Abstract
Background and purpose:
Icariin may be the active ingredient in Herba Epimedii, a Chinese herb commonly used for treatment of osteoporosis. The present study aims to delineate the mechanism(s) by which icariin prevents bone loss after ovariectomy (OVX) in vivo and stimulates osteoblastic functions in vitro.
Experimental approach:
Ovariectomized or sham-operated C57BL/6 mice were treated with vehicle, 17β-oestradiol or icariin for 6 weeks. Total and trabecular bome mineral density (BMD) as well as polar stress-strain index of distal femur were measured by peripheral computed tomography. The mRNA expressions of OPG and RANKL in tibia were studied by RT-PCR. Interactions between the oestrogen receptor (ER) antagonist ICI182,780 and icariin were studied in UMR 106 cells. The functional transactivation of ERα and ERβ as well as ERα phosphorylation by icariin were also assessed.
Key results:
Icariin suppressed the loss of bone mass and strength in distal femur and increased the mRNA expression ratio of OPG/RANKL in tibia, following OVX. Icariin increased ER-dependent cell proliferation, alkaline phosphatase (ALP) activity, gene expression of OPG and the OPG/RANKL ratio in UMR 106 cells. Icariin did not activate ERE-luciferase activity in UMR 106 cells, via the ERα or the ERβ-mediated pathway, but it did increase ERα phosphorylation at Ser118.
Conclusions and implications:
Our results indicate that icariin exerts anabolic effects in bone possibly by activating ER in a ligand-independent manner. Its ability to prevent OVX-induced bone loss without inducing uterotrophic effects supports its use as an alternative regimen for management of postmenopausal osteoporosis.
Keywords: icariin, osteoprotegerin, RANKL, estrogen receptors, BMD
Introduction
Oestrogen or hormone replacement therapy (HRT) was the gold standard for the prevention of osteoporosis. However, the time of onset, duration, dose and regimen of HRT for optimal prevention of osteoporosis remain uncertain (Reginster and Devogelaer, 2006). Most importantly, recent findings of the Women's Health Initiative (WHI) and the Million Women Study (MWS) indicated HRT increased the risk of postmenopausal women to develop breast cancer, stroke, thrombosis and cardiovascular disease (Rossouw et al., 2002; Beral, 2003; Gambacciani et al., 2007). These findings have led to the advice that HRT should not be considered first-line therapy for the prevention of osteoporosis. As different side effects were reported to be associated with the use of other therapeutic agents, such as bisphosphonates, selective estrogen receptor modulators (SERMs), teriparatide and calcitonin (Papaioannou et al., 2007), alternative approaches for prevention or treatment of osteoporosis are worth exploring.
Herba Epimedii is one of the most frequently prescribed herbs in traditional Chinese medicine formula for treatment of osteoporosis in China. Our previous study indicated that extracts of Herba Epimedii could increase trabecular bone mineral density (BMD) in ovariectomized rats, stimulate osteoblastic cell proliferation and differentiation in UMR 106 cells. Such extracts also induced the expression of mRNA for osteoprotegrin (OPG, a soluble, decoy receptor that binds the receptor activator of nuclear factor-κB ligand (RANKL) and the ratio of OPG to RANKL [a membrane-bound tumour necrosis factor ligand family] expression in UMR 106 cells, suggesting that it could modulate the process of osteoclastogenesis (Xie et al., 2005).
Icariin, a marker flavonoid glycoside in Herba Epimedii, is believed to be the major active ingredient that accounts for its bone protective actions (Figure 1). A 24-month randomized, double-blind and placebo-controlled trial by Zhang et al. (2007) demonstrated that a preparation containing 60-mg icariin, 15-mg daidzein and 3-mg genistein could reduce bone loss in late postmenopausal women. A recent study by Nian et al. (2009) reported that treatment of ovariectomized rat with icariin could improve BMD and bone strength and prevent the suppression of serum Ca, phosphorus and 17β-oestradiol induced by ovariectomy (OVX). In addition, icariin was shown to increase cell proliferation and differentiation. Recent studies showed that icariin increased cell proliferation, differentiation, mineralization, osteocalcin secretion as well as expression levels of bone-related proteins in a dose-dependent manner in primary osteoblast cells (Huang et al., 2007). The increase in osteogenic differentiation in pre-osteoblastic MC3T3-E1 cells by icariin was found to be mediated through runt-related transcription factor 2 (Runx2) as well as bone morphogenetic protein (BMP) signalling pathways (Zhao et al., 2008). In addition, icariin could inhibit the formation and bone resorption activities of osteoclasts (Chen et al., 2007; Huang et al., 2007). These results suggest that icariin might prevent bone loss by stimulating bone formation and suppressing bone resorption. However, the understanding of its mechanism of action in bone remains incomplete.
Figure 1.
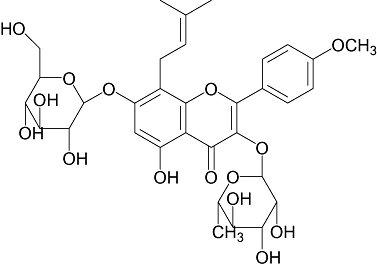
Chemical structure of icariin.
Recent reports indicated that phytoestrogens can attenuate bone loss associated with oestrogen deficiency in both animal and human studies (Dixon, 2004). Certain phytochemicals are being shown to possess differential affinity for and transactivation of oestrogen receptor (ER)α or ERβ (Gutendorf and Westendorf, 2001); nomenclature follows (Alexander et al., 2008). In the classical ER pathway, oestrogens bind to ERs directly to regulate the transcriptional activity of the activation function (AF-2) domain. The ER-ligand complex then interacts with a specific DNA sequence, the oestrogen response element (ERE), to mediate transcription of target genes. On the other hand, ERs can also be activated, independent of ligand binding, by phosphorylation at the activation function (AF-1) domain (Lees et al., 1989; Tora et al., 1989; Tzukerman et al., 1994). For example, epidermal growth factor (EGF) was shown to induce ERα phosphorylation at Ser 118 via the mitogen-activated protein kinase (MAPK) pathway (Chen et al., 2002). In addition, ginsenoside Rg1, the active ingredient in ginseng, exerts its oestrogenic actions in the absence of direct interaction with ER by activation of tyrosine kinase and MEK-mediated pathways, followed by MAPK-dependent phosphorylation of ERα at Ser 118, in human breast cancer cells (Lau et al., 2008; 2009;).
In the present study, we hypothesized that icariin would act as a phytoestrogen in preventing bone loss induced by oestrogen deficiency and promoting osteoblastic activities. We have studied its ability to prevent OVX-induced bone loss in C57BL/6 mice and its actions in rat osteoblast-like UMR 106 cells. In addition, its induction of functional transactivation of ERα and ERβ as well as ERα phosphorylation was also characterized.
