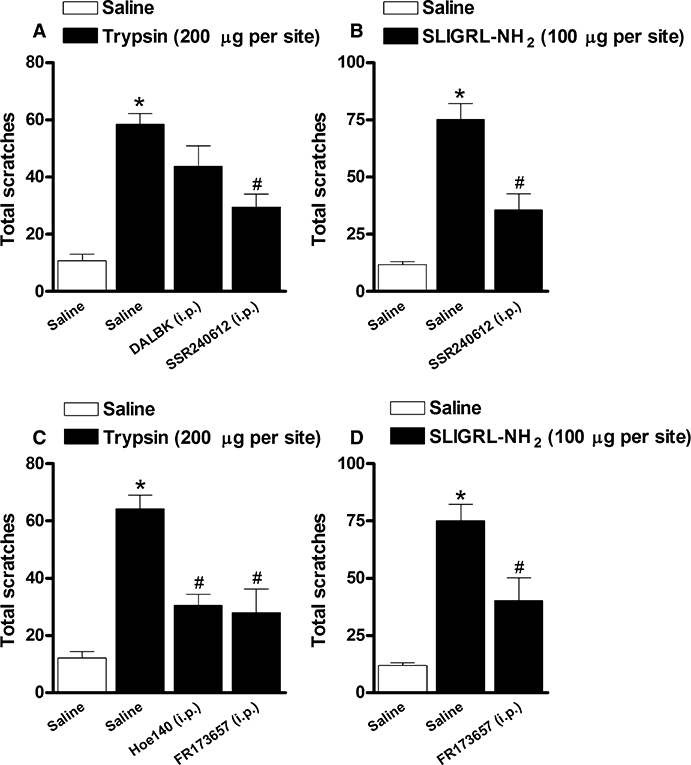
(A, B) Effect of the treatment with the selective kinin B1 receptor antagonists des-Arg9-Leu8-bradykinin (DALBK; 150 nmol·kg−1, i.p., 30 min) or SSR240612 (1 mg·kg−1, i.p., 30 min) on the scratching behaviour induced by (A) trypsin (200 µg·site−1) or (B) SLIGRL-NH2 (100 µg·site−1) in Swiss mice. (C, D) Effect of the treatment with the selective kinin B2 receptor antagonists Hoe 140 (50 nmol·kg−1, i.p., 30 min) or FR173657 (30 mg·kg−1, i.p., 30 min) on the scratching behaviour induced by (C) trypsin (200 µg·site−1) or (D) SLIGRL-NH2 (100 µg·site−1) in Swiss mice. Each column represents the mean of six to 10 animals and the vertical bars represent the SEM. Significantly different when compared with saline group (*P < 0.05) and SLIGRL-NH2- or trypsin-treated group (#P < 0.05).
Next, we assessed the sensitivity of the pruriceptive effect produced by trypsin or SLIGRL-NH2 to some clinically used anti-pruritic treatments. The systemic administration of the corticoid dexamethasone (0.5 mg·kg−1, s.c., 4 h) abolished both trypsin- and SLIGRL-NH2-induced itching (107 ± 11% and 76 ± 13% of inhibition respectively) (Figure 1C and D). Nonetheless, the treatment with the selective histamine H1 receptor antagonist pyrilamine (10 mg·kg−1, s.c., 30 min), at a dose effective against compound 48/80-induced scratching (data not shown), was not able to inhibit the itching induced by both PAR-2 agonists (Figure 1C and D), suggesting that PAR-2 activation-elicited scratching behaviour was not dependent on histamine release from mast cells.