Methods
Animal studies
Animal care and experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of The Hong Kong Polytechnic University. Female C57BL/6J mice were purchased from Laboratory Animal Services Centre (the Chinese University of Hong Kong, Hong Kong, China). Animals were randomly separated into four groups including sham+vehicle (sham, n= 8), OVX+vehicle (OVX, n= 8), OVX+17β-oestradiol (E2, n= 8) and OVX+icariin (icariin, n= 8). The animals (1-month-old) were either ovariectomized or sham-operated. After recovery from surgery for 18 days, they were given orally vehicle, 17β-oestradiol (4 µg·g−1·day−1) or icariin (0.3 mg·g−1·day−1) for 6 weeks. The dosages were chosen based on a previous study (Bao et al., 2005). Animals were fed with diet containing 0.6% calcium and 0.65% phosphorus (TD 98005, Teklad, Madison, WI, USA) throughout the course of the studies. Before the end of the experimental period, the mice were housed individually in a metabolic cage for collection of urine. On the day of killing, blood was collected from the orbital venous sinus of mice. The uterus was excised and the uterine index (uterus/body weight ratio) was calculated by normalizing the weight of uterus to the final body weight of mice. Samples of bone, including femur and tibia, were obtained for peripheral computed tomography (pQCT) analysis and the study of bone-specific mRNA expression.
Biochemical assays of serum and urine samples
Calcium concentration of serum and urine were determined by an o-cresolphthalein complexing method using a commercial kit (Wako Pure Chemical Industries Ltd., Osaka, Japan). Urinary calcium concentration was normalized to creatinine concentration and was expressed as the ratio of urinary calcium to creatinine (Ca/Cr). The inorganic phosphorus concentration in serum and urine were determined by a p-methylaminophenol reduction method using a commercial kit (Wako Pure Chemical Industries Ltd., Osaca, Japan). Urinary phosphorus concentration was normalized to creatinine concentration and was expressed as the phosphorus to creatinine ratio (P/Cr).
BMD analysis by pQCT
Peripheral computed tomography scanning was performed using XCT-2000 (StraTec Medizintechnik GmbH, Pforzheim, Germany). Femurs were placed on a plastic holder and oriented at the centre of the scanning area. Long axis of diaphysis was adjusted parallel to the scanning direction. The distal end (1.5 mm away from the apex) was scanned at a voxel size of 0.3 mm2. BMD (in mg·ccm−1) and polar stress-strain index (polar-SSI) (in mm3) of the distal femur were measured. The SSI is an index of torsional bone strength (Lind et al., 2001).
Real-time quantitative RT-PCR analysis
The femur was excised and immediately frozen in liquid nitrogen and stored at –80°C until required. The frozen femur was put in a RNase-free mortar and pestle which contained liquid nitrogen and ground to a fine powder immersed in liquid nitrogen. The frozen powder was transferred into a tube containing Trizol and total RNA was isolated, according to the manufacturer's protocol. Total RNA was reverse-transcribed in 20 µL of a reaction mixture that contained reverse transcription buffer, deoxynucleotide triphosphate mixture, random primers and MultiScribe™ reverse transcriptase, using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), at 25°C for 10 min, 37°C for 2 h and 85°C for 5 s. The sequences of the PCR primers for OPG, RANKL and the housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) are listed in Table 1. PCR was carried out in a 20 µL reaction mixture containing 10 µL iQ™ SYBR Green Supermix (Bio Rad Laboratories, Hercules, CA, USA) and 0.5 µL of cDNA template. The PCR was performed in an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems) using the following cycle parameters: one cycle of 95°C for 1 min, and 40 cycles of 95°C for 20 s, different Tm temperature for 20 s and 72°C 18 s. Upon completion, a melting curve was examined. Standard curves were generated using serially diluted solutions of cDNA derived from control sample. The target gene transcripts in each sample were normalized on the basis of its GAPDH.
Table 1.
Primers used for real-time RT-PCR
| Gene name | Primers |
|---|---|
| OPG mouse | F: TGAGTGTGAGGAAGGGCGTTA |
| R: CCATCTGGACATTTTTTGCAAA | |
| RANKL mouse | F: GCACACCTCACCATCAATGCT |
| R: GGTACCAAGAGGACAGAGTGACTTTA | |
| GAPDH mouse | F: GACCACAGTCCATGCCATCAC |
| R: GCTGTTGAAGTCGCAGGAGAC | |
| OPG rat | F: GACGAGATTGAGAGAACGAG |
| R: GGTGCTTGACTTTCTAGGTG | |
| RANKL rat | F: TCAGGAGTTCCAGCTATGAT |
| R: CCATCAGCTGAAGATAGTCC | |
| GAPDH rat | F: TACATTTTGCTGATGACTGG |
| R: TGAATGGTAGGAGCTTGACT |
GAPDH, glyceraldehydes-3-phosphate dehydrogenase; OPG, osteoprotegrin; RANKL, nuclear factor-κB ligand.
Culture of rat osteoblastic UMR 106 cells
UMR 106 cells (ATCC no. CRL-1661) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin 100 U·mL−1 and streptomycin 100 ug·mL−1 at 37°C in a humidified atmosphere of 95% air and 5% CO2. Medium was replaced every 3 days. At 80–90% confluence, cells were seeded in 96-well or 6-well plate at a density of 3 × 103 or 1 × 105 cells per well, respectively, for different assays. After 48 h, the medium was changed to phenol red-free DMEM supplemented with 1% dextran-charcoal-stripped serum (sFBS) for 24 h. Cells were then treated with icariin (10−12 M to 10−6 M), 17β-oestradiol (10−8 M) or vehicle in the presence or absence of ICI182,780 (10−6 M) for 48 h.
Cell proliferation assay
The MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was used as an indirect colorimetric measurement of cell proliferation. Briefly, after treatment with icariin (1 × 10−12 M to 1 × 10−6 M), 17β-oestradiol (10−8 M) or vehicle in the presence or absence of ICI182,780 (1 × 10−6 M) for 48 h, the medium was discarded and replaced with 100 µL of MTS/PMS solution (Promega, Madison, WI, USA). After incubation at 37°C for 1 h, an absorbance at 490 nm was measured on a microplate reader (Bio-Rad Laboratories Inc., CA, USA).