Scratching behaviour induced by PAR-2 agonists in kinin B1 receptor and B2 receptor knockout mice
To check the relevance of both kinin B1 and B2 receptors to the scratching behaviour induced by PAR-2 activators, we have employed kinin B1 (B1R−/−) and B2 receptor (B2R−/−) genetically deficient mice and the corresponding wild-type littermates (C57BL/6 strain). As shown in Figure 2, trypsin (200 µg·site−1) or SLIGRL-NH2 (100 µg·site−1) i.d. injections provoked significant scratching behaviour in C57BL/6 wild-type mice, when compared with saline-treated group (Figure 2A–D), in a manner essentially similar to that elicited in Swiss mice. Nevertheless, when trypsin (Figure 2A and B) or SLIGRL-NH2 (Figure 2C and D) were injected in B1R−/− or B2R−/− mice, the frequency of scratching bouts was significantly lower than that observed in the wild-type animals. The deletion of B2 receptors almost abolished the scratching response evoked by PAR-2 activation (83 ± 18% and 84 ± 9% against trypsin and SLIGRL-NH2 respectively), while the B1 receptor deficiency only partially inhibited the scratching behaviour (53 ± 9% and 42 ± 14%, for trypsin and SLIGRL-NH2 models respectively).
Overt nociception induced by trypsin in kinin B1 receptor and B2 receptor knockout mice
In order to assess whether kinin receptors may also contribute to pain evoked by PAR-2 activation, we examined the overt nociception induced by the i.pl. injection of trypsin, a non-selective PAR-2 activator, in either B1 or B2receptor knockout mice. As previously described (Paszcuk et al., 2008), trypsin injection into the mouse hindpaw (300 µg·paw−1) evoked a marked nociceptive response (mostly licking behaviour) in C57BL/6 wild-type mice (Figure 2E and F). On the other hand, the responses induced by i.pl. trypsin administration in B1R−/− or B2R−/− were significantly diminished when compared with the control animals (Figure 2E and F). In contrast to that observed in the scratching behaviour models, the inhibition of nociceptive response caused by B1 receptor deficiency was greater than that caused by B2 receptor deletion (73 ± 8% and 55 ± 15%, for B1R−/− and B2R−/− respectively) (Figure 2E and F).
Scratching behaviour induced by different pruritic agents in kinin B1 receptor and B2 receptor knockout mice
In this set of experiments, the relevance of kinin receptors for the scratching behaviour induced by i.d. injection of different pruritic stimuli was investigated using both kinin B1R−/− and B2R−/− mice. It is possible to observe from Figure 3 that genetic deletion of kinin B2 receptors abolished the increase in the scratching bouts evoked by sodium deoxycholate acid (100 µg·site−1), chloroquine (200 µg·site−1) or compound 48/80 (10 µg·site−1), achieving 96 ± 13%, 78 ± 8% and 92 ± 9% of inhibition respectively (Figure 3A–C). On the other hand, in B1R−/− mice, the scratching response induced by compound 48/80, but not by sodium deoxycholate acid or chloroquine, was significantly inhibited in comparison with wild-type animals (50 ± 17% of inhibition) (Figure 3C).
Figure 3.
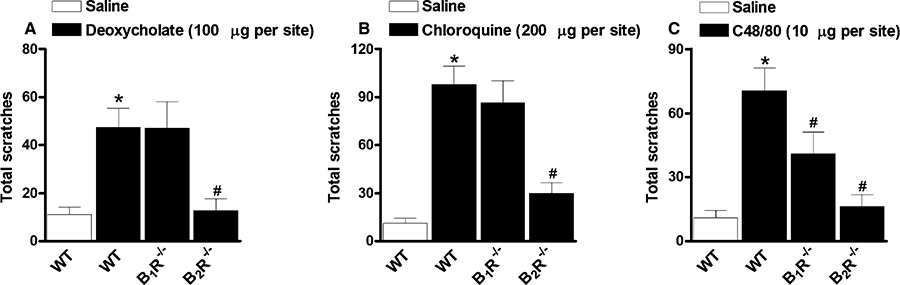
Scratching behaviour induced by (A) deoxycholate (100 µg·site−1, i.d.), (B) chloroquine (200 µg·site−1, i.d.) or (C) compound 48/80 (C48/80; 10 µg·site−1, i.d.) in kinin B1 (B1R−/−) or B2 (B2R−/−) receptor deficient mice. Each column represents the mean of six to 10 animals and the vertical bars represent the SEM. Significantly different when compared with wild-type (WT) saline group (*P < 0.05) and WT deoxycholate-, chloroquine- or compound 48/80-treated group (#P < 0.05).