Alkaline phosphatase (ALP) activity assay
Cells were harvested after treatment and lysed with 100 µL Nonidet P-40 lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40) supplemented with protease inhibitors (2 ug·mL−1 aprotinin, 2 ug·mL−1 leupeptin and 1 mM PMSF) by incubating on ice for 20 min. The supernatant, centrifuged at 12 000×g and 4°C for 5 min, was stored at –80°C until analysis. Intracellular ALP activity was determined using a method described previously (Chen and Wong, 2006). Briefly, the sample was mixed with 1 mL ALP reagent (Stanbio Laboratory, Boerne, TX, USA) and the absorbance change at 405 nm in 3 min was recorded. According to the manufacturer's instruction, ALP activity was calculated as: ALP (U·L−1) = (total volume/sample volume) × (absorbance change in 3 min/0.01875). To normalize the result, Bradford protein assay was carried out and ALP activity was expressed as units of activity (U)·L−1·(µg protein)−1.
Gene expression of OPG and RANKL
The cells were plated in 6-well plates and treated with icariin (10−12 M to 10−6 M), 17β-oestradiol (10−8 M) or vehicle for 48 h. Total RNA was isolated from the cell layer and the gene expression of OPG, RANKL and GAPDH assessed as described above. The sequences of the PCR primers for OPG, RANKL and GAPDH are listed in Table 1.
Transient transfection and ER-mediated luciferase activity assay
Cells were seeded in a 12-well plate at a density of 65 000 cells per well and cultured in phenol red-free DMEM supplemented with 1% dextran-charcoal-stripped serum for 48 h. The cells were transfected by Lipofectamine™ 2000 reagent. ER-α, ER-β and ERE-containing luciferase reporter plasmid vERETkluc were kindly provided by Dr. Vincent Giguere (McGill University, Montreal, Quebec, Canada). 0.4 µg ER-α or ER-β plasmid, 0.4 µg vERETkluc, together with 0.1 µg internal control reporter plasmid pRL-TK, a Renilla luciferase control vector, was cotransfected into the cells in triplicate. Five hours after transfection, cells were treated with vehicle, ICI182,780 (10−8 M), 17β-oestradiol (10−8 M) or icariin (10−8 M) for 24 h. After treatment, the cells were lysed and the luciferase activity was measured using the Dual Luciferase Reporter assay System and the signal was detected by TD-20/20 Luminometer (Turner Design, Sunnyvale, CA, USA). The oestrogen promoter activity was expressed as firefly luciferase values normalized to pRL-TK Renilla luciferase values.
Immunoblotting
Treated cells were harvested and lysed with Nonidet P-40 lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40) supplemented with protease inhibitors (2 ug·mL−1 aprotinin, 2 ug·mL−1 leupeptin and 1 mM PMSF) and phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM NaF). Protein concentrations were determined using the Bradford assay. Equal amounts of proteins were separated by SDS-PAGE on a 10% reducing gel at a constant voltage (200 V) for about 1 h, and transblotted onto PVDF membranes (Immobilin-P, Millipore Corp., Danvers, MA, USA). Immuno-detection was performed after blocking the non-specific binding sites on the membrane with 5% skimmed milk. The blots was probed with monoclonal rabbit anti-human phospho-ERα at Ser 118 (1:2000) or anti-human ERα (1:3000), and followed by incubation with goat anti-rabbit conjugated with horseradish peroxidase (1:2000). The antigen-antibody complexes were then detected with enhanced chemiluminescence (ECL) reagent and visualized by the Lumi-Imager using Lumi Analyst version 3.10 software (Roche, Mannheim, Germany).
Statistical analysis
The in vivo data were analysed by one-way anova and the in vitro data were analysed by the non-paired Student's t-test between control group and each treatment group. The GraphPad Prism version 4.4 software was used. Results are reported as means ± SEM. A P-value <0.05 was considered statistically significant.
Materials
All reagents for cell culture, RT-PCR and plasmid transient transfection kit were purchased from Life Technologies Inc. (Carlsbad, CA, USA) unless otherwise indicated. pRL-TK plasmid and Dual Luciferase Reporter Assay System were from Promega (Madison, WI, USA). Icariin was purchased from LKT Laboratories Inc. (St. Paul, MN, USA). 17β-Oestradiol was purchased from Sigma-Aldrich (St. Louis, MO, USA). ICI182,780 was purchased from Tocris Cookson Ltd. (Avonmouth, Bristol, UK). Antibodies directed against ER and horseradish peroxidase-conjugated anti-rabbit IgG antibody were from Santa Cruz biotechnology (CA, USA). Antibody against phospho-ERα (Ser 118) was purchased from Upstate (Millipore). ECL detection reagents were obtained from Pierce (Rockford, IL, USA). Primers were obtained from Tech Dragon Limited (Hong Kong, China).
Results
Effects of icariin on body weight, uterine index and biochemical parameters in OVX mice
The OVX-induced increase in weight gain in mice was prevented by treatment with 17β-oestradiol, but not by icariin (Table 2). To assess any trophic effects on uterus exerted by 17β-oestradiol or icariin, the uterus to body weight ratio was determined. The uterine index was significantly reduced in OVX mice, suggesting that the surgery was successful. In contrast to 17β-oestradiol, icariin did not increase uterus index in OVX mice (Table 2). Serum Ca levels were not altered by OVX or treatment with 17β-oestradiol or icariin, while serum phosphorus (P) levels were significantly increased by icariin in OVX mice (P < 0.05). Urinary Ca excretion was suppressed in OVX mice treated with 17β-oestradiol (vs. OVX, P < 0.05) and with icariin (vs. OVX, P < 0.01) while urinary P excretion was suppressed in OVX mice in response to treatment with icariin (vs. sham, P < 0.05).
Table 2.
Effects of 17β-oestradiol (E2) and icariin on body weight, uterine index and biochemical parameters in ovariectomized mice
| Weight gain (% of change) | Uterus index (mg·g−1) | Serum Ca (mg·L−1) | Serum P (mg·L−1) | Urinary Ca/Cr (mg·mg−1) | Urinary P/Cr (mg·mg−1) | |
|---|---|---|---|---|---|---|
| Sham | 3.46 ± 0.93 | 1.69 ± 0.11 | 77.5 ± 1.4 | 63.1 ± 1.2 | 0.74 ± 0.05 | 11.32 ± 0.40 |
| OVX | 7.94 ± 0.59** | 0.36 ± 0.06*** | 77.6 ± 1.0 | 58.9 ± 1.7 | 0.93 ± 0.09 | 9.23 ± 0.90 |
| E2 | 2.41 ± 0.08∧∧∧ | 2.69 ± 0.18***∧∧∧ | 77.4 ± 1.2 | 63.7 ± 3.2 | 0.67 ± 0.04∧ | 9.82 ± 0.58 |
| Icariin | 5.23 ± 1.15 | 0.43 ± 0.04*** | 72.3 ± 1.6 | 69.6 ± 3.7∧ | 0.59 ± 0.03∧∧ | 8.66 ± 0.55* |
P < 0.05,
P < 0.01,
P < 0.001 versus sham;
P < 0.05,
P < 0.01,
P < 0.001 versus OVX.