Effect of the treatment with the selective kinin B1 or B2 receptor antagonists on the scratching behaviour induced by PAR-2 agonists in mice
Initially, we sought to assess the effects of systemic kinin B1 and B2 receptor antagonists on the scratching behaviour induced by PAR-2 activation in mice. For this purpose, a series of different selective kinin B1 or B2 receptor antagonists were systemically tested on the scratching induced either by trypsin or SLIGRL-NH2. As shown in Figure 4, treatment i.p. of mice with the selective non-peptide kinin B1 receptor antagonist SSR240612 (1 mg·kg−1, 30 min), but not the selective peptide antagonist DALBK (150 nmol·kg−1, 30 min), produced a considerable inhibition of both trypsin- and SLIGRL-NH2-induced pruritus (60 ± 9% and 62 ± 11% of inhibition respectively) (Figure 4A and B). As shown in the Figure 4C, the scratching behaviour induced by trypsin was markedly inhibited by i.p. injection of the selective kinin B2 receptor antagonists Hoe 140 (50 nmol·kg−1, 30 min) or FR173657 (30 mg·kg−1, 30 min) (63 ± 7% and 69 ± 17% respectively). Likewise, FR173657 (30 mg·kg−1, i.p., 30 min) treatment also inhibited SLIGRL-NH2-induced scratching behaviour (Figure 4D) (54 ± 17%).
Effect of peripheral or central blockade of kinin B1 or B2 receptors on the scratching behaviour induced by PAR-2 agonists
In order to investigate the involvement of intradermal kinin receptors in SLIGRL-NH2-induced pruritus, separate groups of animals were treated locally with the selective kinin B1 or B2 receptor antagonists Hoe 140 and DALBK respectively. Figure 5 shows that local co-injection of Hoe 140 (3 nmol·site−1) significantly prevented the scratching behaviour response induced by SLIGRL-NH2 (51 ± 15% inhibition) (Figure 5D). On the contrary, DALBK (0.3 nmol·site−1, co-injected) treatment was not able to alter this response (Figure 5A). Further, to check the possible involvement of central pathways in the modulatory actions of kinin receptors on the scratching induced by the PAR-2 activator, the animals were treated by i.t. or i.c.v. routes with the selective kinin receptor antagonists. As shown in the Figure 5, the i.t. treatment with the selective kinin B1 or B2 receptor antagonists, DALBK (25 pmol·site−1, 15 min) or Hoe140 (100 pmol·site−1, 15 min), reduced the scratching behaviour induced by SLIGRL-NH2 (54 ± 17% and 76 ± 13% respectively) (Figure 5B and E). Conversely, when injected by i.c.v. route, neither DALBK (25 pmol·site−1, i.c.v.) nor Hoe 140 (100 pmol·site−1, i.c.v.) was effective in inhibiting the pruritus induced by SLIGRL-NH2 (Figure 5C and F).
Figure 5.
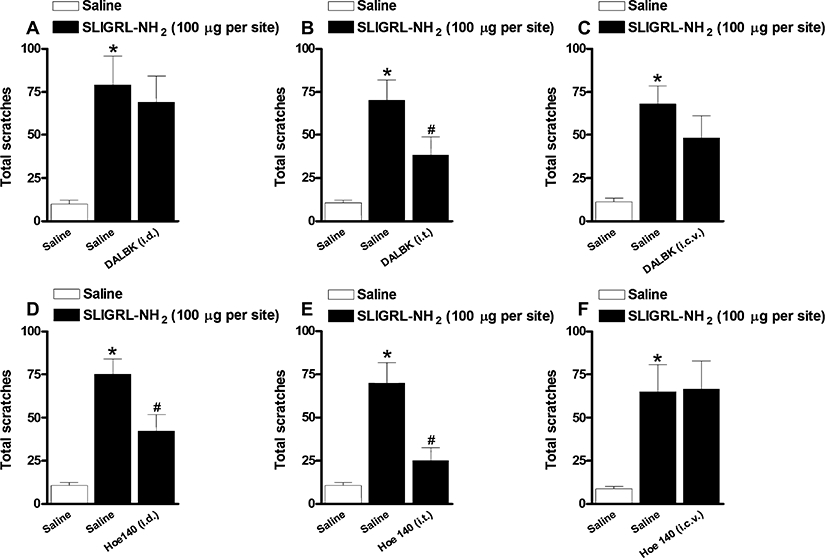
(A, B, C) Effect of (A) intradermal (0.3 nmol·site−1, i.d.), (B) intrathecal (25 pmol·site−1, i.t.) or (C) intracerebroventricular (25 pmol·site−1, i.c.v.) treatment with the selective kinin B1 receptor antagonist DALBK on the scratching behaviour induced SLIGRL-NH2 (100 µg·site−1) in Swiss mice. (D, E, F) Effect of (D) intradermal (3 nmol·site−1, i.d.), (E) intrathecal (100 pmol·site−1, i.t.) or (F) intracerebroventricular (100 pmol·site−1, i.c.v.) treatment with the selective kinin B2 receptor antagonist Hoe 140 on the scratching behaviour induced SLIGRL-NH2 (100 µg·site−1) in Swiss mice. Each column represents the mean of six to 10 animals and the vertical bars represent the SEM. Significantly different when compared with saline group (*P < 0.05) and SLIGRL-NH2-treated group (#P < 0.05).
Discussion and conclusions
Pruritus, in spite of being a self-defensive mechanism, is a common symptom present in several skin or systemic diseases such as atopic dermatitis, psoriasis, renal failure and cholestasis. In spite of much effort, there are, so far, no available pharmacotherapies for the treatment of itching (Ikoma et al., 2006; Paus et al., 2006; Steinhoff et al., 2006). Thus, the need to understand the mechanisms underlying pruritus neurotransmission has as its main focus the discovery of new molecular targets intended to lead to the development of effective anti-pruritic drugs. In this study, we provide convincing evidence implicating both kinin receptors, especially the B2 receptor subtype, in the acute scratching behaviour induced by different pruriginous stimuli in mice. We have made the following major findings: (i) kinin B1 and B2 receptor deficient mice exhibited lower frequency of scratching bouts after the i.d. administration of different pruriginous agents, when compared with wild-type littermates; and (ii) the pretreatment of mice with the selective kinin B1 or B2 receptor antagonists, given by different routes, prevented the scratching behaviour induced by PAR-2 activators.
Recent studies have demosntrated that a single i.d. injection of the selective PAR-2 activating peptide (SLIGRL-NH2) or the non-selective PAR-2 agonists, tryptase or trypsin, is capable of eliciting scratching behaviour in mice (Shimada et al., 2006; Ui et al., 2006; Costa et al., 2008). Our first set of data (Figure 1) confirmed the previous reports, showing that the i.d. injections of SLIGRL-NH2 or trypsin were able to evoke scratching behaviour when applied at the back of the mouse neck. Also, we have shown that pruritus induced by SLIGRL-NH2 was predominantly triggered by PAR-2 activation, as the scratching behaviour was effectively prevented by the selective PAR-2 receptor antagonist FSLLRY-NH2 (Figure 1B). Previously, we had demonstrated that FSLLRY-NH2 treatment almost abolished trypsin-induced itching response (Costa et al., 2008). Herein, the scratching response induced by SLIGRL-NH2 or by trypsin was observed in different mouse strains (Swiss and C57/BL6) allowing us to suggest that i.d. injections of PAR-2 activators can be used as reproducible models to evaluate pruritus in mice.
Our next step was to verify the effects of treatment with a pair of drugs representing the most common classes of clinically used anti-pruritic treatments on the scratching behaviour induced by PAR-2 activators. As noted, the pretreatment with the corticoid dexamethasone greatly reduced both trypsin- and SLIGRL-NH2-induced itching behaviour in mice. Nonetheless, these pruriceptive responses were resistant to the treatment with the selective histamine H1 receptor antagonist pyrilamine (Figure 1C and D). Earlier works have demonstrated that PAR-2 activation-induced itching behaviour was not sensitive to anti-histamine treatment (Shimada et al., 2006; Costa et al., 2008; Tsujii et al., 2008). Therefore, one may infer that PAR-2-mediated scratching behaviour might be used as an experimental model of pruritus aiming at the screening of new therapeutic approaches that could be further applied to the clinical conditions resistant to anti-histamine drugs.