Results are mean ± SEM, n= 7–8 mice per group.
OVX, ovariectomy.
Effects of icariin on total and trabecular BMD of distal femur and bone strength in ovariectomized mice
As shown in Table 3, OVX decreased total BMD (–15%), trabecular BMD (–16%) and polar SSI (–61%) in distal femur of mice (vs. sham, P < 0.01). Treatment of OVX mice with 17β-oestradiol or icariin significantly reversed the OVX-induced changes in all three measures (total BMD, trabecular BMD and polar SSI) in distal femurs. These results indicated that the effects of icariin on bone were similar to those of 17β-oestradiol and thus icariin could restore the bone loss in OVX mice, induced by oestrogen deficiency.
Table 3.
Effects of 17β-oestradiol (E2) and icariin on bone mineral density and bone strength at distal femur in ovariectomized mice
| Total BMD (mg·ccm−1) | Trabecular BMD (mg·ccm−1) | Polar SSI (mm3) | |
|---|---|---|---|
| Sham | 462 ± 11 | 461 ± 14 | 0.83 ± 0.11 |
| OVX | 393 ± 8*** | 386 ± 10** | 0.32 ± 0.05** |
| E2 | 486 ± 17∧∧∧ | 494 ± 23∧∧∧ | 0.71 ± 0.06∧ |
| Icariin | 454 ± 7∧∧ | 443 ± 6.39∧ | 0.64 ± 0.04∧∧ |
P < 0.01,
P < 0.001 versus sham;
P < 0.05,
P < 0.01,
P < 0.01 versus OVX.
Results are mean ± SEM, n= 7–8 mice per group.
BMD, bone mineral density; OVX, ovariectomy.
Effects of icariin on expression of mRNA for OPG and RANKL in femur
Osteoprotegrin and RANKL are identified as the dominant and final mediators of osteoclastogenesis (Bord et al., 2003). The secretion of OPG by osteoblastic cells could block the interaction of RANKL with its functional receptor RANK expressed on the osteoclastic cell surface, thereby inhibiting osteoclastogenesis. Treatment of OVX mice with 17β-oestradiol or icariin significantly decreased RANKL mRNA expression (Figure 2B), but did not significantly affect OPG mRNA expression (Figure 2A). Both 17β-oestradiol and icariin significantly increased the ratio of OPG/RANKL in the femur of OVX mice (Figure 2C). This result suggested that icariin might modulate the process of osteoclastogenesis via its actions on RANKL expression in OVX mice.
Figure 2.
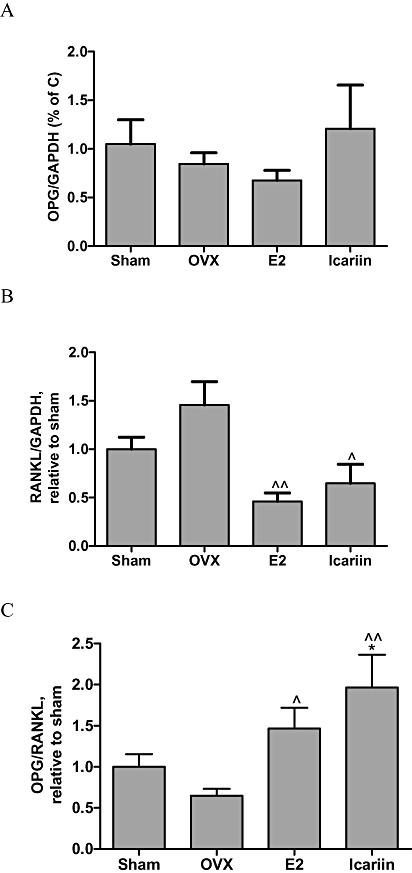
Effects of icariin on OPG and RANKL mRNA expressions in femur from OVX mice, treated with vehicle, 17β-oestradiol (E2, 4 µg·g−1·day−1) or icariin (0.3 mg·g−1·day−1) for 6 weeks. Total RNA was isolated and real-time RT-PCR was performed to determine the mRNA expressions of (A) OPG, (B) RANKL and (C) OPG/RANKL, which were normalized to that of GAPDH. Results were expressed as mean ± SEM. *P < 0.05 versus sham; ∧P < 0.05; ∧∧P < 0.01 versus OVX, n= 5–8. GAPDH, glyceraldehydes-3-phosphate dehydrogenase; OPG, osteoprotegrin; OVX, ovariectomy; RANKL, nuclear factor-κB ligand.
Effects of icariin on cell proliferation and ALP activity in UMR 106 cells
Icariin (10−10 M to 10−6 M) significantly increased cell proliferation by approximately 1.36 to 1.51-fold in UMR 106 cells (P < 0.05), as did 17β-oestradiol (10−8 M) (P < 0.01; Figure 3A). ALP activity in UMR 106 cells was increased by 17β-oestradiol by 1.2-fold (P < 0.001) (Figure 3B), as did icariin (10−14 to 10−8 M) (P < 0.01). These results indicated that icariin could induce osteoblastic cell proliferation and differentiation in a dose-dependent manner.
Figure 3.
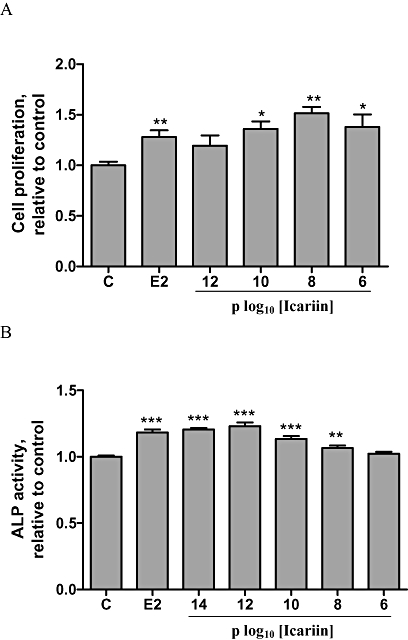
Effects of icariin on cell proliferation and alkaline phosphatase (ALP) activity in UMR 106 cells. (A) UMR 106 cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (10−12–10−6 M) for 48 h. Cell proliferation rate was assessed by the MTS assay. Results were obtained from three independent experiments and were expressed as mean ± SEM. *P < 0.05; **P < 0.01 versus control (C). (B) UMR 106 cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (10−14–10−6 M) for 48 h. The lysates were used for analysis of ALP activity. Results were obtained from three independent experiments in triplicate and were expressed as mean ± SEM. **P < 0.01; ***P < 0.001 versus control (C).