The kallikrein-kinin system has consistently been involved in the pathophysiology of itching (Rajakulasingam et al., 1991; Hayashi and Majima, 1999; Schmelz et al., 2003; Hosogi et al., 2006; Costa et al., 2008). Confirming and extending these previous findings, the results of the present study clearly implicate both kinin B1 and B2 receptors in the scratching behaviour induced by PAR-2 activators in mice. Accordingly, the scratching behaviour induced by trypsin or SLIGRL-NH2 was almost completely abolished in B2R−/− mice, while the genetic deletion of B1 receptor produced a partial but significant reduction of these responses (Figure 2A–D).
Interestingly, we also verified that the deletion of either kinin B1 and B2 receptors significantly reduced the overt nociception caused by i.pl. trypsin injection (Figure 2E–F). B1 or B2 receptor deficient mice are known to display hypoalgesia against the acute overt nociception induced by capsaicin, formalin, phorbol 12-myristate 13-acetate or by high intensity heat stimuli (Boyce et al., 1996; Rupniak et al., 1997; Pesquero et al., 2000; Ferreira et al., 2008). An overall analysis of our data suggests a greater involvement of B1 receptors in the nociceptive response caused by PAR-2 activation, while B2 receptors seem to be preferentially associated with PAR-2-mediated scratching behaviour. In addition, our data also permit us to propose that distinct mechanisms seem to mediate the pruriceptive and nociceptive behaviours caused by PAR-2 activators in mice. Thus, we might suggest that B1 receptors and B2 receptors would differently modulate the nociceptive and pruriceptive pathways. In spite of these suggestions, complementary studies are necessary to make clear the precise mechanisms underlying the actions of kinins in these two models.
The predominant role of the B2 receptors in pruriceptive responses (in relation to the B1 receptor involvement) was also confirmed in three additional acute scratching behaviour models. B2R−/− animals displayed a reduced itching behaviour after the i.d. injection of sodium deoxycholate acid, chloroquine or compound 48/80, when compared with wild-type or B1R−/− animals (Figure 3). The results presented here are consistent with those in a previous publication showing that sodium deoxycholate acid-evoked scratching behaviour was prevented by the treatment with selective kinin B2 receptor (Hoe 140 or FR173657) but not B1 receptor (DALBK) antagonists (Hayashi and Majima, 1999). Although B1 receptor deletion did not alter the pruriceptive responses generated by sodium deoxycholate acid or chloroquine, it was able to significantly reduce the scratching behaviour triggered by compound 48/80 (Figure 3A–C).
Next, we evaluated the effects of selective kinin B1 or B2 receptor antagonists in the scratching behaviour induced by PAR-2 activators in mice. The present study demonstrated that the i.p. treatment with the selective peptide (Hoe 140) or non-peptide (FR173657) kinin B2 receptor antagonists did inhibit both trypsin- and SLIGRL-NH2-induced scratching behaviour. Also, the scratching responses induced by these same pruriginous agents were reduced by the selective non-peptide kinin B1 receptor antagonist SSR240612 (Figure 4A–D). Such results are in accordance with our previous data showing that trypsin-induced itching behaviour was reduced by SSR240612 or FR173657 treatments (Costa et al., 2008). Despite the effectiveness of SSR240612, i.p. treatment with the selective peptide kinin B1 receptor antagonist DALBK was not able to reduce trypsin-elicited itching (Figure 4A). This fact might be related to the pharmacokinetic properties of DALBK, which is a peptide (Campos et al., 2006).