Effects of icariin on OPG and RANKL mRNA expressions in UMR 106 cells
As shown in Figure 4, 17β-oestradiol induced a 2.5-fold increase in OPG mRNA expression (P < 0.001) (Figure 4A) but did not significantly increase RANKL mRNA expression in UMR 106 cells (Figure 4B). In contrast, icariin not only significantly increased OPG mRNA expression in UMR 106 cells at all concentrations (10−12 to 10−6 M) tested (Figure 4A, P < 0.001), but also significantly increased RANKL mRNA expression in UMR 106 cells at 10−10 M to 10−6 M (Figure 4B, P < 0.05). The overall effects of icariin or 17β-oestradiol on the ratio of OPG/RANKL mRNA expression in UMR 106 cells are shown in Figure 4C. The results clearly indicated that icariin, at all concentrations tested, or 17β-oestradiol (10−8 M) significantly increased the OPG/RANKL ratio in UMR 106 cells (P < 0.001), suggesting that icariin might modulate the process of osteoclastogenesis via its actions on OPG and RANKL expression.
Figure 4.
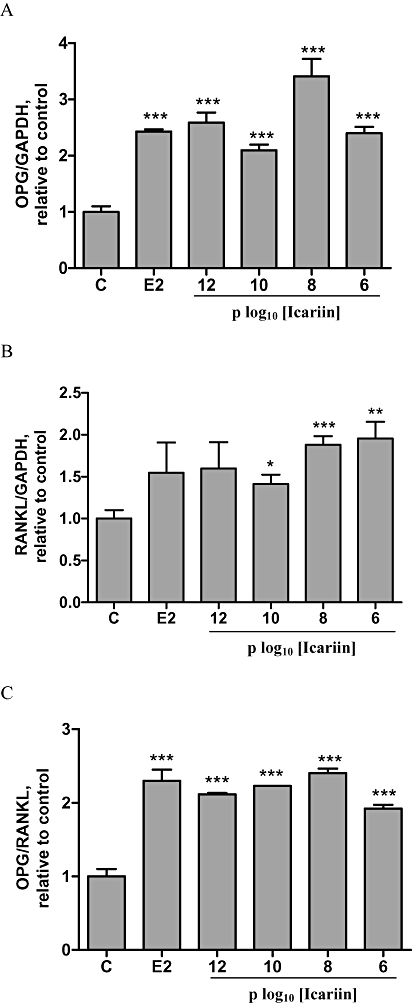
Effects of icariin on OPG and RANKL mRNA expressions in UMR 106 cells. UMR 106 cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (10−12–10−6 M) for 48 h. Total RNA was isolated and real-time RT-PCR was performed to determine the mRNA expressions of (A) OPG, (B) RANKL and (C) OPG/RANKL, which were normalized with that of GAPDH. Results were obtained from two independent experiments in triplicate and expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus control (C). GAPDH, glyceraldehydes-3-phosphate dehydrogenase; OPG, osteoprotegrin; RANKL, nuclear factor-κB ligand.
Effects of ICI182,780 on icariin-induced cell proliferation, ALP activity and OPG/RANKL gene expression in UMR 106 cells
To determine if the stimulatory effects of icariin on cell proliferation, ALP activity, OPG and RANKL gene expression were dependent on the ERs, UMR 106 cells were co-incubated with 1 µM ICI182,780, a specific ER antagonist, for 48 h. As shown in Figure 5A, 10−8 M icariin or 17β-oestradiol significantly induced UMR 106 cell proliferation (P < 0.01) and their stimulatory effects were abolished by co-treatment with ICI182,780. Similarly, the stimulatory effects of 10−8 M icariin or 17β-oestradiol on ALP activities (Figure 5B) as well as on the ratio of OPG/RANKL (Figure 5C) were abolished by co-treatment with ICI182,780. These results suggest that the stimulatory effects of icariin on osteoblastic functions were ER-dependent.
Figure 5.
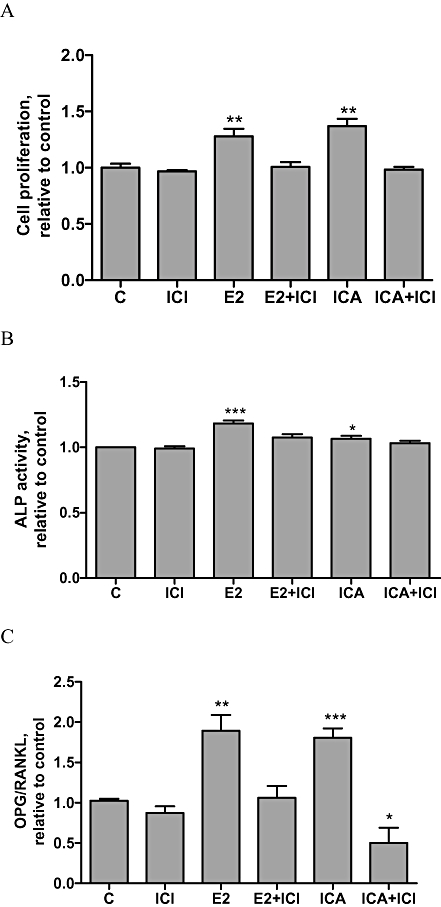
Effects of ICI182,780 on icariin induced cell proliferation, ALP activities and OPG/RANKL expression in UMR 106 cells. UMR 106 cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (ICA, 10−8 M) in the presence or absence of ICI182,780 (ICI) for 48 h. A. Cell proliferation rate was assessed by the MTS assay. Results were obtained from three independent experiments and were expressed as mean ± SEM. **P < 0.01 versus control (C). B. Cell lysates were used for ALP activity measurement. Results were obtained from three independent experiments in triplicate and were expressed as mean ± SEM. *P < 0.05; ***P < 0.001 versus control (C). C. OPG/RANKL ratio. Results were obtained from two independent experiments in triplicate and expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus control (C). ALP, Alkaline phosphatase; OPG, osteoprotegrin; RANKL, nuclear factor-κB ligand.
Effects of icariin on ERα or ERβ-mediated, ERE-dependent, luciferase activities in UMR 106 cells
To determine if icariin could activate ERE-dependent transcriptional activities in UMR 106 cells via ERα or ERβ, UM R 106 cells transiently co-transfected with ERα or ERβ and ERE-luciferase reporter plasmids were treated with either 10−8 M icariin or 17β-oestradiol. At this concentration, 17β-oestradiol significantly increased the ERE-dependent luciferase activities via ERα (Figure 6A) or ERβ (Figure 6B) in UMR106 cells. In contrast, 10−8 M icariin was unable to induce ERE-dependent luciferase activities via ERs (Figure 6A,B). The results indicated that this concentration of icariin did not induce ER-dependent bone anabolic actions via the activation of ERE-dependent transcription in UMR 106 cells.
Figure 6.