To examine the involvement of dermal kinin receptors in SLIGRL-NH2-induced itching, we assessed the local (i.d.) effects of the selective kinin B1 or B2 receptor antagonists. The peripheral blockade of B2 receptors by the i.d. co-treatment with Hoe 140 prevented PAR-2 activating peptide-induced scratching behaviour (Figure 5A), emphasizing the contribution of kinin B2 receptors located in the skin. Indeed, studies using different methodological approaches have found that BK given i.d. may evoke itching sensation in humans in certain circumstances (Schmelz et al., 2003; Hosogi et al., 2006). Also, i.pl. injections of BK or Tyr8-BK (preferential and selective B2 receptor agonists respectively) caused overt nociception in rodents essentially through the activation of constitutively and functionally expressed B2 receptors in peripheral tissues (Boyce et al., 1996; Griesbacher et al., 1998; Campos et al., 1999; Ferreira et al., 2004). However, it is worth mentioning that i.d. injections of BK did not cause a significant increase in the number of scratching bouts when injected at the back of the mouse neck in our paradigm (data not shown). In this study, i.d. co-treatment with the selective kinin B1 receptor antagonist DALBK was not able to interfere with the pruriceptive response evoked by SLIGRL-NH2 (Figure 5B), excluding the involvement of peripherally expressed B1 receptors. In fact, the peripheral injection of B1 receptor agonists rarely induces nociception in naïve animals (Perkins and Kelly, 1994; Ganju et al., 2001; Fox et al., 2003; Ferreira et al., 2004); and priming stimuli are needed to elicit their nociceptive actions (Campos et al., 1995; De Campos et al., 1998; Ma, 2001). In spite of these results, both kinin B1 and B2 receptors, present in the skin, mediated capsaicin-induced ear oedema in mice, suggesting that they could be useful targets in the treatment of some skin inflammatory diseases (Pietrovski et al., 2009).
As a final goal of this study, we sought to investigate the possible involvement of central nervous system pathways in the modulatory actions of kinin receptors in the SLIGRL-NH2-induced scratching behaviour in mice by the use of selective antagonists directly injected into central structures. Noticeably, the i.t. administration of the following kinin receptor antagonists, DALBK (B1 receptor) or Hoe140 (B2 receptor), reduced the pruriceptive response evoked by SLIGRL-NH2. Conversely, neither DALBK nor Hoe140 injected by i.c.v route inhibited this behavioural response. Our findings are supported by evidence showing that both kinin B1 and B2 receptors are functionally expressed at the level of the spinal cord (Chapman and Dickenson, 1992; Corrêa and Calixto, 1993; Pesquero et al., 2000; Ferreira et al., 2002; 2004; Fox et al., 2003). Of relevance, i.t. injection of des-Arg9-BK or Tyr8-BK (selective kinin B1R and B2R agonists respectively) causes thermal and mechanical hyperalgesia in mice (Ferreira et al., 2002; Fox et al., 2003). Moreover, i.t. treatment with kinin B1 or B2 receptor antagonists was effective against the overt nociception caused by formalin in rodents (Chapman and Dickenson, 1992; Ferreira et al., 2002). Corroborating these data, it has been shown that B1R−/− mice show hypoalgesia in chemical models of nociception, probably related with a reduction in dependent activity facilitation (wind-up phenomenon) of spinal nociceptive reflexes (Pesquero et al., 2000). As the pruriceptive behaviour induced by PAR-2 activators is very short, it peaked at 10 min and lasted up to 40 min (data not shown), it seems unlikely that B1 or B2 receptor expression in the spinal cord depends on de novo protein synthesis and the involvement of constitutively expressed kinin receptors is more plausible. Indeed, in the present work, the i.d. injection of SLIGRL-NH2 was not able to induce an increase in the expression of either kinin B1 or B2 receptor protein in the spinal cord, as assessed by Western blot analysis (data not shown).
As a great novelty of this study, we have demonstrated that kinins acting on both receptors, particularly on the B2 receptor subtype, play a critical role in controlling pruriceptive signalling. Firstly, the deletion of kinin B1 or B2 receptors prevented the scratching behaviour triggered by different pruriginous stimuli in mice. Secondly, the treatment of mice with the selective kinin B1 or B2 receptor antagonists potently inhibited PAR-2 activators-induced scratching behaviour, when given by systemic, local and i.t. routes. The findings presented here are in accordance with previous studies showing that the activation of BK B2R is a downstream event of PAR-2 activation in pain (Kawabata et al., 2006; Paszcuk et al., 2008). These pieces of evidence support the notion that selective kinin receptor antagonists, mainly against the B2 receptor subtype, might represent new attractive therapeutic options for treating pruriginous conditions, especially those resistant to anti-histamine treatment.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Apoio aos Núcleos de Excelência (PRONEX) and the Fundação de Apoio à Pesquisa do Estado de Santa Catarina (FAPESC). R.C. and M.N.M are MSc Pharmacology students funded by CAPES. D.M.M. is a Pharmacy & Biochemistry undergraduate student supported by CNPq. E.M.M. is a recipient of a post-doctoral grant from CNPq. The authors gratefully thank Ana Paula Luiz and Maíra Bicca for i.t. and i.c.v. injections, respectively, Maria Martha Campos and Juliana Fabris Lima Garcia for their assistance in English corrections of this manuscript.
Glossary
Abbreviations:
- PAR-2
proteinase-activated receptor-2
Conflict of interest
The authors state no conflicts of interest.
References
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 2nd Edition (2007 revision) Br J Pharmacol. 2007;150(Suppl. 1):S1–S168. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S, Rupniak N, Carlson EJ, Webb J, Borkowski JA, Hess F, et al. Nociception and inflammatory hyperalgesia in B2 bradykinin receptor knockout mice. Immunopharmacology. 1996;33:333–335. doi: 10.1016/0162-3109(96)00101-4. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Inflamatory pain: kinins and antagonists. Cur Op Anesthesiol. 2001;14:519–526. doi: 10.1097/00001503-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MM, Mata LV, Calixto JB. Expression of B1 kinin receptors mediating paw edema and formalin-induced nociception. Modulation by glucocorticoids. Can J Physiol Pharmacol. 1995;73:812–819. doi: 10.1139/y95-110. [DOI] [PubMed] [Google Scholar]
- Campos MM, Leal PC, Yunes RA, Calixto JB. Non-peptide antagonists for kinin B1 receptors: new insights into their therapeutic potential for the management of inflammation and pain. Trends Pharmacol Sci. 2006;27:646–651. doi: 10.1016/j.tips.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Campos ROP, Alves RV, Ferreira J, Kyle DJ, Chakravarty S, Mavunkel BJ, et al. Oral antinociception and oedema inhibition produced by NPC 18884, a non-peptidic bradykinin B2 receptor antagonist. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;360:278–286. doi: 10.1007/s002109900080. [DOI] [PubMed] [Google Scholar]
- Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992;219:427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- Corrêa CR, Calixto JB. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera CU, Dery O, Mcconalogue K, Bohm SK, Khitin LM, Caughey GH, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R, Fernandes ES, Menezes-De-Lima O, Jr, Campos MM, Calixto JB. Effect of novel selective non-peptide kinin B(1) receptor antagonists on mouse pleurisy induced by carrageenan. Peptides. 2006;27:2967–2975. doi: 10.1016/j.peptides.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- De Campos RO, Henriques MG, Calixto JB. Systemic treatment with Mycobacterium bovis bacillus Calmette–Guerin (BCG) potentiates kinin B1 receptor agonist-induced nociception and oedema formation in the formalin test in mice. Neuropeptides. 1998;32:393–403. doi: 10.1016/s0143-4179(98)90062-2. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Campos MM, Araújo R, Bader M, Pesquero JB, Calixto JB. The use of kinin B1 and B2 receptor knockout mice and selective antagonists to characterize the nociceptive responses caused by kinins at the spinal level. Neuropharmacology. 2002;43:1188–1197. doi: 10.1016/s0028-3908(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Trichês KM, Medeiros R, Cabrini DA, Mori MA, Pesquero JB, et al. The role of kinin B1 receptors in the nociception produced by peripheral protein kinase C activation in mice. Neuropharmacology. 