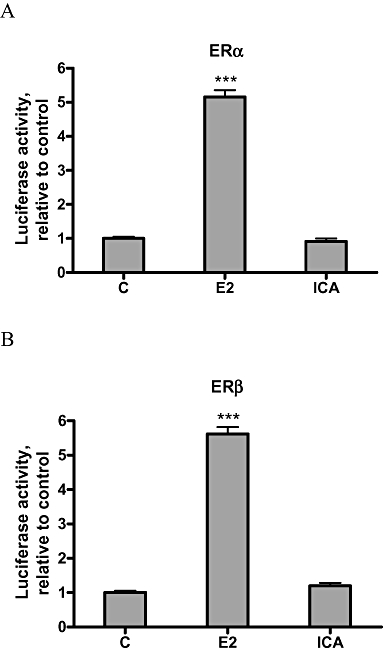
Effects of icariin on ERα or ERβ-mediated ERE-dependent luciferase activity in UMR 106 cells. Cells were co-transfected with 0.4 µg ERα or ERβ plasmid, 0.4 µg ERE-containing luciferase reporter plasmid and 0.1 µg pRL-TK luciferase internal reporter plasmid using the Lipofectamine™ 2000 reagent according to the manufacture's instructions. Transfected cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (ICA; 10−8 M) for 24 h. Activities of luciferase encoded by experimental and internal control plasmid were measured sequentially with the DLR assay reagents. The ERE firefly luciferase activities were normalized for pRL-TK Renilla luciferase values. 100% represents the ERE luciferase activity of the control. Results were obtained from three independent experiments and expressed as mean ± SEM. ***P < 0.001 versus control (C). ER, oestrogen receptor; ERE, oestrogen response element.
Effects of icariin on ERα phosphorylation at Ser 118 in UMR 106 cells
As icariin was unable to activate ERE-dependent transcription via ERα or ERβ, we hypothesized that it might activate ER in a ligand-independent manner. As shown in Figure 7A, treatment of UMR 106 cells with 10−8 M icariin or 17β-oestradiol for 24 h increased ERα phosphorylation at Ser 118, a major site for ligand-independent activation of ERα. Icariin or 17β-oestradiol also significantly increased the ratio of phospho-ERα to ERα (pERα/ERα) expression in UMR 106 cells by 74% and 53%, respectively (Figure 7B), suggesting that icariin could activate ERα by phosphorylation.
Figure 7.
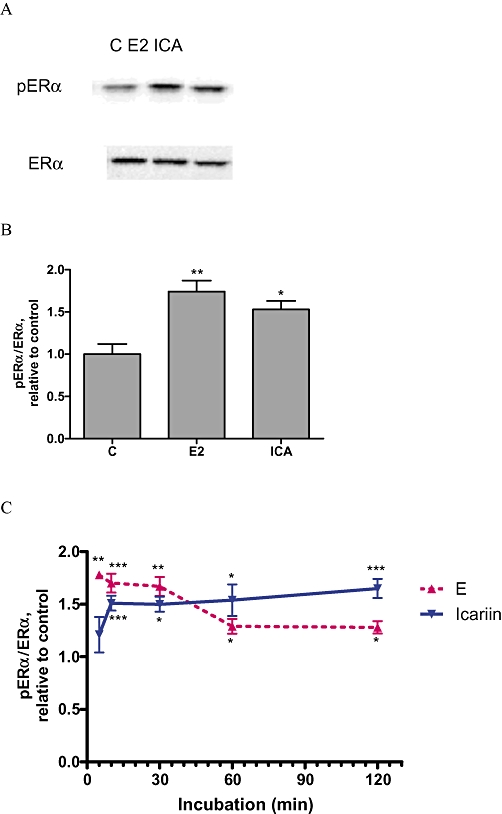
Effects of icariin on phosphorylation of ERα at Ser 118 in UMR 106 cells. (A and B) Cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (ICA; 10−8 M) for 24 h. Proteins extracted from cell lysates were transblotted onto a membrane and probed with anti-phospho-ERα at serine 118 residue (pERα) and anti-ERα (ERα) primary antibodies followed by the corresponding secondary antibodies. Relative intensity of chemiluminescence was measured and phospho-ERα to ERα ratio was calculated. Protein blots of pERα and ERα (A) and a graphical representation of the ratio pERα/ERα (B) were shown. C. The time course of ERα phosphorylation induced by treatment with 10−8 M icariin. Cells were treated with vehicle (C), 17β-oestradiol (E2; 10−8 M) or icariin (ICA; 10−8 M) for. 5 min, 10 min, 30 min, 1 h and 2 h. Results were obtained from three independent experiments and expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus control (C). ER, oestrogen receptor.
To determine the time course of activation of ERα by phosphorylation in response to short-term treatment with icariin, the relative degree of ER phosphorylation at Ser 118 was measured. As shown in Figure 7C, 17β-oestradiol significantly increased pERα/ERα by about 70% within 10 min (P < 0.001), the activation dropped sharply at 30 min and remained up-regulated for the course of treatment in UMR 106 cells. Similarly, icariin was able to significantly increase pERα/ERα at 10 min (P < 0.001) and the stimulation was sustained throughout 120 min of incubation in UMR 106 cells (Figure 7C).
Discussion
The present study systematically evaluated the osteoprotective effects and mechanism of actions of icariin in ovariectomized mice and in rat osteoblast-like UMR 106 cells. Our results clearly demonstrated that icariin suppressed OVX-induced increase in urinary Ca excretion, loss in bone mass and bone strength, as well as increasing the expression ratio of OPG/RANKL mRNA in OVX mice. In addition, our study showed that icariin mimicked 17β-oestradiol in stimulating cell proliferation, ALP activity and OPG/RANKL mRNA expression via ER in UMR 106 cells, suggesting that it could exert oestrogen-like effects in promoting osteoblastic functions and inhibiting osteoclastogenesis.
Our study demonstrated treatment of OVX mice with icariin at 0.3 mg·g−1·day−1 for 6 weeks significantly increased total BMD and trabecular BMD of the distal femur. These results are in agreement with our previous animal study (Xie et al., 2005) as well as a recently reported clinical study by Zhang et al. (2007). In our previous study, treatment of OVX rats with an extract of Herba Epimedii containing icariin for 3 months suppressed serum ALP levels and urinary deoxypyridinoline levels, increased tibial trabecular bone area and decreased trabecular separation at the proximal tibial metaphysis (Xie et al., 2005), suggesting that icariin was effective in preventing bone loss induced by OVX. Zhang et al. (2007) showed that daily oral administration of a preparation containing 60 mg icariin, 15 mg daidzein and 3 mg genistein, to late postmenopausal women for 24 months significantly increased BMD at the neck of the femur and in lumbar spine. The increase in BMD in the icariin treated group in their study was accompanied by the suppression of urinary deoxypyridinoline levels. These studies suggest that the bone-protective action of icariin is likely to be mediated by the suppression of the bone resorption process. These results were also in agreement with previously reported studies in which icariin suppressed osteoclastic differentiation and activities in vitro (Huang et al., 2007) and inhibited osteoclast formation induced by RANKL and macrophage-colony sitmulating factor in mouse bone marrow cultures (Chen et al., 2007). Using pQCT analysis, our study also demonstrated that icariin increased polar SSI in OVX mice. As polar SSI is a measure of torsional bone strength (Lind et al., 2001), the results indicated that icariin increased not only BMD but also bone strength, of distal femur in OVX mice.