2008;54:597–604. doi: 10.1016/j.neuropharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Fox A, Wotherspoin G, McNair K, Hudson L, Patel S, Gentry C, et al. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain. 2003;104:683–691. doi: 10.1016/S0304-3959(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Ganju P, Davis A, Patel S, Nunez X, Fox A. p38 stress-activated protein kinase inhibitor reverses bradykinin B(1) receptor-mediated component of inflammatory hyperalgesia. Eur J Pharmacol. 2001;421:191–199. doi: 10.1016/s0014-2999(01)01048-2. [DOI] [PubMed] [Google Scholar]
- Griesbacher T, Amann R, Sametz W, Diethart S, Juan H. The nonpeptide B2 receptor antagonist FR173657: inhibition of effects of bradykinin related to its role in nociception. Br J Pharmacol. 1998;124:1328–1334. doi: 10.1038/sj.bjp.0701938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Majima M. Reduction of sodium deoxycholic acid-induced scratching behaviour by bradykinin B2 receptor antagonists. Br J Pharmacol. 1999;126:197–204. doi: 10.1038/sj.bjp.0702296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kawao N, Kitano T, Matsunami M, Satoh R, Ishiki T, et al. Colonic hyperalgesia triggered by proteinase-activated receptor-2 in mice: involvement of endogenous bradykinin. Neurosci Lett. 2006;402:167–172. doi: 10.1016/j.neulet.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice: some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Ma QP. The expression of bradykinin B1 receptors on primary sensory neurones that give rise to small calibre sciatic nerve fibres in rats. Neuroscience. 2001;107:665–673. doi: 10.1016/s0306-4522(01)00387-6. [DOI] [PubMed] [Google Scholar]
- Ma QP, Heavens R. Basal expression of bradykinin B(1) receptor in the spinal cord in humans and rats. Neuroreport. 2000;12:2311–2314. doi: 10.1097/00001756-200108080-00006. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Paszcuk AF, Quintão NLM, Fernandes ES, Juliano L, Chapman K, Andrade-Gordon P, et al. Mechanisms underlying the nociceptive and inflammatory responses induced by trypsin in the mouse paw. Eur J Pharmacol. 2008;581:204–215. doi: 10.1016/j.ejphar.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MN, Kelly D. Interleukin-1b induced-desArg9-bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr, Walther T, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrovski EF, Otuki MF, Regoli D, Bader M, Pesquero JB, Cabrini DA, et al. The non-peptide kinin receptor antagonists FR 173657 and SSR 240612: preclinical evidence for the treatment of skin inflammation. Regul Pept. 2009;152:67–72. doi: 10.1016/j.regpep.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Quintão NL, Passos GF, Medeiros R, Paszcuk AF, Motta FL, Pesquero JB, et al. Neuropathic pain-like behavior after brachial plexus avulsion in mice: the relevance of kinin B1 and B2 receptors. J Neurosci. 2008;28:2856–2863. doi: 10.1523/JNEUROSCI.4389-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulasingam K, Polosa R, Holgate ST, Howarth PH. Comparative nasal effects of bradykinin, kallidin and [Des-Arg9]-bradykinin in atopic rhinitic and normal volunteers. J Physiol. 1991;437:577–587. doi: 10.1113/jphysiol.1991.sp018612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153(Suppl. 1):S263–S282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NM, Boyce S, Webb JK, Williams AR, Carlson EJ, Hill RG, et al. Effects of the bradykinin B1 receptor antagonist des-Arg9[Leu8. bradykinin and genetic disruption of the B2 receptor on nociception in rats and mice. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, Mcalpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Lee JB, Kuraishi Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci. 2008;108:385–388. doi: 10.1254/jphs.08200sc. [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Ui1 H, Lee JB, Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J Pharmacol Sci. 2009;109:388–395. doi: 10.1254/jphs.08332fp. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol Ther. 2009;123:292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Ferazzini M, D'andrea MR, Buddenkotte J, Steinhoff M. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 2003;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- Wothersponn G, Winter J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci Lett. 2000;294:175–178. doi: 10.1016/s0304-3940(00)01561-5. [DOI] [PubMed] [Google Scholar]