The suppression of OVX-induced increase in urinary Ca level by icariin was found to be greater than that in mice treated with oestradiol, in the present study. An increase in urinary Ca excretion in OVX mice reflects the combined effects of an increase in bone resorption and a decrease in urinary Ca reabsorption induced by oestrogen deficiency. Thus, our results indicated that the actions of icariin might mimic oestrogen in suppressing OVX-induced increase in bone resorption and inducing oestrogen-dependent Ca reabsorption. Further study will be needed to clarify the possible additional effects of icariin on bones and renal Ca transport that contribute to the conservation of bone mass in OVX mice.
Our study alsoshowed that icariin exerted bone protective effects in OVX mice without exerting a trophic effect on of the uterus. These findings were also in agreement with previous studies (Xie et al., 2005; Zhang et al., 2007). The latter study showed that the endometrial thickness in late postmenopausal women were not affected by treatment with an icariin-containing preparation for 24 months. These results indicate that icariin is able to exert selective effects on bone without exerting unwanted oestrogenic effects on the uterus.
The direct effects of icariin on osteoblastic cell proliferation and differentiation have been reported by others in primary cultures of rat calvaria-derived osteoblasts (Huang et al., 2007) and mouse osteoblasts (Zhao et al., 2008), pre-osteoblastic MC3T3-E1 cells (Chen et al., 2007) as well as human osteoblasts derived from human marrow mesenchymal stem cells (Yin et al., 2007). These studies suggest that icariin might prevent bone loss by modulating the process of bone remodelling. The induction of osteogenic differentiation in pre-osteoblastic MC3T3-E1 cell by icariin was found to be mediated by Runx 2 as well as BMP signalling pathways (Zhao et al., 2008). Moreover, icariin inhibition of osteoclast formation induced by RANK and macrophage-colony stimulating factor in mouse bone marrow culture was associated with the down-regulation of tartrate-resistant acid phosphatase, RANK and calcitonin receptors (Chen et al., 2007). Icariin enhanced the differentiation and proliferation of primary osteoblasts by increasing the mRNA expression of ALP, osteoclacin, COL-1 and OPG and decreasing the mRNA expression of RANKL. In addition, icariin decreased superoxide generation and actin ring formation, required for osteoclast survival and bone resorption activity (Huang et al., 2007). Icariin stimulated angiogenesis by activating the MEK/ERK and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells (Chung et al., 2008). However, the signalling pathway involved in the bone protective effects of icariin is not very clear.
The results of the present study confirmed the stimulation by icariin of proliferation and ALP activities, in a dose-dependent manner, in rat osteoblast-like UMR 106 cells. These results are also in agreement with those reported in our previous study in which Herba Epimedii extracts stimulated osteoblastic cell proliferation and differentiation (Xie et al., 2005). In addition, our present results showed that icariin stimulated OPG and RANKL mRNA expression in UMR 106 cells, dose-dependently. As its stimulatory effects on OPG mRNA expression were stronger than those on RANKL mRNA expression, the effects of icariin at 10−12 M to 10−6 M on the OPG/RANKL ratio were also stimulatory (Figure 4C). These results suggest that icariin might mimic oestrogen to suppress the process of osteoclastogenesis through its direct actions on modulating the expression of OPG and RANKL in osteoblastic cells.
The ratios of OPG/RANKL mRNA expression in femur were also found to be altered by in vivo administration of icariin or 17β-oestradiol in OVX mice in the present study. Both icariin and 17β-oestradiol significantly increased in the ratio of OPG/RANKL by suppression of RANKL mRNA. It is of interest to note that OPG mRNA expression was not significantly induced in OVX mice in response to 6 weeks of treatment with 17β-oestradiol or icariin. In contrast, 17β-oestradiol or icariin treatment significantly decreased the RANKL mRNA expression in femur.
As the in vivo and in vitro bone protective effects of icariin mimicked those of 17β-oestradiol, we hypothesized that the actions of icariin were mediated through the activation of ERs. We found that the stimulatory effects of icariin on cell proliferation, differentiation as well as OPG/RANKL ratio in UMR 106 cells could be abolished by co-treatment with ICI182,780, providing evidence for the involvement of the ERs in mediating the actions of icariin. However, the transfection study indicated that icariin failed to induce ERE-dependent luciferase activities via either ERα nor ERβ in UMR 106 cells, suggesting that the actions of icariin might be different from those of 17β-oestradiol. Recent studies (Klein-Hitpass et al., 1986; Webb et al. (1995; Safe (2001; Levy et al. (2008 indicated that ERE is not the only regulatory element of genes to be regulated by ERs and only about one-third of oestrogen-responsive genes contain ERE sequences. Alternative elements such as activator protein 1 (AP-1) (Webb et al., 1995) and Sp1 (Safe, 2001) are also found to be essential for 17β-oestradiol and SERMs to regulate the full spectrum of genes. Thus, the results of the present study indicated that the ER-dependent actions of icariin in UMR 106 cells might be ERE-independent.
Recent studies have shown that ERs could be stimulated in the absence of ligand binding, by modulating different signal transduction pathways such as the mitogen-activated protein kinase (MAPK)-mediated pathways (Kato et al., 1995; Bunone et al., 1996; Lau et al., 2008). The activation of unliganded ERα via MAPK cascade resulted in ERα phosphorylation at several serine residues -Ser 118, Ser 104 Ser 167 – within the AF-1 domain of ERα, which in turns modulates the transcriptional activity (Chen et al., 2002). To determine if icariin activate ER ligand-independently, the ratio of phosphorylated ERα (Ser118) to total ERα expression in UMR 106 cells in response to icariin treatment was measured. ER phosphorylation at Ser118 was chosen as this serine, Ser118, is a highly conserved residue and represents the major site of phosphorylation in response to agents such as EGF (Chen et al., 2002) and ginsenoside Rg1 (Lau et al., 2008; 2009;). Our results clearly indicated that icariin activated ERα phosphorylation in UMR 106 cells within 10 min of incubation, suggesting that icariin could rapidly activate ER in osteoblastic cells, in a ligand-independent manner.
Our in vitro study indicated that the oestrogen-like activities of icariin in UMR 106 cells were ERE-independent and might be mediated through ligand-independent activation of ER. Kousteni et al. (2002) suggested that ligands activating kinase-mediated actions of ER could reverse the loss of bone mass and strength in OVX mice without significant stimulatory effects on reproductive organs and that kinase-mediated actions of the ER are important for inducing osteoblast differentiation (Kousteni et al., 2007). The fact that icariin could reverse bone loss in OVX mice without uterotrophic effects suggest that it might induce osteoblast differentiation via such kinase-mediated actions of ER. Furher studies will be needed to determine if icariin could activate osteoblastic differentiation via kinase-mediated actions of ERs.
Icariin is an active ingredient identified in Herba Epimedii, a Chinese herb commonly used for treatment of osteoporosis in classical Chinese medicine formulation. The present study clearly demonstrated that this compound protected against bone loss associated with oestrogen deficiency, without exerting uterotrophic effects, directly stimulated ER-dependent osteoblastic functions and might modulate osteoclastogenesis via the regulation of OPG and RANKL mRNA expression in bone cells. Mechanistic studies indicated that the oestrogenic effects of icariin on osteoblastic cells were ERE-independent and involved activation of ER by rapid phosphorylation. Our study provides the evidence to support the use of icariin as an effective candidate for management of postmenopausal osteoporosis. Further studies will be needed to elucidate the mechanism(s) by which icariin activates ERs, as well as other ER-independent pathways in bone cells.
Acknowledgments
This work was supported by the Areas of Excellence Scheme Established under the University Grants Committee of the Hong Kong Special Administrative Region, Chins (AOE/P-10/01), the Hong Kong Polytechnic University Central Research Fund (GYE-47) and the Earmarked Research Grant Council (PolyU 5402/04M, N_PolyU536/04), HKSAR. We thank the support of the State Key Laboratory of Chinese Medicine and Molecular Pharmacology, HKSAR.
Glossary
Abbreviations:
- ALP
alkaline phosphatase
- AP-1
activator protein 1
- BMD
bone mineral density
- BMP
bone morphogenetic protein
- ER
oestrogen receptor
- ERE
oestrogen response element
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- HRT
hormone replacement therapy
- MAPK
mitogen-activated protein kinase
- MWS
Million Women Study
- OPG
osteoprotegrin
- OVX
ovariectomy
- polar-SSI
polar stress-strain index
- RANKL
nuclear factor-κB ligand
- Runx2
runt-related transcription factor 2
- SERMs
selective estrogen receptor modulators
- sFBS
charcoal-stripped fetal bovine serum
- WHI
Women's Health Initiative
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao JR, Wang JW, Li SF, Zhao W, Zhang Q, Yan Y. Effects of icariin on ovariectomized osteoporotic rats. Wei Sheng Yan Jiu. 2005;34:191–193. [PubMed] [Google Scholar]
- Beral V, Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Washbrook E, Sarwar N, Gaynor JB, Pace PE, Thirunuvakkarasu V, et al. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- Chen KM, Ge BF, Liu XY, Ma PH, Lu MB, Bai MH, et al. Icariin inhibits the osteoclast formation induced by RANKL and macrophage-colony stimulating factor in mouse bone marrow culture. Pharmazie. 2007;62:388–391. [PubMed] [Google Scholar]
- Chen WF, Wong MS. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Br J Nutr. 2006;95:1039–1047. doi: 10.1079/bjn20061735. [DOI] [PubMed] [Google Scholar]
- Chung BH, Kim JD, Kim CK, Kim JH, Won MH, Lee HS, et al. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun. 2008;14(376) doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Dixon RA. Phytoestrogens. Annu Rev Plant Biol. 2004;55:225–261. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Genazzani AR. The HRT misuse and osteoporosis epidemic: a possible future scenario. Climacteric. 2007;10:273–275. doi: 10.1080/13697130701511277. [DOI] [PubMed] [Google Scholar]
- Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- Huang J, Yuan L, Wang X, Zhang TL, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–840. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU. An estrogen-responsive element derived from the 5′ flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986;46:1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien C, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC. Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol. 2007;27:1516–1530. doi: 10.1128/MCB.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WS, Chan RYK, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERα pathway. J Steriod Biochem Mol Biol. 2008;108:64–71. doi: 10.1016/j.jsbmb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lau WS, Chen WF, Chan RYK, Guo DA, Wong MS. Involvement of Mitogen-Activated Protein Kinase Pathway (MAPK) in the estrogen-like action of ginsenoside Rg1 in human breast cancer (MCF-7) cells. Brit J Pharm. 2009;156:1136–1146. doi: 10.1111/j.1476-5381.2009.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JA, Fawell SE, Parker MG. Identification of two transactivation domains in the mouse oestrogen receptor. Nuclei Acids Res. 1989;17:5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Tatomer D, Herber CB, Zhao X, Tang H, Sargeant T, et al. Differential regulation of native estrogen receptor-regulatory elements by estradiolestradiol, tamoxifen, and raloxifene. Mol Endocrinol. 2008;22:287–303. doi: 10.1210/me.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Lind L, Larsson S, Orberg J. Torsional testing and peripheral quantitative computed tomography in rat humerus. Bone. 2001;29:265–270. doi: 10.1016/s8756-3282(01)00576-2. [DOI] [PubMed] [Google Scholar]
- Nian H, Ma MH, Nian SS, Xu LL. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine. 2009;16:320–326. doi: 10.1016/j.phymed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging. 2007;24:37–55. doi: 10.2165/00002512-200724010-00003. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Devogelaer JP. Raloxifene reduces fractures in postmenopausal women with osteoporosis. Clin Orthop Relat Res. 2006;443:48–54. doi: 10.1097/01.blo.0000200234.99436.24. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Tora L, White J, Bron C, Tasset D, Webster N, Scheer E. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tzukerman MT, Esty A, Santiso-Mere D, Danielian P, Parker MG, Stein RB. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY, Yao XS, et al. The osteoprotective effect of Herba Epimedii (HEP) extract in vivo and in vitro. Evidence Based Comp Alt Med. 2005;2:353–361. doi: 10.1093/ecam/neh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Chen Z, Liu Z, Ma Q, Dang G. Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chin Med J. 2007;120:204–210. [PubMed] [Google Scholar]
- Zhang G, Qin L, Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res. 2007;22:1072–1079. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ohba S, Shinkai M, Chung U, Nagamune T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Comm. 2008;369:444–448. doi: 10.1016/j.bbrc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Safe S. Transcriptional activation of genes by 17β-estradiol through estrogen receptor-SP1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]


